Abstract
Background
Serum neuron-specific enolase (NSE) had been associated with survival of several cancers. However, its prognostic significance for colorectal cancer (CRC) has not been effectively discussed. We aimed to investigate the relationship between baseline serum NSE and the overall survival (OS) of colorectal adenocarcinoma (CRAD) patients.
Methods
A retrospective study had been conducted by including 564 histopathology confirmed CRAD patients between January 2013 and December 2018 from Yunnan Provincial Cancer hospital, China. Cox proportional hazards model was used to estimate the crude and adjusted associations between serum NSE measured at diagnosis and the OS of the patients. Restricted cubic spline (RCS) was further applied to delineate dose-response trend of the NSE-OS association.
Results
After controlling for possible confounding factors, baseline serum NSE was significantly associated with OS in CRAD: when dichotomizing by the median, patients with higher baseline serum NSE (NSE >= 12.93 ng/mL) were observed a worse prognosis (hazard ratio, HR: 1.82, 95% CI [1.30–2.55], p < 0.01). Stratified analysis by tumor stage revealed a stronger NSE-OS association in advanced CRAD patients. RCS disclosed a prominent dose-response relationship in NSE-OS association for all CRAD patients: along with the increase of baseline serum NSE, the adjusted HR of CRAD patients increased gradually. This dose-response trend is also evident in advanced stage CRAD patients, but not in early stage CRAD patients.
Conclusions
Serum NSE measured at diagnosis might be a useful prognostic indicator for CRAD, especially for advanced stage patients.
Keywords: Neuron-specific enolase (NSE), Colorectal adenocarcinoma (CRAD), Prognosis, Overall survival (OS), Survival analysis
Introduction
Worldwide, cancer is the leading cause of death. GLOBOCAN 2020 estimated that there were 19.3 million new cancer cases and 10 million deaths globally, with colorectal cancer (CRC) accounted for approximately one-tenth of new cancer cases and deaths (Sung et al., 2021). Adenocarcinomas which originate from colorectal glandular epithelial cells accounted for overwhelmingly majority (95–98%) of CRC (Fleming et al., 2012). Disparities exist for the incidence of CRC: higher in men and in developed countries (Sung et al., 2021). The main established risk factors for CRC include: obesity and physical inactivity (Karahalios et al., 2016; Robsahm et al., 2013), unhealthy diet (characterized in high intake of red and processed meats, low intake of fiber, whole grains, and calcium) (Zhao et al., 2017; Norat et al., 2017), smoking (Ordóñez-Mena et al., 2018), alcohol consumption (Fedirko et al., 2011), family history of CRC (Jasperson et al., 2010), and inflammatory bowel diseases (Olén et al., 2017). In China, along with economic transition and Westernized dietary pattern and lifestyle, the incidence of CRC has been persistently increasing (Arnold et al., 2017).
CRC has been ranked the second leading cause of death among cancers (Sung et al., 2021). Although there have been remarkable improvements in the diagnosis and treatment of CRC in the past decades, the overall survival (OS) of CRC is less optimistic: currently, the 5-year OS for all stages CRC patients is 65%, the sixth lowest among all cancers (Siegel et al., 2022). The 5-year OS for CRC patients who underwent surgery in the United States was 54.79% (Zhang et al., 2020). In China, although the age-standardized 5-year relative survival rate for CRC gradually elevated from 2003 to 2015, by the year of 2018, it was still below 57% (Zeng et al., 2018). Therefore, identifying useful prognostic markers is of great clinical importance for CRC patients.
