Abstract
As the great majority of gene expression (GE) biodosimetry studies have been performed using blood as the preferred source of tissue, searching for simple and less-invasive sampling methods is important when considering biodosimetry approaches. Knowing that whole saliva contains an ultrafiltrate of blood and white blood cells, it is expected that the findings in blood can also be found in saliva. This human in vivo study aims to examine radiation-induced GE changes in saliva for biodosimetry purposes and to predict radiation-induced disease, which is yet poorly characterized. Furthermore, we examined whether transcriptional biomarkers in blood can also be found equivalently in saliva. Saliva and blood samples were collected in parallel from radiotherapy (RT) treated patients who suffered from head and neck cancer (n = 8) undergoing fractioned partial-body irradiations (1.8 Gy/fraction and 50–70 Gy total dose). Samples were taken 12–24 h before first irradiation and ideally 24 and 48 h, as well as 5 weeks after radiotherapy onset. Due to the low quality and quantity of isolated RNA samples from one patient, they had to be excluded from further analysis, leaving a total of 24 saliva and 24 blood samples from 7 patients eligible for analysis. Using qRT-PCR, 18S rRNA and 16S rRNA (the ratio being a surrogate for the relative human RNA/bacterial burden), four housekeeping genes and nine mRNAs previously identified as radiation responsive in blood-based studies were detected. Significant GE associations with absorbed dose were found for five genes and after the 2nd radiotherapy fraction, shown by, e.g., the increase of CDKN1A (2.0 fold, P = 0.017) and FDXR (1.9 fold increased, P = 0.002). After the 25th radiotherapy fraction, however, all four genes (FDXR, DDB2, POU2AF1, WNT3) predicting ARS (acute radiation syndrome) severity, as well as further genes (including CCNG1 [median-fold change (FC) = 0.3, P = 0.013], and GADD45A (median-FC = 0.3, P = 0.031)) appeared significantly downregulated (FC = 0.3, P = 0.01–0.03). A significant association of CCNG1, POU2AF1, HPRT1, and WNT3 (P = 0.006–0.04) with acute or late radiotoxicity could be shown before the onset of these clinical outcomes. In an established set of four genes predicting acute health effects in blood, the response in saliva samples was similar to the expected up- (FDXR, DDB2) or downregulation (POU2AF1, WNT3) in blood for up to 71% of the measurements. Comparing GE responses (PHPT1, CCNG1, CDKN1A, GADD45A, SESN1) in saliva and blood samples, there was a significant linear association between saliva and blood response of CDKN1A (R2 = 0.60, P 0.0004). However, the GE pattern of other genes differed between saliva and blood. In summary, the current human in vivo study, (I) reveals significant radiation-induced GE associations of five transcriptional biomarkers in salivary samples, (II) suggests genes predicting diverse clinical outcomes such as acute and late radiotoxicity as well as ARS severity, and (III) supports the view that blood-based GE response can be reflected in saliva samples, indicating that saliva is a mirror of the body for certain but not all genes and, thus, studies for each gene of interest in blood are required for saliva.
INTRODUCTION
In a radiological or nuclear scenario, there is a need for early and high throughput diagnostics to identify highly exposed individuals within the first days to initiate appropriate treatment and increase the prognosis (1). In the absence of physical dosimeters (e.g., in case of terrorist attacks or other scenarios when badge dosimeters are not routinely worn by those likely to be exposed), biological measurements after radiation exposure are used for individual dose estimates and prediction of later occurring acute health effects. Gene expression (GE) analysis has already been shown to be suitable for early (2, 3) and high-throughput minimally invasive radiation biodosimetry (4–6). Nevertheless, most studies dealing with biomarkers for diagnostics and screening purposes based on GE have been performed using blood as the preferred tissue source.
Over the last two decades, saliva as an alternative biofluid has become increasingly interesting as an easily accessible and non-invasive source of human biomarkers (7–10). It has been shown to contain RNA biomarkers for prediction and diagnosis of several diseases especially of the oral cavity, such as oral cancer (11–13) and general disorders of the salivary glands (14, 15). Particularly in emergency situations such as a large-scale radiological accident or nuclear mass casualty scenario, the bottleneck of sampling could be overcome by using easily accessible biosamples such as saliva for high-throughput biodosimetry. Saliva has numerous advantages over other types of biosamples such as blood: non-invasive and straightforward sample collection (possibly by the patient himself or an untrained person), easy and repeatable sampling of the elderly and children (16), far less discomfort to subjects and simplified logistics of sample collection as well as low costs of collection (no venipuncture). Saliva aggregates information from several bodily sources. Because saliva also contains plasma ultra-filtrate and white blood cells (17), this indicates that most compounds, including the robust and indisputable radiation-induced biomarkers expressed in the blood, may also be represented in saliva. This led to the aphorism that saliva is a “mirror of the body” (18, 19). Collecting saliva samples represents an easy, fast, and non-invasive alternative to blood collection for diagnostic screening. So far, there is very limited published data in the field of biodosimetry using saliva (20, 21). A pilot study compared GE changes in blood and saliva and showed that saliva has the potential to provide promising gene-based biomarkers during head and neck radiotherapy (21). Previous metabolomic studies in mice and non-human primate models have already identified radiation as well as dose-specific biomarkers from saliva (22, 23). After the association of radiation-induced GE changes in saliva (radiation-to-gene association), a further step would be to ask about the clinical consequences of these deregulated genes. Are GE changes also associated with later occurring health effects (gene-to-effect prediction)? If this applies, this approach could offer a tool to predict acute and late radiotoxicity in irradiated patients, supporting clinicians in individualizing the therapy regimen.
In previous work, we have shown that methodologic improvements could mitigate the drawbacks of non-sterile saliva samples, such as low RNA yield and high levels of non-human RNA. Based on those findings, a robust workflow was developed to process human whole saliva (not salivary supernatant) for GE analysis, introducing a modified cDNA synthesis aiming at the poly(A)+ -tail and a pre-amplification step prior to qRT-PCR (24). Further efforts were made to advance this workflow. We demonstrated that the quality and quantity of RNA isolates is highly robust considering potential confounding factors such as demographic/epidemiologic parameters (e.g., sex, age, cigarette consumption, or oral hygiene) and the saliva sampling time, making the approach of saliva collection even more attractive for further biomarker studies (25).
The current pilot study addressed the following aspects: (I) Examining the applicability of the newly developed GE working pipeline for identifying radiation-induced biomarkers (radiation-to-gene-association). (II) Identifying genes (mRNA) in saliva samples that are associated with consecutive clinical outcomes in terms of acute and late radiotoxicity (such as radiation-induced mucositis) occurring in patients during RT (gene-to-effect-association). Here, we also examined a four gene set predicting the ARS severity in blood after irradiation (26, 27). (III) Verifying that saliva is a “mirror of the body” by performing saliva and blood GE measurements in the same patients and at the same time points.
MATERIALS AND METHODS
Patients, Sample Collection, Radiotherapy, Ethical Approval
Eight head-and-neck cancer patients (all male, average age 59 ± 6.8 years, Table 1) with indicated local radiotherapy (partial-body irradiation, PBI) and without previous (or concomitant) radio- as well as chemotherapy were sequentially enrolled for blood collection over five weeks. Clinical follow-ups have been carried out for more than three months, depending on the clinical course. All patients underwent treatment with a comparable scheme of radiotherapy, allowing comparability due to the corresponding irradiation field size and dose rate. The prescribed dose was between 50 and 70 Gy and applied within 25 to 33 fractions over 35 to 45 days. Using LINAC with a dose rate of 300 MU/min (Varian Medical Systems Inc., Palo Alto, CA) and the treatment planning system Eclipse (Varian Medical Systems), the single dose per fraction was 2.0 or 2.121 Gy (Table 1). The average whole-body dose absorbed by blood per fraction was 0.08 Gy. No shielding was used during irradiation (28).
TABLE 1.
Overview of the Included Samples, Demographic and Epidemiologic Characteristics of the Patients (Age, Sex, Morbidity) as well as Radiotherapy (RT) Regimen Analyses and Recorded Radiotoxicity Grades According to RTOG/EORTC Late Radiation Morbidity Criteria: Acute (Grade 1–2) and Late (Grade 1–3) Toxicity as well as Late Toxicity Location
| Patient ID | Age (years) | Sex | Morbidity | Partial body Irradiation (25 fractions over 5 weeks) | Equivalent absorbed body dose | Recorded acute and late toxicity | Sampling time points (# RT fraction) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor localization | Tumor grade | Dose per fraction | Prescribed total dose (Gy) | Irradiated blood volume (dm3) | Absorbed blood dose per fraction (Gy) | Acute toxicity (Grade) | Late toxicity (Grade) | Late toxicity location | 0 | 1 | 2 | 25 | |||
| P1 | 50 | male | Submandibular gland | 3 | 2 | 50 | 1 | 0.11 | 1 | early death | none | x | x | x | x |
| P2 | 60 | male | Skin | 3 | 2 | 60 | 1 | 0.09 | 1 | early death | none | x | x | x | x |
| P3 | 70 | male | Tonsil | 2 | 2.1 | 70 | 0.4 | 0.07 | 2 | 2 | s.c./mucosal | x | x | x | x |
| P4 | 50 | male | Base of tongue | 2 | 2.1 | 50 | 0.5 | 0.11 | 2 | 3 | s.c./mucosal | x | x | x | |
| P5 | 50 | male | Supraglottis | 3 | 2.1 | 50 | 0.7 | 0.09 | 2 | 2 | s.c./mucosal | x | x | x | |
| P6 | 66 | male | Hypopharynx | 2 | 2 | 66 | 0.5 | 0.08 | 1 | 1 | s.c./mucosal | x | x | x | |
| P7 | 66 | male | Larynx | 3 | 2 | 66 | 0.5 | 0.09 | 1 | 1 | s.c./mucosal | x | x | x | |
| P8 | 60 | male | Disseminated neoplasm | 2 | 2 | 60 | 0.4 | 0.08 | 1 | 1 | s.c./mucosal | x | x | x | |
Notes. Further, the sampling time points are depicted for each patient according to radiotherapy fractions (RT1 = 24 h after the start of Radiotherapy, RT1 ≈ 48 h after the start of radiotherapy, RT25 ≈ 5 weeks after the start of radiothearpy). m – male, s.c. - subcutaneous.
