Abstract
Background and Purpose
Many patients who gained successful recanalization by endovascular treatment (EVT) with acute large vessel occlusion (LVO) did not have the favorable outcome. The study aimed to assess the association between H-type hypertension and clinical prognosis in patients with LVO after receiving EVT.
Methods
Our study enrolled patients from the Endovascular Treatment With versus Without Tirofiban for Stroke Patients with Large Vessel Occlusion (RESCUE BT) Trial. H-type hypertension is defined as patients with hypertension and homocysteine (Hcy) ≥10µmol/L. The primary outcome was a favorable functional outcome, defined as a score of 0–2 on the modified Rankin Scale (mRS) at 90 days. The secondary outcomes were mortality, successful recanalization, futile recanalization, and symptomatic intracerebral hemorrhage (sICH).
Results
The plasma homocysteine level was recorded for 215 patients with hypertension in our study. Among those patients, 172 patients (80%) were founded with Hcy ≥10µmol/L (H-type hypertension), and 43 patients (20%) with Hcy <10µmol/L (non-H-type hypertension). The probability of favorable outcome decreased with homocysteine increasing in patients with hypertension. H-type hypertension was associated with a low probability of favorable outcome (adjusted odds ratio (aOR), 0.38 [95% confidence interval (CI), 0.18–0.80]; p = 0.01) at 90 days. The effects of H-type hypertension on mortality (aOR, 1.90 [95% CI, 0.67–5.39]; p = 0.23) and sICH (aOR, 0.55 [95% CI, 0.13–2.29]; p = 0.41) were not significant.
Conclusion
Our findings suggest that patients with H-type hypertension have a lower likelihood of achieving favorable outcomes but do not have an increased mortality rate within 90 days.
Keywords: H-type hypertension, clinical prognoses, large vessel occlusion
Introduction
Acute large vessel occlusive (LVO) of ischemic stroke could cause severe disability and death. Endovascular treatment (EVT) is the primary treatment for patients with LVO when administered within 24 hours of symptom onset.1 Currently, 90% of successful recanalization has been achieved by EVT for patients with LVO, but the rate of functional independence at 90 days is just 50%.2,3 Therefore, the focus is not on increasing the successful recanalization but rather on further improving the good prognosis of patients.
Homocysteine, a sulfur-containing amino acid, derives from methionine metabolism.4 A number of studies had suggested that high homocysteine concentrations were closely associated with an increased risk of both the occurrence and recurrence of ischemic stroke.5–9 Numerous registry studies showed that high homocysteine was associated with early neurology deficit and worse clinical prognosis.8,10,11 Hypertension has also been noted as a risk factor for ischemic stroke.12 A previous survey revealed that elevated homocysteine was found in nearly 75% of hypertension patients in Asia.13 The association of H-type hypertension (homocysteine and hypertension) with clinical outcomes has also been investigated in patients with stroke. Patients with H-type hypertension had less likelihood to achieve functional independence and high rates of mortality.14,15 Prior studies almost investigated the relationship between H-type hypertension and clinical prognosis in patients with small-vessel stroke or separately demonstrated the effect of homocysteine and hypertension on stroke prognosis. However, there is a lack of research exploring the effect of H-type hypertension on unfavorable outcomes and mortality in patients who have ischemic stroke and underwent EVT due to LVO.
Therefore, we aimed to investigate the relationship between H-type hypertension and clinical prognosis after EVT in patients with ischemic stroke due to LVO based on the Endovascular Treatment With versus Without Tirofiban for Stroke Patients with Large Vessel Occlusion (RESCUE BT) Trial.16
Methods
Study Population
Our study was a substudy of the Endovascular Treatment With versus Without Tirofiban for Stroke Patients with Large Vessel Occlusion (RESCUE BT) Trial (Trial Registration chictr.org.cn, ChiCTR-INR-17014167),17 which was a multicenter, randomized, double-blind, placebo-controlled trial. Consecutive patients were included and randomly assigned to the tirofiban or placebo group in China from October 10, 2018, to October 31, 2021. Among those patients, 215 patients were included in our study, 172 patients were defined as having H-type hypertension (Hcy defined as ≧10 µmol/L), and 43 patients were defined as not having H-type hypertension (Hcy defined as <10 µmol/L). The medical ethics committee of the Chinese Ethics Committee of Registering Clinical Trials approved the trial. Besides, the written informed consent was obtained from patients or their legal representatives.
