Abstract
Objective
To analyze the prevalence and molecular characteristics of 16S rRNA methylase genes in clinical isolates of carbapenem-resistant Enterobacterales, for clinical doctors provide a reference basis for the rational use of drugs.
Methods
The Enterobacterales isolated from our hospital from 2020 to 2022 were selected and identified by VITEK 2 Compact automatic bacterial identification instrument. Resistance genes were detected by polymerase chain reaction.
Results
A total of 180 carbapenem-resistant Enterobacterales were isolated, of which 158 (87.8%) were resistant to at least one aminoglycoside. The resistance rates to gentamicin, tobramycin and amikacin were 85.0%,82.8% and 54.4%, respectively. Compared with 16S rRNA methyltransferase negative isolates, the positive isolates were more sensitive to trimethoprim-sulfamethoxazole, tetracycline and minocycline, but had higher resistance rates to aztreonam, tobramycin, gentamicin, amikacin and ciprofloxacin. The resistance rates of 16S rRNA methyltransferase gene positive strains to most commonly used antibiotics were more than 80%. But the rates for colistin and tigecycline were less than 10%. There were 114 strains (63.3%) positive for 16S rRNA methyltransferase genes, mainly rmtB, accounting for 70.2%. The positive rates of other armA, rmtA and armA+rmtB genes were 22.8%, 4.4% and 2.6%, respectively. No rmtC, rmtD, rmtE and npmA genes were detected. In addition, 175 of the 180 carbapenem-resistant Enterobacterales carried at least one carbapenemase genes. The blaKPC was the main one (115, 65.7%). There were 111 (61.7%) strains carried both carbapenemase and 16S rRNA methyltransferase genes, simultaneously. Compared with 16S rRNA methyltransferase negative strains, the positive strains carried more blaKPC genes and less blaNDM genes, with P values of 0.034 and 0.003, respectively.
Conclusion
blaKPC and rmtB genes are the main resistance mechanisms of Enterobacterales to carbapenems and aminoglycosides in our hospital. It is necessary to strengthen the detection of multi-drug resistant strains to provide scientific basis for clinical rational drug use.
Keywords: enterobacterales, carbapenemase, 16S rRNA methylase
Introduction
The latest CHINET data show that in recent years, the isolation rate of carbapenem-resistant Enterobacterales (CRE) in China has remained at a high level, especially the resistance rate of Klebsiella to carbapenems is 21.7 ~ 23.1%,1 which shows a wide range of resistance to antibiotics commonly used in clinical practice. Patients with CRE infection have severe clinical manifestations and high mortality. CRE has been ranked as one of the three most urgent antimicrobial resistance threats.2 According to data from the China Antimicrobial Resistance Surveillance Network, Escherichia coli, Enterobacter cloacae and other Enterobacteriaceae bacteria were highly sensitive to aztreonam-avibactam, amikacin, colistin, polycolistin B and tigecycline, with sensitivity rates ranging from 87.1% to 95.5%.3 The recommended treatment for CRE infection is aminoglycosides, tigecycline, colistin, and ceftazidime-avibactam (avycaz) alone or in combination.4 Studies have shown that the combination of aminoglycosides can improve the therapeutic effect of CRE5 and the treatment failure rate of aminoglycosides is low.6 This shows that aminoglycosides are still effective antibiotics for clinical combination treatment of CRE. However, in recent years, the production of 16S rRNA methyltransferase (RMTase) leads to high-level aminoglycoside drug resistance and wide spread worldwide, and RMTase often coexists with extended-spectrum β-lactamases (ESBLs) or carbapenemases,7 which brings challenges to the treatment of infectious diseases and the control of drug-resistant bacteria. Resistance phenotypes and genotypes may be different in different regions. In this study, we investigated the molecular epidemiology of 16S rRNA methyltransferase gene in CRE strains isolated from a hospital in Nanjing to better understand its prevalence, guide clinicians to use drugs scientifically and rationally, and formulate preventive measures.