In recent years, it has been found that many serum tumor markers were closely associated with the survival of CRC: such as indicators of systemic inflammatory response (C-reactive protein; neutrophil-to-lymphocyte ratio, NLR) (Koike et al., 2008; Templeton et al., 2014); nutritional status (albumin) (Chiang et al., 2017), and tumor burden (carcinoembryonic antigen, CEA; carbohydrate antigen, CA19-9, CA24-2) (Rao et al., 2021; Tampellini et al., 2015). Lately, studies have reported serum enzymes may also be related to cancer survival, like alkaline phosphatase and cholinesterase (Xiao et al., 2019; Ran et al., 2022). At the same time, neuron-specific enolase (NSE) has attracted study interest. The highly acidic soluble brain protein 14-3-2 was first described by Moore and McGregor in 1965 and had been renamed NSE since the neuron-specific protein has exhibited enolase activity (Marangos, Zomzely-Neurath & York, 1976). NSE is a cell-specific isoform of the glycolytic enzyme enolase and a highly specific marker of neurons and peripheral neuroendocrine cells and thus is used to identify neuroendocrine cells and diagnose malignant tumors (Isgrò, Bottoni & Scatena, 2015). NSE is one of the most reliable serum markers for the diagnosis and prognosis of small-cell lung cancer (SCLC), and a meta-analysis of 16 studies showed that elevated pretreatment serum NSE predicted poorer OS for SCLC patients, with a combined HR of 1.78 (Tian et al., 2020). In addition, serum NSE has also been associated with increased risk of neuroendocrine tumors, adult neuroblastoma, melanoma, seminoma, renal cell carcinoma, Merkel cell tumor, carcinoid tumor, anaplastic and immature teratoma, and malignant pheochromocytoma (Isgrò, Bottoni & Scatena, 2015).
Endocrine differentiation can exist, and the presence of focal endocrine cells was common in colorectal adenocarcinoma (CRAD) (Shia et al., 2002; Gulubova & Vlaykova, 2008). Endocrine differentiation can affect the expression of several neuroendocrine markers, such as NSE, chromogranin A, and synaptophysin. Among these, NSE and chromogranin A have been considered the most common markers that reflect neuroendocrine differentiation in CRC (Atasoy et al., 2003). Higher level of serum NSE had been associated with poorer tumor differentiation (Baudin et al., 1998). In a recently published study, Luo et al. (2020) disclosed that NSE level was significantly associated with tumor stage, lymph node, and distant metastasis of CRC patients, suggesting that NSE could be used as a progression biomarker of CRC. Therefore, it is reasonable to suspect that serum NSE may be associated with survival outcomes of CRC. However, although the prognostic relevance of NSE had been discussed for several types of cancer, such as SCLC, non-small-cell lung cancer (NSCLC), prostate cancer (PC), small cell carcinoma of the urinary bladder (SCCB), and pancreatic neuroendocrine tumors (NETs) (Bremnes et al., 2003; Pujol et al., 2001; Yao et al., 2016; Fan et al., 2017; Naito et al., 2017), its association with CRC survival has not been effectively explored so far.
We intended to investigate the hypothetical prognostic significance of NSE in CRC in a large sample of Chinese patients retrieved by using retrospective design.
Materials and Methods
Study design
Study subjects were collected from the Third Affiliated Hospital of Kunming Medical University, which is also the provincial cancer hospital of Yunnan province in southwest China. We retrospectively identified patients who were hospitalized from January 1 2013 to December 31 2018, and were firstly diagnosed as CRC by histopathology. Considering the predominant proportion of colorectal adenocarcinoma in CRC, we only included this specific histopathological type.
Patient information was obtained from the digitalized hospital information system (HIS) which updated on daily basis from late 2012 onward. The following key variables of study relevance were extracted: gender, age at diagnosis, ethnicity, smoking history, alcohol drinking history, body mass index (BMI), chemotherapy, curative operation, clinical stage, serum blood indicators (NSE, NLR, albumin, alpha-fetoprotein, carcinoembryonic antigen, CA125, CA19-9). Survival information was ascertained by using computer-assisted telephone interviewing follow-up system built by the hospital. The deadline for follow-up was set as December 31, 2019. The study protocol was reviewed and approved by the Ethics Review Board of Kunming Medical University (Approval number: KMMU2021MEC109). Because of the retrospective nature, informed consents had been waived.
Variables and definitions
Death of the patient due to any cause was the primary outcome. Survival time was defined as the date of the patient’s histopathological diagnosis to the date of death or the last valid follow-up, whichever comes first. Baseline serum NSE was measured within 7 days before or after the diagnosis. If multiple tests are available during this period, the one closest to the date of diagnosis will be chosen. Serum NSE was treated differently for multiple analytical purposes: either as a binary variable dichotomized by using the median (12.93 ng/mL) as the cut-off value to guarantee ideal statistical power, or as an ordinal variable divided by quartiles, or as a quantitative variable in its original form.