Treatment-related radiation toxicity was recorded for each patient (Table 1). Acute toxicity grading was performed according to the worst grade of symptoms recorded during treatment or up to three months after the end of the radiotherapy using the CTCAE v4.0 (29). Late toxicity grades were classified as the worst grade of symptoms that persisted more than three months after the end of the radiotherapy scheme using the RTOG grading system (30). Patients P1 and P2 died due to the rapid progression of cancer and not due to radiation toxicity. Patients with oral mucositis or xerostomia received improved mouthwash, artificial saliva and/or Cevimeline (hydrochloride) for stimulating secretion by the salivary glands and treating symptoms of dry mouth.
Twenty-four peripheral whole blood samples (2.5 ml each) were obtained via venipuncture using the PAXgene Blood RNA system (BD Diagnostics, PreAnalytiX GmbH, Hombrechtikon, Switzerland). In parallel, 24 whole saliva samples were collected using ORAgene®RNA (catalog number: RE-100) vial collection kits from DNA Genotek used according to the manufacturer’s instructions (DNA Genotek Inc., Kanata, Ontario, Canada). The kit is an all-in-one system for unstimulated sampling (e.g., no saliva secretion stimulation with sugar or drugs), stabilization, and transportation of RNA from whole saliva. From all donors, blood and whole saliva were sampled before the first radiation treatment, which served as a control sample prior to the irradiation (reference), and ideally after 24 h, 48 h, and 5 weeks, i.e., after 1st, 2nd, and 25th radiotherapy fraction (Table 1). No specific oral hygiene, eating, drinking, or smoking habits were followed. Saliva and blood samples were stored at room temperature overnight and placed in a freezer (−20°C) for storage. All samples and data were obtained with informed consent from the donors, processed anonymously without exception, and only used for this specific purpose. Sampling was carried out in accordance with the institutional guidelines and regulations. Informed consent was obtained from each individual, and the local Ethical Committee of the University Hospital in Hradec Kralove (Czech Republic) approved experimentation with human subjects according to The Code of Ethics of the World Medical Association – Declaration of Helsinki (approval no: 201401-S15P). All data were handled according to the European General Data Protection Regulation.
RNA Extraction and Quality/Quantity Control
Total RNA, comprising a mixture of human and bacterial RNA, was isolated from whole saliva samples following a combination of the ORAgene® RNA purification protocol (31) and the mirVana™ kit protocol (Invitrogen™, ThermoFisher Scientific, Carlsbad, CA 92008; USA/Life Technologies, Darmstadt, Germany) as described in detail elsewhere (24). In brief, the samples were heated at 50°C (1 h), three aliquots (of 1,000 μl) were generated, incubated at 90°C (15 min), cooled to room temperature, 40 μl ORAgene® neutralizer solution (1/25 of total volume) was added, incubated on ice, centrifuged at 13,000 g (3 min) and the cell-free clear supernatant was collected for further processing. We then continued processing using the mirVana™ kit protocol (32) by adding the Lysis/Binding Solution. The mirVana™ kit isolated total RNA, including human and bacterial RNA species, by combining a Phenol-Chloroform RNA precipitation with further processing using silica membranes. After several washing procedures to purify RNA from other residual debris, DNA residuals were digested on the membrane (RNAse-free DNAse Set, Qiagen, Hilden, Germany). RNA was eluted with 100 μl RNAse free water in a collection tube, and the aliquots were pooled for each sample. To increase the input RNA amount for downstream gene expression analysis, sample volumes were reduced by evaporating at 45°C for 90 min, followed by re-elution with 30 μl of RNase-free water.
Blood samples were processed during another study (28). In brief, the PAXGene tubes containing the whole blood (n = 24) were thawed, washed, and centrifuged according to the PAXgene Blood RNA system protocol (BD Diagnostics, PreAnalytiX GmbH, Hombrechtikon, Switzerland). Cells in the supernatant were lysed (Proteinase K; BD Diagnostics, PreAnalytiX GmbH, Hombrechtikon, Switzerland), then the Lysis/Binding Solution was added, and further steps were performed according to the mirVana™ kit protocol described above.
The quality and quantity of isolated total RNA was measured spectrophotometrically using NanoDrop™ One Microvolume UV-Vis spectrophotometer (NanoDrop, PeqLab Biotechnology, Erlangen, Germany). RNA integrity was assessed by the 4200 TapeStation System (Life Science Group, Penzberg, Germany), and DNA contamination was checked via conventional PCR using β-actin primers.
cDNA Synthesis and Pre-Amplification
To ensure equal human RNA input for cDNA-synthesis as a prerequisite for comparability among samples when performing quantitative RT-PCR, 18S rRNA (Hs99999901_g1) as surrogate for human RNA and pan-bacterial 16S rRNA (Ba04230899_s1) as a surrogate for bacterial contamination were quantified after reverse transcription via the High-capacity cDNA reverse transcription kit (33) (Applied Biosystems™, Life Technologies, Darmstadt, Germany). Using the RNA concentration from repeated NanoDrop™ measurements and the calculated 18S/16S rRNA ratio (to reconstruct the human portion of the total RNA amount including human and bacterial RNA parts) for each sample [ratio 2^(Ct18S rRNA - Ct16S rRNA)], a defined amount of human RNA (4 ng) could be reverse transcribed in a second cDNA synthesis via the SuperScript® III First-Strand Synthesis System with Oligo (dT)20 primers (25).
Due to high bacterial contamination and low amounts of human RNA, samples from patient P8 were discarded. To detect low-abundance mRNA species, pre-amplification was required to increase the amount of specific cDNA targets synthesized with the SuperScript® III First-Strand Synthesis System. Ten cycles of pre-amplification were performed according to the TaqMan® PreAmp Master Mix (Thermo Fisher Scientific Baltics UAB, Vilnius, Lithuania) (34). In the present work, 13 different TaqMan® Gene Expression Assays (4 genes for normalization purposes and 9 genes for detecting radiation-induced target genes) were utilized and pooled to enable the multiplex amplification of specific cDNA targets. ACTB (Hs01060665_g1), ATP6 (Hs02596862_g1), B2M (Hs00187842_m1), and HPRT1 (Hs02800695_m1) were used as an internal control for normalization purposes. PHPT1 (Hs03645225_m1), CCNG1 (Hs00171112_m1), CDKN1A (Hs00355782_m1), GADD45A (Hs00169255_m1), SESN1 (Hs00902782_m1), FDXR (Hs01031617_m1), DDB2 (Hs00172068_m1), POU2AF1 (Hs01573371_m1), and WNT3 (Hs00902257_m1) known as radiation-induced targets in blood were detected as well (2, 26–28, 35–37).
Real-time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
For human (18S rRNA) and pan-bacterial (16S rRNA) primer probe designs (for ratio calculation; see above), cDNA from a high-capacity cDNA reverse transcription kit was used. For the nine primer probe designs representing previously identified biomarkers of radiation exposure in the blood (PHPT1, CCNG1, CDKN1A, GADD45A, SESN1, FDXR, DDB2, POU2AF1, and WNT3), SuperScript™ III First-Strand Synthesis SuperMix was used in combination with a 10× pre-amplification for the detection of each gene in each saliva sample. For blood samples, cDNA from a High-capacity cDNA reverse transcription kit was used. The experiments for analyzing blood samples were performed during another study (28). That’s why, the blood gene expression (GE) data of CDKN1A, PHPT1, CCNG1, GADD45, and SESN1 for the comparison in the results part Task III part 1 was used from the mentioned study. The qRT-PCR reaction contained the TaqMan® Universal PCR Master Mix and one of the inventoried TaqMan® Gene Expression Assays for separate detection of transcripts. The qRT-PCR for the genes 18S rRNA and 16S rRNA was performed similarly. All measurements were run in duplicate, using a 96-well-format TaqMan® qRT-PCR platform and the QuantStudio™ 12K OA Real-Time PCR System (Thermo Fisher Scientific Inc., Waltham, MA). After the calculation of input and normalization using ACTB, ATP6, and B2M in saliva samples, as well as HPRT1 in blood samples, fold change (FC) differences in GE were calculated by the −ΔΔCt-approach [cycle threshold (Ct)] relative to unexposed samples of the same patient used as the calibrator.