Materials and Methods
Species Identification, Antimicrobial Susceptibility Testing, and Confirmation of Carbapenemase Production
Carbapenem-resistant enterobacterales isolates collected at the Nanjing Pukou People’s Hospital during a 3-year period between January 2020 and December 2022 were included in the study,180 non-repetitive enterobacterales isolates were received. All the 180 isolates were reidentified by MALDI-TOF MS (bioMérieux, France). Antimicrobial susceptibility test was performed using the VITEK-2 COMPACT system (bioMérieux, France). The CRE isolates were defined as strains resistant to either of the carbapenems, namely, imipenem or meropenem, or both, with a minimum inhibitory concentration (MIC) of 4µg/mL. The existence of the carbapenemase genes (KPC, NDM, OXA, IMP, and VIM) was confirmed by PCR. Quality control and interpretation of the results were based on 2020 CLSI breakpoints (CLSI, 2020) for all the antimicrobial agents with the exception of tigecycline. Tigecycline MICs were interpreted using the European Committee for Antimicrobial Susceptibility Testing (EUCAST) criteria (EUCAST, 2020). Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC27853 were used as quality control (QC) strains.
Detection of Drug Resistance Genes
The DNA was extracted through boiling bacterial colonies in sterile distilled water for 10 minutes. Single PCR was used to analyze carbapenemase genes (blaKPC, blaIMP, blaVIM, blaNDM, and blaOXA-48) and 16S rRNA methylase genes(armA, rmtA, rmtB, npmA, rmtC, and rmtD) with specific primers for each one, as previously reported, the primers and references to PCR conditions are shown in Table 1.8–11 The PCR products were subjected to 1.5% agarose gel electrophoresis. After electrophoresis, they were stained with ethidium bromide solution for 15 min. The results were observed and recorded in UV gel imager.
Table 1.
Primers of Carbapenemase and 16S rRNA Methylase Genes Used in This Study
| Target gene | Sequence (5’ to 3’) | Amplicon size/bp | Reference |
|---|---|---|---|
| blaKPC | F:ATGTCACTGTATCGCCGTCT | 893 | [8] |
| R:TTTTCAGAGCCTTACTGCCC | |||
| blaNDM | F:CAGCACACTTCCTATCTC | 292 | [8] |
| R:CCGCAACCATCCCCTCTT | |||
| blaOXA-48 | F:TTGGTGGCATCGATTATCGG | 743 | [8] |
| R:GAGCACTTCTTTTGTGATGGC | |||
| blaIMP | F: CTACCGCAGCAGAGTCTTTG | 587 | [8] |
| R:AACCAGTTTTGCCTTACCAT | |||
| blaVIM | F: ATGGTGTTTGGTCGCATATC | 509 | [8] |
| R:TGGGCCATTCAGCCAGATC | |||
| armA | F:ATTCTGCCTATCCTAATTGG | 315 | [9] |
| R:ACCTATACTTTATCGTCGTC | |||
| rmtA | F:CTAGCGTCCATCCTTTCCTC | 635 | [9] |
| R:TTGCTTCCATGCCCTTGCC | |||
| rmtB | F:ATGAACATCAACGATGCCCT | 769 | [10] |
| R:CCTTCTGATTGGCTTATCCA | |||
| rmtC | F:CGAAGAAGTAACAGCCAAAG | 711 | [9] |
| R:ATCCCAACATCTCTCCCACT | |||
| rmtD | F:GGGCTGAATCCTGTCTACCTCG | 741 | [11] |
| R:CGTTCTCGCCCAGTATTTC | |||
| rmtE | F:ATGAATATTGATGAAATGGTTGC | 818 | [11] |
| R:TGATTGATTTCCTCCGTTTTTG | |||
| npmA | F:AAGCACTTTCATACTGACG | 980 | [11] |
| R:CAATTTTGTTCTTATTAGC |
Statistical Analysis of Data
We used WHONET 5.6 software and SPSS software (version 22.0) for data analysis. The WHONET 5.6 software was used to analyze the bacterial drug susceptibility results. Chi-square tests were used to test the association of a set of counts between CRE isolates groups. All tests with a p-value <0.05 were taken as significant.