We also retrieved baseline NLR, albumin (ALB), alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), CA125, CA19-9 as control variables, in consideration that systemic nutritional status, inflammatory response, and tumor biomarkers of CRC might be influential confounding factors. These baseline serum blood indicators were also measured within 7 days prior or post cancer diagnosis. They were included into the analysis as binary variables using the following recommended cut-offs in normal individuals: 35 U/L for ALB, 8.78 ug/L for AFP, 5 ug/L for CEA, 35 kU/L for CA125, 37 kU/L for CA19-9. Moreover, extensive evidence had shown that lower levels of ALB and higher levels of CEA, CA125, and CA19-9 were significantly associated with poor prognosis for CRC patients when the above cut-off values were used (Lai et al., 2011; Ramphal et al., 2019; Li et al., 2023). In addition, considering that the blood tests were extracted 7 days before or after the time of diagnosis, which may introduce variability to test results between patients, therefore we also included the measurement time (ranges from −7 to 7 days) as an important controlling variable throughout the analysis.
Statistical analysis
Descriptive statistics were used to display and compare the general characteristics of CRAD patients. Survival curves for CRAD patients with different baseline serum NSE levels were plotted and tested by Kaplan-Meier method and log-rank test. Univariate and multivariate Cox proportional hazards (CPH) models were used to estimate crude and adjusted hazard ratios (HRs) together with 95% confidence intervals (CIs) for baseline serum NSE and the OS of CRAD. To estimate the dose-response relationship of the association, we preliminarily incorporated baseline NSE as an ordinal variable in the multivariate Cox model. Subsequently, the nonlinear relationship between baseline NSE on its original continuous form and the OS was evaluated using a restricted cubic spline (RCS) curve based on CPH model. RCS with three knots was used and the reference value was the median of NSE (Harrell, 2015). The reason to choose RCS instead of other splines is because it has previously been shown that the RCS can be used to approximate complex hazard functions in the context of time-to-event data (Rutherford, Crowther & Lambert, 2015). Variables that attained less rigorous significance (p < 0.10) in the univariate Cox model were included in the subsequent multivariate Cox model. For multivariate analysis, a two-tailed p < 0.05 was deemed statistically significant. Statistical analyses were performed in R software (Version 4.1.3; R Core Team, 2022).
Results
Characteristics of study subjects
We initially identified 3,217 patients with confirmed histopathological diagnosis of CRAD between 2013 and 2018. After careful review, 564 patients with complete information were included in the final analysis. The general characteristics of the study subjects were summarized in Table 1: most patients were male (60.28%); the average age at diagnosis was 59.15 years with a standard deviation (SD) of 11.79; 35.99% and 33.87% of patients reported smoking and alcohol drinking history; 180 (31.91%) were in stages I–II and 384 (68.09%) were in stages III–IV; the median survival time of all patients was 788.00 days (Inter-quartiles range, IQR: 923.00 days); 54.61% patients had received chemotherapies and 43.62% received curative operations.
Table 1. General characteristics of 564 CRAD patients.