Outcome Assessment
The primary outcome was a favorable outcome defined as a modified Rankin Scale (mRS, range, 0 to 6 points, with higher scores indicating greater disability)18 score of 0 to 2 at 90 days. We also examined the following dichotomizations of the mRS score: 0 to 1 versus 2 to 6, 0 to 3 versus 4 to 6, and 0 to 5 versus 6 (mortality). The imaging outcome, including recanalization status, was evaluated using the modified Thrombolysis in Cerebral Infarction (mTICI) score,19 which ranges from 0 (no reperfusion) to 3 (complete reperfusion) by DSA. Successful recanalization was defined as mTICI 2b-3. Symptomatic intracerebral hemorrhage (sICH) was evaluated according to the Heidelberg Bleeding Classification20(any intracerebral hemorrhage on CT and neurological deterioration of ≥4 points using the NIHSS or ≥2 points using the 11 NIHSS subcategories) within 48 hours. In addition, futile recanalization21 was defined as an mRS score of 3 to 6 in patients with successful recanalization.
Statistical Analysis
Statistical analyses were performed using SPSS (version 23, IBM Corp., Armonk, NY) and RStudio (version 1.3.1093) statistical software. A two-tailed p-value <0.05 (two-tailed) was defined as statistically significant. Analysis of univariate data was performed using the Mann–Whitney U-test for continuous variables (median and interquartile range [IQR]) and the chi-squared test or Fisher’s exact test for categorical variables (percentages).
A restricted cubic spline regression model was used to explore the nonlinear association between plasma total homocysteine level and favorable outcomes among patients with hypertension. The association between H-type hypertension and clinical outcomes was assessed using univariate and multivariate logistic regression. In the multivariate logistic regression model, because the sample size of the non-H-type hypertension is 43, the four most important adjusted confounding factors, including age, baseline NIHSS, baseline ASPECTS, and onset to puncture time, were included. Furthermore, supportive analyses were performed by IPTW (Inverse Probability of Treatment Weighting) analysis and the PSM (propensity score matching analysis) based on logistic regression in our study. IPTW model adjusted for age, sex, diabetes, cerebral infarction, atrial fibrillation, smoking, hyperlipidemia, occlusion site, stroke etiology, baseline NIHSS, baseline ASPECT, oral anticoagulant, mono-antiplatelet treatment, onset to puncture time, and tirofiban. Weighted standardized differences were used to evaluate the similarity of H-type hypertension and non-H-type hypertension after application of the IPTW. For propensity score matching analysis, we performed a 1:2 matching based on the nearest-neighbor matching algorithm with a caliper width of 0.2 of the propensity score with age, sex, oral anticoagulant, mono-antiplatelet treatment, onset to puncture time, occlusion site, stroke etiology, baseline NIHSS, baseline ASPECT, tirofiban, and medical history, such as diabetes, smoking, hyperlipidemia, and cerebral infarction, as covariates.
The predictors of favorable outcome and mortality were explored in patients with H-type hypertension according to baseline differences between two groups (favorable outcome versus unfavorable outcome and mortality versus survival) and prior studies. Furthermore, we also explored the effect of H-type hypertension in different groups; thus, subgroup analysis was performed based on age, sex, baseline NIHSS, baseline ASPECT, and stroke etiology, adjusting for age, baseline NIHSS, baseline ASPECT, and onset to puncture time.
Results
Baseline Characteristics
There were 524 patients with hypertension among the 948 patients in the RESCUE BT Trial. Of these hypertensive patients, 215 had homocysteine data and were included in our study. One hundred seventy-two (80%) patients were H-type hypertension, 43 (20%) were non-H-type hypertension (in Figure S1). The median (interquartile range) age, NIHSS, and ASPECT for the cohorts were 69 (61–75), 16 (11–19), and 8 (7–9), respectively.
Baseline characteristics details were shown in Table 1. H-type hypertension was frequent in the male group (65.1% vs 37.2%, p = 0.001). Besides, the onset to puncture time was longer in H-type hypertension cohorts compared with non-H-type hypertension cohorts (400 vs 283, p = 0.03). Baseline characteristics after propensity score matching and IPTW were shown in Tables S1 and S2.
Table 1.