Results
General Characteristics of CRE Isolates
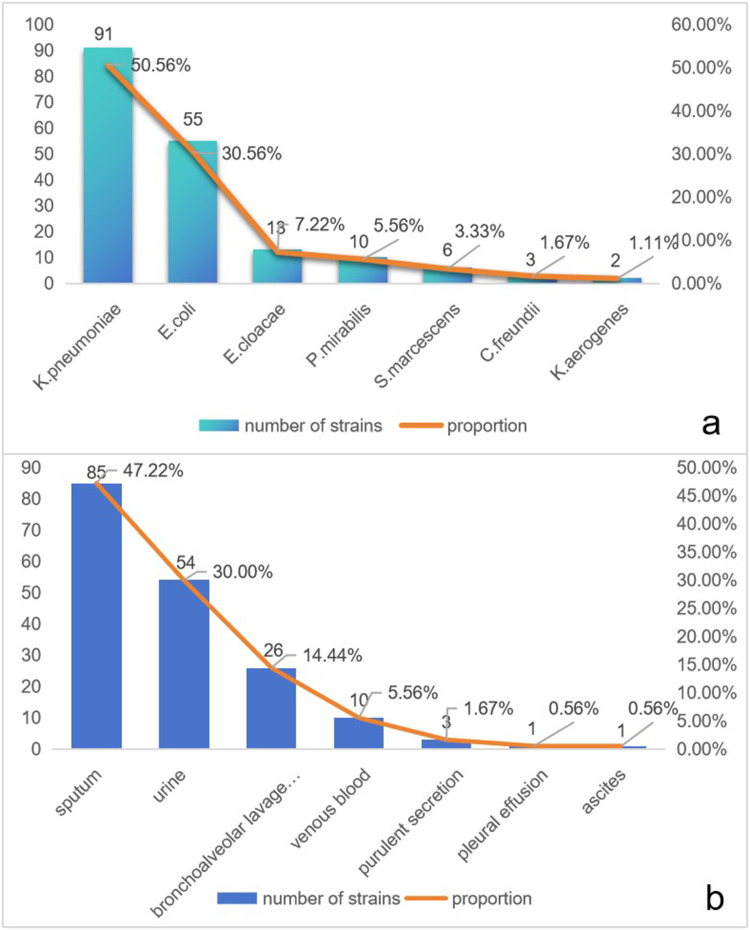
After eliminating duplicate strains, we detected 180 strains of CRE at this hospital between January 2020 and December 2022. Among the 180 CRE strains, K. pneumoniae accounted for the highest proportion, which was 50.1% (91/180), followed by E. coli 30.6% (55/180), E. cloacae 7.2% (13/180), P. mirabilis 5.6% (10/180), S. marcescens 3.3% (6/180), C. freundii 1.7% (3/180), and K. aerogenes 1.1% (2/180)(Figure 1a). Those strains had been isolated from sputum (47.22%), urine (30.00%), bronchoalveolar lavage fluid (14.44%), venous blood (5.56%), purulent secretion (1.67%), pleural effusion (0.56%) and ascites (0.56%)(Figure 1b).
Figure 1.
Distribution and specimen types of CRE isolates, (a) Strain distribution of the 180 CRE strains. (b) Specimen distribution of 180 CRE strains.
Prevalence of Genes in CRE
PCR analysis of 16S rRNA methylase genes revealed that 63.3% (114/180) of the CRE isolates were found to carry at least one 16S rRNA methylase gene, with rmtB, armA and rmtA being detected alone in 80, 26, and 5 strains, respectively, and with rmtB and armA in combination in 3 strains. However, rmtC, rmtD, rmtE and npmA were not detected in these strains. Based on the presence of 16S rRNA methylase genes in these CRE strains, CRE strains were divided into two groups (16S rRNA methylase genes-positive strains and 16S rRNA methylase genes-negative strains). Carbapenemase genes were detected in 175 of 180 CRE strains, including blaKPC (n= 115), blaNDM (n = 43), blaIMP (n = 5), blaNDM+blaKPC (n = 11), blaIMP+blaKPC (n = 1). Compared with 16S rRNA methylase genes-negative CRE strains, the positive strains carried more blaKPC gene and less blaNDM gene, with P values of 0.034 and 0.003, respectively (Tables 2–4) (Figure 2).
Table 2.
