Abstract
Objective:
This study assessed the relationship between electrophysiological measures of the electrically evoked compound action potential (eCAP) and speech perception scores measured in quiet and in noise in post-lingually deafened adult cochlear implant (CI) users. It tested the hypothesis that how well the auditory nerve (AN) responds to electrical stimulation is important for speech perception with a CI in challenging listening conditions.
Design:
Study participants included 24 post-lingually deafened adult CI users. All participants used Cochlear® Nucleus™ CIs in their test ears. In each participant, eCAPs were measured at multiple electrode locations in response to single-pulse, paired-pulse, and pulse-train stimuli. Independent variables included six metrics calculated from the eCAP recordings: the electrode-neuron interface (ENI) index, the neural adaptation (NA) ratio, NA speed, the adaptation recovery (AR) ratio, AR speed, and the amplitude modulation (AM) ratio. The ENI index quantified the effectiveness of the CI electrodes in stimulating the targeted AN fibers. The NA ratio indicated the amount of NA at the AN caused by a train of constant-amplitude pulses. NA speed was defined as the speed/rate of NA. The AR ratio estimated the amount of recovery from NA at a fixed time point after the cessation of pulse-train stimulation. AR speed referred to the speed of recovery from NA caused by previous pulse-train stimulation. The AM ratio provided a measure of AN sensitivity to AM cues. Participants’ speech perception scores were measured using Consonant-Nucleus-Consonant (CNC) word lists and AzBio sentences presented in quiet, as well as in noise at signal-to-noise ratios (SNRs) of +10 and +5 dB. Predictive models were created for each speech measure to identify eCAP metrics with meaningful predictive power.
Results:
The ENI index and AR speed individually explained at least 10% of the variance in most of the speech perception scores measured in this study, while the NA ratio, NA speed, the AR ratio, and the AM ratio did not. The ENI index was identified as the only eCAP metric that had unique predictive power for each of the speech test results. The amount of variance in speech perception scores (both CNC words and AzBio sentences) explained by the eCAP metrics increased with increased difficulty in the listening condition. Over half of the variance in speech perception scores measured in +5 dB SNR noise (both CNC words and AzBio sentences) was explained by a model with only three eCAP metrics: the ENI index, NA speed, and AR speed.
Conclusions:
Of the six electrophysiological measures assessed in this study, the ENI index is the most informative predictor for speech perception performance in CI users. In agreement with the tested hypothesis, the response characteristics of the AN to electrical stimulation are more important for speech perception with a CI in noise than they are in quiet.
Keywords: cochlear implant, auditory nerve, electrically evoked auditory compound action potential, speech perception
INTRODUCTION
Over 730,000 individuals who are deaf or severely hard-of-hearing have received a cochlear implant (CI) (National Institute on Deafness and Other Communication Disorders, 2021). Despite general improvements in auditory perception after receiving a CI (e.g., Bittencourt et al., 2012; Boisvert et al., 2020; Hey et al., 2020; Rasmussen et al., 2022), there remains large variability in speech recognition performance among CI patients (e.g., Holden et al., 2013; Blamey et al., 2013; Goudey et al., 2021). While some CI patients can converse without lip-reading, others can only perceive environmental sounds (e.g., Holden et al., 2013; Wei et al., 2017; Han et al., 2019; Boisvert et al., 2020; Lin et al., 2022; Zhang et al., 2022). Identifying factors accounting for the observed speech perception variability among CI users has been a highly active area of research (e.g., Blamey et al., 1996, 2013; Lazard et al., 2012; Holden et al., 2013, 2016; Kaandorp et al., 2017; James et al., 2019; Zhao et al., 2020; Goudey et al., 2021; Heutink et al., 2021). In general, better speech perception outcomes for CI users have been associated with shorter duration of deafness (Lazard et al., 2012; Blamey et al., 1996, 2013; Zhao et al., 2020; Bernhard et al., 2021; Goudey et al., 2021), better residual hearing before CI (Lazard et al., 2012; Blamey et al., 2013; Boisvert et al., 2020; Zhao et al., 2020; Goudey et al., 2021), closer electrode-to-neuron distance (Finley et al., 2008; Holden et al., 2013, 2016; Heutnik et al., 2021), right-ear implantation (Kraaijenga et al., 2018; Liang et al., 2020; Goudey et al., 2021), and better cognitive function (Holden et al., 2013; Kaandorp et al., 2017; Mussoi & Brown, 2019). However, factors investigated in these studies can only explain approximately 10–40% of the variance in speech perception scores among CI users (Blamey et al., 1996, 2013; Lazard et al., 2012; Holden et al., 2013; James et al., 2019; Zhao et al., 2020; Goudey et al., 2021). Therefore, further studies to identify additional key factors accounting for the speech perception variability among CI users are urgently needed.
CIs provide auditory information to implanted patients by converting acoustic signals into sequences of electrical pulses (i.e., pulse trains) that stimulate nearby auditory nerve (AN) fibers. Subsequently, AN fibers transmit the information to higher-level neural structures for further processing and interpretation. Theoretically, how well electrical stimulation is encoded and processed by the AN should be an important factor for speech perception outcomes in CI users. Results reported in the auditory literature support this theory. Specifically, the presence of AN response to electrical stimulation (i.e., the presence of the electrically evoked compound action potential, eCAP), faster recovery from refractoriness, and faster growth of eCAP amplitudes with increasing stimulation level are associated with better speech perception in CI users (for a review, see van Eijl et al., 2017). More recently, it has been shown that better speech perception outcomes are associated with higher effectiveness of the CI electrodes in stimulating the targeted AN fibers (i.e., electrode-to-neuron interface, ENI, Bierer, 2010; Skidmore et al., 2021; Arjmandi et al., 2022), faster recovery from neural adaptation (NA) induced by prior stimulation (He et al., 2022c), and greater number and discharge synchrony of excited AN fibers (Dong et al., 2022). Based on these promising results, we took a bottom-up approach in this study to determine/identify peripheral factors that are of potential importance for speech perception outcomes in CI users.
In this study, electrophysiological measures of the eCAP were used to assess the quality of the ENI and several aspects of temporal responsiveness of the AN to electrical stimulation. The eCAP is a neural response that is generated by a population of AN fibers responding synchronously to electrical stimulation (Brown et al., 1990; He et al., 2017). Like the ENI, eCAP measures in response to single-pulse and paired-pulse stimulation are affected by electrode position, intracochlear resistance, and the number of activated AN fibers (e.g., Eisen & Franck, 2004; Shepherd et al., 2004; Brown et al., 2010; Ramekers et al., 2014; Schvartz-Leyzac & Pfingst, 2016; Pfingst et al., 2015, 2017; He et al., 2018; Schvartz-Leyzac et al., 2020). Therefore, eCAPs measured in response to single-pulse and paired-pulse stimulation can be considered as a functional readout for the quality of the ENI (Skidmore et al., 2022a). The temporal response properties of the AN evaluated in this study included NA and recovery from NA (i.e., adaptation recovery, AR) induced by trains of biphasic pulses with constant amplitudes (e.g., Wilson et al., 1997; Hay-McCutcheon et al., 2005; Hughes et al., 2012; Zhang et al., 2013; Ramekers et al., 2015; He et al., 2016; Mussoi & Brown, 2019; He et al., 2022a, b, c, d) and the sensitivity to sinusoidal amplitude modulation (AM) cues implemented in the pulse-train stimulation (Nourski et al., 2007; Tejani et al., 2017; Riggs et al., 2021). NA and AR of the AN were selected as measures of interest because of their essential roles in accurately encoding speech sounds (Delgutte, 1980; Johnson, 1980; Delgutte & Kiang, 1984). AN sensitivity to AM cues was selected as a measure of interest because temporal cues are particularly important for speech perception in CI users and they are encoded in the AM of pulse trains delivered by the CI (Wilson et al., 1991).
The relationship between speech perception outcomes and each of these eCAP metrics has been evaluated in different studies. For example, Skidmore et al. (2021) assessed the correlation between Consonant-Nucleus-Consonant (CNC) word (Peterson & Lehiste, 1962) and AzBio sentence (Spahr et al., 2012) scores measured in quiet and the quality of the ENI, as quantified by the ENI index estimated based on eCAP results, in post-lingually deafened adult CI users. Their results showed a significant, positive correlation between CNC word and AzBio sentence scores and the ENI index (r = 0.49 – 0.73, p ≤ 0.005, N = 24). He et al. (2022c) investigated the effects of NA and AR of the AN on CNC word scores measured in quiet and in noise at a signal-to-noise ratio (SNR) of +10 dB in 18 post-lingually deafened adult CI users. Their results did not demonstrate a statistically significant relationship between NA of the AN and CNC word scores measured in either testing condition, which is consistent with the results reported by Zhang et al. (2013). The speed of AR was found to account for 14% of the variability in CNC word scores measured in quiet and 17% of the variability in CNC word scores measured in noise with a SNR of +10 dB (He et al., 2022c). In contrast, the results from Mussoi & Brown (2019) did not demonstrate a significant relationship between speed of AR and speech perception scores. However, it should be pointed out that AR of the AN was only evaluated at one electrode location for each participant in Mussoi & Brown (2019), while He et al. (2022c) evaluated AR of the AN at 3–4 electrodes for each participant. Results of a recent study showed that correlating eCAP metrics measured at one electrode location with speech scores can lead to inaccurate conclusions (He et al., 2022d). In addition, the speed of AR was quantified using different methods in these two studies. These methodological differences might account for the inconsistent results reported in Mussoi & Brown (2019) and He et al. (2022c). Finally, He et al. (2022d) evaluated the association between AN sensitivity to AM cues and CNC word scores measured in quiet and in noise at a SNR of +10 dB. Their results revealed a nonsignificant relationship between these measures.