Baseline Characteristics of Patients with or Without H-Type Hypertension
| Baseline Characteristic | All | Non-H-type hypertension (n=43) | H-Type hypertension (n=172) | P value |
|---|---|---|---|---|
| Age, median (IQR), y | 69 (61–75) | 68 (64–76) | 69 (59–75) | 0.780 |
| Sex, No (%) | ||||
| Female | 87 (40.5) | 27 (62.8) | 60 (34.9) | 0.001 |
| Male | 128 (59.5) | 16 (37.2) | 112 (65.1) | |
| Medical history No. (%) | ||||
| Diabetes | 63 (29.3) | 11 (25.6) | 52 (30.2) | 0.55 |
| Cerebral infarction | 45 (20.9) | 12 (27.9) | 33 (19.2) | 0.21 |
| Atrial fibrillation | 61 (28.4) | 15 (34.9) | 46 (26.7) | 0.29 |
| Hyperlipidemia | 31 (14.4) | 6 (14.0) | 25 (14.5) | 0.92 |
| Smoking | 61 (28.4) | 8 (18.6) | 53 (30.8) | 0.11 |
| Prestroke antithrombotictreatment No. (%) | ||||
| Oral anticoagulant | 26 (12.1) | 5 (11.6) | 21 (32.2) | 0.92 |
| Mono-antiplatelet treatment | 12 (5.6) | 5 (11.6) | 7 (4.1) | 0.05 |
| Stroke etiology No. (%) | ||||
| LAA | 124 (57.7) | 21 (48.8) | 103 (59.9) | 0.40 |
| CE | 70 (32.6) | 17 (39.5) | 53 (30.8) | |
| Other | 21 (9.8) | 5 (11.6) | 16 (9.3) | |
| NIHSS score, median (IQR) | 16 (11–19) | 16 (12–20) | 15 (11–19) | 0.38 |
| Onset to puncture(minutes)(IQR) | 380 (232–614) | 283 (210–510) | 400 (246–682) | 0.03 |
| Imaging characteristics | ||||
| ASPECTS score, median (IQR) | 8 (7–9) | 8 (7–9) | 8 (6–9) | 0.78 |
| Occlusion site – No. (%) | ||||
| Intracranial internal carotid artery | 48 (22.3) | 6 (14) | 42 (24.4) | 0.18 |
| M1 middle cerebral artery segment | 139(64.7) | 33 (76.7) | 106 (61.6) | |
| M2 middle cerebral artery segment | 28 (13.0) | 4 (9.3) | 24 (14.0) | |
| Tirofiban (%) | 102 (47.4) | 17 (39.5) | 85 (49.4) | 0.25 |
Abbreviations: LAA, Large artery atherosclerosis; CE, Cardioembolism; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta stroke program early CT score.
Outcomes of H-Type Hypertension versus Non-H-Type Hypertension
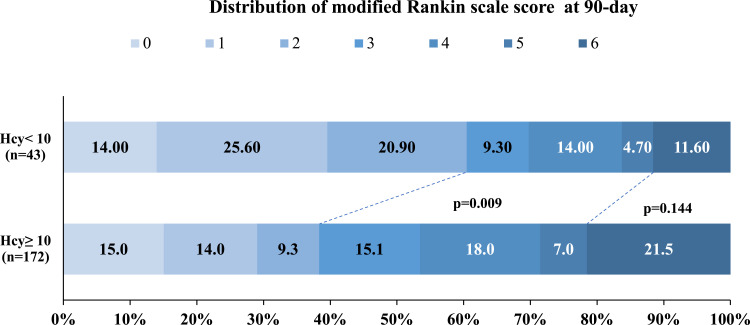
Following 90 days, the distribution of mRS for the two groups was described in Figure 1. There was a low rate of achieving favorable outcome at 90 days in patients with H-type hypertension compared with patients without (38.4% vs 60.5%, p = 0.009) (in Table 2). Patients in the H-type hypertension group had numerically higher mortality (21.5% vs 11.6%), but the difference did not reach statistical significance. In addition, the rates of sICH were similar between the H-type and non-H-type hypertension groups (5.3% vs 7.1%, p = 0.64).
Figure 1.
Distribution of Scores on the modified Rankin Scale at 90 Days Shown is the distribution of the scores on the modified Rankin Scale at 90 days according to H-type hypertension in all patients.
Table 2.
Association of H-Type Hypertension with Clinical Outcomes
| variables | Non-H-Type Hypertension (n=43) | H-Type Hypertension (n=172) | P value | Unadjusted OR, P | Adjusted OR‡, P | IPTW OR, P | PSM OR, P |
|---|---|---|---|---|---|---|---|
| mRS0-1(%) | 17 (39.5) | 50 (29.1) | 0.19 | 0.63 (0.31–1.26), 0.19 | 0.64 (0.30–1.34), 0.24 | 0.64 (0.31–1.32), 0.22 | 0.58 (0.27–1.25), 0.17 |
| mRS0-2(%) | 26 (60.5) | 66 (38.4) | 0.009 | 0.41 (0.21–0.81), 0.01 | 0.38 (0.18–0.80), 0.01 | 0.33 (0.16–0.70), 0.004 | 0.35 (0.16–0.74), 0.006 |
| mRS0-3(%) | 30 (69.8) | 92 (53.5) | 0.05 | 0.41 (0.19–0.90), 0.03 | 0.44 (0.20–0.98), 0.04 | 0.42 (0.19–0.94), 0.03 | 0.41 (0.19–0.90), 0.03 |
| Successful recanalization (%) | 41 (95.3) | 151 (87.8) | 0.15 | 0.35 (0.08–1.56), 0.17 | 0.39 (0.09–1.80), 0.23 | 0.43 (0.09–1.97), 0.28 | 0.32 (0.07–1.53), 0.16 |
| Futile recanalization (%)† | 15 (36.6) | 86 (57.0) | 0.02 | 2.29 (1.13–4.68), 0.02 | 2.50 (1.16–5.41), 0.02 | 2.96 (1.34–6.52), 0.007 | 2.79 (1.26–6.15), 0.01 |
| sICH with 48h (%) | 3 (7.1) | 9 (5.3) | 0.64 | 0.72 (0.19–2.79), 0.64 | 0.55 (0.13–2.29) 0.41 | 0.66 (0.15–2.89), 0.59 | 0.86 (0.38–1.93), 0.71 |
| Any ICH with 48h (%) | 13 (31) | 49 (28.7) | 0.77 | 0.90 (0.43–1.87), 0.77 | 0.77 (0.36–1.65), 0.50 | 0.94 (0.43–2.06), 0.87 | 0.49 (0.09–2.53), 0.39 |
| Mortality (%) | 5 (11.6) | 37 (21.5) | 0.14 | 2.08 (0.78–5.67), 0.15 | 1.90 (0.67–5.39), 0.23 | 2.08 (0.69–6.23), 0.19 | 2.07 (0.71–6.03), 0.18 |
Notes: †In the Futile recanalization group, the number of non-H-type hypertension patients is 41, and the number of H-type hypertension patients is 151. ‡Adjusting for age, baseline NIHSS, baseline ASPECTS, onset to puncture.