Prevalence of 16S rRNA Methylase Genes Among CRE Clinical Strains
| Isolates | Negative Strains | ArmA | rmtA | rmtB | ArmA+rmtB | Total |
|---|---|---|---|---|---|---|
| K.pneumoniae | 25 | 13 | 3 | 48 | 2 | 91 |
| E.coli | 19 | 7 | 2 | 26 | 1 | 55 |
| E.cloacae | 10 | 2 | 0 | 1 | 0 | 13 |
| P.mirabilis | 4 | 3 | 0 | 3 | 0 | 10 |
| S.marcescens | 5 | 1 | 0 | 0 | 0 | 6 |
| C.freundii | 1 | 0 | 0 | 2 | 0 | 3 |
| K.aerogenes | 2 | 0 | 0 | 0 | 0 | 2 |
| Total | 66 | 26 | 5 | 80 | 3 | 180 |
Table 3.
Prevalence of 16S rRNA Methylase Genes and Carbapenemase Genes Among CRE Clinical Strains
| 16S RMTases | Carbapenemase | Total | |||||
|---|---|---|---|---|---|---|---|
| Negative Strains | KPC | NDM | IMP | NDM+KPC | IMP+KPC | ||
| Negative strains | 2 | 36 | 24 | 1 | 3 | 0 | 66 |
| ArmA | 0 | 17 | 6 | 1 | 2 | 0 | 26 |
| rmtA | 0 | 3 | 2 | 0 | 0 | 0 | 5 |
| rmtB | 3 | 58 | 10 | 2 | 6 | 1 | 80 |
| ArmA+rmtB | 0 | 1 | 1 | 1 | 0 | 0 | 3 |
| Total | 5 | 115 | 43 | 5 | 11 | 1 | 180 |
Table 4.
Distribution of Carbapenemase Resistance Genes in 16S rRNA Methylase Genes- Positive and Negative Strains
| 16S RMTases | Carbapenemase | |||||
|---|---|---|---|---|---|---|
| Negative Strains | KPC | NDM | IMP | NDM+KPC | IMP+KPC | |
| 16S + (n=114) | 3 (2.6) | 79 (69.3) | 19 (16.7) | 4 (3.5) | 8 (7.0) | 1 (0.9) |
| 16S - (n=66) | 2 (3.0) | 36 (54.5) | 24 (36.4) | 1 (1.5) | 3 (4.5) | 0 |
| P-values | 0.609 | 0.034 | 0.003 | 0.394 | 0.375 | 0.633 |
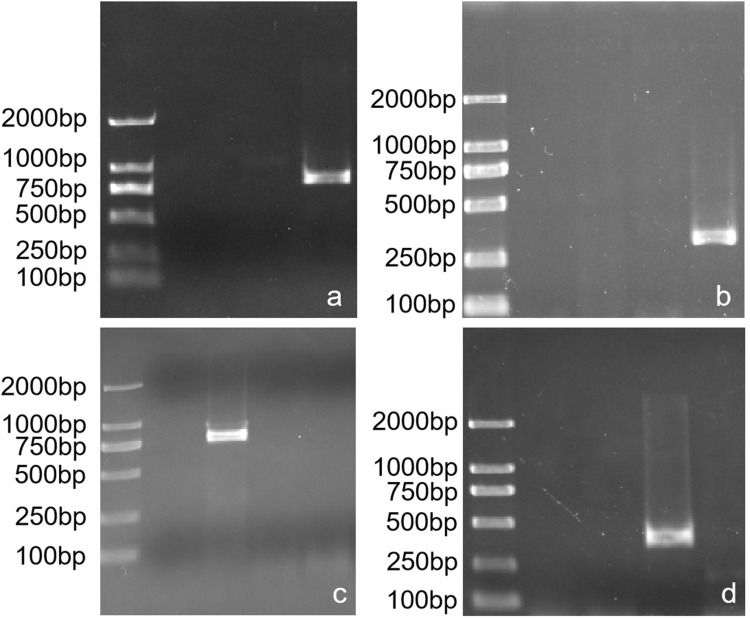
Figure 2.
Electrophoresis map for PCR products of carbapenemase and 16S RMTases genes (a) Electrophoresis map of blaKPC gene product; (b) Electrophoresis map of blaNDM gene product; (c) Electrophoresis map of rmtB gene product; (d) Electrophoresis map of armA gene product.
