Abstract
The tau protein undergoes pathological changes in Alzheimer’s disease and other tauopathies that eventually lead to functional impairments. Over the years, several therapeutic approaches have been examined to slow or halt the progression of tau pathology but have yet to lead to an approved disease-modifying treatment. Of the drugs in clinical trials that directly target tau, immunotherapies are the largest category and mostly consist of antibodies in different stages of development. There is a reasonable optimism that at least some of these compounds will have a clinically meaningful efficacy. This view is based on the significant although modest efficacy of some antibodies targeting amyloid-β in Alzheimer’s disease and the fact that tau pathology correlates much better with the degree of dementia than amyloid-β lesions. In Alzheimer’s disease, clearing pathological tau may therefore improve function later in the disease process than when removing amyloid-β. This review provides a brief update on the active and passive clinical tau immunization trials with insight from preclinical studies. Various epitopes are being targeted and some of the antibodies are said to target extracellular tau but because almost all of pathological tau is found intracellularly, the most efficacious antibodies should be able to enter the cell.
Keywords: Alzheimer’s disease, tauopathies, tau protein, immunotherapy, vaccine, antibody, clinical trials
Introduction
There have been various developments in the field of tau immunotherapies since my previous review article on this topic published in your journal in 2018. Of the eight ongoing clinical trials at that time, three antibody trials have been discontinued but seven additional ones have started and are still ongoing (Table 1). At this time, there are two tau vaccines and nine tau antibodies in clinical trials in Phase I to III. While there are several possible reasons for why some of these approaches have failed, none have been safety related. In total, 14 antibodies have entered trials and two vaccines. The vaccines were among the earliest that entered trials but have not advanced as fast as some of the antibodies. Both are still in Phase II. Of the five antibodies that failed, two were discontinued in Phase I and three in Phase II.
Table 1:
Tau Immunotherapies that entered clinical trials
| Drug | Tau epitope | Antibody subclass | Clinical trials | Trial Status | Company |
|---|---|---|---|---|---|
| Active immunization | |||||
| AADvac-1 | Tau294–305 | N/A | NCT02579252, NCT03174886, NCT04445831 | Phase II | Axon Neuroscience SE |
| ACI-35 | p-Tau396/404 | N/A | NCT04445831 | Phase II | AC Immune SA, Janssen |
| Passive immunization | |||||
| APNmAb005 | Conformational epitope | - | NCT05344989 | Phase I | Aprinoia Therapeutics |
| Bepranemab | Tau235–246 | IgG4 | NCT03464227, NCT03605082, NCT04185415, NCT04658199, NCT04867616 | Phase II | UCB Biopharma, Genentech |
| BIIB076 | Mid-region | IgG1 | NCT03056729 | Discontinued | Biogen, Neurimmune, Eisai |
| E2814 | HVPGG motif, Tau299–303 and 362–366 | IgG1 | NCT04231513, NCT04971733, NCT05269394, NCT01760005 | Phase II/III | Eisai |
| Gosuranemab | Tau8–19 | IgG4 |
NCT02460094, NCT02658916, NCT03068468, NCT03658135, NCT03352557 |
Discontinued | Biogen, Bristol- Meyers Squibb |
| JNJ-63733657 | p-Tau217 | - |
NCT03375697 NCT03689153, NCT05407818, NCT04619420 |
Phase I | Janssen |
| Lu AF87908 | p-Tau396 | IgG1 | NCT04149860 | Phase I | H. Lundbeck A/S |
| MK-2214 | Unspecified, possibly p-Tau413 | - | jRCT2031220627, NCT05466422 |
Phase I | Merck |
| PNT001 | Cis p-Tau231 | - |
NCT04096287, NCT04677829 |
Phase I | Pinteon Therapeutics |
| PRX005 | Epitope in R1, R2 and R3 microtubule-binding repeats | - | Not registered | Phase I | Prothena |
| RG7345 | p-Tau422 | - | NCT02281786 | Discontinued | Roche |
| Semorinemab | N-terminus of all six isoforms | IgG4 |
NCT03289143, NCT03828747 |
Phase II | AC Immune SA, Genentech |
| Tilavonemab | Tau25–30 | IgG4 |
NCT02494024, NCT03413319, NCT03391765, NCT03744546, NCT02880956, NCT03712787 |
Discontinued | Abbvie, C2N Diagnostics LLC |
| Zagotenemab | Conformational epitope, Tau7–9 and 312–342 | - |
NCT02754830, NCT03019536, NCT03518073 |
Discontinued | Eli Lilly |
We have written several reviews on this topic since the 2018 publication [1–6]. For details on the animal studies that justified bringing these antibodies and vaccines to clinical trials, please refer to those prior reviews, although the preclinical work on some of them has yet to be reported in peer reviewed journals. It is also notable that when reported, some of the experimental design focuses on prevention of tau seeding, which may not prevent tau toxicity [5;7], and the efficacy of some of them is not supported by functional studies.
Here I will provide a brief overview of the status of the individual antibodies and vaccines that have entered trials since this approach was first reported to be efficacious as a vaccine and as an antibody [8–11] (Table 1). Possible reasons for why some of these potential treatments have failed will be discussed as well, and an overview will be provided of the various factors to be considered when designing therapeutic tau antibodies.
Discontinued clinical trials on tau antibodies
RG7345 recognizes phospho-serine 422 and was discontinued while being tested in healthy subjects that are thought to not have this epitope [12]. With that in mind, target engagement could not have been an issue so a poor pharmacokinetic profile may have been the reason. BIIB076 was described to bind to the mid-domain of tau and had shown target engagement in Phase I but its development was halted for business reasons [13].
The three antibodies that failed in Phase II had all been described to work only extracellularly, two bound solely to the N-terminus and one to a conformational discontinuous epitope consisting of the N-terminus and amino acids in the three hundred range [14–16]. All apparently engaged their epitope but none showed any indication of functional improvement to justify larger Phase III trials. Specifically Gosuranemab is an IgG4 antibody that binds to amino acids 15–22 of tau. Over 99% of the tau protein is found intracellularly and the extracellular fraction is thought to consist mostly of amino acids 150–250 [5;17-20]. Most recently, the majority of tau in the brain interstitial fluid was shown to be fragmented with a similar pattern of at least ten distinct fragments spanning the entire tau protein in three different mouse models of tauopathy [21]. Therefore, even though Gosuranemab showed a clear target engagement in the trials, clearing a very small extracellular fraction of tau was always unlikely to improve neuronal function. The same goes for Tilavonemab, another IgG4 antibody against amino acids 25–30 of the tau protein that also was described to only work extracellularly. It showed target engagement in clinical trials without functional improvements. The third antibody, Zagotenemab, differs from the other two because of its discontinuous epitope. Its antibody subclass was not described but it is the humanized version of the MC1 antibody. That antibody recognizes early forms of tau aggregates on tissue sections but it had not been useful to detect tau in biological fluids, perhaps because of its low affinity and its epitope may not be prominent extracellularly. With that in mind, it was said to work only extracellularly but it is unclear if it showed any target engagement in clinical trials. None of the findings from its trials have been published. It was discontinued in Phase II because it did not improve cognition, which was the primary outcome.