Abbreviations: sICH, symptomatic intracerebral hemorrhage; ICH, intracerebral hemorrhage; OR, odds ratio; IPTW, Inverse Probability of Treatment Weighting; PSM, propensity score matching analysis.
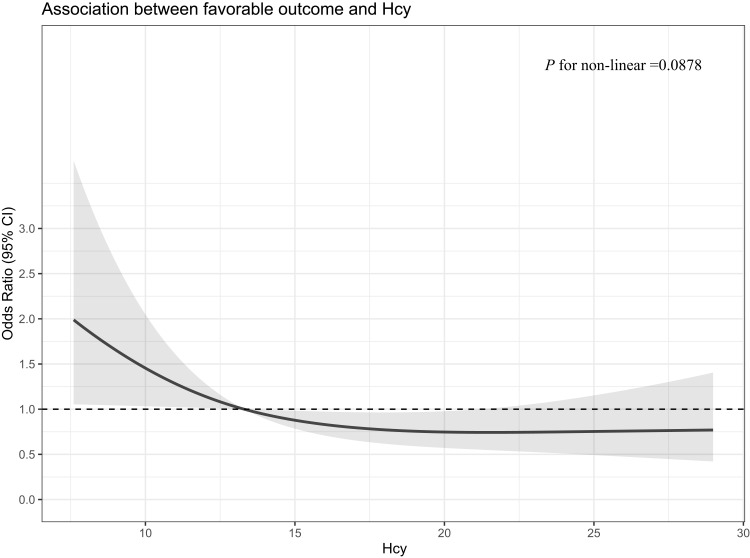
Figure 2 showed that the probability of favorable outcome at 90 days decreased linearly with increasing homocysteine (P for non-linear = 0.0878) in patients with hypertension. The association of H-type hypertension and clinical outcomes was also explored using multivariate logistic regression, propensity score matching, and the IPTW model. In comparison with non-H-type hypertension, H-type hypertension was associated with less opportunity of favorable outcome (aOR, 0.38 [95% confidence interval (CI), 0.18–0.80], p = 0.01; IPTW OR, 0.33 [95% CI, 0.16–0.70], p = 0.004; PSM OR, 0.35 [95% CI, 0.16–0.74], p = 0.006). H-type hypertension could increase the risk of futile recanalization (aOR, 2.50 [95% confidence interval (CI), 1.16–5.41], p = 0.02; IPTW OR, 2.96 [95% CI, 1.34–6.52], p = 0.07; PSM OR, 2.79 [95% CI, 1.26–6.15], p = 0.01) (in Table 2).
Figure 2.
Association between plasma homocysteine levels and favorable outcome in patients with hypertension.