| Characteristics | All patients (N = 564) | Lower group (NSE < 12.93 ng/mL, N = 282) | Higher group (NSE >= 12.93 ng/mL, N = 282) | Statistic | p value |
|---|---|---|---|---|---|
| Sex | χ2 = 1.90 | 0.17 | |||
| Female | 224 (39.72)c | 120 (53.57)c | 104 (46.43)c | ||
| Male | 340 (60.28)c | 162 (47.65)c | 178 (52.35)c | ||
| Age at diagnosis (Year) | 59.15 (11.79)a | 58.71 (11.81)a | 59.60 (11.77)a | t = −0.89 | 0.37 |
| Ethnicity | χ2 = 0.07 | 0.79 | |||
| Minorities | 64 (11.35)c | 31 (48.44)c | 33 (51.56)c | ||
| Han majority | 500 (88.65)c | 251 (50.20)c | 249 (49.80)c | ||
| Smoking history | χ2 = 2.78 | 0.10 | |||
| No | 361 (64.01)c | 171 (47.37)c | 190 (52.63)c | ||
| Yes | 203 (35.99)c | 111 (54.68)c | 92 (45.32)c | ||
| Alcohol drinking history | χ2 = 2.29 | 0.13 | |||
| No | 373 (66.13)c | 178 (47.72)c | 195 (52.28)c | ||
| Yes | 191 (33.87)c | 104 (54.45)c | 87 (45.55)c | ||
| BMI (kg/m2) | 22.49 (3.05)a | 22.68 (3.07)a | 22.30 (3.03)a | t = 1.47 | 0.14 |
| Chemotherapy | χ2 = 1.03 | 0.31 | |||
| No | 256 (45.39)c | 134 (52.34)c | 122 (47.66)c | ||
| Yes | 308 (54.61)c | 148 (48.05)c | 160 (51.95)c | ||
| Curative operation | χ2 = 4.15 | <0.05 | |||
| No | 318 (56.38)c | 147 (46.23)c | 171 (53.77)c | ||
| Yes | 246 (43.62)c | 135 (54.88)c | 111 (45.12)c | ||
| Clinical stage | χ2 = 10.57 | <0.01 | |||
| Stage I–II | 180 (31.91)c | 108 (60.00)c | 72 (40.00)c | ||
| Stage III–IV | 384 (68.09)c | 174 (45.31)c | 210 (54.69)c | ||
| Survival length (Day) | 788.00 (923.00)b | 977.00 (932.00)b | 546.50 (782.25)b | Z = 6.66 | <0.01 |
| NLR (Unit free) | 2.10 (1.32)b | 2.04 (1.24)b | 2.17 (1.45)b | Z = −1.90 | 0.06 |
| ALB (U/L) | 44.90 (5.45)b | 44.90 (5.01)b | 44.90 (5.63)b | Z = −0.89 | 0.37 |
| AFP (ug/L) | 2.85 (1.63)b | 2.78 (1.44)b | 3.01 (1.88)b | Z = −1.59 | 0.11 |
| CEA (ug/L) | 5.63 (19.96)b | 3.81 (8.71)b | 8.84 (35.93)b | Z = −5.60 | <0.01 |
| CA125 (kU/L) | 13.57 (10.93)b | 12.86 (8.97)b | 14.88 (12.50)b | Z = −2.87 | <0.01 |
| CA19-9 (kU/L) | 13.88 (24.09)b | 12.61 (18.44)b | 16.89 (43.03)b | Z = −3.43 | <0.01 |
| NSE (ng/mL) | 12.93 (4.63)b | – | – | – | – |
Notes:
Mean (SD).
Median (IQR).
Frequency (Proportion).
Patients were divided into two groups by using the median of serum NSE (12.93 ng/mL): the lower group (NSE < 12.93 ng/mL) and the higher group (NSE >= 12.93 ng/mL). We compared characteristics of the two groups, and statistical tests showed the differences in curative operation, clinical stage, CEA, CA125, and CA19-9. Specifically, we found that patients with higher levels of serum NSE group tended to not have curative surgery, be in stage III–IV, and have higher CEA, CA125 and CA19-9 (both p < 0.05, Table 1). Additionally, the median survival time for patients of lower NSE level was 1.79-fold longer than patients of higher NSE level (977.00 vs 546.50 days, p < 0.01 by rank-sum test).