Even though the results of these previous studies are informative and make a scientific contribution to the literature, they have two limitations. First, none of these studies measured all these important eCAP metrics and speech perception scores in the same group of study participants. As a result, no study has included these eCAP metrics in a multiple regression model to predict speech perception scores. Rather, most studies cited above reported correlations between a single eCAP measure and a speech perception score. These correlation analyses do not account for other eCAP metrics that may explain some of the same variance in speech perception scores. Therefore, the results from correlation analyses may not reflect the unique explanatory power of individual eCAP metrics. Consequently, the results of these studies do not provide conclusive information about which eCAP metrics have the greatest predictive power for speech perception outcomes in CI patients. In addition, speech perception tests implemented in these studies are not representative of current audiological practice for CI patients. Specifically, both CNC word lists and AzBio sentence lists are recommended as speech perception tests for assessing clinical outcomes in adult CI users (American Academy of Audiology, 2019). In addition, assessing speech perception performance in both quiet and in noise testing conditions is recommended (Adunka et al., 2018). The noise conditions with SNRs of +10 and +5 dB are most commonly used in clinical practice for CIs (Carlson et al., 2018). Previous studies either tested speech perception performance only in quiet (Zhang et al., 2013; Skidmore et al., 2021), using another speech test (Mussoi & Brown, 2019), or did not include the noise condition with a SNR of +5 dB (He et al., 2022a, c, d). These caveats limit the clinical application of these previously reported results.
There are some pieces of evidence suggesting that the importance of faithful neural encoding of auditory information at the AN to speech perception may increase as the listening condition becomes more challenging. For example, competing background noise has a much larger, negative effect on speech perception performance in patients with auditory neuropathy spectrum disorder, a disorder characterized by dyssynchrony in AN fiber activity, than in patients with typical sensorineural hearing loss (Starr et al., 1998; Kraus et al., 2000; Shallop, 2002; Zeng & Liu, 2006; Rance et al., 2007; Berlin et al., 2010; Walker et al., 2016). Improving neural synchrony in AN fiber activity using electrical stimulation of a CI could reduce the difference in the magnitude of the noise effect on speech perception performance between these two patient populations (for reviews, see Myers & Nicholson, 2021; Bo et al., 2022). Second, the speed of AR of the AN accounted for more variability in CNC word scores measured at +10 dB SNR than measured in quiet (He et al., 2022c). Unfortunately, these interesting results do not provide conclusive information about the relative importance of AN responsiveness to electrical stimulation to speech perception outcomes in different listening conditions or which AN response properties are more important for listening in challenging conditions than other properties. There is a scientific and clinical need to address these two knowledge gaps.
To address the study limitations and knowledge gaps described above, this study evaluated the association between six eCAP metrics and speech perception scores (CNC words and AzBio sentences) measured in quiet and in noise at SNRs of +10 and +5 dB in post-lingually deafened adult CI users. The primary objective of this study was to identify eCAP metrics that were important predictors for CI speech perception outcomes. We hypothesized that how well the AN encodes and processes electrical stimulation is more important for understanding speech in more challenging listening conditions compared to quiet listening conditions. This hypothesis was formulated based on results showing that listeners with degraded AN function (i.e., patients with auditory neuropathy spectrum disorder and elderly listeners) experience excessive difficulty in understanding speech in the presence of competing background noise (Starr et al., 1998; Kraus et al., 2000; Shallop, 2002; Zeng & Liu, 2006; Rance et al., 2007; Berlin et al., 2010; Harris et al., 2021). According to our hypothesis, we expected that the amount of variance in speech perception scores explained by the eCAP metrics (i.e., R2s of the predictive models) would be greater for results measured with higher levels of competing background noise.
MATERIALS AND METHODS
Study Participants
Study participants included 24 post-lingually deafened adult CI users. All participants were implanted with a Cochlear™ Nucleus® device (Cochlear Ltd., Sydney, NSW, Australia) with a full electrode insertion. All study participants utilized the advanced combination encoder (ACE) coding strategy in their sound processors. Only one ear was tested for each participant. For bilateral CI users, the test ear was selected pseudo-randomly. Detailed demographic information of the study participants is provided in Table 1. Written informed consent was obtained from all study participants at the time of data collection. The study was approved by the Biomedical Institutional Review Board (IRB) at The Ohio State University (IRB study #: 2017H0131).
TABLE 1.
Demographic information of all subjects who participated in this study.
| Subject Number | Sex | Ear Tested | AAT (yrs) | AAI (yrs) | Internal Device and Electrode Array | Etiology of Hearing Loss |
|---|---|---|---|---|---|---|
|
| ||||||
| A03 | M | L | 61.8 | 58.8 | CI512 | SHL |
| A06 | M | L | 69.0 | 60.7 | CI512 | Meniere’s |
| A07 | M | R | 52.7 | 43.3 | CI24RE (CA) | Unknown |
| A08 | F | R | 67.6 | 54.4 | CI24RE (CA) | Hereditary |
| A09 | F | L | 38.0 | 31.5 | CI24RE (CA) | Trauma |
| A11 | M | R | 80.7 | 77.5 | CI24RE (CA) | Noise |
| A19 | F | R | 54.7 | 44.7 | CI24RE (CA) | Rubella |
| A20 | M | R | 62.7 | 60.3 | CI522 | Trauma |
| A21 | F | R | 24.8 | 23.6 | CI532 | Unknown |
| A24 | M | R | 36.8 | 25.6 | CI24RE (CA) | Hereditary |
| A29 | F | R | 59.6 | 48.5 | CI24RE | Hereditary |
| A30 | F | R | 65.6 | 64.9 | CI532 | Unknown |
| A34 | M | L | 70.4 | 68.7 | CI532 | Trauma |
| A35 | F | L | 76.6 | 72.4 | CI422 | Noise |
| A37 | F | R | 28.7 | 15.2 | CI24RE (CA) | Unknown |
| A40 | M | R | 59.5 | 59.0 | CI532 | Unknown |
| A41 | F | R | 79.8 | 73.3 | CI24RE | Hereditary |
| A50 | M | L | 78.4 | 77.7 | CI622 | Noise |
| A52 | M | L | 67.6 | 65.2 | CI532 | Tumor |
| A54 | M | L | 69.7 | 69.3 | CI622 | Noise |
| A61 | F | R | 84.0 | 80.7 | CI532 | Unknown |
| A65 | F | L | 58.7 | 54.1 | CI532 | Unknown |
| A67 | F | R | 57.3 | 56.9 | CI622 | Noise |
| A70 | M | R | 69.8 | 68.3 | CI632 | Trauma |
AAT, age at testing; AAI, age at implantation; 24RE (CA), Freedom Contour Advance electrode array; SHL, sudden hearing loss
eCAP Measurements and Metrics
The procedures for obtaining the eCAP were the same as those used in our recent studies (Skidmore et al., 2021; Riggs et al., 2021; He et al., 2022a, b). All eCAPs were obtained using the Advanced Neural Response Telemetry function via the Custom Sound EP (v. 5.1 or 6.0) software interface (Cochlear Ltd, Sydney, NSW, Australia). The stimulus consisted of one or more symmetric, cathodic-leading, biphasic pulses with an interphase gap of 7 μs and a pulse phase duration of 25 μs/phase. For measuring NA of the AN, trains of pulses with increasing stimulation durations were presented at individual electrode locations at a rate of 900 pulses per second (pps). For measuring AR of the AN, the stimulus was a 100-ms pulse train with a pulse rate of 900 pps presented to individual CI electrodes. For measuring AN sensitivity to AM cues, the stimulus was a 200-ms pulse train with a carrier rate of 2000 pps that was sinusoidally amplitude modulated at 20 Hz with a modulation depth of 100% (Riggs et al., 2021). All stimuli were presented in a monopolar-coupled stimulation mode to individual CI electrodes via an N6 sound processor connected to a programming pod.
The ENI index
The ENI index is a number between 0 and 100 that represents the overall quality of the ENI, where larger numbers represent better ENIs. The ENI index was calculated for each participant using the model created by Skidmore et al. (2021). Briefly, the ENI index was a compound metric composed of four parameters derived from eCAPs evoked by single-pulse and paired-pulse stimulation. The four parameters included the lowest stimulation level that evoked an eCAP (i.e., the eCAP threshold), the slope of the eCAP amplitude growth function, the latency of the first negative peak (i.e., N1 latency) in the recorded waveform with the largest eCAP amplitude, and the absolute refractory period estimated from the eCAP refractory recovery function. Each of the four parameters was recorded at three electrode locations along the length of the electrode array (i.e., one basal, one middle and one apical electrode). These twelve measures (4 parameters x 3 electrode locations) were inputs into the model that was created using linear regression with elastic net regularization to produce the ENI index. The ENI index was calculated for each participant with the following equation
| (1) |
where is a vector of twelve model parameters (i.e., regression coefficients), is a vector of the twelve eCAP measures, is a model parameter representing the ordinate intercept, represents the vector transpose, and and are the minimum and maximum values of among all participants in the model training dataset, respectively. Additional details regarding the ENI index (originally called the cochlear nerve index), including a detailed description of its development and values for the constant parameters in Equation 1 (i.e., , and ), are contained in Skidmore et al. (2021). To summarize, the ENI index represents the overall quality of the ENI across the entire electrode array, and it increases with decreasing eCAP thresholds, N1 latencies and absolute refractory periods and increasing slope of the eCAP amplitude growth function.
Neural adaptation of the AN
Details of using eCAP measures to assess NA of the AN have been reported in He et al. (2022a). Briefly, eCAPs were recorded in response to a single pulse and in response to 18 pulse-train stimuli of increasing durations presented at 900 pps. The pulse-train stimuli consisted of trains of 2, 3, 4, 5, 6, 7, 8, 40, 41, 42, 43, 44, 45, 85, 86, 87, 88, and 89 pulses. Smaller eCAP amplitudes were evoked by the longer pulse-trains due to the AN fibers gradually adapting to the constant-amplitude stimulus.