Predictors of Clinical Outcomes
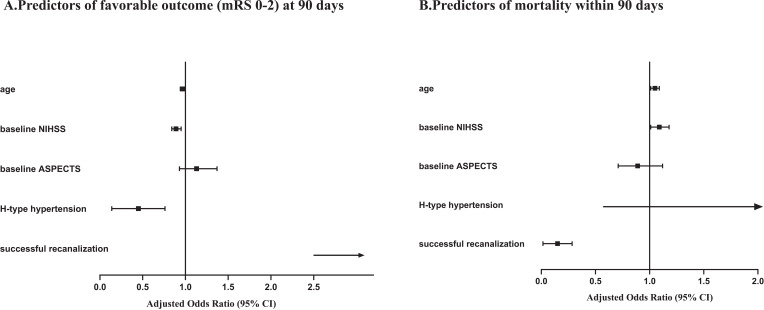
The multivariate analysis suggested that H-type hypertension (aOR, 0.38 [95% CI, 0.18–0.79]; p = 0.01), age (aOR, 0.97 [95% CI, 0.94–1]; p = 0.047), the lower baseline NIHSS (aOR, 0.89 [95% CI, 0.84–0.95]; p < 0.001), and successful recanalization (aOR, 19.90 [95% CI, 2.46–161.34]; p = 0.005) were independent factors for favorable outcome (in Figure 3A). Age (aOR, 1.05 [95% CI, 1.01–1.09]; p = 0.02), baseline NIHSS score (aOR, 1.09 [95% CI, 1.01–1.18]; p = 0.02), and successful recanalization (aOR,0.11 [95% CI, 0.04–0.30]; p < 0.001) were associated with mortality within 90 days (in Figure 3B).
Figure 3.
Predictors for favorable outcome: (A) (mRS score 0–2) and (B) mortality at 90 days. Adjusting for age, baseline NIHSS, baseline ASPECTS, successful recanalization.
Furthermore, in patients with H-type hypertension, high baseline NIHSS was mildly associated with a low probability of favorable outcome (aOR, 0.90 [95% CI, 0.84–0.97]; p = 0.003) and a high probability of mortality (aOR, 1.08 [95% CI, 1.00–1.17]; p = 0.04). Successful recanalization was significantly associated with a high probability of favorable outcome (aOR, 15.16 [95% CI, 1.89–121.79]; p = 0.01) and a low probability of mortality (aOR, 0.16 [95% CI, 0.06–0.43]; p < 0.001) (in Table 3).
Table 3.
Predictors for Favorable Outcome and Mortality in H-Type Hypertension Cohorts
| Variable | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Favorable outcome | ||||
| Baseline NIHSS score | 0.91 (0.85–0.96) | 0.002 | 0.90 (0.84–0.97) | 0.003 |
| ASPECTS score | 1.29 (1.05–1.57) | 0.02 | 1.15 (0.93–1.42) | 0.18 |
| Successful recanalization | 15.12 (1.98–115.56) | 0.009 | 15.16 (1.89–121.79) | 0.01 |
| Mortality | ||||
| Baseline NIHSS score | 1.08 (1.00–1.16) | 0.04 | 1.08 (1.00–1.17) | 0.04 |
| ASPECTS score | 0.80 (0.65–0.99) | 0.04 | 0.88 (0.70–1.70) | 0.26 |
| Successful recanalization | 0.15 (0.06–0.39) | <0.001 | 0.16 (0.06–0.43) | <0.001 |
Subgroup Analyses
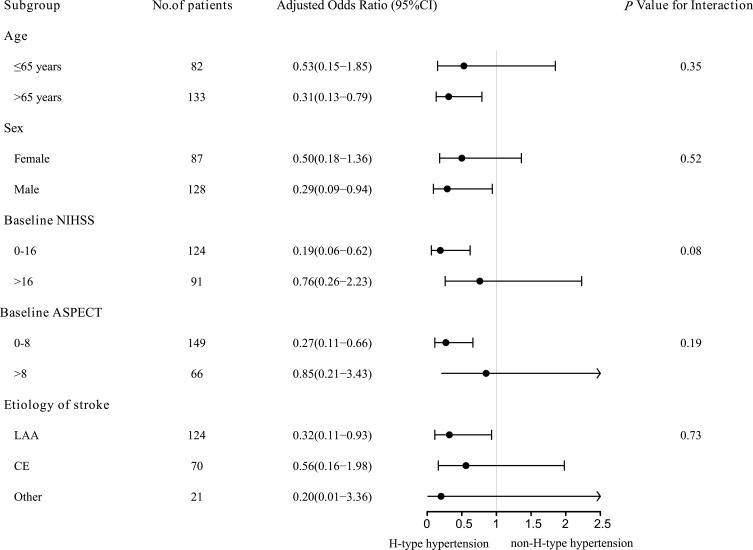
Subgroup analyses were performed according to baseline characteristics to explore the effect of H-type hypertension in different patients (in Figure 4). The results of the subgroup suggested that the potential benefit of non-H-type hypertension as compared with H-type hypertension may have been greater in the elderly, male, low baseline NIHSS score, low baseline ASPECT, and large artery atherosclerosis (LAA) cohorts.
Figure 4.
Subgroup analyses of the effects of H-type hypertension.
Discussion
In this study conducted in China, patients with H-type hypertension were associated with a low probability of favorable outcome in patients receiving EVT due to LVO. The opportunity for favorable outcome slowly decreased because of homocysteine increasing among hypertension cohorts. Nevertheless, the association of H-type hypertension with mortality was not significant during the follow-up.