Antibiotic Susceptibilities of CRE
Of the 180 CRE isolates, 158 (87.8%) were resistant to at least one specified aminoglycoside drug. The resistance rates of gentamicin, tobramycin and amikacin were 85.0% (153/180), 82.8% (149/180) and 54.4% (98/180), respectively. The 98 strains resistant to amikacin were all resistant to gentamicin and tobramycin. Compared with 16S rRNA methylase genes-negative isolates, 16S rRNA methylase genes-positive isolates were more sensitive to trimethoprim-sulfamethoxazole, tetracycline and minocycline, but had higher drug resistance to amikacin, gentamicin, tobramycin, amtronam and ciprofloxacin (P < 0.05), the difference was statistically significant. The resistance rate of 16S rRNA methylase genes-positive CRE strains to the commonly used clinical antibiotics cefoxitin, piperacillin/tazobactam, cefotaxime, cefepime, amtronam, imipenem, meropenem, tobramycin, amikacin, gentamicin, ceftazidime, ciprofloxacin was higher than 80%, while the resistance rate to colistin and tigecycline was lower than 10% (Table 5).
Table 5.
Antimicrobial Resistance Rate of 180 CRE Clinical Strains
| Antimicrobial Agents | 16S+(n=114) | 16S-(n=66) | P-values | ||
|---|---|---|---|---|---|
| Strain Number | Percentage | Strain Number | Percentage | ||
| Cefoxitin | 114 | 100 | 66 | 100 | – |
| Piperacillin-tazobactam | 114 | 100 | 66 | 100 | – |
| Cefotaxime | 114 | 100 | 66 | 100 | – |
| Cefepime | 114 | 100 | 66 | 100 | – |
| Aztreonam | 114 | 100 | 59 | 89.4 | 0.001 |
| Imipenem | 114 | 100 | 65 | 98.5 | 0.367 |
| Meropenem | 114 | 100 | 66 | 100 | – |
| Tobramycin | 114 | 100 | 35 | 53.0 | 0.000 |
| Amikacin | 98 | 85.9 | 0 | 0 | 0.000 |
| Gentamicin | 114 | 100 | 39 | 59.1 | 0.000 |
| Ceftazidime | 114 | 100 | 66 | 100 | – |
| Ciprofloxacin | 110 | 96.5 | 58 | 87.9 | 0.029 |
| Trimethoprim-sulfamethoxazole | 64 | 56.1 | 57 | 86.4 | 0.000 |
| Tetracycline | 56 | 49.1 | 55 | 83.3 | 0.000 |
| Minocycline | 32 | 28.1 | 49 | 74.2 | 0.000 |
| Colistin | 7 | 6.1 | 2 | 3.0 | 0.294 |
| Tigecycline | 2 | 1.8 | 1 | 1.5 | 0.696 |
Discussion
Carbapenems are often the last resort for the treatment of multi-drug resistant (MDR) gram-negative infections. In recent years, with the increasingly frequent use of carbapenems, CRE strains have emerged widely around the world,12 and compared with patients infected with carbapenem sensitive strain, CRE infected patients face a greater risk of death.13 Studies had shown that tigecycline combined with aminoglycosides (amikacin or gentamicin) has a synergistic effect on CRE both in vitro and in animal models, suggesting that the combined administration of these drugs is a promising approach for the treatment of CRE infection.14 However, 16S rRNA methylases have been identified as a source of acquired resistance to aminoglycosides. All of them methylate the target of aminoglycosides, namely the 16S rRNA, and consequently confer high-level and broad-spectrum resistance to all clinically relevant aminoglycosides. The specific mechanism of resistance is the addition of a 3CH motif provided by S-adenosylmethionine (SAM) to specific residues in the A-site of 16S rRNA, catalyzed by 16S RMTase, and a significant reduction in the binding capacity of methylated 16S rRNAs to aminoglycosides, leading to extensive and high levels of resistance to a wide range of aminoglycosides.15 At present, 16S rRNA methyltransferase has been found in Gram-negative bacteria in many countries.16
This study showed that 87.8% of CRE were resistant to at least one of the specified aminoglycosides. Amikacin, tobramycin, and gentamicin resistance rates were 54.4%, 82.8%, and 85.0%, respectively, and all 98 amikacin-resistant strains were resistant to both gentamicin and tobramycin, which is consistent with the results of a study in China.7 However, it was slightly lower than the 92% found in a national surveillance study in Greece, and they found that gentamicin was the most sensitive aminoglycoside in the in vitro experiments of CRE, with a resistance rate of only 57%, whereas the amikacin resistance rate was 82%,17 which is not consistent with our study. The reasons analyzed may be related to overuse of drugs in hospitals, geographical and cultural differences, health level and sanitary conditions of the country. The present study also showed that 16S rRNA methyltransferase positive isolates were more sensitive to trimethoprim-sulfamethoxazole, tetracycline and minocycline but had a higher rate of resistance to piperacillin/tazobactam, amitrazine, gentamicin, amikacin, and ciprofloxacin, as compared to 16S rRNA methyltransferase negative isolates. The 16S rRNA methyltransferase-positive strains were resistant to all clinically used antimicrobial drugs, such as cefoxitin, piperacillin/tazobactam, cefotaxime, cefepime, amitrazam, imipenem, meropenem, tobramycin, amikacin, gentamicin, ceftazidime, and ciprofloxacin except for colistin and tigecycline. It can be seen that aminoglycoside resistant CRE strains are extensively resistant strains, which should be paid attention to. Fortunately, both tigecycline and colistin had good antibacterial activity against these bacteria, and the susceptibility rate of bacteria was ≥93.9%. They were should be carefully selected when choosing antibacterial drug combinations based on drug sensitivity.