Statistical Analysis
Descriptive and analytical statistics were performed using SAS (release 9.4, Cary, NC). Associations with GE were either examined using linear (for continuous variables such as age) or logistic regression models (for categorical variables such as acute or late radiotoxicities). Acute toxicity was examined with grade 1 vs. grade 2. Due to the reduced patient number, we merged late toxicity grades into binary categories to increase the power. For late toxicity, grades 1 and 2 were merged into one category, and grades 3 and 4 into a second category. Significant GE differences at specific time points were calculated using either parametrical (t test) or non-parametric tests, where applicable. To compare frequencies of patients showing the same direction of differential gene expression (DGE) response in saliva and blood, a FC > |1.2| was introduced to define up- or downregulation or non-response (lying below 1.2) to partial-body irradiation. A fold change of 1.2 was chosen to allow for high sensitivity. Statistical analysis was performed separately for each of the first two radiotherapy fractions and for some comparisons combined to increase the power. If mean GE values in these comparisons revealed a similar GE tendency (up- or downregulation) in both radiotherapy fractions and became statically significant after merging them, they were reported in this study. Further calculations and graphical presentations were performed using Excel 2010 (Microsoft) and Sigma Plot 14.5 (Jandel Scientific, Erkrath, Germany).
RESULTS
Saliva Sample RNA Quantity and Quality Control
An average of 15.1 μg (SD ± 21.4) total RNA per 2 ml of saliva could be isolated in 24 samples from seven patients. The A260/A280 nm ratio was measured at a mean of 1.9. A mean RNA integrity number (RIN) of 5.3 (SD ± 1.5) was detected and all saliva samples showed gel-like image bands of human 28S rRNA and 18S rRNA, which did not indicate severe degradation. The β-actin PCR could not detect DNA contamination in all samples (data not shown). The mean average (± SD) 16S/18S rRNA ratio of 41,516 ± 147,552 indicated a high bacterial abundance over human total RNA (Fig. 1). All four samples from patient P8 had to be excluded from GE and further analysis due to high bacterial contamination (16S rRNA raw Ct value of 20.8 on average, min 17.6) and low amounts of human RNA (18S rRNA raw Ct value of 37.4 on average, max 38.1, data not shown). All other samples (n = 24) fulfilled previously detected quality and quantity criteria for GE analysis in saliva samples [e.g. 18S rRNA Ct value < 30 and RNA integrity number (RIN) ≥ 5; (24, 25)].
FIG. 1.
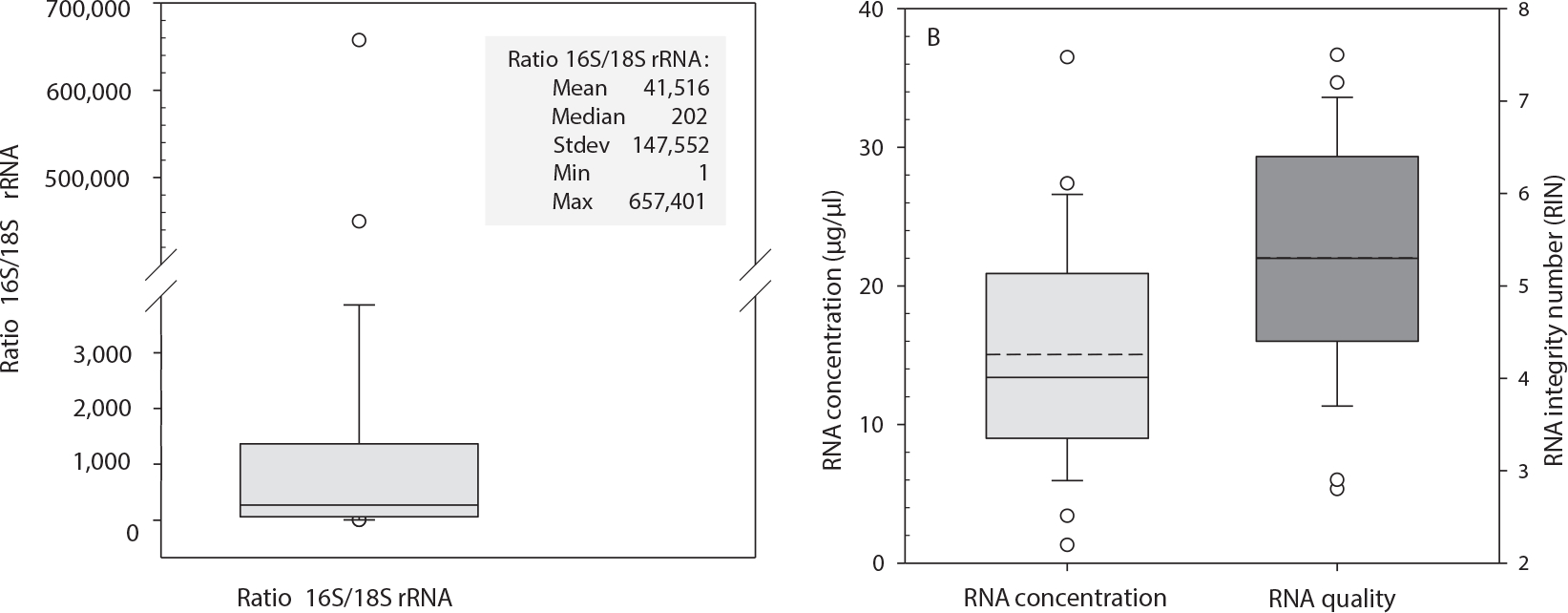
The box plot in panel A displays the ratio of bacterial 16S rRNA and human 18S rRNA for all whole saliva samples (n = 24). Solid lines represent the median and circles the outliers. The inserted table shows the calculated ratio between raw Ct values of human 18S rRNA and bacterial 16S rRNA as an indicator of bacterial contamination in relation to human RNA. Descriptive statistics: mean, median, standard deviation (stdev), minimum (min) and maximum (max). The box plots in panel B represent the concentration (μg/μl) of 24 RNA isolates (left side). The right side shows the quality of isolated RNA using RNA integrity numbers (RIN) for saliva samples (total n = 24). Dashed lines represent the mean, solid lines the median, and circles the outliers.
Task I: Examining for Radiation-Induced Genes during Radiotherapy
Median DGE of all patients of CDKN1A was significantly increased in saliva samples after the 2nd dose fraction, presenting a median FC of 1.9 (P = 0.017), which then rose after the 25th radiotherapy fraction showing a median FC of 2.2 (not statistically significant, Fig. 2, Supplementary Table S1;2 https://doi.org/10.1667/RADE-23-00176.1.S1). With increasing radiation dose, a downregulation of median CCNG1 DGE was observed. No persistent pattern of median DGE with dose could be observed for PHPT1, GADD45A, and SESN1 (Fig. 2).
FIG. 2.
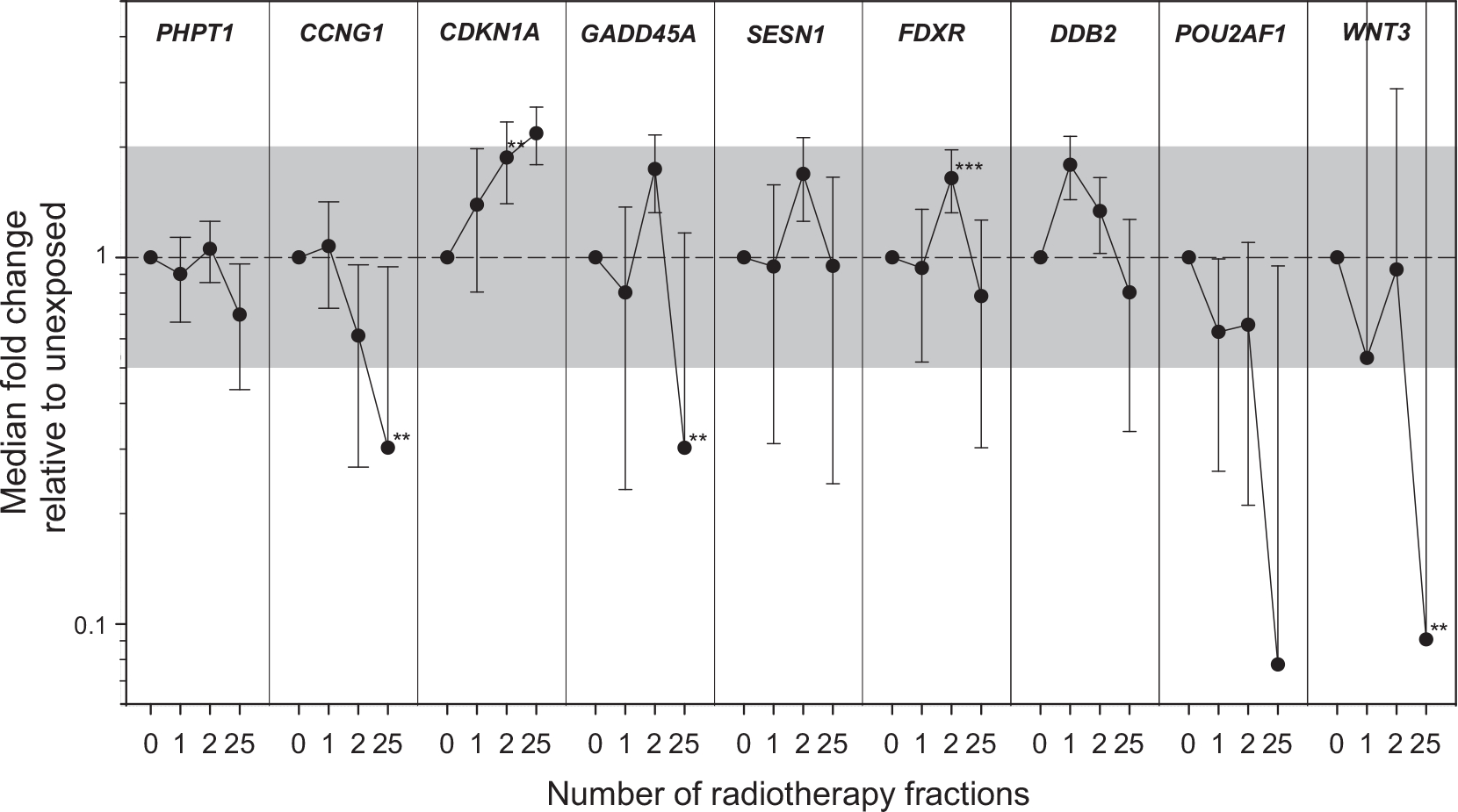
Aggregated data of DGE in saliva for all 9 genes (PHPT1, CCNG1, CDKN1A, GADD45, SESN1, FDXR, DDB2, POU2AF1, and WNT3) is shown over time of the radiotherapy scheme (number of radiotherapy fractions). GE is given as fold change (FC) relative to unexposed (normalized against a combination of ACTB/ATP6/B2M). Symbols reflect the median (N = 7), and error bars the standard error of the mean (SEM). The superimposed grey area refers to a FC < |2|. Significant changes in GE relative to unexposed are indicated with asterisks (**P < 0.02). Individual plots per donor and gene are shown in Supplementary Fig. S1 (https://doi.org/10.1667/RADE-26-00176.1.S1).