Baseline serum NSE and the OS of CRAD
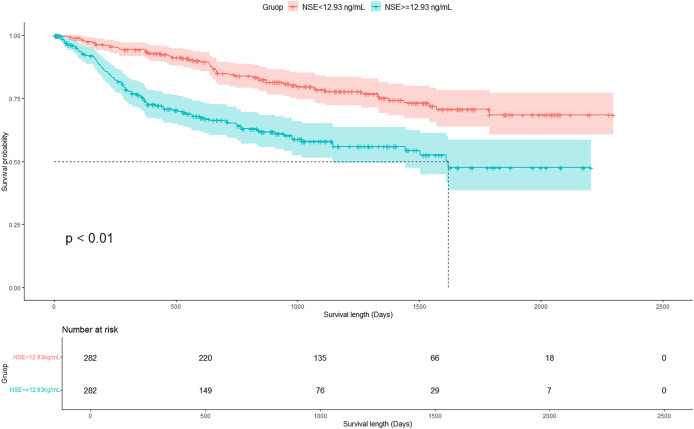
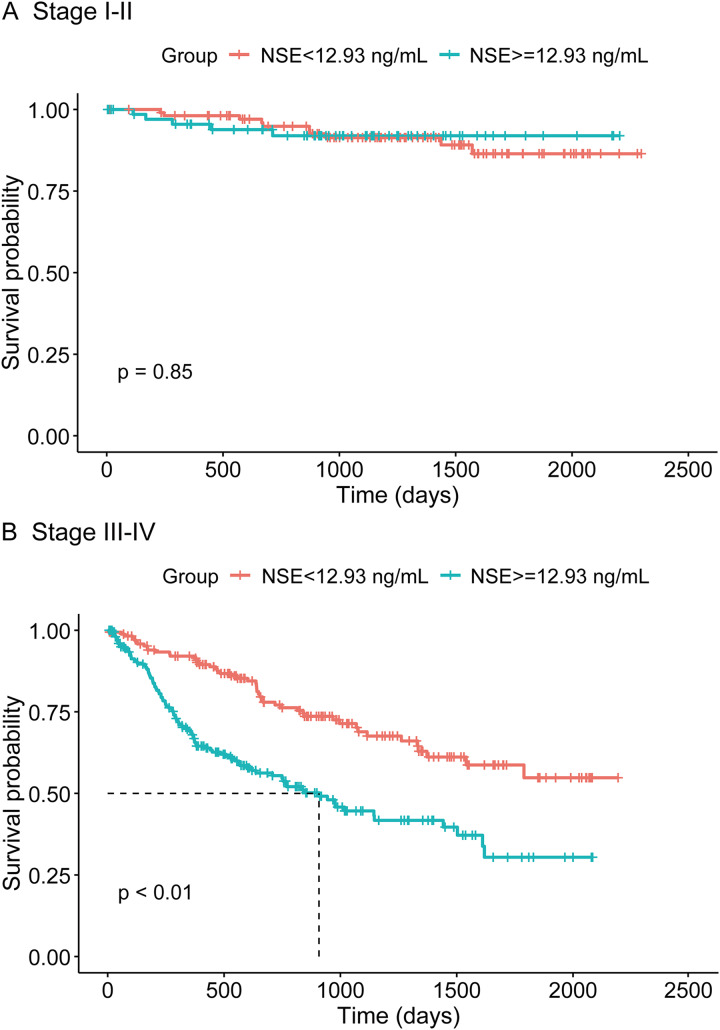
Figure 1 displays the survival curves of the two groups of CRAD patients with different baseline serum NSE levels. OS was significantly better for patients with lower NSE level than patients with higher NSE level (p < 0.01 by log-rank test). Stratified analysis by clinical stage revealed that, for early stage CRAD patients (stage I–II), baseline NSE level was not significantly associated with OS (p = 0.85 by log-rank test), whereas for patients of advanced stage (stage III–IV), baseline NSE level was prominently related to OS (p < 0.01 by log-rank test), with a better OS observed for patients of lower baseline NSE (Fig. 2).
Figure 1. Kaplan-Meier survival curves for CRAD patients with different baseline serum NSE levels.
Figure 2. (A and B) Kaplan-Meier survival curves for CRAD patients with different baseline serum NSE levels, stratified by clinical stage.