The NA ratio
The NA ratio is a measure of the amount of NA that occurs in response to a train of constant amplitude pulses, with smaller NA ratios indicating greater NA. The NA ratio was calculated at individual electrode locations as the average eCAP amplitude evoked by the pulse-train stimuli divided by the eCAP amplitude evoked by a single pulse presented at the same stimulation level as the individual pulses in the pulse-trains. Specifically, it was calculated according to the following equation
| (2) |
where is the eCAP amplitude evoked by the last pulse of the th pulse train and is the eCAP amplitude evoked by a single pulse presented at the same stimulation level as the pulses in the pulse-train. The NA ratio was calculated based on eCAP results measured at four electrode locations along the electrode array, and then averaged together to create one measure for each participant in this study.
NA speed
NA speed was estimated by fitting a two-parameter power law to all the data points (i.e., normalized eCAP amplitudes evoked by a single pulse and pulses 2–8, 40–45, and 85–89 of the pulse-train). Therefore, variations in eCAP amplitudes (e.g., Hughes et al., 2012; Ramekers et al., 2015; He et al., 2022a) were accounted for in the function fitting procedure. The specific power law function used for estimating NA speed was
| (3) |
where represents the eCAP amplitude evoked by the last pulse of a pulse-train of duration ) divided by the eCAP amplitude evoked by a single pulse () presented at the same stimulation level as the pulses in the pulse-train. and are unitless exponents that represent the speed of NA occurring within the first 8-ms of stimulation (i.e., short phase) and between 8 and 100 ms of stimulation (i.e., long phase), respectively. indicates the relative contribution of the short phase and the long phase to the overall fit of the function. For this study, NA speed was calculated by averaging the absolute values of estimated at four electrode locations along the electrode array. The sign of reflects the direction of change in eCAP amplitude with increasing duration of pulse-train stimulation. In most individuals eCAP amplitudes decrease with increasing duration of pulse-train stimulation due to NA. This results in a negative value of . However, eCAP amplitudes increase with longer pulse-train stimulation in a limited number of individuals (He et al., 2016, 2022a), which results in a positive value of . Nevertheless, the sign does not affect the speed of adaptation, only the direction. Therefore, the absolute value of was used for calculating NA speed, with larger values indicating faster NA.
Neural Adaptation Recovery of the AN
Details of using eCAP measures to assess the amount and the speed of AR are reported in He et al. (2022b). Briefly, eCAPs were recorded in response to a probe pulse presented at 1.054, 2, 4, 8, 16, 32, 64, 128 and 256 ms after the offset of the last pulse of a 100-ms pulse-train masker presented at 900 pps. As the masker-probe-interval (MPI) increased, AN fibers gradually recovered from the NA induced by the pulse-train masker, which resulted in gradually increased eCAP amplitudes at longer MPIs.
The AR ratio
The AR ratio quantifies the amount of recovery from NA induced by a constant-amplitude pulse-train. Larger AR ratios indicate greater recovery from NA. The AR ratio was calculated by dividing the amplitude of the eCAP to the probe pulse presented after 256 ms of the end of the pulse-train stimulation (i.e., MPI = 256 ms) by the eCAP amplitude evoked by a single-pulse stimulus presented at the same stimulation level. In this study, the AR ratio was calculated at four electrode locations along the electrode array, and then averaged together to create one measure for each participant.
AR speed
AR speed was estimated using a mathematical model with up to three exponential components in the form of
| (4) |
where represented the normalized eCAP amplitude (re: the eCAP amplitude evoked by a single pulse) evoked by the probe pulse at an MPI of t. , and were fitting coefficients and and were the time constants for early enhancement, slow decay, and slow recovery, respectively. Detailed descriptions and explanations of these three phases have been reported in He et al. (2022b). Briefly, the early enhancement phase likely represents the faciliatory effect of the residual partial-depolarization mechanism (Middlebrooks, 2004) and the slow decay phase likely represents the decay of this faciliatory effect (Miller et al., 2011). The slow recovery phase is dominated by AR (Nourski et al., 2007; Miller et al., 2011; He et al., 2022b). For this study, AR speed was calculated by averaging the values of estimated at four electrode locations along the electrode array. Larger averaged indicated slower recovery from NA.
The AM ratio
The AM ratio is a measure of AN sensitivity to AM cues, with larger AM ratios indicating greater AN sensitivity to AM cues. The AM ratio was calculated using the same procedures as detailed in Riggs et al. (2021). Briefly, eCAPs evoked by twenty pulses within the last two cycles of an AM pulse train were measured. eCAPs evoked by single pulses with probe levels corresponding to the stimulation levels of the twenty pulses of the AM pulse train were also measured. The AM ratio was then calculated at individual electrode locations according to the following equation
| (5) |
where is a vector of eCAP amplitudes evoked by the AM pulse train and is a vector of eCAP amplitudes evoked by single pulses presented at the same stimulation levels as the pulses in the AM pulse train. The AM ratio was calculated at seven electrode locations along the electrode array, and then averaged together to create one measure for each participant in this study.
Speech Measures
Speech perception performance was evaluated using CNC word (Peterson & Lehiste, 1962) and AzBio sentence (Spahr et al., 2012) lists presented in quiet and in two noise conditions. All auditory stimuli were presented in a sound-proof booth via a speaker placed one meter in front of the participant at zero degrees azimuth. The target stimulus was always presented at 60 dB(A) sound pressure level (SPL). For the noise conditions, speech-shaped noise was presented concurrently with the target stimulus at 50 dB(A) SPL or 55 dB(A) SPL (i.e., SNR of +10 dB or +5 dB, respectively).
Statistical Analyses
For each speech perception test result measured in each listening condition, three types of predictive models were created. First, six individual models were created by using simple linear regression with each eCAP metric (i.e., ENI index, NA ratio, NA speed, AR ratio, AR speed and AM ratio) as the only predictor to identify the relationships between each eCAP metric and each speech measure. The R2 for each individual model indicated the variance in speech scores explained by that metric without adjusting for the other eCAP metrics. Second, a complete model was created using multiple linear regression with all six eCAP metrics as predictors. The R2 for the complete model quantified the contribution of AN responsiveness to speech perception scores by indicating the variance in speech scores explained by all six eCAP metrics together. Third, a reduced model was created using all subsets selection. All possible combinations of the eCAP metrics were examined and the combination of eCAP metrics with the lowest Akaike Information Criterion (Akaike, 1974) was selected as the reduced model. The R2 for the reduced model indicated the variance in speech scores explained by the eCAP metrics selected in the model. The assumptions of regression analyses including normality and constant variance were evaluated by examining the residuals of all models created in this study and no violations were detected. Despite the lack of strong correlations among the six eCAP metrics (see Figure 1), the variance inflation factor (VIF) was computed for the full models to assess the potential multicollinearity among the predictor variables. The largest VIF score among the eCAP metrics was 1.60 (see Figure 1), which is smaller than the conservative rule of thumb suggesting any concern about multicollinearity (i.e., VIF > 3). Therefore, the statistical models used in this study were appropriate. The p-values obtained in the regression analyses were adjusted for multiple comparisons using the False Discovery Rate (Benjamini & Hochberg, 1995). All statistical modeling was performed with R v. 4.2 (R Core Team, 2022) using α = 0.05 for statistical significance. It should be pointed out that the significance of the results was not evaluated exclusively based on p-values but also based on its impact on clinical outcomes following the recommended best practice in the field of biostatistics (Wasserstein & Lazar, 2016).
Figure 1.