For FDXR, median DGE increased significantly (1.6 fold, P = 0.002) after the 2nd radiotherapy fraction (Fig. 2, Supplementary Fig. S1B; https://doi.org/10.1667/RADE-23-00176.1.S2 and Supplementary Table S1; https://doi.org/10.1667/RADE-23-00176.1.S2). Expected although insignificant upregulation of DDB2 and insignificant downregulation of POU2AF1 and WNT3 was found after the 1st and/or 2nd radiotherapy fraction. After the 25th radiotherapy fraction, a significant downregulation of many genes including CCNG1 (median FC = 0.3, P = 0.013), GADD45A (median FC = 0.3, P = 0.031) and WNT3 (median FC = 0.1, P = 0.017) could be observed. PHPT1 appeared insignificantly downregulated. Only CDKN1A was upregulated, and SESN1 was unchanged from control values (Fig. 2, Supplementary Fig. S1A and Supplementary Table S1).
Task II: Examining for Radiation-Induced Genes Associated with and Predicting Clinical Outcomes
Three head and neck cancer patients recorded a grade 2 and four a grade 1 acute toxicity. Concerning late toxicity grading, one patient showed the highest grade of 3, two grade 2, and two grade 1 (Table 1). All late toxicities were located subcutaneously and/or mucosal. Two patients died due to the rapid progression of cancer and not due to radiation toxicity. No late effects (>3 months after the end of the radiotherapy scheme) could be evaluated for these two patients. The 1st and 2nd radiotherapy fraction measurements were merged and reported for those genes, showing DGE going in the same direction after both radiotherapy fractions (Table 2). The merged set of raw data is provided within Supplementary Table S2 (https://doi.org/10.1667/RADE-23-00176.1.S3).
TABLE 2.
Overview of the GE Results (Normalized Ct Values) and the Significant Correlations with Acute (Grade 1–2) and Late Toxicity Grade (Categories of Grade 1–2 and 3–4)
| Normalized Ct value | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| n | mean | stdev | min | max | FC | p-value | ||
| Acute toxicity - grade | ||||||||
| CCNG1 | ||||||||
| RT 1st&2nd fraction combined | grade 1 | 8 | 10.5 | 0.8 | 9.3 | 11.7 | Ref | |
| grade 2 | 6 | 11.4 | 0.5 | 10.8 | 12.4 | 0.54 | 0.04 | |
| DDB2 | ||||||||
| RT 1st&2nd fraction combined | grade 1 | 8 | 10 | 1.3 | 7.7 | 11.7 | Ref | |
| grade 2 | 6 | 11.2 | 0.6 | 10.5 | 12.2 | 0.44 | 0.06 | |
| Late toxicity - grade | ||||||||
| HPRT1 | ||||||||
| RT 1st&2nd fraction combined | grade 1–2 | 8 | 12.2 | 0.5 | 11.4 | 13.2 | Ref | |
| grade 3–4 | 6 | 13.2 | 0.7 | 12.5 | 14 | 0.50 | 0.006 | |
| POU2AF1 | ||||||||
| RT 1st&2nd fraction combined | grade 1–2 | 7 | 12.7 | 1.7 | 10.1 | 15.4 | Ref | |
| grade 3–4 | 5 | 15.5 | 1.1 | 14.2 | 17.2 | 0.14 | 0.02 | |
| WNT3 | ||||||||
| pre-exposure | grade 1–2 | 3 | 14.7 | 1.1 | 13.6 | 16.2 | Ref | |
| grade 3–4 | 3 | 17.8 | 0.9 | 16.6 | 18.8 | 0.12 | 0.04 | |
Notes. Fold changes were calculated with grade 1 in acute toxicity and grade 1–2 in late toxicity as the reference. Provided are numbers (n) per category and corresponding descriptive statistics: mean, standard deviation (stdev), minimum (min) and maximum (max), fold changes difference (FC) with corresponding reference. Only genes that showed significant or borderline significant results are depicted. Data for all genes is shown in Supplemental Table S2 (https://doi.org/10.1667/RADE-23-00176.1.S3).
A weakly significant association of CCNG1 (P = 0.04) and a borderline significant association for DDB2 (P = 0.06) was found for grade 2 relative to grade 1 acute toxicity. The DGE of both genes was about twofold downregulated (Table 2, Supplementary Table S2; https://doi.org/10.1667/RADE-23-00176.1.S3).
A significant association of merged binary late toxicity grades was detected for HPRT1 (P = 0.006, 0.5 fold) and POU2AF1 (P = 0.02, 0.1 fold) after 1st and 2nd radiotherapy fraction combined, and before irradiation for WNT3 (P = 0.04, 0.1 fold) (Table 2). HPRT1, POU2AF1 and WNT3 DGE remained downregulated after 1st and 2nd radiotherapy fraction but did not reach significance (Supplementary Table S2; https://doi.org/10.1667/RADE-23-00176.1.S3).
Except for HPRT1 (tumor grade 2 vs. 3; P = 0.01), tumor grading did not appear significantly associated with DGE in all genes and over all time points examined (data not shown).
Task III: Comparing GE Changes in Saliva vs. Blood
1. Comparing radiation-induced DGE of CDKN1A, PHPT1, CCNG1, GADD45, and SESN1 in saliva vs. blood.
For each patient, an upregulation of CDKN1A DGE after radiation exposure could be detected in both saliva as well as in blood samples at all time points except for two patients (P4 and P6 after the first radiotherapy fraction, Fig. 3A). Plotting corresponding CDKN1A DGE of saliva and blood samples from each patient and time point resulted in a significant association (rsq = 0.6, P = 0.0004, Fig. 3B).
FIG. 3.