Ongoing clinical trials on tau immunotherapies
Active immunotherapy
The two tau vaccines in clinical trials have shown an excellent safety profile but have not advanced as rapidly through the different trial phases as some of the antibodies (Table 1).
AADvac1:
AADvac1 was the first tau immunotherapy that entered clinical trials [22–28]. The vaccine contains a tau peptide of amino acids 294–305 of the tau protein that is linked to keyhole limpet haemocyanin and is administered in an aluminum hydroxide adjuvant to boost its immunogenicity. Four Phase I to II trials on it have been completed, three in AD patients and one in patients with non-fluent agrammatic variant progressive aphasia (naPPA), which is a tauopathy that relates to AD and frontotemporal dementia. The AD trials have shown a strong immunogenicity and an excellent safety profile with at least some hints of efficacy based on evaluation of biomarkers, brain imaging and functional outcome that justifies larger stratified studies with sufficient statistical power to assess its clinical efficacy. The outcome of the naPPA study has yet to be reported.
ACI-35:
ACI-35 targets the p-tau396/404 epitope with the tau peptide incorporated into liposomes to improve its immunogenicity [29;30]. Its Phase I to II trials in AD patients have confirmed its safety but its immunogenicity was rather weak, which led to its modification by adding a second adjuvant and a T-cell helper epitope (ACI-35.030) or a carrier protein (JACI-35.054). The ACI-35.030 version led to a stronger antibody response than the JACI-35.054 version and is advancing to be tested in a larger AD population.
Passive immunotherapy
The antibodies that remain in trials target diverse tau epitopes and typically are of either the IgG1 subclass or the IgG4 subclass (Table 1). The former conveys effector function that should facilitate microglial phagocytosis of the antibody-tau complex, whereas the latter is neutral in that regard and thereby avoids possible inflammatory side effects associated with microglial activation.
APNmAb005:
APNmAb005 is a recent entry into this field. It has been described to recognize oligomeric and insoluble tau in various tauopathies [31]. Its subclass has not been revealed and it is currently being tested in healthy subjects in a Phase I safety study.
Bepranemab:
Bepranemab is an IgG4 antibody that recognizes amino acids 235–250 of the tau protein [32–34]. It has been said to bind to extracellular tau. Phase I trials in healthy subjects and patients with progressive supranuclear palsy (PSP) have not revealed any safety issues but some of the findings have yet to be reported and the PSP study is continuing as an open-label extension study. The antibody is currently in a Phase II trial in AD patients that is scheduled to end in 2025.
E2814:
E2814 is an IgG1 antibody that binds to HVPGG amino acids that are in the second and fourth repeat of the microtubule-binding domain of tau [35;36]. It has been reported to bind to extracellular tau. It is currently in Phase II/III trials in AD patients with familial mutations in the amyloid precursor protein or presenilins that are scheduled to end in 2027. The tau antibody will be administered with or without Lecanemab, the Aβ targeting antibody that was recently approved by the FDA. Earlier Phase I/II trials in healthy subjects and familial AD patients appeared to be safe with favorable pharmacokinetics and target engagement in CSF but only preliminary results have been reported.
JNJ-63733657:
JNJ-63733657 is an IgG1 antibody that binds to p-tau217 [37]. Three Phase I trials in healthy individuals and AD patients have been completed. Data from one of these trials has been reported showing a good safety profile and target engagement with similar pharmacokinetics in patients and healthy subjects. The antibody is currently in a Phase II study in AD patients that is scheduled to end in 2025.
Lu AF87908:
Lu AF87908 is an IgG1 antibody that was raised against the p-Ser396/404 epitope and it primarily binds to the pSer396 region [38–43]. Its Phase I study in healthy subjects and AD patients ended in July 2023 and its findings will presumably be released in the near future.
MK-2214:
MK-2214 does not have a reported epitope or subclass but it may be derived from a mouse antibody that binds to p-tau413 [44;45]. Two Phase I trials are ongoing in healthy subjects and patients with AD or mild cognitive impairment (MCI) that are supposed to end in 2024.
PNT001:
PNT001 binds to a unique cis conformation of tau around p-tau231 [46–49]. This form of tau is reported by the original developer of this antibody to be only found under pathological conditions and to be highly toxic. Its subclass has not been published. A phase I trial in healthy subjects did not reveal any safety issues but a second Phase I trial in patients with acute traumatic brain injury (TBI) was terminated early for unknown reasons.
PRX005:
PRX005 is an IgG1 antibody that binds to the microtubule binding region in both 3R and 4R tau isoforms [50]. It is currently in Phase I trials in healthy subjects and AD patients with no safety issues reported to date.
Semorinemab:
Semorinemab is an IgG4 antibody directed at the N-terminus of all six isoforms of the tau protein [51–54]. It has been reported to target extracellular tau. A Phase I trial in healthy subjects and AD patients did not reveal any safety concerns. A subsequent Phase II study in patients with prodromal or mild AD confirmed an excellent safety profile but it missed both primary and secondary efficacy endpoints. A second Phase II study in patients with moderate AD was completed in August 2023. Top line results showed that the antibody slowed decline on one cognitive test and reduced CSF tau but other cognitive or functional tests did not show efficacy and tau PET signal was not altered. It is not clear at this point if the antibody will advance into Phase III studies.
Mechanistic considerations for therapeutic tau antibodies.
Although there are no proven reasons for the failures of the antibodies that have been discontinued, one can speculate about likely reasons based on what we know about tau biology and chemistry.
Extracellular vs intracellular tau:
First of all, antibodies that only work extracellularly are less likely to have clinical benefits than antibodies that can work both extra- and intracellularly. This is based on the fact that only a tiny fraction of tau is found extracellularly (much less than one percent, [5;17]), even considering that tau levels in brain interstitial fluid are about ten times higher than in CSF, both in humans and mice [55;56]. As mentioned before, most of tau in CSF and brain interstitial fluid is fragmented [18–21]. A caveat there is that it is not clear whether antibodies said to target only extracellular tau were tested sufficiently to exclude intracellular targeting. Note also that antibody humanization can dramatically change antibody properties, including their ability to enter cells ([7], see Antibody Charge section).