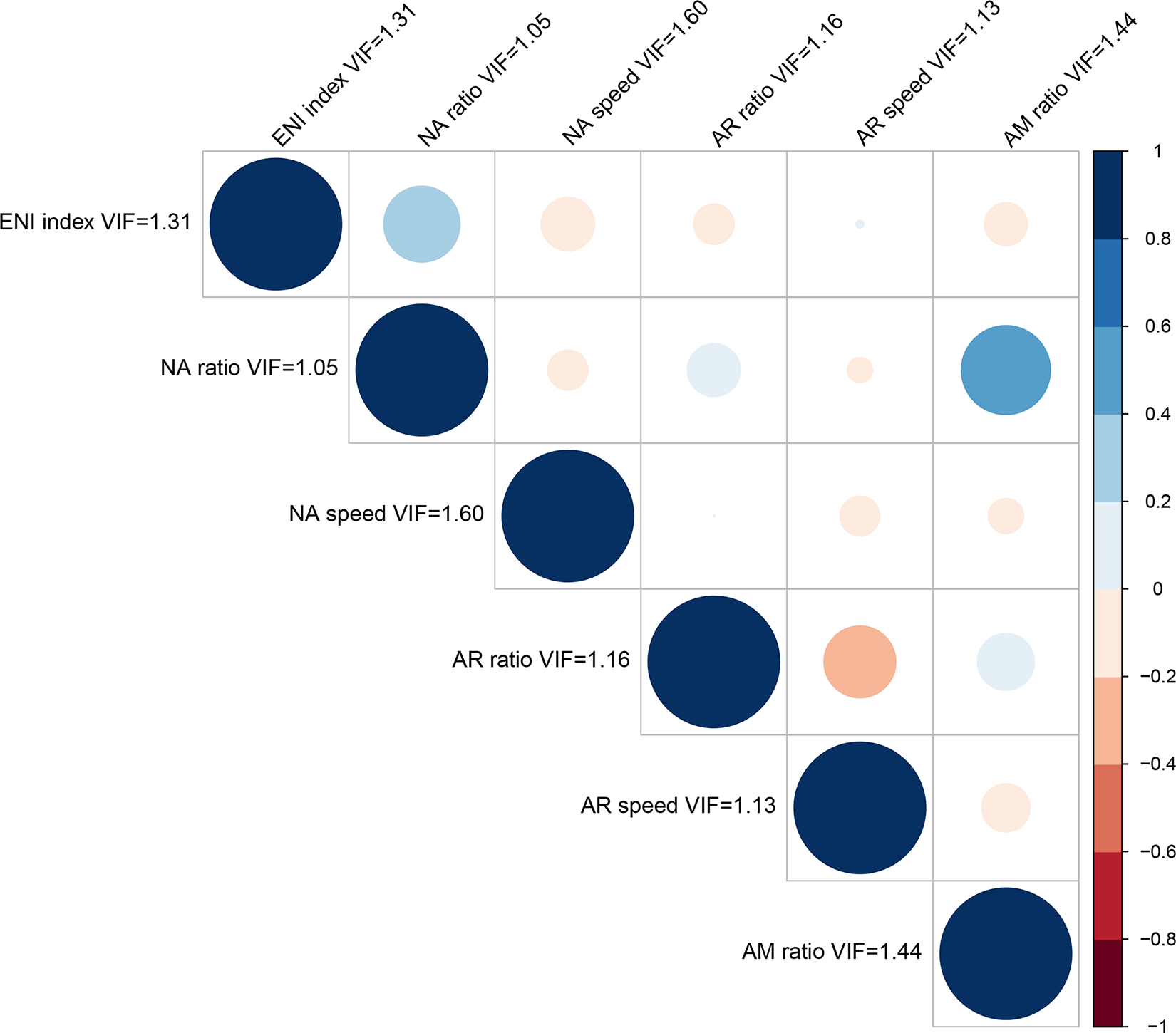
Results of Pearson correlations between each of the electrically evoked compound action potential (eCAP) metrics. The color and size of the circles represent the direction and magnitude of the correlation. The variance inflation factor (VIF) is also shown for each eCAP metric. ENI, electrode neuron interface; NA, neural adaptation; AR, adaptation recovery; AM, amplitude modulation
RESULTS
Results of CNC word tests and AzBio sentence tests as a function of six eCAP metrics for each of the three listening conditions are shown in Figure 2 and Figure 3, respectively. Overall, speech perception performance appears to decrease with increasing level of competing background noise. Additionally, visual inspection of these figures revealed substantial variations in the relationship between each eCAP metric and each speech test result.
Figure 2.
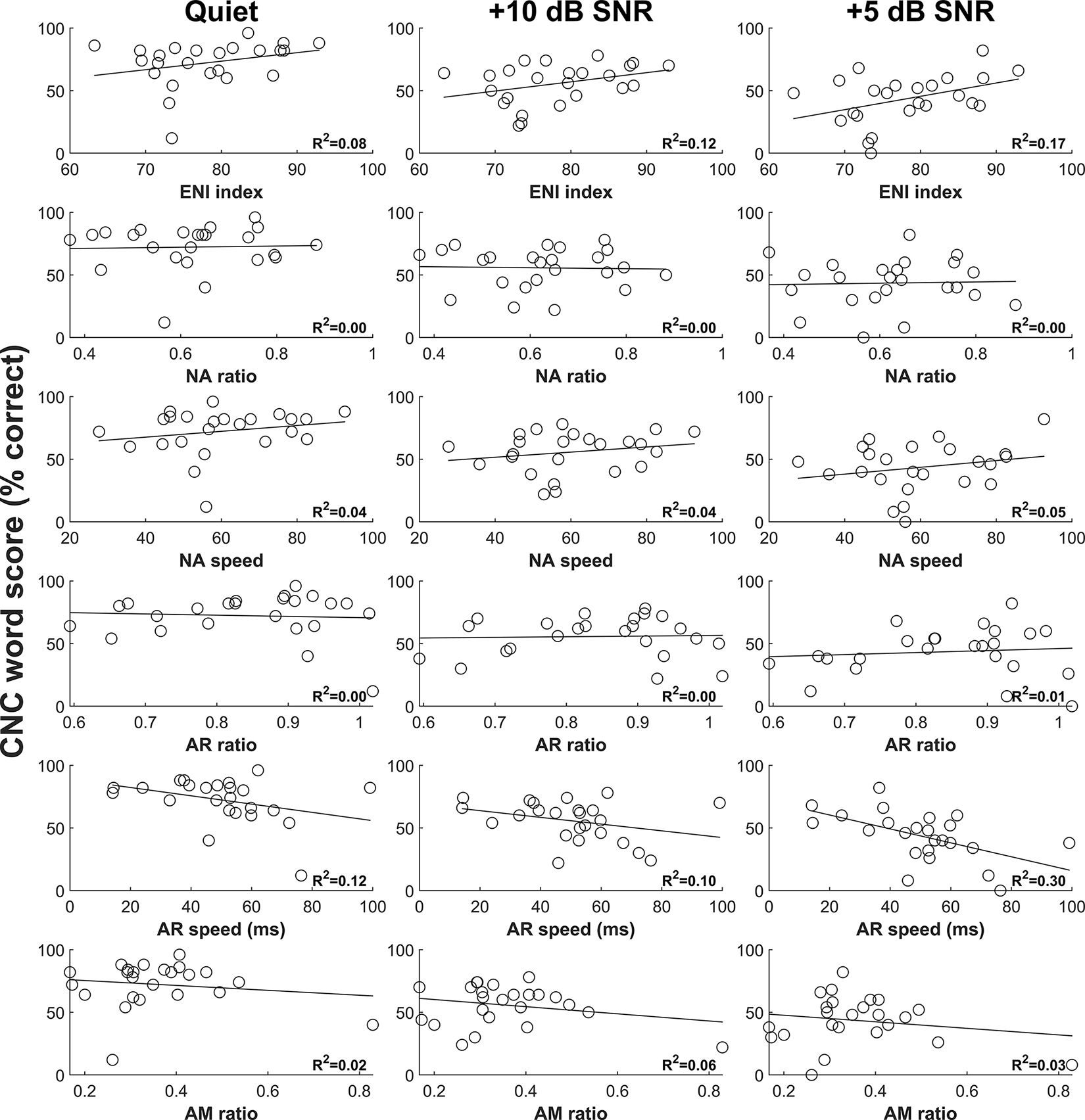
Results of CNC word tests as a function of six eCAP metrics for three listening conditions. Each circle represents the result for an individual study participant. The variance in CNC word scores explained by the eCAP metric calculated from simple linear regression is provided in the lower right-hand corner of each panel. CNC, Consonant-Nucleus-Consonant; eCAP, electrically evoked compound action potential.
Figure 3.
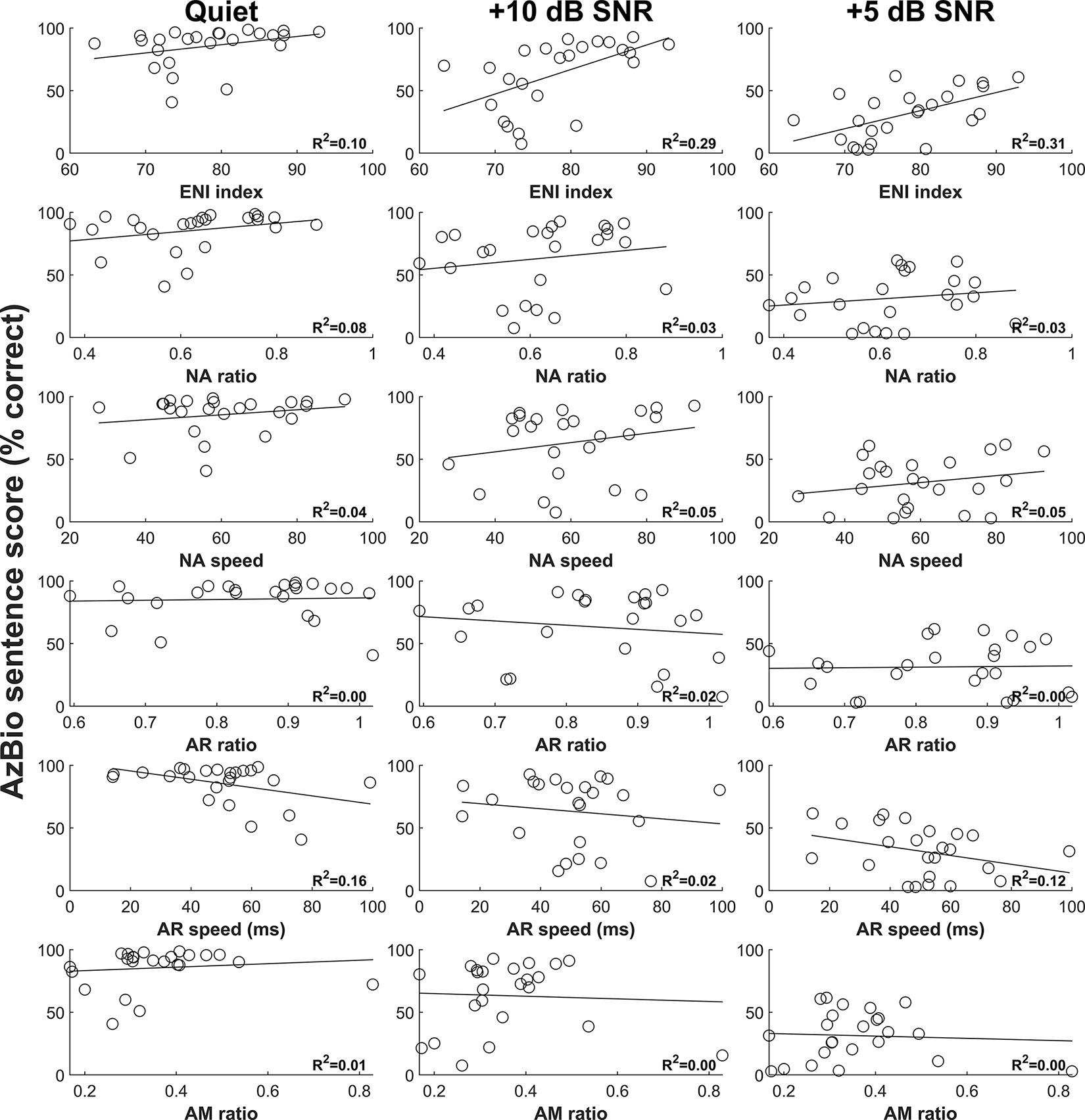
Results of AzBio sentence tests as a function of six eCAP metrics for three listening conditions. Each circle represents the result for an individual study participant. The variance in AzBio sentence scores explained by the eCAP metric calculated from simple linear regression is provided in the lower right-hand corner of each panel. eCAP, electrically evoked compound action potential.