In a previous study, 75% of patients with hypertension in Asia were observed to have higher plasma homocysteine levels.22 Accumulative evidence has demonstrated that elevated homocysteine was considered a risk factor for ischemic stroke23 and atherosclerotic plaques.24 Hypertension has also been widely investigated as a risk factor for stroke.25,26 H-type hypertension could increase the probability five times of cardiovascular disease compared to patients with simple hypertension.27 Chongke Zhong et al supported that H-type hypertension was a predictor of poor clinical outcome.22 The H-type hypertension group had a higher opportunity of occurrence of stroke recurrence or all-cause mortality (22.83% vs 10.24%) than the simple hypertension group during the 1-year follow-up.15 Compared with prior studies, our study enrolled patients with ischemic stroke who underwent EVT due to anterior circulation LVO. In our study, H-type hypertension also indicated an unfavorable outcome and futile recanalization.
Indeed, H-type hypertension correlated with worse clinical outcome could be explained by elevated homocysteine induced oxidative stress for the vascular endothelial cells to impair the blood–brain barrier and aggravated the injury by the production of vasodilator, nitric oxide, in hypertension.28,29 Compared with non-H-type hypertensive patients, CD4+ T-cell percentage in peripheral blood was significantly decreased in H-type hypertensive patients.30 Treg cells were considered to have a protective impact on hypertension.31 The decrease of total CD4+ T cells makes blood pressure protective Treg cells further decrease, and pro-inflammatory cytokines increase comparatively, which will aggravate hypertension and target organ damage.30 Prior studies suggested that hypertension and homocysteine might have a certain synergistic harmful effect on functional recovery for patients with ischemic stroke.32–34 Besides, the rates of diabetes were frequent in the H-type hypertension group (30.2% vs 25.6%), yet the difference did not arrive statistically significant in our findings. Huang et al35 and Yang et al36 assessed the association between H-type hypertension and diabetes and found that H-type hypertension might increase the risk of a worse prognosis in patients with diabetes. Further, Chen et al had proved H-type hypertension (aOR, 1.543 [95% CI, 1.411–1.688]; p < 0.001) was associated with a high risk of atherosclerotic plaques. Patients with LAA were less likely to achieve functional independence at 3 months compared to those in the cardioembolism cohort.37 In our study, the large artery atherosclerosis of the etiology of stroke was higher in H-type hypertension (59.9% vs 48.8%) than without elevated homocysteine group.
Perini et al38 provided evidence that homocysteine increasing in the acute phase of stroke was not a predictor of mortality. Additionally, the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial39 also suggested that reducing the level of homocysteine after stroke had no impact on clinical outcome during a follow-up of 2 years. Nevertheless, numerous studies evaluated the relationship between homocysteine and mortality and found that high concentrations of homocysteine were associated with higher mortality from stroke and cardiovascular disease.40,41 And, in an analysis based on 3799 ischemic stroke patients, after a follow-up of 48 months, elevated homocysteine levels in the acute phase were associated with mortality.8 Zhang et al32 aimed to investigate whether serum homocysteine contributed to the risk of mortality in primary stroke patients, which demonstrated that high homocysteine concentration had a negative effect on increasing rates of all-cause mortality (relative risk (RR), 1.75 [95% CI, 1.3–2.4]; p < 0.001). Still, our results also supported the reports of Naess et al42 that measured the homocysteine following 6 years and revealed mortality related to homocysteine levels (hazard ratio 1.04, p = 0.02). In our findings, the mortality was high in H-type hypertension compared with non-H-type hypertension (21.5% vs 11.6%, p = 0.14), but the differences have no statistical significance. One possible reason is the small sample size, which may limit statistical power to detect the effect of H-type hypertension on mortality. Large clinical trials are needed to elucidate the results.
This study has several limitations that must be considered. First, as a post-hoc analysis of the RESCUE BT study, some bias might be unavoidable, even PSM and IPTW statisticals were performed in our study. Second, a small sample size could underestimate the effect of H-type hypertension on predicting clinical prognosis. Further prospective studies are needed to validate our findings. Third, the homocysteine levels were measured at the admission of the acute phase of the stroke. However, without serial measurements, the relationship between homocysteine change and prognosis could not be observed, which might guide the treatment for stroke.
Conclusion
H-type hypertension had a negative impact on the favorable outcome compared to the non-H-type hypertension. Future randomized controlled studies are needed to confirm whether homocysteine levels can be used as a potential target to improve the prognosis of patients with LVO.
Acknowledgments
We thank all the coinvestigators of RESCUE BT for their dedication to this study.
Funding Statement
The Second Affiliated Hospital of Army Medical University Talent Development Special Project (2022XKRC002), Outstanding Scientist Family of Chongqing City, National Natural Science Foundation of China (No. 82371334), The Natural Science Foundation of Xiamen (3502Z202374018).