Although extracellular tau may be involved in the spread of tau pathology through anatomically connected regions, focusing solely on clearing it while leaving intracellular tau intact can only be expected to have a modest impact. The best chance of success for extracellular tau antibodies would be in presymptomatic cases before the burden of intracellular tau becomes high enough to result in functional impairments. The reason to focus on extracellular tau seems to be based on the assumption that targeting tau intracellularly would be associated with adverse reactions that could derail further development of the antibodies. I am not aware of any studies that support this argument. In contrast, numerous studies by my laboratory and others have showed that tau antibodies that work both extra- and intracellularly are not associated with any particular toxicity specifically linked to their ability to enter cells [7;9;46;57-69]. This may in part be related to the fact that most of the antibody-tau interactions within the cell are likely to take place within the endosomal-lysosomal system following endosomal uptake of the antibodies, primarily via receptor-mediated uptake (about 80%) and to some extent via non-specific bulk endocytosis (about 20%) [7;57-60;62;66]. The antibodies will then meet their tau target when autophagosomes with tau aggregates fuse with endosomes containing the antibodies. In the lysosomes, efficacious antibodies will then disassemble tau aggregates to allow access of lysosomal enzymes to degrade them. Not all antibodies found with tau in lysosomes are effective. Depending on their affinity and/or epitope, some may make the aggregates more compact and thereby more difficult to degrade. A cytosolic pathway via the proteasome is likely to be also in play. We have shown that some antibodies leak into the cytosol, presumably from bursting or leaky endosomes [59]. Mallery et al identified a high affinity Fc binding site on TRIM21, a ubiquitin E3 ligase that facilitates ubiquitination and thereby subsequent proteosomal clearance of antibody-target complex [70]. It was first reported to be involved in promoting clearance of antibody-virus complexes and later of antibody-tau complexes [65;69;70]. It is highly unlikely that a high affinity binding site for the Fc-moiety of antibodies can exist intracellularly without a biological role of antibodies inside cells. As a matter of fact, most if not all cells have extracellular receptors that recognize the Fc region of antibodies. This feature may have evolved to allow antibody inside cells to neutralize viruses and possibly other intracellular pathogens. When I was writing my first grant on this topic, that was the argument I used to justify targeting tau intracellularly with antibodies [71].
The dramatic difference in the amount of tau inside vs outside cells is likely to be even more pronounced in primary tauopathies compared to AD. In contrast to AD that presents with increased levels of tau in CSF, primary tauopathies such as progressive supranuclear palsy, corticobasal degeneration and Pick’s disease do not have increased tau levels in CSF compared to controls [72]. This makes it even less likely for tau antibodies that solely work extracellularly to be effective in those tauopathies. With this in mind, it is not clear why extracellular tau antibodies were tested in primary tauopathies (Gosuranemab and Tilavonemab) in trials that were eventually discontinued, or why another extracellular antibody (Bepranemab) is being examined in progressive supranuclear palsy.
Tau epitope:
Various tau epitope have and are being targeted and it is hard to say which one may be best (Table 1). Based on studies by us and others, subtle differences in binding within the same tau region can greatly influence efficacy, which suggests that serendipity may eventually guide us to the most efficacious antibodies in human patients. However, targeting certain regions of tau is likely to be more efficacious than others.
In our initial studies, we focused on phospho-tau epitopes because this posttranslational modification is most strongly associated with pathological tau. One of the most prominent phospho-tau epitope consists of p-tau396/404. In our initial active vaccination studies that region turned out to be also very immunogenic which led us to successfully show its efficacy in clearing pathological tau and improving function in tauopathy mice, followed by similar findings using the prototype antibody against this region, PHF1 [8–11], whose efficacy was confirmed by others [73]. Notably, targeting this region is being examined in one of the active immunization trials (ACI-35) and one of the passive immunization trials (Lu AF87908). Other phospho-tau epitopes that are being examined include p-tau217 (JNJ-63733657), cis p-tau231 (PNT001; its mouse version was shown to work intracellularly), and possibly p-tau413 (MK-2214). One antibody targeting p-tau422 failed early in healthy subjects, presumably for pharmacokinetic reasons. This particular epitope has been described to be only found in tauopathy and not in healthy subjects so the failure cannot have been related to lack of target engagement.
The other vaccine/antibodies in trials or that have failed target normal tau epitopes apart from Zagotenemab that failed and targeted a discontinuous conformational tau epitope that is only found in pathological tau. As mentioned above, antibodies that target the N-terminus have been discontinued because they did not result in functional improvements (Gosuranemab and Tilavonemab). The third one targeting this epitope has failed in one trial in prodromal and early AD while resulting in some functional improvements in moderate AD (Semorinemab). Its further development is being considered but it is hard to see it being very effective if it only acts extracellularly as described for the mouse antibody. As mentioned before, not only is extracellular tau a tiny fraction of intracellular tau but most of extracellular tau has been described to lack the N-terminus, which should further reduce its efficacy [18–20]. With this in mind, several antibodies that later entered clinical trials target the mid-domain of tau (Bepranemab and JNJ-63733657) and thereby the largest fraction of extracellular tau, or the microtubule binding region (E2814 and PRX005) which is closely linked to tau aggregation. Of these, Bepranemab and E2814 have been described to work only extracellularly. Since Bepranemab targets the largest extracellular tau fragment, it is more likely to work than other extracellular tau antibodies against tau epitopes found in smaller amounts outside cells. Like the N-terminus, the microtubule binding region is only a small fraction of extracellular tau but this antibody has already advanced to Phase II/III trials based on it safety, favorable pharmacokinetic profile and target engagement in CSF. It should be noted though that target engagement does not predict efficacy but is a required feature to advance the antibody into larger trials.
Of the other antibodies in trials, the epitope of APNmAb005 has not been reported.
Antibody subclass:
For antibodies that have revealed subclass, only two have been examined in clinical trials, IgG1 and IgG4 (see Table 1). IgG1 antibodies have full effector function and are selected to enhance efficacy by promoting microglial phagocytosis of antibody-tau complexes. Conversely, IgG4 antibodies are devoid of effector function and are selected for safety reasons to minimize adverse inflammatory reactions. There are not many reports examining this issue for tau antibodies. An early study looked at this for two antibodies but their binding sites differed as well so no firm conclusions can be derived from that study [74]. Later, this issue was examined in culture for an antibody that binds to the same tau region as the AADvac1 antibody. It concluded that an IgG1 subclass was preferable but there was not a major difference in the efficacy of the subclasses, the strong effect of tau on microglial cytokine release made it difficult to evaluate subclass effect on that inflammatory effect, and microglia in culture behave differently from the same cells in vivo [75]. This was also looked into for Semorinemab, reaching a different conclusion that an IgG4 subclass was preferable because the IgG1 version was toxic in culture based on it causing MAP2 fragmentation in the cells [51]. However, the IgG1 subclass of Semorinemab showed target engagement at a lower dose than its IgG4 subclass and both had an excellent safety profile in vivo [52]. A third study examining tau antibody subclasses showed a similar effect of mouse IgG2a (closest to human IgG1) and mouse IgG1 (less effector function) on clearing several soluble and insoluble phospho-tau epitopes as quantified on western blots, but IgG1 seemed to be better at clearing one phospho-tau epitope on immunostained brain sections [76]. My laboratory looked into this more closely recently by studying cellular uptake and efficacy of all of the mouse subclasses (IgG1, IgG2a, IgG2b and IgG3) in culture models [68]. The IgG1 and IgG2a were the most efficacious and the IgG3 was the least efficacious. Mouse IgG2a and IgG3 are equivalent to human IgG1 and IgG4. None of the subclasses were specifically linked to toxicity although for one of the tau antibodies, all of the bivalent IgGs were toxic compared to the parent single domain antibody. This suggests that for that particular antibody, a bivalent binding stabilized a toxic conformation of the tau protein. We are examining that intriguing possibility further in in vivo studies. In summary, apart from culture studies, there is no in vivo evidence that an IgG4 subclass is any safer than an IgG1 subclass but at least one in vivo study indicates that Semorinemab on an IgG1 backbone is more efficacious than that antibody on an IgG4 backbone [52]. Keeping in mind that extracellular tau is only a miniscule fraction of total tau it is unlikely that microglial clearance of tau-IgG1 complexes will have notable adverse reactions.