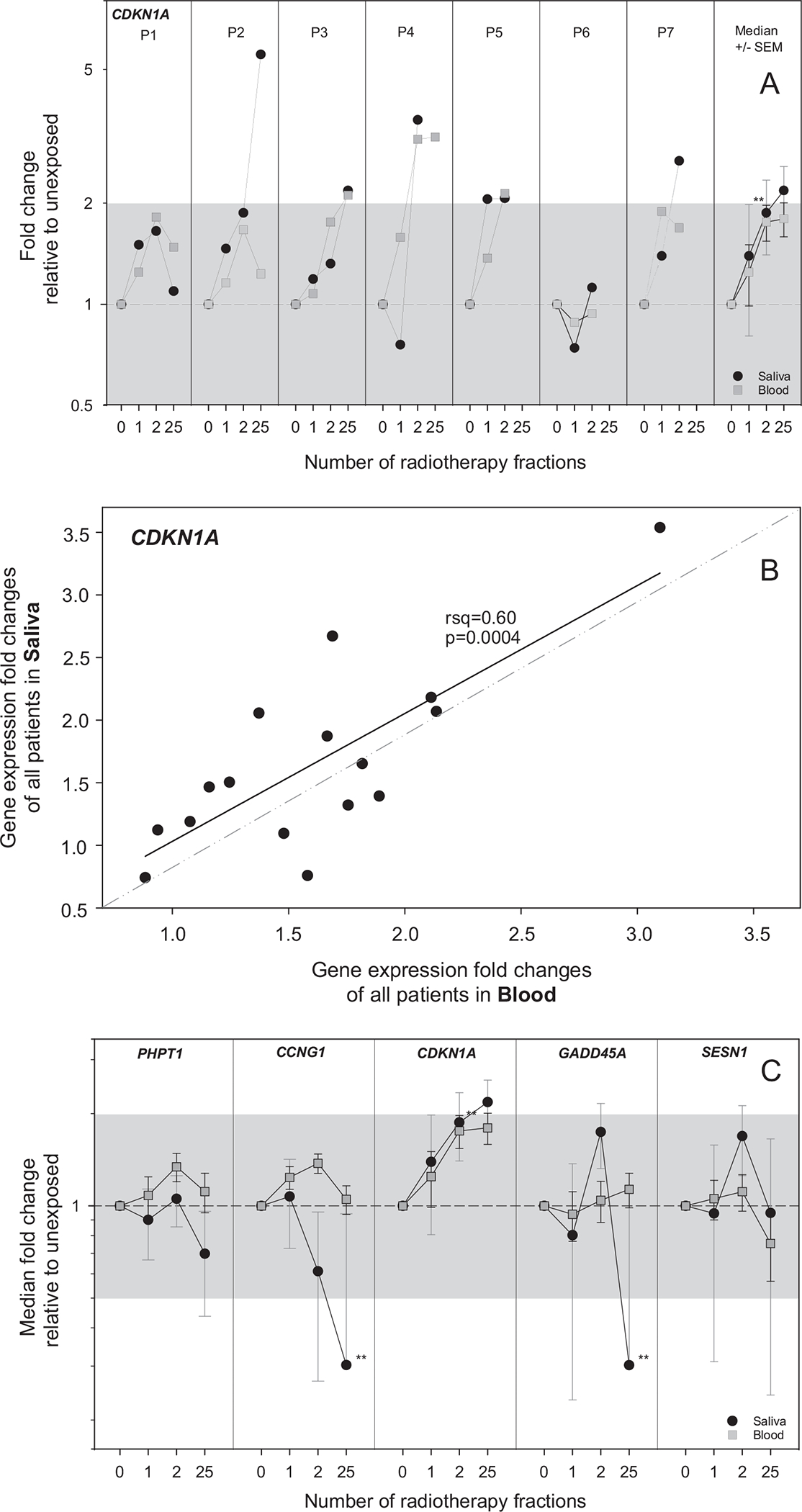
This figure focuses on the comparison of radiation-induced DGE of CDKN1A, PHPT1, CCNG1, GADD45, and SESN1 in saliva versus blood (task III-1). Panel A: the DGE of CDKN1A is shown by way of example for each patient in separate panels over time of the radiotherapy scheme (number of radiotherapy fractions). GE is given as fold change (FC) relative to unexposed (normalized against a combination of ACTB/ATP6/B2M in saliva samples and HPRT1 in blood samples). The black circles represent GE results from saliva samples, and gray squares represent GE results from blood samples. In the right panel, data of all patients for CDKN1A is aggregated. Symbols reflect the median (N = 7), and error bars the standard error of the mean (SEM). The superimposed gray areas refer to a FC < |2|. Significant changes in GE relative to unexposed are indicated with asterisks (**P < 0.02). Panel B: FC values obtained with RNA from saliva samples and those obtained with RNA from blood samples for each measurement were correlated with linear regression analysis (calculated R2 and P values are provided). Outliers from 95% confidence interval were excluded. Panel C: Equivalently shows aggregated data for PHPT1, CCNG1, CDKN1A, GADD45, and SESN1. Individual plots per donor and gene are shown in Supplementary Fig. S1 (https://doi.org/10.1667/RADE-26-00176.1.S1).
For genes PHPT1, CCNG1, GADD45, and SESN1, median DGE values of saliva and blood samples did not correspond significantly and DGE for some time points appeared even significantly different (Fig. 3C).
The frequency of similarly up- or downregulated genes in saliva and blood FC > |1.2| differed among genes. The overall conformity (including 1st, 2nd, and 25th radiotherapy fraction measurements) reached a maximum of 76.5% for CDKN1A and a minimum of 23.5% regarding SESN1 (Table 3). Intermediate overall conformities of 47.1%, 35.3% and 29.4% were calculated for PHPT1, CCNG1 and GADD45, respectively (Table 3).
TABLE 3.
Overview of the Simple Direction of Response (Up- or Downregulation, FC > |1.2|) or Non-Response for PHPT1, CCNG1, CDKN1A, GADD45, and SESN1 in Blood and Saliva Samples for each Patient over Three Time Points after the Start of Radiotherapy (RT) (RT1, RT2, RT25)
| Gene | Patient | Direction of FC | Overall conformity Saliva - Blood | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT 1 | RT 2 | RT 25 | |||||||||||||||||
| Saliva | Blood | Saliva | Blood | Saliva | Blood | 1st + 2nd RT | all RT fractions | ||||||||||||
| up | down | up | down | up | down | up | down | up | down | up | down | yes (%) | no (%) | undefinded (%) | yes (%) | no (%) | undefinded (%) | ||
| CDKN1A | N2 | X | X | X | X | o | x | ||||||||||||
| N3 | x | o | X | X | X | X | |||||||||||||
| N4 | O | O | X | X | X | X | |||||||||||||
| N5 | x | x | X | X | x | ||||||||||||||
| N7 | X | X | X | X | |||||||||||||||
| N8 | x | o | O | O | 11/14 | 1/14 | 2/14 | 13/17 | 1/17 | 3/17 | |||||||||
| N9 | X | X | X | X | 78.6 | 7.1 | 14.3 | 76.5 | 5.9 | 17.6 | |||||||||
| PHPT1 | N2 | x | o | O | O | x | o | ||||||||||||
| N3 | x | o | X | X | O | O | |||||||||||||
| N4 | O | O | x | x | x | x | |||||||||||||
| N5 | x | o | o | x | x | ||||||||||||||
| N7 | x | x | o | x | |||||||||||||||
| N8 | O | O | X | X | 7/14 | 2/14 | 5/14 | 8/17 | 3/17 | 6/17 | |||||||||
| N9 | X | X | X | X | 50.0 | 14.3 | 35.7 | 47.1 | 17.6 | 35.3 | |||||||||
| CCNG1 | N2 | x | o | x | o | X | X | ||||||||||||
| N3 | x | o | X | X | x | o | |||||||||||||
| N4 | o | x | x | x | x | x | |||||||||||||
| N5 | x | x | x | x | x | ||||||||||||||
| N7 | X | X | X | X | |||||||||||||||
| N8 | x | o | x | o | 5/14 | 3/14 | 6/14 | 6/17 | 4/17 | 7/17 | |||||||||
| N9 | X | X | X | X | 35.7 | 21.4 | 42.9 | 35.3 | 23.5 | 41.2 | |||||||||
| GADD45A | N2 | X | X | x | x | x | o | ||||||||||||
| N3 | x | o | x | o | x | o | |||||||||||||
| N4 | o | x | x | o | x | x | |||||||||||||
| N5 | x | x | X | X | x | ||||||||||||||
| N7 | o | x | X | X | |||||||||||||||
| N8 | x | o | x | o | 5/14 | 2/14 | 7/14 | 5/17 | 3/17 | 9/17 | |||||||||
| N9 | X | X | X | X | 35.7 | 14.3 | 50.0 | 29.4 | 17.6 | 52.9 | |||||||||
| SESN1 | N2 | x | x | o | x | o | x | ||||||||||||
| N3 | o | o | x | o | o | x | |||||||||||||
| N4 | x | o | x | o | x | o | |||||||||||||
| N5 | x | o | x | o | x | ||||||||||||||
| N7 | x | x | o | o | |||||||||||||||
| N8 | x | o | x | o | |||||||||||||||
| N9 | o | o | x | x | 4/14 | 2/14 | 8/14 | 4/17 | 2/17 | 11/17 | |||||||||
| 28.6 | 14.3 | 57.1 | 23.5 | 11.8 | 64.7 | ||||||||||||||
Notes. FC ≥ |1.2| was considered as an up- or down-regulation represented by the symbol “x”, FC ≤ |1.2| was considered as an no de-regulation represented by the symbol “o”. The column “overall conformity Saliva - blood” provides a summary per gene for early radiotherapy fractions (RT1, RT2) and for all radiotherapy cycles (RT1, RT2, RT25). Conformity or no conformity in deregulation or undefined response between corresponding blood and saliva samples from the same patient at the same time point is depicted.
2. Comparing radiation-induced DGE of FDXR, DDB2, POU2AF1 and WNT3 in saliva vs. blood.
Previous work identified a set of four genes (FDXR, DDB2, POU2AF1, WNT3) which predicts the hematological acute radiation syndrome (H-ARS) severity within the first three days after irradiation. These four genes were not measured in blood samples within this study, but the radiation-induced upregulation of FDXR and DDB2 as well as the downregulation of POU2AF1 and WNT3 was shown in several previous studies (3, 26, 27, 38, 39).