Individual Models
The results of each individual model assessing the relationship between an individual eCAP metric and a speech test result indicated that the ENI index and AR speed generally explained more variance in speech perception scores as individual predictors than the NA ratio, NA speed, the AR ratio, and the AM ratio. Specifically, the ENI index explained 8 – 17% of the variance in CNC word scores and 10 – 31% of the variance in AzBio sentence scores, and AR speed explained 10 – 30% of the variance in CNC word scores and 2 – 16% of the variance in AzBio sentence scores. In contrast, the NA ratio, NA speed, the AR ratio, and the AM ratio explained an average of 2% of the variance in each of the speech tests as individual predictors (see Table A1 and Table A2, Supplemental Digital Content 1, which contain the detailed results of the individual models for CNC word scores AzBio sentence scores, respectively). For all speech tests, better speech perception performance was associated with better quality of the ENI and faster recovery from neural adaptation.
Complete Models
The results of the complete models revealed that the variance in scores on each speech test explained by the eCAP metrics increased with increased difficulty in the listening condition (see Table B1 and Table B2, Supplemental Digital Content 2, which contain the detailed results of the complete models for CNC word scores and AzBio sentence scores, respectively). In quiet, the eCAP metrics explained 29% and 36% of the variance in CNC word scores and AzBio sentence scores, respectively. In comparison, the eCAP metrics explained 33% and 42% of the variance in CNC word scores and AzBio sentence scores measured in noise at a SNR of +10 dB, respectively. In the most difficult listening condition (i.e., +5 dB SNR), the eCAP metrics explained over half of the variance in both CNC word scores (R2 = 0.57) and AzBio sentence scores (R2 = 0.51).
Reduced Models
The results of each reduced model that only included the eCAP metrics that contributed unique power to predicting CNC word scores and AzBio sentence scores are provided in Table 2 and Table 3, respectively. As shown in the tables, the ENI index was the only eCAP metric that was selected in each of the six reduced models. Therefore, the ENI index contributed unique predictive power for each of the six speech test results that was independent of the other eCAP metrics. Along with the ENI index, NA speed and AR speed were selected in both reduced models for speech scores measured in +5 dB SNR noise. In this noise condition, these three eCAP metrics explained 53% and 51% of the variance in CNC word scores and AzBio sentence scores, respectively. Also, the R2 values of the reduced models increased with increased difficulty in the listening condition.
TABLE 2.
Results of statistical models that only included eCAP metrics that contributed unique predictive power to explaining variance in CNC word scores measured in three listening conditions.
| Listening condition | eCAP metric | Regression coefficient | T value | P value | FDR P value | R2 |
|---|---|---|---|---|---|---|
|
| ||||||
| Quiet | 0.20 | |||||
| ENI index | 0.68 | 1.45 | 0.161 | 0.184 | ||
| AR speed | −0.33 | −1.79 | 0.087 | 0.163 | ||
|
| ||||||
| +10 dB SNR | 0.29 | |||||
| ENI index | 0.68 | 1.67 | 0.111 | 0.163 | ||
| AR speed | −0.30 | −1.87 | 0.077 | 0.163 | ||
| AM ratio | −30.21 | −1.35 | 0.193 | 0.193 | ||
|
| ||||||
| +5 dB SNR | 0.53 | |||||
| ENI index | 1.18 | 2.92 | 0.008 | 0.032 | ||
| NA speed | 0.30 | 1.62 | 0.122 | 0.163 | ||
| AR speed | −0.53 | −3.41 | 0.003 | 0.024 | ||
eCAP, electrically evoked compound action potential; CNC, Consonant-Nucleus-Consonant; SNR, signal-to-noise ratio; ENI, electrode neuron interface; NA, neural adaptation; AR, adaptation recovery; AM, amplitude modulation; FDR, false discovery rate.
TABLE 3.
Results of statistical models that only included eCAP metrics that contributed unique predictive power to explaining variance in AzBio sentence scores measured in three listening conditions.
| Listening condition | eCAP metric | Regression coefficient | T value | P value | FDR P value |
R2 |
|---|---|---|---|---|---|---|
|
| ||||||
| Quiet | 0.26 | |||||
| ENI index | 0.66 | 1.69 | 0.105 | 0.105 | ||
| AR speed | −0.33 | −2.17 | 0.042 | 0.098 | ||
|
| ||||||
| +10 dB SNR | 0.38 | |||||
| ENI index | 2.15 | 3.39 | 0.003 | 0.011 | ||
| NA speed | 0.53 | 1.81 | 0.084 | 0.098 | ||
|
| ||||||
| +5 dB SNR | 0.51 | |||||
| ENI index | 1.57 | 3.79 | 0.001 | 0.007 | ||
| NA speed | 0.36 | 1.86 | 0.078 | 0.098 | ||
| AR speed | −0.32 | −2.01 | 0.059 | 0.098 | ||
eCAP, electrically evoked compound action potential; SNR, signal-to-noise ratio; ENI, electrode neuron interface; NA, neural adaptation; AR, adaptation recovery; AM, amplitude modulation; FDR, false discovery rate.
Summary of Study Results
The ENI index and AR speed individually explained up to 30% of the variance in speech perception scores measured in this study. The amount of variance in speech scores (both CNC words and AzBio sentences) explained by the eCAP metrics in the completed and reduced models increased with increased levels of competing background noise. The ENI index was the only eCAP metric that was selected in each of the six reduced models. Over half of the variance in speech scores (both CNC words and AzBio sentences) measured in +5 dB SNR noise was explained by only three eCAP metrics: the ENI index, NA speed, and AR speed. These main findings from all the statistical models created in this study are provided in Table 4.
TABLE 4.
Summary of results from statistical models created in this study.
| Speech test | Listening condition | R2 of complete model | eCAP metrics selected in reduced model | R2 of reduced model |
|---|---|---|---|---|
| CNC words | Quiet | 0.29 | ENI index AR speed |
0.20 |
| CNC words | +10 dB SNR | 0.33 | ENI index AR speed AM ratio |
0.29 |
| CNC words | +5 dB SNR | 0.57 | ENI index NA speed AR speed |
0.53 |
| AzBio sentences | Quiet | 0.36 | ENI index AR speed |
0.26 |
| AzBio sentences | +10 dB SNR | 0.42 | ENI index NA speed |
0.38 |
| AzBio sentences | +5 dB SNR | 0.51 | ENI index NA speed AR speed |
0.51 |
eCAP, electrically evoked compound action potential; CNC, Consonant-Nucleus-Consonant; SNR, signal-to-noise ratio; ENI, electrode neuron interface; NA, neural adaptation; AR, adaptation recovery; AM, amplitude modulation.
DISCUSSION
Comparison of eCAP Metrics
The primary objective of this study was to identify eCAP metrics that were important predictors for speech perception scores in post-lingually deafened adult CI users. Overall, the results of this study showed that the ENI index was the most sensitive predictor of speech perception scores in this patient population. This was most clearly shown by the result of the ENI index being the only eCAP metric selected in all reduced models. This suggested that the ENI index contributed explanatory power to the predictive capability of all the models that was independent of the other eCAP metrics. Overall, this result demonstrating the value of the ENI index in predicting speech perception scores is generally consistent with what has been reported in the literature. Specifically, better speech perception outcomes have been reported in CI users with higher quality ENIs as estimated by electrode placement (Finley et al., 2008; Heutink et al., 2021), size of the AN in imaging results (Kang et al., 2010), psychophysical detection thresholds (Garadat et al., 2013), focused stimulation thresholds (Long et al., 2014), and eCAP measures (Skidmore et al., 2021).
While not the most sensitive predictor of speech scores in this study, AR speed also had meaningful predictive power in the models. This was shown by AR speed being selected in five of the reduced models. This result is also supported by our recent study in which we reported a moderate, negative correlation between CNC word scores measured in quiet and in noise and the speed of AR averaged across multiple electrodes for each participant (He et al., 2022c).
In contrast to the ENI index and AR speed, the other eCAP metrics (i.e., the NA ratio, NA speed, the AR ratio and the AM ratio) explained limited variances (i.e., less than 9%) in each of the speech perception scores measured in this study as individual predictors. These results are consistent with the null results from other studies (Zhang et al., 2013; He et al., 2022c, d). Additionally, the NA ratio and the AR ratio were not selected in any of the reduced models, and the AM ratio was selected in only one reduced model, which suggested that, in general, these eCAP measures did not contribute a meaningful amount of unique information to the predictive models. However, NA speed was selected in the reduced model for CNC words measured in +5 dB SNR noise and for AzBio sentences tested at SNRs of +10 and +5 dB. Therefore, a measure of the speed of NA may provide beneficial, predictive information for speech perception scores measured in challenging listening conditions, even if it is not a robust predictor of speech perception scores as an individual predictor.