Antibody affinity:
Generally speaking, higher affinity of an antibody for its target is often thought to translate to better efficacy in neutralizing or clearing that target. This may be the case for many targets but our experience is that it does not always apply to antibody-mediated clearance of pathological tau. For example, our 4E6 antibody targeting the p-tau396/404 region has a rather low affinity for this target (around 10−7 M) and is very effective in clearing pathological tau in culture and in vivo resulting in functional improvements [7;58;62;64;66]. On the other hand, our 6B2 antibody which targets the same region with a high affinity (up to 10−10 M), albeit a slightly different binding site, is ineffective in clearing tau and does not improve function [7;58;62;64;66]. Both can be found within neurons in the endosomal-lysosomal system after administration. A possible explanation for their contrasting effects is that the relatively modest 4E6 binding to tau may loosen up tau aggregates within the lysosomes and thereby allow better access of lysosomal enzymes to degrade tau, whereas the strong binding of 6B2 may render the aggregates more compact and thereby more difficult to degrade. This is likely epitope dependent, considering that for a couple of our antibodies that specifically or selectively recognize tau that is truncated an amino acid 421, higher affinity conveys better efficacy [77;78].
Antibody charge:
To consider the influence of overall charge of an antibody on its properties such as uptake into neurons has not received much attention in the neurodegeneration field but it is a key consideration in other fields such as for cancer antibodies to try to improve their access into tumors. Our initial findings that antibodies can enter neurons and bind to pathological tau was first met with disbelief or indifference although it was already clear from other fields such as in virology that antibodies could have those properties [9]. This has been the case in the tau immunotherapy field as well with several groups showing uptake of monoclonal tau antibodies into neurons [7;9;46;57-69]. Neuronal uptake of antibodies has also been reported in models of Aβ amyloidosis, Parkinson’s disease and amyotrophic lateral sclerosis [79–85]. Autoantibodies that can be detrimental have also been shown to be taken up into neurons [86–92]. Over the years, we have shed some light on this issue and implicated Fc receptors as a major pathway for uptake of tau antibodies into neurons, and showed that differences in uptake can at least in part be explained by charge differences [7;57-60;62;66]. In our hands, antibodies that have a close to a neutral overall charge (isoelectric point (IEP)) are taken up to a larger extent than very basic or acidic antibodies [7]. In light of the fact that over 99% of tau, including its pathological forms, is found within cells [5;17], in particular neurons, the charge of antibodies needs to be taken into consideration when advancing antibodies within or from preclinical studies to clinical trials. Notably, antibody humanization typically replaces most of the mouse sequence with a human sequence, which can result in a completely difference charge of the antibody. We noted this for our 4E6 mouse antibody that has an IEP around 6.5 but its chimeric version with most of the mouse scaffold replaced with a human scaffold has an IEP around 9.0, and unlike the mouse version does not enter neurons and thereby cannot neutralize the toxicity of or clear intracellular tau [7]. With this in mind, it is entirely unclear if the tau antibodies in clinical trials that were described not to enter neurons have maintained such restricted ability in their humanized versions. It would be helpful if the drug companies with antibodies in trials would published their detailed characterization of their humanized antibodies and if/how their properties may differ from their mouse counterparts.
Antibody size:
All of the tau antibodies in clinical trials are whole antibodies that consist of an Fc moiety that is linked to two Fab arms with an overall molecular weight of around 150 kDa (Figure 1). This is also the case for most of the preclinical studies on potentially therapeutic tau antibodies. Their key advantages are a long half-life of 1–4 weeks and the ability to bind to two targets at the same time. Their key disadvantage is large size that may limit uptake into the brain and cells and prevent them from accessing cryptic epitopes that may have therapeutic relevance. Their Fc-region that can convey effector function such as to enhance microglial phagocytosis of tau-antibody complexes can be seen as an advantage to enhance efficacy or a disadvantage by leading to adverse inflammation. Antibody fragments like Fabs (50 kDa), single chain variable fragments (scFvs, 25 kDa) and single domain antibodies (sdAbs, VHHs, 15 kDa) have shorter half-lives and would typically be administered as single units that prevents the avidity enhancement that can be obtained by a bivalent antibody. However, their smaller size compared to whole antibodies should allow greater brain entry and perhaps enhanced cellular uptake to access the largest pool of pathological tau. sdAbs in particular should be able to access novel cryptic epitopes that may provide a therapeutic advantage. Their single unit facilitates cellular folding, which renders them ideally suited for gene therapy that may end up being the preferred approach to prophylactically target tau in familial tauopathies.
Figure 1:
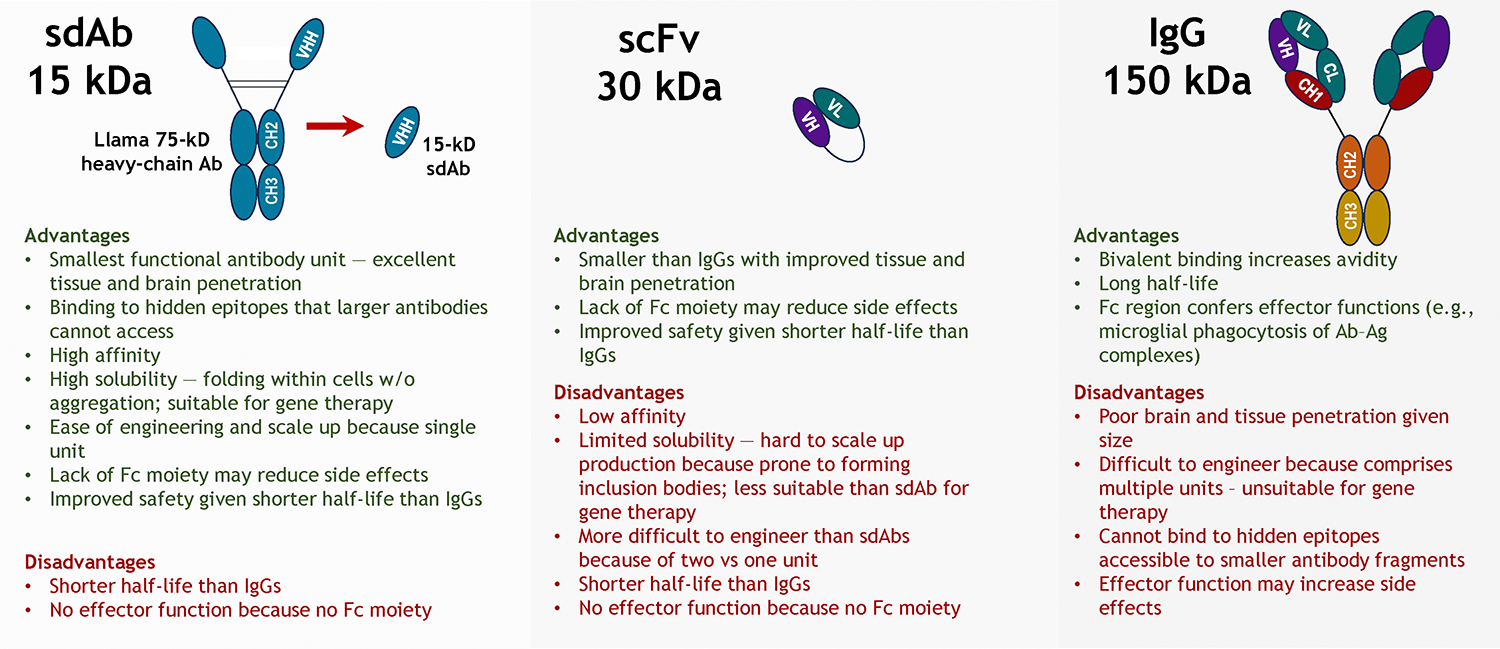
Advantages and disadvantages of whole antibodies versus antibody fragments.