Data Sharing Statement
The datasets used and/or analysed during the current study were available from the corresponding author on reasonable request.
Ethics Approval Statements
The RESCUE BT trial was designed in compliance with the Declaration of Helsinki and has been registered on the Chinese Clinical Trial Registry (chictr.org.cn, ChiCTR-INR-17014167). The study was approved by the ethics committee of the Xinqiao Hospital, Army Medical University, and all participating centers. The informed consent was obtained for all participants.
Disclosure
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. 2020;368(6983). [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 4.Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. Serum homocysteine level is related to cerebral small vessel disease in a healthy population. Neurology. 2019;92(4):e317–e325. doi: 10.1212/WNL.0000000000006816 [DOI] [PubMed] [Google Scholar]
- 5.Iso H, Moriyama Y, Sato S, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109(22):2766–2772. doi: 10.1161/01.CIR.0000131942.77635.2D [DOI] [PubMed] [Google Scholar]
- 6.Homocysteine Studies C. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015 [DOI] [PubMed] [Google Scholar]
- 7.Shi Z, Liu S, Guan Y, et al. Changes in total homocysteine levels after acute stroke and recurrence of stroke. Sci Rep. 2018;8(1):6993. doi: 10.1038/s41598-018-25398-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Z, Guan Y, Huo YR, et al. Elevated total homocysteine levels in acute ischemic stroke are associated with long-term mortality. Stroke. 2015;46(9):2419–2425. doi: 10.1161/STROKEAHA.115.009136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han L, Wu Q, Wang C, et al. Homocysteine, ischemic stroke, and coronary heart disease in hypertensive patients: a population-based, prospective cohort study. Stroke. 2015;46(7):1777–1786. doi: 10.1161/STROKEAHA.115.009111 [DOI] [PubMed] [Google Scholar]
- 10.Kwon HM, Lee YS, Bae HJ, Kang DW. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke. 2014;45(3):871–873. doi: 10.1161/STROKEAHA.113.004099 [DOI] [PubMed] [Google Scholar]
- 11.Wu XQ, Ding J, Ge AY, Liu FF, Wang X, Fan W. Acute phase homocysteine related to severity and outcome of atherothrombotic stroke. Eur J Intern Med. 2013;24(4):362–367. doi: 10.1016/j.ejim.2013.01.015 [DOI] [PubMed] [Google Scholar]
- 12.Perry HM Jr, Davis BR, Price TR, et al. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: the systolic hypertension in the elderly program (SHEP). JAMA. 2000;284(4):465–471. doi: 10.1001/jama.284.4.465 [DOI] [PubMed] [Google Scholar]
- 13.Liu LS; Writing Group of Chinese Guidelines for the Management of H. [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(7):579–615. Polish. [PubMed] [Google Scholar]
- 14.Li J, Jiang S, Zhang Y, et al. H-type hypertension and risk of stroke in Chinese adults: a prospective, nested case-control study. J Transl Intern Med. 2015;3(4):171–178. doi: 10.1515/jtim-2015-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T, Zhu J, Fang Q, et al. Association of h-type hypertension with stroke severity and prognosis. Biomed Res Int. 2018;2018:8725908. doi: 10.1155/2018/8725908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Investigators RBT, Qiu Z, Li F, et al. Effect of intravenous tirofiban vs placebo before endovascular thrombectomy on functional outcomes in large vessel occlusion stroke: the rescue bt randomized clinical trial. JAMA. 2022;328(6):543–553. doi: 10.1001/jama.2022.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Z, Li F, Sang H, et al. Endovascular treatment with versus without tirofiban for stroke patients with large vessel occlusion: the multicenter, randomized, placebo-controlled, double-blind rescue bt study protocol. Int J Stroke. 2022;17(10):1151–1155. doi: 10.1177/17474930211069510 [DOI] [PubMed] [Google Scholar]
- 18.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6 [DOI] [PubMed] [Google Scholar]
- 19.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 21.de Rueda M E, Parrilla G, Manzano-Fernandez S, et al. Combined multimodal computed tomography score correlates with futile recanalization after thrombectomy in patients with acute stroke. Stroke. 2015;46(9):2517–2522. doi: 10.1161/STROKEAHA.114.008598 [DOI] [PubMed] [Google Scholar]
- 22.Zhong C, Lv L, Liu C, et al. High homocysteine and blood pressure related to poor outcome of acute ischemia stroke in Chinese population. PLoS One. 2014;9(107498):e107498. doi: 10.1371/journal.pone.0107498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Li Y, Chen Y, Feng L, Nie Z. Homocysteine level and risk of different stroke types: a meta-analysis of prospective observational studies. Nutr, Metab Cardiovasc Dis. 2014;24(11):1158–1165. doi: 10.1016/j.numecd.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Wang F, Zheng Y, Zeng Q, Liu H. H-type hypertension is an important risk factor of carotid atherosclerotic plaques. Ann Clin Exp Hypertens. 2016;38(5):424–428. doi: 10.3109/10641963.2015.1116547 [DOI] [PubMed] [Google Scholar]