It is worth mentioning that each of the eCAP metrics included in this study represent objective measures of specific neural response properties. Therefore, in addition to identifying eCAP metrics that are useful for predicting speech perception scores with a CI, the results of this study also emphasize the relative importance of different aspects of AN function to speech perception with a CI. For example, the results of this study suggest that the speed of NA and AR at the level of the AN is more important for speech perception with a CI than the amount of NA and AR that occur during electrical stimulation.
Comparison of Listening Conditions
This study tested the hypothesis that AN responsiveness to electrical stimulation is especially important for speech perception in challenging listening conditions. The results of this study are consistent with this hypothesis. Specifically, the variances in speech perception scores explained by the eCAP metrics all together (i.e., R2s of the complete models) were higher for speech perception scores measured in higher levels of noise for both word and sentence scores. These results suggest that AN responsiveness to electrical stimulation is more important for speech perception in noise compared to speech perception in quiet. In agreement with this idea, some human listeners with impaired AN function (e.g., patients with auditory neuropathy spectrum disorder or elderly listeners) can have excellent speech perception outcomes in quiet listening conditions but experience excessive difficulty in understanding speech in the presence of competing background noise (Starr et al., 1998; Kraus et al., 2000; Shallop, 2002; Zeng & Liu, 2006; Rance et al., 2007; Berlin et al., 2010; Harris et al., 2021). Moreover, the temporal precision of speech onset cues has been shown to be particularly important for speech perception in noisy listening conditions (McLaughlin et al., 2013). Therefore, we postulate that less information from the peripheral auditory system may be needed to understand speech in quiet than in noise. Consequently, increased listening effort may sufficiently compensate for degraded auditory input to the central auditory system in quiet but not in noise (e.g., Winn et al., 2015). However, this postulation has not been verified and therefore remains as speculation. A future study will evaluate the relative contribution of peripheral and central factors to speech perception with a CI in quiet and in noise.
Clinical Application and Implication
Results of this study showed that the ENI index was the most informative predictor for speech perception performance in post-lingually deafened adult CI users. This finding generally aligns with the positive relationship between the quality of ENI and speech perception outcomes reported in CI patients (Finley et al., 2008; Kang et al., 2010; Garadat et al., 2013; Long et al., 2014; Heutink et al., 2021; Skidmore et al., 2021). As a result, the ENI index can potentially be used as a biomarker for predicting CI clinical outcomes.
Results of this study also suggest the importance of enhancing the quality of the ENI for improving speech perception outcomes in CI patients. The quality of the ENI is negatively impacted by poor AN function (Skidmore et al., 2021), bone and tissue growth caused by intracochlear surgical trauma (Seyyedi & Nadol, 2014; Kamakura & Nadol, 2016), and large distances between CI electrodes and their target AN fibers (Finley et al., 2008; Heutink et al., 2021). These factors are affected by electrode array design and surgical practice. For example, lateral wall electrode arrays generally result in a larger distance to the AN compared to perimodiolar electrode arrays. Technologies/strategies that can reduce surgical trauma and/or improve placement of the electrode array in the cochlea should lead to enhanced ENI quality, and, therefore, result in improved CI outcomes. For example, dexamethasone-eluting electrode arrays and using robotics-assistance to provide a slow, steady electrode insertion during CI surgery are two novel technologies/strategies that could potentially result in improved CI outcomes by enhancing the quality of the ENI.
Study Limitations
One potential limitation of the present study is that only 24 post-lingually deafened adult participants were included in the study. Therefore, the variance in speech perception performance explained by the eCAP metrics in this study cannot be assumed to represent the variance explained in the entire CI patient population. Results of studies including data from hundreds or thousands of CI users (e.g., Blamey et al., 1996, 2013; Lazard et al., 2012; Holden et al., 2013; James et al., 2019; Goudey et al., 2021; Heutink et al., 2021) might provide a better estimate of the variance explained by different factors. However, the purpose of this study was to identify the most relevant eCAP predictors of speech outcomes for adult CI users, which was accomplished in this study. A future study will evaluate the variance in speech perception scores accounted for by the ENI index and AR speed in a large sample of patients to obtain a more representative estimate of the variance in speech perception scores explained by these two eCAP metrics.
The other potential limitation of the present study is that exclusively eCAP measures were included in the predictive models. Other factors, such as cognition, cortical sensitivity to within-stimulus acoustic changes, etiology of hearing loss, and duration of deafness, have been shown to be correlated with speech perception outcomes in CI patients (e.g., Lazard et al., 2012; Blamey et al., 2013; Holden et al., 2013; Kaandorp et al., 2017; Mussoi & Brown, 2019; Zhao et al., 2020; Bernhard et al., 2021; Goudey et al., 2021; McGuire et al. 2021; Van Heteren et al., 2022). However, the objective of this study was not to create a model that could explain as much variance in speech perception scores as possible. Rather, the objective was to identify the most relevant eCAP predictors of speech. These results provided a foundation for future studies that combine a small subset of eCAP measures (e.g., ENI index and AR speed) with other factors (e.g., cortical encoding and processing of electrical stimulation, cognitive measures, etc.) to better understand the contribution of each level of the neural system to speech perception outcomes in CI patients.
CONCLUSIONS
Of the six eCAP-derived metrics included in this study, the quality of the ENI is the most sensitive predictor of speech perception scores in post-lingually deafened adult CI users, followed by the speed of recovery from NA. A predictive model with three eCAP metrics can explain approximately half of the variance in speech perception scores measured with competing background noise presented at +5 dB SNR. The responsiveness of the AN to electrical stimulation considerably impacts speech perception outcomes with a CI, especially in difficult listening conditions.
Supplementary Material
Footnotes
Conflict of Interest: None.
LIST OF SUPPLEMENTAL DIGITAL CONTENT
Supplemental Digital Content 1. Tables with detailed results of the individual models. pdf
Supplemental Digital Content 2. Tables with detailed results of the complete models. pdf
REFERENCES
- Adunka OF, Gantz BJ, Dunn C, Gurgel RK, & Buchman CA (2018). Minimum reporting standards for adult cochlear implantation. Otolaryngology–Head and Neck Surgery, 159(2), 215–219. 10.1177/0194599818764329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723. 10.1109/tac.1974.1100705 [DOI] [Google Scholar]
- American Academy of Audiology. (2019). Clinical practice guidelines: Cochlear implants. https://www.audiology.org/wp-content/uploads/2021/05/CochlearImplantPracticeGuidelines.pdf [DOI] [PubMed]
- Arjmandi MK, Jahn KN, & Arenberg JG (2022). Single-Channel focused thresholds relate to vowel identification in pediatric and adult cochlear implant listeners. Trends in Hearing, 26, 233121652210953. 10.1177/23312165221095364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, Wilensky D, Li L, Mattingly KR, Taylor-Jeanfreau J, Keats BJB, John P St., Montgomery E, Shallop JK, Russell BA, & Frisch SA. (2010). Multi-site diagnosis and management of 260 patients with auditory neuropathy/dys-synchrony (auditory neuropathy spectrum disorder*). International Journal of Audiology, 49(1), 30–43. 10.3109/14992020903160892 [DOI] [PubMed] [Google Scholar]
- Bernhard N, Gauger U, Romo Ventura E, Uecker FC, Olze H, Knopke S, Hänsel T, & Coordes A (2021). Duration of deafness impacts auditory performance after cochlear implantation: A meta-analysis. Laryngoscope Investigative Otolaryngology, 6(2), 291–301. 10.1002/lio2.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA (2010). Probing the electrode-neuron interface with focused cochlear implant stimulation. Trends in Amplification, 14(2), 84–95. 10.1177/1084713810375249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt AG, Torre AAGD, Bento RF, Tsuji RK, & Brito R de. (2012). Prelingual deafness: Benefits from cochlear implants versus conventional hearing aids. International Archives of Otorhinolaryngology, 16(3), 387–390. 10.7162/S1809-97772012000300014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey P, Arndt P, Bergeron F, Bredberg G, Brimacombe J, Facer G, Larky J, Lindström B, Nedzelski J, Peterson A, Shipp D, Staller S, & Whitford L (1996). Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiology & Neuro-Otology, 1(5), 293–306. 10.1159/000259212 [DOI] [PubMed] [Google Scholar]
- Blamey P, Artieres F, Baskent D, Bergeron F, Beynon A, Burke E, Dillier N, Dowell R, Fraysse B, Gallégo S, Govaerts PJ, Green K, Huber AM, Kleine-Punte A, Maat B, Marx M, Mawman D, Mosnier I, O’Connor AF, & O’Leary S (2013). Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiology and Neurotology, 18(1), 36–47. 10.1159/000343189 [DOI] [PubMed] [Google Scholar]
- Bo D, Huang Y, Wang B, Lu P, Chen W, & Xu Z (2022). Auditory and speech outcomes of cochlear implantation in children with auditory neuropathy spectrum disorder: A systematic review and meta-analysis. Annals of Otology, Rhinology & Laryngology, 000348942210922. 10.1177/00034894221092201 [DOI] [PubMed] [Google Scholar]