Conclusions
Tau immunotherapies are the largest segment of clinical trials on therapies that directly target the tau protein. Although a few tau antibodies have failed in trials, several other tau antibodies remain in Phase I-III stages of examination to determine if they can directly slow or halt the progression of tau pathology in human patients. Two tau vaccines are also continuing in trials with additional antibodies and vaccines likely to enter clinical testing in the near future. It took about 24 years from the first in vivo report of a successful Aβ vaccination in a mouse model to the approval of a therapeutic Aβ antibody [93]. A similar lag time from the first in vivo report of a successful tau vaccination in a mouse model to an approval of a therapeutic tau antibody would mean that we could expect an approval of a tau antibody in 2030–31 [8;9]. As outlined in this review, targeting tau is likely to be more complex than targeting Aβ. Specifically, pathological tau protein is primarily found intracellularly whereas Aβ lesions are mostly extracellular. In addition, the tau protein is about 10 times the size of Aβ that greatly increases possible binding sites for the antibodies, and it undergoes various posttranslational modifications that exceed such changes for Aβ. Collectively, these differences render tau a more complicated target than Aβ. However, greater mechanistic insight into how these therapies work as reviewed here is likely to speed up the development of efficacious tau antibodies to slow or halt the progression of all tauopathies.
Acknowledgements:
EMS is currently supported by NIH grants R01 AG032611, R01 NS077239, RF1 NS120488 and R56 AG083436.
Footnotes
Conflict of Interest:
EMS is an inventor on patents on tau immunotherapies that are assigned to New York University. Some of those patents are licensed to H. Lundbeck A/S.
References
- [1].Congdon EE, Ji C, Tetlow AM, Jiang Y, Sigurdsson EM (2023) Tau-targeting therapies for Alzheimer disease: current status and future directions. Nat Rev Neurol 19, 715–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ji C, Sigurdsson EM (2021) Current Status of Clinical Trials on Tau Immunotherapies. Drugs 81, 1135–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Congdon EE, Jiang Y, Sigurdsson EM (2022) Targeting tau only extracellularly is likely to be less efficacious than targeting it both intra- and extracellularly. Semin Cell Dev Biol 126, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sandusky-Beltran LA, Sigurdsson EM (2020) Tau immunotherapies: Lessons learned, current status and future considerations. Neuropharmacology 175, 108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sigurdsson EM (2019) Alzheimer’s therapy development: A few points to consider. Prog Mol Biol Transl Sci 168, 205–217. [DOI] [PubMed] [Google Scholar]
- [6].Congdon EE, Sigurdsson EM (2018) Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol 14, 399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Congdon EE, Chukwu JE, Shamir DB, Deng J, Ujla D, Sait HBR, Neubert TA, Kong XP, Sigurdsson EM (2019) Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy. EBioMedicine 42, 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Asuni AA, Quartermain D, Sigurdsson EM (2006) Tau-based Immunotherapy for Dementia. Alzheimer’s & Dementia 2, S40. [Google Scholar]
- [9].Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM (2007) Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 27, 9115–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM (2010) Passive tau immunotherapy diminishes functional decline and clears tau aggregates in a mouse model of tauopathy. Alzheimer’s & Dementia 6, S578. [Google Scholar]
- [11].Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM (2011) Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem 118, 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alzheimer Research Forum, Drugs in Clinical Trials: RG7345, https://www.alzforum.org/therapeutics/rg7345, Last updated Nov 20, 2015, Accessed October 26, 2023.
- [13].Alzheimer Research Forum, Drugs in Clinical Trials: BIBB076, https://www.alzforum.org/therapeutics/biib076, Last updated July 28, 2022, Accessed October 26, 2023.
- [14].Alzheimer Research Forum, Drugs in Clinical Trials: Gosuranemab, https://www.alzforum.org/therapeutics/gosuranemab, Last updated January 17, 2022, Accessed October 26, 2023.
- [15].Alzheimer Research Forum, Drugs in Clinical Trials: Tilavonemab, https://www.alzforum.org/therapeutics/tilavonemab, Last updated February 7, 2023, Accessed October 26, 2023.
- [16].Alzheimer Research Forum, Drugs in Clinical Trials: Zagotenemab, https://www.alzforum.org/therapeutics/zagotenemab, Last updated October 29, 2021, Accessed October 26, 2023.