- 25.Cressman MD, Gifford RW Jr. Hypertension and stroke. J Am Coll Cardiol. 1983;1(2):521–527. doi: 10.1016/S0735-1097(83)80083-7 [DOI] [PubMed] [Google Scholar]
- 26.Du X, Cruickshank K, McNamee R, et al. Case-control study of stroke and the quality of hypertension control in north west england. BMJ. 1997;314(7076):272–276. doi: 10.1136/bmj.314.7076.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease. The European concerted action project. JAMA. 1997;277(22):1775–1781. doi: 10.1001/jama.1997.03540460039030 [DOI] [PubMed] [Google Scholar]
- 28.Stamler JS, Osborne JA, Jaraki O, et al. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. 1993;91(1):308–318. doi: 10.1172/JCI116187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamura T, Kitamura A, Moriyama Y, et al. Plasma level of homocysteine is correlated to extracranial carotid-artery atherosclerosis in non-hypertensive Japanese. J Cardiovasc Risk. 1999;6(6):371–377. doi: 10.1177/204748739900600603 [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Zheng H, Huang J, Shen Y, Luo M. T-cell subsets are associated with serum homocysteine concentration in patients with essential hypertension. Ann Clin Exp Hypertens. 2017;39(4):377–381. doi: 10.1080/10641963.2016.1267189 [DOI] [PubMed] [Google Scholar]
- 31.Kvakan H, Kleinewietfeld M, Qadri F, et al. Regulatory t cells ameliorate angiotensin ii-induced cardiac damage. Circulation. 2009;119(22):2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782 [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Sun K, Chen J, et al. High plasma homocysteine levels contribute to the risk of stroke recurrence and all-cause mortality in a large prospective stroke population. Clin Sci. 2009;118(3):187–194. doi: 10.1042/CS20090142 [DOI] [PubMed] [Google Scholar]
- 33.Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E. Heart outcomes prevention evaluation I. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the hope 2 trial. Stroke. 2009;40(4):1365–1372. doi: 10.1161/STROKEAHA.108.529503 [DOI] [PubMed] [Google Scholar]
- 34.Xu G, Liu X, Wu W, Zhang R, Yin Q. Recurrence after ischemic stroke in Chinese patients: impact of uncontrolled modifiable risk factors. Cerebrovasc Dis. 2007;23(2–3):117–120. doi: 10.1159/000097047 [DOI] [PubMed] [Google Scholar]
- 35.Huang T, Ren J, Huang J, Li D. Association of homocysteine with type 2 diabetes: a meta-analysis implementing Mendelian randomization approach. BMC Genomics. 2013;14(1):867. doi: 10.1186/1471-2164-14-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang N, Yao Z, Miao L, et al. Novel clinical evidence of an association between homocysteine and insulin resistance in patients with hypothyroidism or subclinical hypothyroidism. PLoS One. 2015;10:0125922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Zeng G, Zeng H, et al. Endovascular treatment for acute basilar artery occlusion due to different stroke etiologies of large artery atherosclerosis and cardioembolism. Eur Stroke J. 2022;7(3):238–247. doi: 10.1177/23969873221101285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perini F, Galloni E, Bolgan I, et al. Elevated plasma homocysteine in acute stroke was not associated with severity and outcome: stronger association with small artery disease. Neurol Sci. 2005;26(5):310–318. doi: 10.1007/s10072-005-0505-7 [DOI] [PubMed] [Google Scholar]
- 39.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the vitamin intervention for stroke prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565–575. doi: 10.1001/jama.291.5.565 [DOI] [PubMed] [Google Scholar]
- 40.Sacco RL, Anand K, Lee HS, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the northern Manhattan study. Stroke. 2004;35(10):2263–2269. doi: 10.1161/01.STR.0000142374.33919.92 [DOI] [PubMed] [Google Scholar]
- 41.Cui R, Moriyama Y, Koike KA, et al. Serum total homocysteine concentrations and risk of mortality from stroke and coronary heart disease in Japanese: the JACC study. Atherosclerosis. 2008;198(2):412–418. doi: 10.1016/j.atherosclerosis.2007.09.029 [DOI] [PubMed] [Google Scholar]
- 42.Naess H, Nyland H, Idicula T, Waje-Andreassen U. C-reactive protein and homocysteine predict long-term mortality in young ischemic stroke patients. J Stroke Cerebrovasc Dis. 2013;22(8):e435–440. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study were available from the corresponding author on reasonable request.