In the present study, the overall detection rate of the 16S RMTase gene in CRE clinical isolates (63.3%) was much higher than that found in Greece,17 but slightly lower than the 66.7% of CR-hvKP isolates reported to carry the 16S rRNA methyltransferase gene in our country,18 and in a teaching hospital in the Northeast, the prevalence of 16S RMTase gene in CRKP isolates has reached 75%, and these differences may be related to the type of specimens collected and the different strain categories.19 In our study, we found that 16S RMTase genes in CRE were mainly rmtB, followed by armA gene, and rmtC, rmtD, rmtE and npmA genes were not detected, which is consistent with previous reports.7 The present study also showed that 16S rRNA methyltransferase-positive strains carried more blaKPC genes compared to 16S rRNA methyltransferase-negative strains. There were 111 strains carried both carbapenemase and 16S rRNA methyltransferase genes, simultaneously, and the rmtB and blaKPC-coupled genotype predominates in our hospital. And that, in our study, the coexistence of three genes included blaNDM+blaKPC+rmtB (6 strains), blaNDM+blaKPC+ArmA (2 strains), blaIMP+blaKPC+rmtB (1 strains), ArmA+rmtB+blaKPC (1 strains), ArmA+rmtB+blaNDM (1 strains) and ArmA+rmtB+blaIMP (1 strains). As early as 2007, armA and blaOXA-23 coupling was first identified in 16S rRNA methyltransferase-producing Acinetobacter baumannii in North America.20 Since then, there have been increasing reports of associations between carbapenemases and RMT enzymes globally. Among carbapenem-resistant A. baumannii found in Athens Hospital, Greece, 93.7% were positive for the armA gene and 98.5% of the positive strains carried the blaOXA-23 gene.21 K. pneumoniae carrying the blaKPC-2 and rmtG genes was found in Brazil,22 and K. pneumoniae carrying the blaNDM-1, blaOXA-48 and armA genes was found in Serbia,23 and in a national survey study conducted in the UK, it was shown that 93.4% of 16S RMTase-positive strains carried the acquired carbapenemase genes, with blaNDM being the most common at 83.1%.24 In a study from Switzerland, the association of blaNDM and ArmA was the most commonly observed, emphasized in the majority (22.3%) of the isolates.25 Genes carried by bacteria were different in different places, but according to literature reports, 16S rRNA methylase gene is located in mobile gene elements such as transposons and plasmids, which can break through geographical and species boundaries and carry out extensive horizontal and clonal propagation.26,27 The emergence of these coupled genotypes will increase the resistance of Enterobacterales to aminoglycosides, carbapenems, and other antibiotics, posing a great challenge for the clinical treatment of infectious diseases.
Conclusion
In our study, the positive rate of 16S rRNA methyltransferase gene in CRE strain was 63.3%, mainly rmtB genotype, and these strains carried more blaKPC carbapenemase genes. These results suggest that blaKPC and 16S rRNA methyltransferase rmtB genes are the main resistance mechanisms of Enterobacterales to carbapenems and aminoglycosides in our hospital. The 16S rRNA methyltransferase gene exists in plasmids, transposons and other mobile genetic elements, and can break through the geographical and species boundaries, coupling and spreading with other drug-resistant genes. Therefore, the monitoring and epidemiological analysis of the 16S rRNA methyltransferase gene and the study of its drug-resistance mechanism can provide a scientific basis for the rational use of drugs in the clinic.