- [17].Han P, Serrano G, Beach TG, Caselli RJ, Yin J, Zhuang N, Shi J (2017) A Quantitative Analysis of Brain Soluble Tau and the Tau Secretion Factor. J Neuropathol Exp Neurol 76, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barthelemy NR, Gabelle A, Hirtz C, Fenaille F, Sergeant N, Schraen-Maschke S, Vialaret J, Buee L, Junot C, Becher F, Lehmann S (2016) Differential Mass Spectrometry Profiles of Tau Protein in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease, Progressive Supranuclear Palsy, and Dementia with Lewy Bodies. J Alzheimers Dis 51, 1033–1043. [DOI] [PubMed] [Google Scholar]
- [19].Barthelemy NR, Fenaille F, Hirtz C, Sergeant N, Schraen-Maschke S, Vialaret J, Buee L, Gabelle A, Junot C, Lehmann S, Becher F (2016) Tau Protein Quantification in Human Cerebrospinal Fluid by Targeted Mass Spectrometry at High Sequence Coverage Provides Insights into Its Primary Structure Heterogeneity. J Proteome Res 15, 667–676. [DOI] [PubMed] [Google Scholar]
- [20].Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, Sullivan M, Crisp MJ, Kasten T, Kirmess KM, Kanaan NM, Yarasheski KE, Baker-Nigh A, Benzinger TLS, Miller TM, Karch CM, Bateman RJ (2018) Tau Kinetics in Neurons and the Human Central Nervous System. Neuron 97, 1284–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barini E, Plotzky G, Mordashova Y, Hoppe J, Rodriguez-Correa E, Julier S, LePrieult F, Mairhofer I, Mezler M, Biesinger S, Cik M, Meinhardt MW, Ercan-Herbst E, Ehrnhoefer DE, Striebinger A, Bodie K, Klein C, Gasparini L, Schlegel K (2022) Tau in the brain interstitial fluid is fragmented and seeding-competent. Neurobiol Aging 109, 64–77. [DOI] [PubMed] [Google Scholar]
- [22].Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M (2014) First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res Ther 6, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, Vince-Kazmerova Z, Katina S, Fialova L, Prcina M, Parrak V, Dal-Bianco P, Brunner M, Staffen W, Rainer M, Ondrus M, Ropele S, Smisek M, Sivak R, Winblad B, Novak M (2017) Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol 16, 123–134. [DOI] [PubMed] [Google Scholar]
- [24].Novak P, Kovacech B, Katina S, Schmidt R, Scheltens P, Kontsekova E, Ropele S, Fialova L, Kramberger M, Paulenka-Ivanovova N, Smisek M, Hanes J, Stevens E, Kovac A, Sutovsky S, Parrak V, Koson P, Prcina M, Galba J, Cente M, Hromadka T, Filipcik P, Piestansky J, Samcova M, Prenn-Gologranc C, Sivak R, Froelich L, Fresser M, Rakusa M, Harrison J, Hort J, Otto M, Tosun D, Ondrus M, Winblad B, Novak M, Zilka N (2021) ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat Aging 1, 521–534. [DOI] [PubMed] [Google Scholar]
- [25].Novak P, Zilka N, Zilkova M, Kovacech B, Skrabana R, Ondrus M, Fialova L, Kontsekova E, Otto M, Novak M (2019) AADvac1, an Active Immunotherapy for Alzheimer’s Disease and Non Alzheimer Tauopathies: An Overview of Preclinical and Clinical Development. J Prev Alzheimers Dis 6, 63–69. [DOI] [PubMed] [Google Scholar]
- [26].Novak P, Schmidt R, Kontsekova E, Kovacech B, Smolek T, Katina S, Fialova L, Prcina M, Parrak V, Dal-Bianco P, Brunner M, Staffen W, Rainer M, Ondrus M, Ropele S, Smisek M, Sivak R, Zilka N, Winblad B, Novak M (2018) FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res Ther 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Paholikova K, Salingova B, Opattova A, Skrabana R, Majerova P, Zilka N, Kovacech B, Zilkova M, Barath P, Novak M (2015) N-terminal truncation of microtubule associated protein tau dysregulates its cellular localization. J Alzheimers Dis 43, 915–926. [DOI] [PubMed] [Google Scholar]
- [28].Alzheimer Research Forum, Drugs in Clinical Trials: AADvac1, https://www.alzforum.org/therapeutics/aadvac1, Last updated June 16, 2021, Accessed October 26, 2023.
- [29].Theunis C, Crespo-Biel N, Gafner V, Pihlgren M, Lopez-Deber MP, Reis P, Hickman DT, Adolfsson O, Chuard N, Ndao DM, Borghgraef P, Devijver H, van LF, Pfeifer A, Muhs A (2013) Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One 8, e72301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Alzheimer Research Forum, Drugs in Clinical Trials: ACI-35, http://www.alzforum.org/therapeutics/aci-35, Last updated January 2, 2023, Accessed October 26, 2023.
- [31].Alzheimer Research Forum, Drugs in Clinical Trials: APNmAb005, https://www.alzforum.org/therapeutics/apnmab005, Last updated February 7, 2023, Accessed October 26, 2023.
- [32].Courade JP, Angers R, Mairet-Coello G, Pacico N, Tyson K, Lightwood D, Munro R, McMillan D, Griffin R, Baker T, Starkie D, Nan R, Westwood M, Mushikiwabo ML, Jung S, Odede G, Sweeney B, Popplewell A, Burgess G, Downey P, Citron M (2018) Epitope determines efficacy of therapeutic anti-Tau antibodies in a functional assay with human Alzheimer Tau. Acta Neuropathol 136, 729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Albert M, Mairet-Coello G, Danis C, Lieger S, Caillierez R, Carrier S, Skrobala E, Landrieu I, Michel A, Schmitt M, Citron M, Downey P, Courade JP, Buee L, Colin M (2019) Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain 142, 1736–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Alzheimer Research Forum, Drugs in Clinical Trials: Bepranemab, https://www.alzforum.org/therapeutics/bepranemab, Last updated January 23, 2023, Accessed October 26, 2023.
- [35].Roberts M, Sevastou I, Imaizumi Y, Mistry K, Talma S, Dey M, Gartlon J, Ochiai H, Zhou Z, Akasofu S, Tokuhara N, Ogo M, Aoyama M, Aoyagi H, Strand K, Sajedi E, Agarwala KL, Spidel J, Albone E, Horie K, Staddon JM, de SR (2020) Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer’s disease. Acta Neuropathol Commun 8, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Alzheimer Research Forum, Drugs in Clinical Trials: E2814, https://www.alzforum.org/therapeutics/e2814, Last updated October 23, 2023, Accessed October 26, 2023.
- [37].Alzheimer Research Forum, Drugs in Clinical Trials: JNJ-63733657, https://www.alzforum.org/therapeutics/jnj-63733657, Last updated January 23, 2023, Accessed October 27, 2023.
- [38].Chukwu JE, Pedersen JT, Pedersen LO, Volbracht C, Sigurdsson EM, Kong XP (2018) Tau Antibody Structure Reveals a Molecular Switch Defining a Pathological Conformation of the Tau Protein. Sci Rep 8, 6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rosenqvist N, Asuni AA, Andersson CR, Christensen S, Daechsel JA, Egebjerg J, Falsig J, Helboe L, Jul P, Kartberg F, Pedersen LO, Sigurdsson EM, Sotty F, Skjodt K, Stavenhagen JB, Volbracht C, Pedersen JT (2018) Highly specific and selective anti-pS396-tau antibody C10.2 targets seeding-competent tau. Alzheimers Dement (N Y ) 4, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Andersson CR, Falsig J, Stavenhagen JB, Christensen S, Kartberg F, Rosenqvist N, Finsen B, Pedersen JT (2019) Antibody-mediated clearance of tau in primary mouse microglial cultures requires Fcgamma-receptor binding and functional lysosomes. Sci Rep 9, 4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Helboe L, Rosenqvist N, Volbracht C, Pedersen LO, Pedersen JT, Christensen S, Egebjerg J, Christoffersen CT, Bang-Andersen B, Beach TG, Serrano GE, Falsig J (2022) Highly Specific and Sensitive Target Binding by the Humanized pS396-Tau Antibody hC10.2 Across a Wide Spectrum of Alzheimer’s Disease and Primary Tauopathy Postmortem Brains. J Alzheimers Dis 88, 207–228. [DOI] [PubMed] [Google Scholar]
- [42].Jacobsen AM, van de Merbel NC, Ditlevsen DK, Tvermosegaard K, Schalk F, Lambert W, Bundgaard C, Pedersen JT, Rosenqvist N (2023) A Quantitative LC-MS/MS Method for Distinguishing the Tau Protein Forms Phosphorylated and Nonphosphorylated at Serine-396. J Am Soc Mass Spectrom 34, 441–451. [DOI] [PubMed] [Google Scholar]
- [43].Alzheimer Research Forum, Drugs in Clinical Trials: Lu-AF87908, https://www.alzforum.org/therapeutics/lu-af87908, Last updated February 28, 2023, Accessed October 27, 2023.