This known upregulation of FDXR and DDB2, as well as downregulation of POU2AF1 and WNT3, was also detected in saliva samples within the current study, which is depicted in Fig. 2. Median FDXR and DDB2 DGE revealed an upregulation either after the 1st or 2nd radiotherapy fraction, while POU2AF1 and WNT3 were downregulated after both radiotherapy fractions (Fig. 2 and Supplementary Table S3; https://doi.org/10.1667/RADE-23-00176.1.S4). All four genes’ median DGE measurements were downregulated after the 25th radiotherapy fraction.
The direction of deregulated DGE of these four genes in all measurements taken after both, the 1st and 2nd radiotherapy fraction combined as well as measurements taken after all radiotherapy fractions, revealed similarities between saliva and the previously observed and validated deregulation in blood based studies ranging between 57–71% and 53–71%, respectively (Table 4).
TABLE 4.
The Table Comprises the Direction of Deregulation of FDXR, DDB2, POU2AF1, and WNT3 in each Patient over Three Time Points after the Start of Radiotherapy (RT) (RT1 ≈ 24 h after Start of RT, RT1 ≈ 48 h after the Beginning of the RT, RT25 ≈ 5 weeks after the start of RT)
| Gene | Patient | Expected deregulation | Direction of FC | Overall conformity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT 1 | RT 2 | RT 25 | all RT fractions | 1st + 2nd RT | ||||||||
| up | down | up | down | up | down | frequency | percentage | frequency | percentage | |||
| FDXR | P1 | up-regulation | X | X | x | |||||||
| P2 | x | X | x | |||||||||
| P3 | X | X | x | |||||||||
| P4 | x | X | ||||||||||
| P5 | X | X | ||||||||||
| P6 | X | X | ||||||||||
| P7 | x | X | 10/17 | 59% | 10/14 | 71% | ||||||
| DDB2 | P1 | up-regulation | X | x | x | |||||||
| P2 | x | X | X | |||||||||
| P3 | X | X | x | |||||||||
| P4 | x | x | ||||||||||
| P5 | x | x | ||||||||||
| P6 | X | X | ||||||||||
| P7 | X | X | 9/17 | 53% | 8/14 | 57% | ||||||
| POU2AF1 | P1 | down-regulation | x | x | X | |||||||
| P2 | X | X | X | |||||||||
| P3 | X | x | X | |||||||||
| P4 | X | |||||||||||
| P5 | x | X | ||||||||||
| P6 | X | X | ||||||||||
| P7 | x | 10/15 | 67% | 7/12 | 58% | |||||||
| WNT3 | P1 | down-regulation | x | X | X | |||||||
| P2 | X | x | X | |||||||||
| P3 | X | x | X | |||||||||
| P4 | x | |||||||||||
| P5 | X | |||||||||||
| P6 | X | X | ||||||||||
| P7 | X | 10/14 | 71% | 7/11 | 64% | |||||||
Notes. An up- or down-regulation is shown regardless of the height of deregulation (fold-change). Knowing that FDXR and DDB2 show a radiation-induced up-regulation and POU2AF1 and WNT3 are down-regulated after radiation exposure, frequency and the percentage represent the overall conformity of GE measurements with the expected deregulation and for early radiotherapy fractions (RT1, RT2) and for all RT fractions.
DISCUSSION
Biofluids such as whole blood are investigated for estimating individual doses in high-throughput biodosimetry and predicting later occurring acute health effects like acute radiation syndrome (40–43). The expression of specific genes in blood as the most collected biofluid so far has already been shown to be modulated in a dose-dependent manner (44, 45). There is strong evidence for gene expression to be used for early (2, 3), high-throughput (6), and minimally invasive radiation biodosimetry (4). The collection of saliva samples could represent an ideal non-invasive alternative to blood considering high-throughput biodosimetry for victims of radiological/nuclear incidents (20, 46). In this human in vivo study with head and neck cancer patients undergoing fractioned radiotherapy in terms of partial-body irradiation, we wanted to show that a combination of GE analysis and saliva as a non-invasive and easily collectible biofluid can be useful for e.g., biodosimetry purposes. Hereby, we had the unique opportunity to collect whole saliva and whole blood samples (as a positive control) from RT patients in parallel and examined nine genes (mRNA) known to be radiation-responsive in blood (28).
Except for one patient, the quality and quantity of RNA isolated from saliva samples was overall sufficient for GE analysis. The samples of the stated patient were discarded because they did not fulfil the quality criteria of 18S rRNA FC < 30 (indicating sufficient amounts of human RNA). Possible reasons may be insufficient sampling compliance (2.5 ml saliva required), samples not shaken vigorously after collection (a prerequisite for conservation to avoid degradation), concomitant oral mucositis and/or xerostomia.
As a first attempt, we examined nine radiation-induced genes previously identified in blood [PHPT1, CCNG1, CDKN1A, GADD45A, SESN1, FDXR, DDB2, POU2AF1, WNT3 (2, 28, 35–37)]. These genes are commonly used for biodosimetry purposes (FDXR, DDB2, CDKN1A, and GADD45A) and partly associated with radiation-induced acute health effects [FDXR, DDB2, POU2AF1, WNT3, (26, 27)]. Two genes (CDKN1A and DDB2) already appeared to be radiation responsive in saliva after head and neck cancer radiotherapy in a previous pilot study (21). In saliva, almost all patients revealed an upregulation of CDKN1A with increasing radiation exposure and downregulation of CCNG1, indicating the existence of radiation-responsive genes in saliva. The upregulated CDKN1A and the downregulated CCNG1 in saliva corresponded with blood measurements within our study and published examinations in blood (28). Almost similar responses of irradiated saliva and blood CDKN1A measurements in all examined patients and time points (R2 = 0.60, P value 0.0004) provided further hints for the reflection of DGE in saliva as the “mirror” of blood. However, responses of PHPT1, GADD45A, and SESN1 with dose in saliva differed from corresponding blood measurements within this study (Fig. 2) and cited work (28). Almost opposing DGE patterns with increasing doses were found for these genes in saliva versus blood of the same patient. That does not argue against their use for biodosimetry purposes, but it emphasizes organ-specific differences in radiation response. Otherwise, with increasing radiation exposure, an expected upregulation of FDXR and DDB2 and a downregulation of POU2AF1 and WNT3 could be found in up to 71% of all examined saliva samples (Fig. 3A and B) in correspondence to cited work (28). Hence, saliva measurements mirror only partially radiation-induced blood responses, and organ-specific responses must be acknowledged. Therefore, every radiation-responsive blood gene must be reevaluated in saliva. Interestingly, after the 25th radiotherapy fraction, all genes except CDKN1A and SESN1 became downregulated (Figs. 2 and 3). A similar pattern was observed on Rhesus macaques, and all four genes (FDXR, DDB2, POU2AF1 and WNT3) examined in blood were down-regulated 35 days after single high-dose radiation exposures (47). Radiation exposures (50–60 Gy fractionated partial-body irradiation including the salivary gland vs. 5–7 Gy total-body irradiation including the hematopoietic system) differed considerably, but the radiation-responsive organs received high-dose radiation exposures. This analogy identified in saliva indicates the usefulness of this biofluid as a surrogate of blood measurements. Within this study, the previously detected upregulation of CDKN1A and DDB2 in saliva in another cohort of eight head and neck cancer patients undergoing radiotherapy could be successfully validated (21).
The documentation of normal tissue responses in our patients, namely acute and late toxicities, allowed us to examine whether radiation-induced genes might also be useful as predictors of diverse clinical outcomes when examined after the 1st and 2nd radiotherapy fraction before normal tissue responses are detected. Several genes (e.g., CCNG1, DDB2, HPRT1, POU2AF1, and WNT3) revealed associations with acute and late toxicities (Table 2). However, these associations were weak or borderline significant. Nevertheless, the DGE of these genes examined after the 1st and 2nd radiotherapy fraction were consistently deregulated in the same direction (up- or downregulated, Supplementary Table S1; https://doi.org/10.1667/RADE-23-00176.1.S2). However, the low sample size made these consistent trends insignificant, and even merging measurements from the 1st and 2nd radiotherapy fraction resulted in weak associations, which must be interpreted cautiously. The low sample size represents a substantial limitation of our study. Our work must be considered as a more explorative type of study for hypothesis generation. Larger studies are planned for validation purposes in the near future. Interestingly, HPRT1 used as a housekeeping gene in several cited studies (28,48) was predictive for late toxicity normal tissue responses in our study (Supplementary Table S2; https://doi.org/10.1667/RADE-23-00176.1.S3). Again, HPRT1 appeared consistently downregulated after 1st and 2nd radiotherapy fraction and the strongest association with late toxicity was found for this gene (P values of 0.006). Nevertheless, the low sample size does not rule out significant findings by chance and requires validation. Interestingly, even pre-exposure HPRT1 appeared downregulated in patients developing higher degrees of late toxicity. This was insignificant, but for WNT3, a tenfold downregulation pre-exposure reached significance (Supplementary Table S1; https://doi.org/10.1667/RADE-23-00176.1.S1). Recently, a gene (CHD5) was independently validated in two different Rhesus macaques cohorts. This gene predicted the radiosensitivity (survival) of lethally irradiated Rhesus macaques (47, 49). It could be hypothesized that the DGE of HPRT1 and WNT3 might present a prone pre-exposure transcriptomic status so irradiation at this status increases the likeli-hood of developing more severe late toxicities. Again, further studies in this regard with increased sample sizes are required. No significant association of tumor grading with DGE could be detected among all genes and time points, possibly due to the underlying disease effect. Nevertheless, these salivary genes could serve as predictive assays for identifying radiotoxic health effects caused by radiotherapy, potentially contributing to medical management decision-making, or could be used for the prognosis of deterministic health effects in victims in radio/nuclear incidents.