Acknowledgments
This work was supported by the Natural Science Foundation of Ningxia (2024AAC03642), the Nanjing Pukou People’s Hospital Project (KJ2022-19), the Open Project Funding Projects from Ningxia Key Laboratory of Clinical and Pathogenic Microbiology (MKLG-2024-13), the Medical Young Backbone Talent Project of General Hospital of Ningxia Medical University.
Ethics Approval and Consent to Participate
The clinical isolates used in our study have been obtained from patients as part of routine hospital procedure, and the study was approved by the Medical Science Research Ethics Committee of the Nanjing Pukou People’s Hospital (2022-SR-017, approved 28 April 2022).
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Yan GUO, Fupin HU, Demei ZHU, et al. Surveillance of bacterial resistance in tertiary hospitals across China: results of CHINET antimicrobial resistance surveillance program in 2022. J Infect Chemother. 2024;24(03):277–286. [Google Scholar]
- 2.WHO. Global priority list of antibiotic resistant bacteria to guide research, discovery, and development of new antibiotics[R/OL]. Available From: https://www.whoint/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed November 19, 2024.
- 3.China Antimicrobial Resistance Surveillance Network. Antimicrobial susceptibility, resistance mechanisms, and molecular characteristics of carbapenem-resistant Enterobacterales (except K.pneumoniae) in China. J Infect Chemother. 2024;24(05):537–544. [Google Scholar]
- 4.Xu Y, Gu B, Huang M, et al. Epidemiology of carbapenem resistant Enterobacteriaceae(CRE) during 2000-2012 in Asia. Asia J Thorac Dis. 2015;7(3):376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WANG Minggui. Strategy for diagnosis and treatment of carbapenem-resistant gram-negative bacterial infections. Chin J Infect Chemother. 2024;24(2):133–134. [Google Scholar]
- 6.Gutiérrez-Gutiérrez B, Salamanca E, De Cueto M, et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT):a retrospective cohort study. Lancet Infect Dis. 2017;17(7):726–734. doi: 10.1016/S1473-3099(17)30228-1 [DOI] [PubMed] [Google Scholar]
- 7.Shen X, Liu L, Yu J, et al. High prevalence of 16S rRNA methyltransferase genes in carbapenem-resistant Klebsiella pneumoniae clinical isolates associated with bloodstream infections in 11 Chinese teaching hospitals. Infect Drug Resist. 2020;13:2189–2197. doi: 10.2147/IDR.S254479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galani I, Karaiskos I, Karantani I, et al; On Behalf Of The Study Collaborators. Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014–2016. Euro Surveill. 2018;23(31). doi: 10.2807/1560-7917.ES.2018.23.30.1700775 [DOI] [PubMed] [Google Scholar]
- 9.Y D, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007;45(1)::88–94. doi: 10.1086/518605 [DOI] [PubMed] [Google Scholar]
- 10.YAN JJ, WU JJ, KO WC, et al. Plasmid-mediated 16S rRNA methylases conferring high-level aminoglycoside resistance in Escherichia coli and Klebsiella pneumoniae isolates from two Taiwanese hospitals. J Antimicrob Chemother. 2004;54(6):1007–1012. doi: 10.1093/jac/dkh455 [DOI] [PubMed] [Google Scholar]
- 11.Davis MA, Baker KN, Orfe LH, et al. Discovery of a gene conferring multiple-aminoglycoside resistance in Escherichia coli. Antimicrob Agents Chemother. 2010;54(6):6):2666–2669. doi: 10.1128/AAC.01743-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe R. Regional dissemination of carbapenem-resistant Enterobacteriaceae accompanying with enhanced resistance in Northern Osaka, Japan. Nihon Saikingaku Zasshi. 2022;77(2):129–138. doi: 10.3412/jsb.77.129 [DOI] [PubMed] [Google Scholar]