- Boisvert I, Reis M, Au A, Cowan R, & Dowell RC (2020). Cochlear implantation outcomes in adults: A scoping review. PLOS ONE, 15(5), e0232421. 10.1371/journal.pone.0232421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Etler CP, O’Brien S, & Oleson JJ (2010). Effects of long-term use of a cochlear implant on the electrically evoked compound action potential. Journal of the American Academy of Audiology, 21(01), 005–015. 10.3766/jaaa.21.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, & Gantz B (1990). Electrically evoked whole-nerve action potentials: Data from human cochlear implant users. The Journal of the Acoustical Society of America, 88(3), 1385–1391. 10.1121/1.399716 [DOI] [PubMed] [Google Scholar]
- Buechner A, Bardt M, Haumann S, Geissler G, Salcher R, & Lenarz T (2022). Clinical experiences with intraoperative electrocochleography in cochlear implant recipients and its potential to reduce insertion trauma and improve postoperative hearing preservation. PLOS ONE, 17(4), e0266077. 10.1371/journal.pone.0266077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ML, Sladen DP, Gurgel RK, Tombers NM, Lohse CM, & Driscoll CL (2018). Survey of the american neurotology society on cochlear implantation: Part 1, candidacy assessment and expanding indications. Otology & Neurotology, 39(1), e12–e19. 10.1097/mao.0000000000001632 [DOI] [PubMed] [Google Scholar]
- Delgutte B (1980). Representation of speech-like sounds in the discharge patterns of auditory-nerve fibers. The Journal of the Acoustical Society of America, 68(3), 843–857. 10.1121/1.384824 [DOI] [PubMed] [Google Scholar]
- Delgutte B (1997). Auditory neural processing of speech in The Handbook of Phonetic Science. (Hardcastle WJ & Laver J, Eds.; pp. 507–538). Oxford: Blackwell. [Google Scholar]
- Delgutte B, & Kiang NYS (1984). Speech coding in the auditory nerve: IV. Sounds with consonant-like dynamic characteristics. The Journal of the Acoustical Society of America, 75(3), 897–907. 10.1121/1.390599 [DOI] [PubMed] [Google Scholar]
- Dong Y, Briaire JJ, Stronks HC, & Frijns JHM (2022). Speech perception performance in cochlear implant recipients correlates to the number and synchrony of excited auditory nerve fibers derived from electrically evoked compound action potentials. Ear and Hearing, Publish Ahead of Print. 10.1097/aud.0000000000001279 [DOI] [PubMed] [Google Scholar]
- Eisen MD, & Franck KH (2004). Electrically evoked compound action potential amplitude growth functions and hiresolution programming levels in pediatric CII implant subjects. Ear and Hearing, 25(6), 528–538. 10.1097/00003446-200412000-00002 [DOI] [PubMed] [Google Scholar]
- Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, Hullar TE, & Skinner MW (2008). Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otology & Neurotology, 29(7), 920–928. 10.1097/mao.0b013e318184f492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garadat SN, Zwolan TA, & Pfingst BE (2013). Using temporal modulation sensitivity to select stimulation sites for processor maps in cochlear implant listeners. Audiology and Neurotology, 18(4), 247–260. 10.1159/000351302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudey B, Plant K, Kiral I, Jimeno-Yepes A, Swan A, Gambhir M, Büchner A, Kludt E, Eikelboom RH, Sucher C, Gifford RH, Rottier R, & Anjomshoa H (2021). A multicenter analysis of factors associated with hearing outcome for 2,735 adults with cochlear implants. Trends in Hearing, 25, 233121652110375. 10.1177/23312165211037525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JJ, Suh M-W, Park MK, Koo J-W, Lee JH, & Oh SH (2019). A predictive model for cochlear implant outcome in children with cochlear nerve deficiency. Scientific Reports, 9(1). 10.1038/s41598-018-37014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Ahlstrom JB, Dias JW, Kerouac LB, McClaskey CM, Dubno JR, & Eckert Mark. A. (2021). Neural presbyacusis in humans inferred from age-related differences in auditory nerve function and structure. The Journal of Neuroscience, 41(50), 10293–10304. 10.1523/jneurosci.1747-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Abbas PJ, Doyle DV, McFayden TC, & Mulherin S (2016). Temporal response properties of the auditory nerve in implanted children with auditory neuropathy spectrum disorder and implanted children with sensorineural hearing loss. Ear and Hearing, 37(4), 397–411. 10.1097/aud.0000000000000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Shahsavarani BS, McFayden TC, Wang H, Gill KE, Xu L, Chao X, Luo J, Wang R, & He N (2018). Responsiveness of the electrically stimulated cochlear nerve in children with cochlear nerve deficiency. Ear and Hearing, 39(2), 238–250. 10.1097/aud.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Skidmore J, Conroy S, Riggs WJ, Carter BL, & Xie R (2022a). Neural adaptation of the electrically stimulated auditory nerve is not affected by advanced age in postlingually deafened, middle-aged, and elderly adult cochlear implant users. Ear and Hearing, 43(4), 1228–1244. 10.1097/aud.0000000000001184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Skidmore J, & Carter BL (2022b). Characteristics of the adaptation recovery function of the auditory nerve and its association with advanced age in postlingually deafened adult cochlear implant users. Ear and Hearing, 43(5), 1472–1486. 10.1097/aud.0000000000001198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Skidmore J, Carter BL, Lemeshow S, & Sun S (2022c). Postlingually deafened adult cochlear implant users with prolonged recovery from neural adaptation at the level of the auditory nerve tend to have poorer speech perception performance. Ear and Hearing, 43(6), 1761–1770. 10.1097/aud.0000000000001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Skidmore J, Koch B, Chatterjee M, Carter BL, & Yuan Y (2022d). Relationships between the auditory nerve sensitivity to amplitude modulation, perceptual amplitude modulation rate discrimination sensitivity, and speech perception performance in postlingually deafened adult cochlear implant users. Ear and Hearing, Publish Ahead of Print. 10.1097/aud.0000000000001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Teagle HFB, & Buchman CA (2017). The electrically evoked compound action potential: From laboratory to clinic. Frontiers in Neuroscience, 11. 10.3389/fnins.2017.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heutink F, Verbist BM, van der Woude W-J, Meulman TJ, Briaire JJ, Frijns JHM, Vart P, Mylanus EAM, & Huinck WJ (2021). Factors influencing speech perception in adults with a cochlear implant. Ear and Hearing, 42(4), 949–960. 10.1097/aud.0000000000000988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey M, Neben N, Stöver T, Baumann U, Mewes A, Liebscher T, Schüssler M, Aschendorff A, Wesarg T, Büchner A, Greenham P, & Hoppe U (2020). Outcomes for a clinically representative cohort of hearing-impaired adults using the Nucleus® CI532 cochlear implant. European Archives of Oto-Rhino-Laryngology, 277(6), 1625–1635. 10.1007/s00405-020-05893-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heydebrand G, & Skinner MW (2013). Factors affecting open-set word recognition in adults with cochlear implants. Ear and Hearing, 34(3), 342–360. 10.1097/aud.0b013e3182741aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden LK, Firszt JB, Reeder RM, Uchanski RM, Dwyer NY, & Holden TA (2016). Factors affecting outcomes in cochlear implant recipients implanted with a perimodiolar electrode array located in scala tympani. Otology & Neurotology, 37(10), 1662–1668. 10.1097/mao.0000000000001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ML, Castioni EE, Goehring JL, & Baudhuin JL (2012). Temporal response properties of the auditory nerve: Data from human cochlear-implant recipients. Hearing Research, 285(1–2), 46–57. 10.1016/j.heares.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CJ, Karoui C, Laborde M-L, Lepage B, Molinier C-É, Tartayre M, Escudé B, Deguine O, Marx M, & Fraysse B (2019). Early sentence recognition in adult cochlear implant users. Ear and Hearing, 40(4), 905–917. 10.1097/aud.0000000000000670 [DOI] [PubMed] [Google Scholar]
- Johnson DH (1980). The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. The Journal of the Acoustical Society of America, 68(4), 1115–1122. 10.1121/1.384982 [DOI] [PubMed] [Google Scholar]
- Kaandorp MW, Smits C, Merkus P, Festen JM, & Goverts ST (2017). Lexical-Access ability and cognitive predictors of speech recognition in noise in adult cochlear implant users. Trends in Hearing, 21, 233121651774388. 10.1177/2331216517743887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura T, & Nadol JB (2016). Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hearing Research, 339, 132–141. 10.1016/j.heares.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang WS, Lee JH, Lee HN, & Lee K-S (2010). Cochlear implantations in young children with cochlear nerve deficiency diagnosed by MRI. Otolaryngology–Head and Neck Surgery, 143(1), 101–108. 10.1016/j.otohns.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Kraaijenga VJC, Derksen TC, Stegeman I, & Smit AL (2018). The effect of side of implantation on unilateral cochlear implant performance in patients with prelingual and postlingual sensorineural hearing loss: A systematic review. Clinical Otolaryngology: Official Journal of ENT-UK; Official Journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery, 43(2), 440–449. 10.1111/coa.12988 [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham MA, Cunningham J, King CD, Koch DB, Nicol TG, McGee TJ, Stein LK, & Wright BA (2000). Consequences of neural asynchrony: A case of auditory neuropathy. Journal of the Association for Research in Otolaryngology, 1(1), 33–45. 10.1007/s101620010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz M, Sönmez H, Joseph G, Büchner A, & Lenarz T (2012). Cochlear implant performance in geriatric patients. The Laryngoscope, 122(6), 1361–1365. 10.1002/lary.23232 [DOI] [PubMed] [Google Scholar]