This study directly compares GE responses between saliva and equivalent whole blood from the same individual in parallel, showing that blood biomarkers are reflected in whole saliva and providing support for the idea that saliva is a “mirror of the body”. This study supports further exploration of human saliva as a more attractive material for expanded biomarker studies such as cancer biomarkers, infectious disease, etc., already detected and validated in blood.
Finally, some limitations of this manuscript need to be considered: Conclusions drawn from our work may be limited given the advanced disease stage of our study group. There is an ongoing debate on the impact of confounders, such as cancer disease, previous therapies, current concomitant treatment, etc., on certain GE markers, although we tried to homogenize the collective concerning radiotherapy regimen, sex, age, etc. We demonstrated in previous work that the GE of six promising candidate genes previously found in a baboon model could be validated in leukemia patients undergoing total-body irradiation (38). To further minimize confounding conditions that may lead to misclassification or high false positive rates for diagnosis of radiation exposure, we collected pre- and post-exposure samples to ensure that irradiation is the only exposure type. Furthermore, the detected genes in saliva were also found in blood samples, providing as a positive control. Nevertheless, a number of potentially confounding factors, such as the development of acute or late radiotoxicity and its treatment, could potentially influence GE as well. Because of the small patient number (n = 7), even statistically significant results must be interpreted carefully and require further validation on a larger cohort, as already stated above.
In summary, the current human in vivo study (I) reveals significant radiation-induced GE associations of five transcriptional biomarkers in salivary samples, (II) suggests genes that may predict diverse clinical outcomes such as acute and late radiotoxicity as well as ARS severity, and (III) supports the view that blood-based GE response can be reflected in saliva samples correspondingly, indicating that saliva is a “mirror of the body” for certain but not all genes. Thus, studies for each blood gene of interest are required for saliva.
Supplementary Material
Supplemental Table S1. The table summarizes the descriptive statistics of all nine genes and patients (n = 7) in saliva samples over the time of the RT scheme (number of RT fractions). In PHPT1, CCNG1, CDKN1A, GADD45, and SESN1, qRT-PCR data from blood samples is additionally shown. GE is given as fold change (FC) relative to unexposed (normalized against a combination of ACTB/ATP6/B2M in saliva samples and HPRT1 in blood samples). Shown are the numbers per group with descriptive statistics [mean, median, standard deviation (stdev), standard error of the mean (sem), minimum (min), maximum (max)] and the corresponding P values (paired t-test).
Supplemental Fig. S1. Shown is the DGE of PHPT1, CCNG1, GADD45, and SESN1 (part A) as well as the DGE of FDXR, DDB2, POU2AF1, and WNT3 (part B) in saliva samples for each patient in separate panels over the time of RT scheme (number of RT fractions). Data from blood samples is also shown for PHPT1, CCNG1, CDKN1A, GADD45, and SESN1. GE is given as fold change (FC) relative to unexposed (normalized against a combination of ACTB/ATP6/B2M in saliva samples and HPRT1 in blood samples). Black circle symbols represent GE results from saliva samples, and grey square symbols represent GE results from blood samples. In the right panel, data of all patients for each gene is aggregated. Symbols reflect the median (n = 7), and error bars the standard error of the mean (SEM). The superimposed grey areas refer to a FC < |2|. Significant changes in GE relative to unexposed are indicated with asterisks (*P < 0.05, **P < 0.02, ***P < 0.005).
Supplemental Table S2. Overview of all GE results (normalized Ct values) and the correlations with acute (grade 1 and 2) and late toxicity grading (categories of grade 1–2 and 3–4). Provided are numbers (n) per category, mean Ct values, and P values for each gene and comparison. Data showing significant or borderline significant results are presented in bold.
Supplemental Table S3. Overview of GE results from nine genes (PHPT1, CCNG1, GADD45, SESN1, FDXR, DDB2, POU2AF1, and WNT3) in saliva samples for each patient (n = 7) over the time of RT scheme (number of RT fractions). In PHPT1, CCNG1, CDKN1A, GADD45, and SESN1, qRT-PCR data from blood samples is additionally shown. The fold-change difference provides the GE values (Ct values) with pre-exposure samples as reference (antilog of inverse log-2 transformed Ct values). In the lower part, median fold-changes are depicted for each gene. Data showing FC > |2| are presented in bold.
ACKNOWLEDGMENTS
We are very thankful for the sophisticated technical support provided by Oliver Wittmann. This work was supported by the German Ministry of Defence and the Czech Ministry of Defence (A long-term developmental plan 1011). SAG and SAA were supported in part by National Institute of Allergy and Infectious Diseases (NIAID) grant U19AI067773. The authors declare that they have no conflicts of interest.
Footnotes
Editor’s note. The online version of this article (DOI: https://doi.org/10.1667/RADE-23-00176.1) contains supplementary information that is available to all authorized users.
REFERENCES
- 1.Chaudhry MA. Biomarkers for human radiation exposure. Vol. 15, J Biomed Sci. 2008. p. 557–63. [DOI] [PubMed] [Google Scholar]
- 2.Badie C, Kabacik S, Balagurunathan Y, Bernard N, Brengues M, Faggioni G, et al. Laboratory intercomparison of gene expression assays. Radiat Res. 2013;180(2):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostheim P, Coker O, Sch€ule S, Hermann C, Combs SE, Trott KR, et al. Identifying a diagnostic window for the use of gene expression profiling to predict acute radiation syndrome. Radiat Res. 2021;195(1):38–46. [DOI] [PubMed] [Google Scholar]
- 4.Paul S, Amundson SA. Development of Gene Expression Signatures for Practical Radiation Biodosimetry. Int J Radiat Oncol Biol Phys. 2008;71(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Garcia L, O’Brien G, Sipos B, Mayes S, Love MI, Turner DJ, et al. Generation of a Transcriptional Radiation Exposure Signature in Human Blood Using Long-Read Nanopore Sequencing. Radiat Res. 2020;193(2):143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Port M, Ostheim P, Majewski M, Voss T, Haupt J, Lamkowski A, et al. Rapid High-Throughput Diagnostic Triage after a Mass Radiation Exposure Event Using Early Gene Expression Changes. Radiat Res. 2019;192(2):208–18. [DOI] [PubMed] [Google Scholar]
- 7.Schulz BL, Cooper-White J, Punyadeera CK. Saliva proteome research: current status and future outlook. Crit Rev Biotechnol. 2013; 33(3):246–59. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DTW. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013; 26(4):781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer CA, Schafer JJ, Yakob M, Lima P, Camargo P, Wong DTW. Saliva diagnostics: utilizing oral fluids to determine health status. Monogr Oral Sci 2014; 24:88–98. [DOI] [PubMed] [Google Scholar]
- 10.Cuevas-Córdoba B, Santiago-García J. Saliva: a fluid of study for OMICS. OMICS 2014; 18(2):87–97. [DOI] [PubMed] [Google Scholar]
- 11.Ghizoni JS, Nichele R, de Oliveira MT, Pamato S, Pereira JR. The utilization of saliva as an early diagnostic tool for oral cancer: microRNA as a biomarker. Vol. 22, Clinical and Translational Oncology. 2020. p. 804–12. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, John MAR, Zhou X, Kim Y, Sinha U, Jordan RCK, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10(24):8442–50. [DOI] [PubMed] [Google Scholar]
- 13.Kaczor-Urbanowicz KE, Martin Carreras-Presas C, Aro K, Tu M, Garcia-Godoy F, Wong DTW. Saliva diagnostics – Current views and directions. Vol. 242, Experimental Biology and Medicine. 2017. p. 459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Cao H, Lin J, Olsen N, Zheng SG. Biomarkers for Primary Sjögren’s Syndrome. Vol. 13, Genomics, Proteomics and Bioinformatics. 2015. p. 219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michael A, Bajracharya SD, Yuen PST, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010; 16(1):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maron JL, Johnson KL, Rocke DM, Cohen MG, Liley AJ, Bianchi DW. Neonatal salivary analysis reveals global developmental gene expression changes in the premature infant. Clin Chem. 2010; 56(3):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calouius PEB. The leukocyte count in saliva. Oral Surgery, Oral Medicine, Oral Pathology 1958. Jan 1 [cited 2023 Aug 21]; 11(1):43–6. [DOI] [PubMed] [Google Scholar]