- [44].Umeda T, Eguchi H, Kunori Y, Matsumoto Y, Taniguchi T, Mori H, Tomiyama T (2015) Passive immunotherapy of tauopathy targeting pSer413-tau: a pilot study in mice. Ann Clin Transl Neurol 2, 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Alzheimer Research Forum, Drugs in Clinical Trials: MK-2214, https://www.alzforum.org/therapeutics/mk-2214, Last updated February 23, 2023, Accessed October 27, 2023.
- [46].Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, Zhou XZ, Lu KP (2015) Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523, 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lu KP, Kondo A, Albayram O, Herbert MK, Liu H, Zhou XZ (2016) Potential of the Antibody Against cis-Phosphorylated Tau in the Early Diagnosis, Treatment, and Prevention of Alzheimer Disease and Brain Injury. JAMA Neurol 73, 1356–1362. [DOI] [PubMed] [Google Scholar]
- [48].Qiu C, Albayram O, Kondo A, Wang B, Kim N, Arai K, Tsai CY, Bassal MA, Herbert MK, Washida K, Angeli P, Kozono S, Stucky JE, Baxley S, Lin YM, Sun Y, Rotenberg A, Caldarone BJ, Bigio EH, Chen X, Tenen DG, Zeidel M, Lo EH, Zhou XZ, Lu KP (2021) Cis P-tau underlies vascular contribution to cognitive impairment and dementia and can be effectively targeted by immunotherapy in mice. Sci Transl Med 13, eaaz7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Alzheimer Research Forum, Drugs in Clinical Trials: PNT001, https://www.alzforum.org/therapeutics/pnt001, Last updated January 26, 2022, Accessed October 27, 2023.
- [50].Alzheimer Research Forum, Drugs in Clinical Trials: PRX005, https://www.alzforum.org/therapeutics/prx005, Last updated February 6, 2023, Accessed October 27, 2023.
- [51].Lee SH, Le Pichon CE, Adolfsson O, Gafner V, Pihlgren M, Lin H, Solanoy H, Brendza R, Ngu H, Foreman O, Chan R, Ernst JA, DiCara D, Hotzel I, Srinivasan K, Hansen DV, Atwal J, Lu Y, Bumbaca D, Pfeifer A, Watts RJ, Muhs A, Scearce-Levie K, Ayalon G (2016) Antibody-Mediated Targeting of Tau In Vivo Does Not Require Effector Function and Microglial Engagement. Cell Rep 16, 1690–1700. [DOI] [PubMed] [Google Scholar]
- [52].Ayalon G, Lee SH, Adolfsson O, Foo-Atkins C, Atwal JK, Blendstrup M, Booler H, Bravo J, Brendza R, Brunstein F, Chan R, Chandra P, Couch JA, Datwani A, Demeule B, DiCara D, Erickson R, Ernst JA, Foreman O, He D, Hotzel I, Keeley M, Kwok MCM, Lafrance-Vanasse J, Lin H, Lu Y, Luk W, Manser P, Muhs A, Ngu H, Pfeifer A, Pihlgren M, Rao GK, Scearce-Levie K, Schauer SP, Smith WB, Solanoy H, Teng E, Wildsmith KR, Bumbaca YD, Ying Y, Fuji RN, Kerchner GA (2021) Antibody semorinemab reduces tau pathology in a transgenic mouse model and engages tau in patients with Alzheimer’s disease. Sci Transl Med 13, eabb2639. [DOI] [PubMed] [Google Scholar]
- [53].Monteiro C, Toth B, Brunstein F, Bobbala A, Datta S, Ceniceros R, Sanabria Bohorquez SM, Anania VG, Wildsmith KR, Schauer SP, Lee J, Dolton MJ, Ramakrishnan V, Abramzon D, Teng E (2023) Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants With Mild-to-Moderate Alzheimer Disease: Lauriet. Neurology 101, e1391–e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Alzheimer Research Forum, Drugs in Clinical Trials: Semorinemab, https://www.alzforum.org/therapeutics/semorinemab, Last updated September 14, 2023, Accessed October 27, 2023.
- [55].Herukka SK, Rummukainen J, Ihalainen J, von Und Zu FM, Koivisto AM, Nerg O, Puli LK, Seppala TT, Zetterberg H, Pyykko OT, Helisalmi S, Tanila H, Alafuzoff I, Hiltunen M, Rinne J, Soininen H, Jaaskelainen JE, Leinonen V (2015) Amyloid-beta and Tau Dynamics in Human Brain Interstitial Fluid in Patients with Suspected Normal Pressure Hydrocephalus. J Alzheimers Dis 46, 261–269. [DOI] [PubMed] [Google Scholar]
- [56].Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, Binder LI, Mandelkow EM, Diamond MI, Lee VM, Holtzman DM (2011) In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci 31, 13110–13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Krishnamurthy PK, Deng Y, Sigurdsson EM (2011) Mechanistic Studies of Antibody-Mediated Clearance of Tau Aggregates Using an ex vivo Brain Slice Model. Front Psychiatry 2, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Congdon EE, Gu J, Sait HB, Sigurdsson EM (2013) Antibody Uptake into Neurons Occurs Primarily via Clathrin-dependent Fcgamma Receptor Endocytosis and Is a Prerequisite for Acute Tau Protein Clearance. J Biol Chem 288, 35452–35465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gu J, Congdon EE, Sigurdsson EM (2013) Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology. J Biol Chem 288, 33081–33095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Krishnaswamy S, Lin Y, Rajamohamedsait WJ, Rajamohamedsait HB, Krishnamurthy P, Sigurdsson EM (2014) Antibody-derived in vivo imaging of tau pathology. J Neurosci 34, 16835–16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Collin L, Bohrmann B, Gopfert U, Oroszlan-Szovik K, Ozmen L, Gruninger F (2014) Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain 137, 2834–2846. [DOI] [PubMed] [Google Scholar]
- [62].Congdon EE, Lin Y, Rajamohamedsait HB, Shamir DB, Krishnaswamy S, Rajamohamedsait WJ, Rasool S, Gonzalez V, Levenga J, Gu J, Hoeffer C, Sigurdsson EM (2016) Affinity of Tau antibodies for solubilized pathological Tau species but not their immunogen or insoluble Tau aggregates predicts in vivo and ex vivo efficacy. Mol Neurodegener 11, 62–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shamir DB, Rosenqvist N, Rasool S, Pedersen JT, Sigurdsson EM (2016) Internalization of tau antibody and pathological tau protein detected with a flow cytometry multiplexing approach. Alzheimers Dement 12, 1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shamir DB, Deng Y, Wu Q, Modak S, Congdon EE, Sigurdsson EM (2020) Dynamics of internalization and intracellular interaction of tau antibodies and human pathological tau protein in a human neuron-like model. Frontiers in Neurology 11, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McEwan WA, Falcon B, Vaysburd M, Clift D, Oblak AL, Ghetti B, Goedert M, James LC (2017) Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. Proc Natl Acad Sci U S A 114, 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wu Q, Lin Y, Gu J, Sigurdsson EM (2018) Dynamic assessment of tau immunotherapies in the brains of live animals by two-photon imaging. EBioMedicine 35, 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chandupatla