- Liang C, Wenstrup LH, Samy RN, Xiang J, & Zhang F (2020). The effect of side of implantation on the cortical processing of frequency changes in adult cochlear implant users. Frontiers in Neuroscience, 14. 10.3389/fnins.2020.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P-H, Wu H-P, Wu C-M, Chiang Y-T, Hsu JS, Tsai C-Y, Wang H, Tseng L-H, Chen P-Y, Yang T-H, Hsu C-J, Chen P-L, Wu C-C, & Liu T-C (2022). Cochlear implantation outcomes in patients with auditory neuropathy spectrum disorder of genetic and non-genetic etiologies: A multicenter study. Biomedicines, 10(7), 1523. 10.3390/biomedicines10071523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Holden TA, McClelland GH, Parkinson WS, Shelton C, Kelsall DC, & Smith ZM (2014). Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. Journal of the Association for Research in Otolaryngology, 15(2), 293–304. 10.1007/s10162-013-0437-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Laughlin M, Reilly RB, & Zeng F-G (2013). Rate and onset cues can improve cochlear implant synthetic vowel recognition in noise. The Journal of the Acoustical Society of America, 133(3), 1546–1560. 10.1121/1.4789940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire K, Firestone GM, Zhang N, & Zhang F (2021). The acoustic change complex in response to frequency changes and its correlation to cochlear implant speech outcomes. Frontiers in Human Neuroscience, 15. 10.3389/fnhum.2021.757254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC (2004). Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. The Journal of the Acoustical Society of America, 116(1), 452–468. 10.1121/1.1760795 [DOI] [PubMed] [Google Scholar]
- Miller CA, Woo J, Abbas PJ, Hu N, & Robinson BK (2011). Neural masking by sub-threshold electric stimuli: Animal and computer model results. Journal of the Association for Research in Otolaryngology, 12(2), 219–232. 10.1007/s10162-010-0249-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussoi BSS, & Brown CJ (2019). Age-Related changes in temporal resolution revisited. Ear and Hearing, 40(6), 1328–1344. 10.1097/aud.0000000000000732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K, & Nicholson N (2021). Cochlear implant behavioral outcomes for children with auditory neuropathy spectrum disorder: A mini-systematic review. American Journal of Audiology, 30(3), 777–789. 10.1044/2021_aja-20-00175 [DOI] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders. (2021). Cochlear Implants. (NIH Publication No. 00–4798). Washington, DC: U.S. Government Printing Office. [Google Scholar]
- Nourski KV, Abbas PJ, Miller CA, Robinson BK, & Jeng F-C (2007). Acoustic–electric interactions in the guinea pig auditory nerve: Simultaneous and forward masking of the electrically evoked compound action potential. Hearing Research, 232(1–2), 87–103. 10.1016/j.heares.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GE, & Lehiste I (1962). Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders, 27(1), 62–70. 10.1044/jshd.2701.62 [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Swiderski DL, Hughes AP, Strahl SB, Sinan M, & Raphael Y (2017). Neurotrophin gene therapy in deafened ears with cochlear implants: Long-term effects on nerve survival and functional measures. Journal of the Association for Research in Otolaryngology, 18(6), 731–750. 10.1007/s10162-017-0633-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Zhou N, Colesa DJ, Watts MM, Strahl SB, Garadat SN, Schvartz-Leyzac KC, Budenz CL, Raphael Y, & Zwolan TA (2015). Importance of cochlear health for implant function. Hearing Research, 322, 77–88. 10.1016/j.heares.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria. https://www.R-project.org [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Klis SFL, & Grolman W (2015). Recovery characteristics of the electrically stimulated auditory nerve in deafened guinea pigs: Relation to neuronal status. Hearing Research, 321, 12–24. 10.1016/j.heares.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SFL, & Grolman W (2014). Auditory-Nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. Journal of the Association for Research in Otolaryngology, 15(2), 187–202. 10.1007/s10162-013-0440-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G, Barker E, Mok M, Dowell R, Rincon A, & Garratt R (2007). Speech perception in noise for children with auditory neuropathy/dys-synchrony type hearing loss. Ear and Hearing, 28(3), 351–360. 10.1097/aud.0b013e3180479404 [DOI] [PubMed] [Google Scholar]
- Rasmussen KMB, West NC, Bille M, Sandvej MG, & Cayé-Thomasen P (2022). Cochlear implantation improves both speech perception and patient-reported outcomes: A prospective follow-up study of treatment benefits among adult cochlear implant recipients. Journal of Clinical Medicine, 11(8), 2257. 10.3390/jcm11082257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs WJ, Vaughan C, Skidmore J, Conroy S, Pellittieri A, Carter BL, Stegman CJ, & He S (2021). The sensitivity of the electrically stimulated auditory nerve to amplitude modulation cues declines with advanced age. Ear and Hearing, 42(5), 1358–1372. 10.1097/aud.0000000000001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz-Leyzac KC, Holden TA, Zwolan TA, Arts HA, Firszt JB, Buswinka CJ, & Pfingst BE (2020). Effects of electrode location on estimates of neural health in humans with cochlear implants. Journal of the Association for Research in Otolaryngology, 21(3), 259–275. 10.1007/s10162-020-00749-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz-Leyzac KC, & Pfingst BE (2016). Across-site patterns of electrically evoked compound action potential amplitude-growth functions in multichannel cochlear implant recipients and the effects of the interphase gap. Hearing Research, 341, 50–65. 10.1016/j.heares.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyyedi M, & Nadol JB Jr (2014). Intracochlear inflammatory response to cochlear implant electrodes in humans. Otology & Neurotology, 35(9), 1545–1551. 10.1097/mao.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallop JK (2002). Auditory neuropathy/dys-synchrony in adults and children. Seminars in Hearing, 23(3), 215–224. 10.1055/s-2002-34474 [DOI] [Google Scholar]
- Shepherd RK, Roberts LA, & Paolini AG (2004). Long-term sensorineural hearing loss induces functional changes in the rat auditory nerve. European Journal of Neuroscience, 20(11), 3131–3140. 10.1111/j.1460-9568.2004.03809.x [DOI] [PubMed] [Google Scholar]
- Skidmore J, Carter BL, Riggs WJ, & He S (2022a). The effect of advanced age on the electrode-neuron interface in cochlear implant users. Ear and Hearing, 43(4), 1300–1315. 10.1097/aud.0000000000001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore J, Ramekers D, Colesa DJ, Schvartz-Leyzac KC, Pfingst BE, & He S (2022b). A broadly applicable method for characterizing the slope of the electrically evoked compound action potential amplitude growth function. Ear and Hearing, 43(1), 150–164. 10.1097/aud.0000000000001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore J, Xu L, Chao X, Riggs WJ, Pellittieri A, Vaughan C, Ning X, Wang R, Luo J, & He S (2021). Prediction of the functional status of the cochlear nerve in individual cochlear implant users using machine learning and electrophysiological measures. Ear and Hearing, 42(1), 180–192. 10.1097/aud.0000000000000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahr AJ, Dorman MF, Litvak LM, Van Wie S, Gifford RH, Loizou PC, Loiselle LM, Oakes T, & Cook S (2012). Development and validation of the AzBio sentence lists. Ear and Hearing, 33(1), 112–117. 10.1097/aud.0b013e31822c2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Winter M, Derebery MJ, Oba S, & Michalewski HJ (1998). Transient deafness due to temperature-sensitive auditory neuropathy. Ear and Hearing, 19(3), 169–179. 10.1097/00003446-199806000-00001 [DOI] [PubMed] [Google Scholar]
- Tejani VD, Abbas PJ, & Brown CJ (2017). Relationship between peripheral and psychophysical measures of amplitude modulation detection in cochlear implant users. Ear and Hearing, 38(5), e268–e284. 10.1097/aud.0000000000000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijl RHM, Buitenhuis PJ, Stegeman I, Klis SFL, & Grolman W (2016). Systematic review of compound action potentials as predictors for cochlear implant performance. The Laryngoscope, 127(2), 476–487. 10.1002/lary.26154 [DOI] [PubMed] [Google Scholar]
- van Heteren JAA, Vonck BMD, Stokroos RJ, Versnel H, & Lammers MJW (2022). The acoustic change complex compared to hearing performance in unilaterally and bilaterally deaf cochlear implant users. Ear and Hearing, 43(6), 1783–1799. 10.1097/AUD.0000000000001248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, McCreery R, Spratford M, & Roush P (2016). Children with auditory neuropathy spectrum disorder fitted with hearing aids applying the american academy of audiology pediatric amplification guideline: Current practice and outcomes. Journal of the American Academy of Audiology, 27(3), 204–218. 10.3766/jaaa.15050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL, & Lazar NA (2016). The ASA statement on p-Values: Context, process, and purpose. The American Statistician, 70(2), 129–133. 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- Wei X, Li Y, Chen B, Gong Y, Fu Q-J, Liu T, Cui D, Su Q, & Shi Y (2017). Predicting auditory outcomes from radiological imaging in cochlear implant patients with cochlear nerve deficiency. Otology & Neurotology, 38(5), 685–693. 10.1097/mao.0000000000001382 [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, & Rabinowitz WM (1991). Better speech recognition with cochlear implants. Nature, 352(6332), 236–238. 10.1038/352236a0 [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, & Zerbi M (1997). Temporal representations with cochlear implants. The American Journal of Otology, 18(6), S30–34. [PubMed] [Google Scholar]
- Winn MB, Edwards JR, & Litovsky RY (2015). The impact of auditory spectral resolution on listening effort revealed by pupil dilation. Ear and Hearing, 36(4), e153–e165. 10.1097/aud.0000000000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F-G, & Liu S (2006). Speech perception in individuals with auditory neuropathy. Journal of Speech, Language, and Hearing Research, 49(2), 367–380. 10.1044/1092-4388(2006/029) [DOI] [PubMed] [Google Scholar]
- Zhang F, Benson C, Murphy D, Boian M, Scott M, Keith R, Xiang J, & Abbas P (2013). Neural adaptation and behavioral measures of temporal processing and speech perception in cochlear implant recipients. PLoS ONE, 8(12), e84631. 10.1371/journal.pone.0084631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen B, Kong Y, Liau N, Wei X, Shi Y, Chen J, Yang M, Dhanasingh A, & Li Y (2022). Analysis of long-term cochlear implantation outcomes and correlation with imaging characteristics in patients with common cavity deformity. Frontiers in Neuroscience, 16. 10.3389/fnins.2022.857855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao EE, Dornhoffer JR, Loftus C, Nguyen SA, Meyer TA, Dubno JR, & McRackan TR (2020). Association of patient-related factors with adult cochlear implant speech recognition outcomes: A meta-analysis. JAMA Otolaryngology–Head & Neck Surgery, 146(7), 613–620. 10.1001/jamaoto.2020.0662 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