- 18.Segal A, Wong DT. Salivary diagnostics: Enhancing disease detection and making medicine better. European Journal of Dental Education. 2008; 12(SUPPL. 1):22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DTW. Salivary biomarkers: Toward future clinical and diagnostic utilities. Vol. 26, Clinical Microbiology Reviews. 2013. p. 781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pernot E, Cardis E, Badie C. Usefulness of saliva samples for biomarker studies in radiation research. Cancer Epidemiology Biomarkers and Prevention. 2014; 23(12):2673–80. [DOI] [PubMed] [Google Scholar]
- 21.Lacombe J, Brooks C, Hu C, Menashi E, Korn R, Yang F, et al. Analysis of Saliva Gene Expression during Head and Neck Cancer Radiotherapy: A Pilot Study. Radiat Res. 2017; 188(1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laiakis EC, Strawn SJ, Brenner DJ, Fornace AJ. Assessment of saliva as a potential biofluid for biodosimetry: A pilot metabolomics study in mice. Radiat Res. 2016; 186(1):92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laiakis EC, Nishita D, Bujold K, Jayatilake MM, Bakke J, Gahagen J, et al. Salivary Metabolomics of Total Body Irradiated Nonhuman Primates Reveals Long-Term Normal Tissue Responses to Radiation. Int J Radiat Oncol Biol Phys. 2019; 105(4):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostheim P, Tichý A, Sirak I, Davidkova M, Stastna MM, Kultova G, et al. Overcoming challenges in human saliva gene expression measurements. Sci Rep. 2020; 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostheim P, Alemu SW, Tichý A, Sirak I, Davidkova M, Stastna MM, et al. Examining potential confounding factors in gene expression analysis of human saliva and identifying potential housekeeping genes. Sci Rep 2022; 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Port M, Herodin F, Valente M, Drouet M, Lamkowski A, Majewski M, et al. First generation gene expression signature for early prediction of late occurring hematological acute radiation syndrome in baboons. Radiat Res. 2016; 186(1):39–54. [DOI] [PubMed] [Google Scholar]
- 27.Port M, Hérodin F, Drouet M, Valente M, Majewski M, Ostheim P, et al. Gene Expression Changes in Irradiated Baboons: A Summary and Interpretation of a Decade of Findings. Radiat Res. 2021; 195(6):501–21. [DOI] [PubMed] [Google Scholar]
- 28.Tichy A, Kabacik S, O’Brien G, Pejchal J, Sinkorova Z, Kmochova A, et al. The first in vivo multiparametric comparison of different radiation exposure biomarkers in human blood. PLoS One. 2018; 13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Institute N. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. [cited 2023 Aug 21]. [Google Scholar]
- 30.Cox JD, Stetz JA, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995. Mar 30 [cited 2023 Aug 21]; 31(5):1341–6. [DOI] [PubMed] [Google Scholar]
- 31.Part I-Laboratory preparation and storage of a saliva/Orage-ne•RNA sample Purification steps Notes. 2017; (3):3–5. [Google Scholar]
- 32.Life Technologies. mirVanaTM miRNA Isolation Kit. 2011; 33. [Google Scholar]
- 33.Applied Biosystems. High Capacity cDNA Reverse Transcription Kits for 200 and 1000 Reactions Protocol (Rev E). Manual. 2010; (06):1–29. [Google Scholar]
- 34.Fisher T, July S. TaqMan PreAmp Master Mix User Guide. 2018; (4384557). [Google Scholar]
- 35.Kabacik S, MacKay A, Tamber N, Manning G, Finnon P, Paillier F, et al. Gene expression following ionising radiation: Identification of biomarkers for dose estimation and prediction of individual response. Int J Radiat Biol. 2011; 87(2):115–29. [DOI] [PubMed] [Google Scholar]
- 36.Manning G, Kabacik S, Finnon P, Bouffler S, Badie C. High and low dose responses of transcriptional biomarkers in ex vivo X-irradiated human blood. Int J Radiat Biol. 2013; 89(7):512–22. [DOI] [PubMed] [Google Scholar]
- 37.Paul S, Barker CA, Turner HC, McLane A, Wolden SL, Amundson SA. Prediction of in vivo radiation dose status in radiotherapy patients using ex vivo and in vivo gene expression signatures. Radiat Res. 2011; 175(3):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Port M, Majewski M, Herodin F, Valente M, Drouet M, Forcheron F, et al. Validating Baboon Ex Vivo and in Vivo Radiation-Related Gene Expression with Corresponding Human Data. Radiat Res. 2018; 189(4):389–98. [DOI] [PubMed] [Google Scholar]
- 39.Agbenyegah S, Abend M, Atkinson MJ, Combs SE, Trott KR, Port M, et al. Impact of Inter-Individual Variance in the Expression of a Radiation-Responsive Gene Panel Used for Triage. Radiat Res. 2018; 190(3):226–35. [DOI] [PubMed] [Google Scholar]
- 40.Rothkamm K, Beinke C, Romm H, Badie C, Balagurunathan Y, Barnard S, et al. Comparison of established and emerging biodosimetry assays. Radiat Res. 2013; 180(2):111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amundson SA. Transcriptomics for radiation biodosimetry: progress and challenges. Int J Radiat Biol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostheim P, Amundson SA, Badie C, Bazyka D, Evans AC, Ghandhi SA, et al. Gene expression for biodosimetry and effect prediction purposes: promises, pitfalls and future directions–key session ConRad 2021. Int J Radiat Biol. 2022; 98(5):843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abend M, Ostheim P, Port M. Radiation-induced gene expression changes used for biodosimetry and clinical outcome prediction: challenges and promises. Cytogenet Genome Res 2023. May 12; 1–8. (doi: 10.1159/000530947) [DOI] [PubMed] [Google Scholar]
- 44.Amundson SA, Do KT, Shahab S, Bittner M, Meltzer P, Trent J, et al. Identification of potential mRNA biomarkers in peripheral blood lymphocytes for human exposure to ionizing radiation. Radiat Res. 2000; 154(3):342–6. [DOI] [PubMed] [Google Scholar]
- 45.Dressman HK, Muramoto GG, Chao NJ, Meadows S, Marshall D, Ginsburg GS, et al. Gene expression signatures that predict radiation exposure in mice and humans. PLoS Med. 2007; 4(4):690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aro K, Wei F, Wong DT, Tu M. Saliva liquid biopsy for point-of-care applications. Vol. 5, Frontiers in Public Health. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwanke D, Fatanmi O, Wise S, Schüle S, Wiegel T, Singh VK, et al. Validating a four-gene set for H-ARS severity prediction in peripheral blood samples of irradiated Rhesus macaques. Radit Res. In press. 2024. [DOI] [PubMed] [Google Scholar]
- 48.Lee SY, Choe YH, Han JH, Hwang G, Choi MY, Thakur G, et al. HPRT1 Most Suitable Reference Gene for Accurate Normalization of mRNA Expression in Canine Dermal Tissues with Radiation Therapy. Genes (Basel) 2022; 13(11):1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostheim P, Majewski M, Gluzman-Poltorak Z, Vainstein V, Basile LA, Lamkowski A, et al. Predicting the radiation sensitivity of male and female rhesus macaques using gene expression. Radiat Res. 2021; 195(1):25–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. The table summarizes the descriptive statistics of all nine genes and patients (n = 7) in saliva samples over the time of the RT scheme (number of RT fractions). In PHPT1, CCNG1, CDKN1A, GADD45, and SESN1, qRT-PCR data from blood samples is additionally shown. GE is given as fold change (FC) relative to unexposed (normalized against a combination of ACTB/ATP6/B2M in saliva samples and HPRT1 in blood samples). Shown are the numbers per group with descriptive statistics [mean, median, standard deviation (stdev), standard error of the mean (sem), minimum (min), maximum (max)] and the corresponding P values (paired t-test).
Supplemental Fig. S1. Shown is the DGE of PHPT1, CCNG1, GADD45, and SESN1 (part A) as well as the DGE of FDXR, DDB2, POU2AF1, and WNT3 (part B) in saliva samples for each patient in separate panels over the time of RT scheme (number of RT fractions). Data from blood samples is also shown for PHPT1, CCNG1, CDKN1A, GADD45, and SESN1. GE is given as fold change (FC) relative to unexposed (normalized against a combination of ACTB/ATP6/B2M in saliva samples and HPRT1 in blood samples). Black circle symbols represent GE results from saliva samples, and grey square symbols represent GE results from blood samples. In the right panel, data of all patients for each gene is aggregated. Symbols reflect the median (n = 7), and error bars the standard error of the mean (SEM). The superimposed grey areas refer to a FC < |2|. Significant changes in GE relative to unexposed are indicated with asterisks (*P < 0.05, **P < 0.02, ***P < 0.005).
Supplemental Table S2. Overview of all GE results (normalized Ct values) and the correlations with acute (grade 1 and 2) and late toxicity grading (categories of grade 1–2 and 3–4). Provided are numbers (n) per category, mean Ct values, and P values for each gene and comparison. Data showing significant or borderline significant results are presented in bold.
Supplemental Table S3. Overview of GE results from nine genes (PHPT1, CCNG1, GADD45, SESN1, FDXR, DDB2, POU2AF1, and WNT3) in saliva samples for each patient (n = 7) over the time of RT scheme (number of RT fractions). In PHPT1, CCNG1, CDKN1A, GADD45, and SESN1, qRT-PCR data from blood samples is additionally shown. The fold-change difference provides the GE values (Ct values) with pre-exposure samples as reference (antilog of inverse log-2 transformed Ct values). In the lower part, median fold-changes are depicted for each gene. Data showing FC > |2| are presented in bold.