RR, Flatley A, Feederle R, Mandelkow EM, Kaniyappan S (2020) Novel antibody against low-n oligomers of tau protein promotes clearance of tau in cells via lysosomes. Alzheimers Dement (NY) 6, e12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Congdon EE, Pan R, Jiang Y, Sandusky-Beltran LA, Dodge A, Lin Y, Liu M, Kuo MH, Kong XP, Sigurdsson EM (2022) Single domain antibodies targeting pathological tau protein: Influence of four IgG subclasses on efficacy and toxicity. EBioMedicine 84, 104249–104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mukadam AS, Miller LVC, Smith AE, Vaysburd M, Sakya SA, Sanford S, Keeling S, Tuck BJ, Katsinelos T, Green C, Skov L, Kaalund SS, Foss S, Mayes K, O’Connell K, Wing M, Knox C, Banbury J, Avezov E, Rowe JB, Goedert M, Andersen JT, James LC, McEwan WA (2023) Cytosolic antibody receptor TRIM21 is required for effective tau immunotherapy in mouse models. Science 379, 1336–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC (2010) Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 107, 19985–19990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sigurdsson EM (2001) Immune Therapy for AD Plaques and Tangles. NIH, 1R01AG020197. [Google Scholar]
- [72].Hu WT, Trojanowski JQ, Shaw LM (2011) Biomarkers in frontotemporal lobar degenerations--progress and challenges. Prog Neurobiol 95, 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O’Neill MJ, Hutton ML, Citron M (2011) Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem 286, 34457–34467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ittner A, Bertz J, Suh LS, Stevens CH, Gotz J, Ittner LM (2015) Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice. J Neurochem 132, 135–145. [DOI] [PubMed] [Google Scholar]
- [75].Zilkova M, Nolle A, Kovacech B, Kontsekova E, Weisova P, Filipcik P, Skrabana R, Prcina M, Hromadka T, Cehlar O, Rolkova GP, Maderova D, Novak M, Zilka N, Hoozemans JJM (2020) Humanized tau antibodies promote tau uptake by human microglia without any increase of inflammation. Acta Neuropathol Commun 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bajracharya R, Brici D, Bodea LG, Janowicz PW, Gotz J, Nisbet RM (2021) Tau antibody isotype induces differential effects following passive immunisation of tau transgenic mice. Acta Neuropathol Commun 9, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Modak SR, Solesio M, Krishnaswamy S, Congdon EE, Sigurdsson EM (2015) Antibodies targeting truncated tau protein reduce tau pathology in primary neuronal and mixed cortical cultures. Soc Neurosci Abstr 579.14. [Google Scholar]
- [78].Modak SR, Sigurdsson EM (2017) Antibodies targeting truncated Asp421 tau protein clear human Alzheimer’s tau and prevent its toxicity in primary neuronal and mixed cortical cultures. Soc Neurosci Abstr 478.19. [Google Scholar]
- [79].Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D (2005) Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 46, 857–868. [DOI] [PubMed] [Google Scholar]
- [80].Tampellini D, Magrane J, Takahashi RH, Li F, Lin MT, Almeida CG, Gouras GK (2007) Internalized antibodies to the A beta domain of APP reduce neuronal A beta and protect against synaptic alterations. J Biol Chem 282, 18895–18906. [DOI] [PubMed] [Google Scholar]
- [81].Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D (2011) Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE 6, e19338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [82].Gustafsson G, Eriksson F, Moller C, da Fonseca TL, Outeiro TF, Lannfelt L, Bergstrom J, Ingelsson M (2017) Cellular Uptake of alpha-Synuclein Oligomer-Selective Antibodies is Enhanced by the Extracellular Presence of alpha-Synuclein and Mediated via Fcgamma Receptors. Cell Mol Neurobiol 37, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pozzi S, Codron P, Soucy G, Renaud L, Cordeau PJ, Dutta K, Bareil C, Julien JP (2020) Monoclonal full-length antibody against TAR DNA binding protein 43 reduces related proteinopathy in neurons. JCI Insight 5, e140420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nguyen L, Montrasio F, Pattamatta A, Tusi SK, Bardhi O, Meyer KD, Hayes L, Nakamura K, Banez-Coronel M, Coyne A, Guo S, Laboissonniere LA, Gu Y, Narayanan S, Smith B, Nitsch RM, Kankel MW, Rushe M, Rothstein J, Zu T, Grimm J, Ranum LPW (2020) Antibody Therapy Targeting RAN Proteins Rescues C9 ALS/FTD Phenotypes in C9orf72 Mouse Model. Neuron 105, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Benkler C, O’Neil AL, Slepian S, Qian F, Weinreb PH, Rubin LL (2018) Aggregated SOD1 causes selective death of cultured human motor neurons. Sci Rep 8, 16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Graus F, Illa I, Agusti M, Ribalta T, Cruz-Sanchez F, Juarez C (1991) Effect of intraventricular injection of an anti-Purkinje cell antibody (anti-Yo) in a guinea pig model. J Neurol Sci 106, 82–87. [DOI] [PubMed] [Google Scholar]
- [87].Fabian RH, Ritchie TC (1986) Intraneuronal IgG in the central nervous system. J Neurol Sci 73, 257–267. [DOI] [PubMed] [Google Scholar]
- [88].Greenlee JE, Burns JB, Rose JW, Jaeckle KA, Clawson S (1995) Uptake of systemically administered human anticerebellar antibody by rat Purkinje cells following blood-brain barrier disruption. Acta Neuropathol (Berl) 89, 341–345. [DOI] [PubMed] [Google Scholar]
- [89].Karpiak SE, Mahadik SP (1987) Selective uptake by Purkinje neurons of antibodies to S-100 protein. Exp Neurol 98, 453–457. [DOI] [PubMed] [Google Scholar]
- [90].Hill KE, Clawson SA, Rose JW, Carlson NG, Greenlee JE (2009) Cerebellar Purkinje cells incorporate immunoglobulins and immunotoxins in vitro: implications for human neurological disease and immunotherapeutics. J Neuroinflammation 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rocchi A, Sacchetti S, De FA, Giovedi S, Parisi B, Cesca F, Holtje M, Ruprecht K, Ahnert-Hilger G, Benfenati F (2019) Autoantibodies to synapsin I sequestrate synapsin I and alter synaptic function. Cell Death Dis 10, 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Goldwaser EL, Acharya NK, Wu H, Godsey GA, Sarkar A, DeMarshall CA, Kosciuk MC, Nagele RG (2020) Evidence that Brain-Reactive Autoantibodies Contribute to Chronic Neuronal Internalization of Exogenous Amyloid-beta1–42 and Key Cell Surface Proteins During Alzheimer’s Disease Pathogenesis. J Alzheimers Dis 74, 345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177. [DOI] [PubMed] [Google Scholar]