- 13.Zhou R, Fang X, Zhang J, et al. Impact of carbapenem resistance on mortality in patients infected with Enterobacteriaceae: a systematic review and meta-analysis. BMJ Open. 2021;11(12):e054971. doi: 10.1136/bmjopen-2021-054971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni W, Yang D, Guan J, et al. In vitro and in vivo synergistic effects of tigecycline combined with aminoglycosides on carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2021;76(8):2097–2105. doi: 10.1093/jac/dkab122 [DOI] [PubMed] [Google Scholar]
- 15.Wachino JI, Doi Y, Arakawa Y. Aminoglycoside resistance: updates with a focus on acquired 16s ribosomal RNA methyltransferases. Infect Dis Clin North Am. 2020;34(4):887–902. doi: 10.1016/j.idc.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W, Hu F. Research updates of plasmid-mediated aminoglycoside resistance 16S rRNA methyltransferase. Antibiotics. 2022;11(7):906. doi: 10.3390/antibiotics11070906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galani I, Nafplioti K, Adamou P, et al. Nationwide epidemiology of carbapenem resistant Klebsiella pneumoniae isolates from Greek hospitals, with regards to plazomicin and aminoglycoside resistance. BMC Infect Dis. 2019;19(1):167. doi: 10.1186/s12879-019-3801-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao W, De Wang L, Li D, et al. High prevalence of 16s rRNA methylase genes among carbapenem-resistant hypervirulent Klebsiella pneumoniae isolates in a Chinese Tertiary Hospital. Microb Drug Resist. 2021;27(1):44–52. doi: 10.1089/mdr.2019.0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin L, Xiao X, Wang X, et al. In vitro antimicrobial susceptibility differences between carbapenem-resistant KPC-2-producing and NDM-1-producing Klebsiella pneumoniae in a teaching hospital in Northeast China. Microb Drug Resist. 2020;26(2):94–99. doi: 10.1089/mdr.2018.0398 [DOI] [PubMed] [Google Scholar]
- 20.Doi Y, Adams JM, Yamane K, Paterson DL. Identification of 16S rRNA methylase-producing Acinetobacter baumannii clinical strains in North America. Antimicrob Agents Chemother. 2007;51(11):4209–4210. doi: 10.1128/AAC.00560-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nafplioti K, Galani I, Angelidis E, et al. Dissemination of international clone II Acinetobacter baumannii strains coproducing OXA-23 carbapenemase and 16S rRNA methylase armA in Athens, Greece. Microb Drug Resist. 2020;26(1):9–13. doi: 10.1089/mdr.2019.0075 [DOI] [PubMed] [Google Scholar]
- 22.Mancini S, Poirel L, Corthesy M, et al. Klebsiella pneumoniae co-producing KPC and RmtG, finally targeting Switzerland. Diagn Microbiol Infect Dis. 2018;90(2):151–152. doi: 10.1016/j.diagmicrobio.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 23.Seiffert SN, Marschall J, Perreten V, et al. Emergence of Klebsiella pneumoniae co-producing NDM-1, OXA-48, CTX-M-15, CMY-16, QnrA and armA in Switzerland. Int J Antimicrob Agents. 2014;44(3):260–262. doi: 10.1016/j.ijantimicag.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 24.Taylor E, Sriskandan S, Woodford N, et al. High prevalence of 16S rRNA methyltransferases among carbapenemase-producing Enterobacteriaceae in the UK and Ireland. Int J Antimicrob Agents. 2018;52(2):278–282. doi: 10.1016/j.ijantimicag.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 25.Fournier C, Poirel L, Despont S, et al. Increasing trends of association of 16S rRNA methylases and carbapenemases in enterobacterales clinical isolates from Switzerland, 2017–2020. Microorganisms. 2022;10(3):615. doi: 10.3390/microorganisms10030615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Chen ZL, LIU JH, et al. Emergence of RmtB methylase-producing Escherichia coli and Enterobacter cloacae isolates from pigs in China. J Antimicrob Chemother. 2007;59(5):880–885. doi: 10.1093/jac/dkm065 [DOI] [PubMed] [Google Scholar]
- 27.Doi Y, Hazen TH, Boitano M, et al. Whole-genome assembly of Klebsiella pneumoniae coproducing NDM-1 and OXA-232 carbapenemases using single-molecule, real-time sequencing. Antimicrob Agents Chemother. 2014;58(10):5947–5953. doi: 10.1128/AAC.03180-14 [DOI] [PMC free article] [PubMed] [Google Scholar]