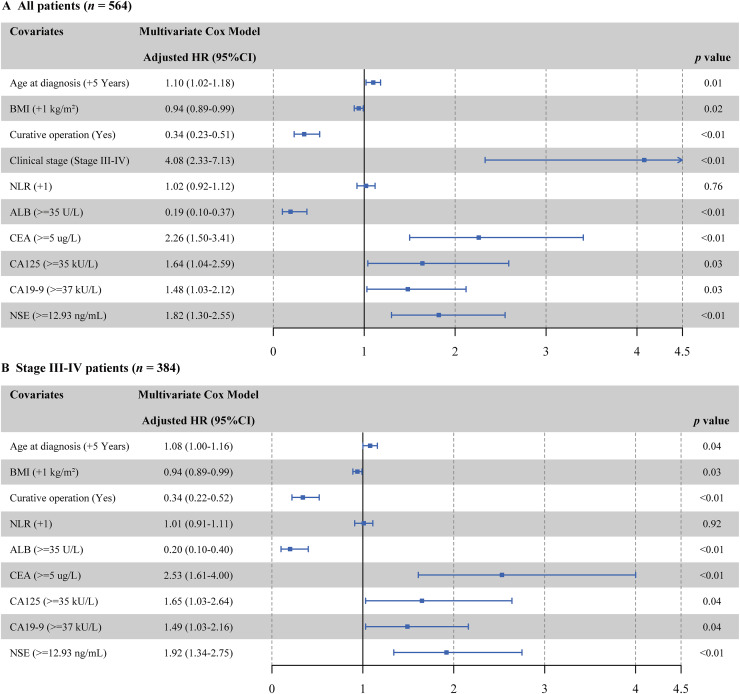
Univariate Cox regression identified 10 potential covariates from 16 candidates: age at diagnosis, BMI, curative operation, clinical stage, NLR, ALB, CEA, CA125, CA19-9, and NSE (see in Table S1). After adjustment by multivariate Cox model, baseline serum NSE remained a significant prognostic factor: the adjusted HR for CRAD patients of higher baseline NSE was 1.82 (95% CI [1.30–2.55], p < 0.01) compared with CRAD patients of lower baseline NSE (Fig. 3A). Subsequently, we performed the multivariate Cox regression in CRAD patients in different clinical stages, and the adjusted HR of serum NSE was not statistically significant in patients with stage I–II CRAD (HR: 1.07 95% CI [0.34–3.37]; p = 0.91). However, among stage III–IV patients, the higher level of serum NSE had a 1.92-fold death hazard than lower level (p < 0.05; Fig. 3B). We further performed a sensitivity analysis in stage III-IV CRAD patients stratified by curative operation, and found the adjusted NSE-OS association was statistically significant, regardless of whether curative operation was performed (both p < 0.01; see in Fig. S1).
Figure 3. (A and B) Multivariate Cox proportional hazards model fitting results for OS of CRAD patients.
Dose-response association between baseline NSE and OS of CRAD
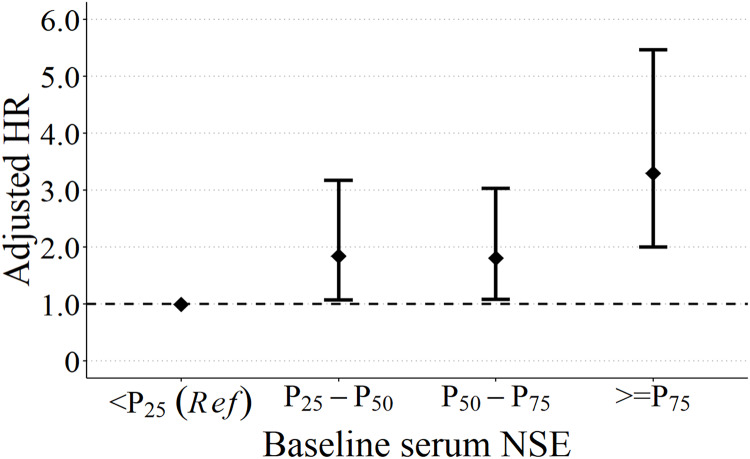
To test the robustness and disclose the trend of this significant NSE-OS association, we divided study subjects into four groups according to quartiles of baseline serum NSE: group 1 (NSE < 11.08 ng/mL), group 2 (11.08 <= NSE < 12.93 ng/mL), group 3 (12.93 <= NSE < 15.72 ng/mL), and group 4 (NSE >= 15.72ng/mL). By taking group 1 as the reference, multivariate Cox model disclosed that, along with the increase of baseline NSE, the death hazard of CRAD patients also increased: the adjusted HRs were 1.84 (95% CI [1.07–3.17], p < 0.05), 1.81 (95% CI [1.08–3.03], p < 0.05), and 3.30 (95% CI [2.00–5.47], p < 0.01) for group 2, group 3, and group 4, respectively (Fig. 4).
Figure 4. Quartiles of baseline NSE and the OS of CRAD patients.
Adjusted by age at diagnosis, BMI, curative operation, clinical stage, NLR, ALB, CEA, CA125, CA19-9.
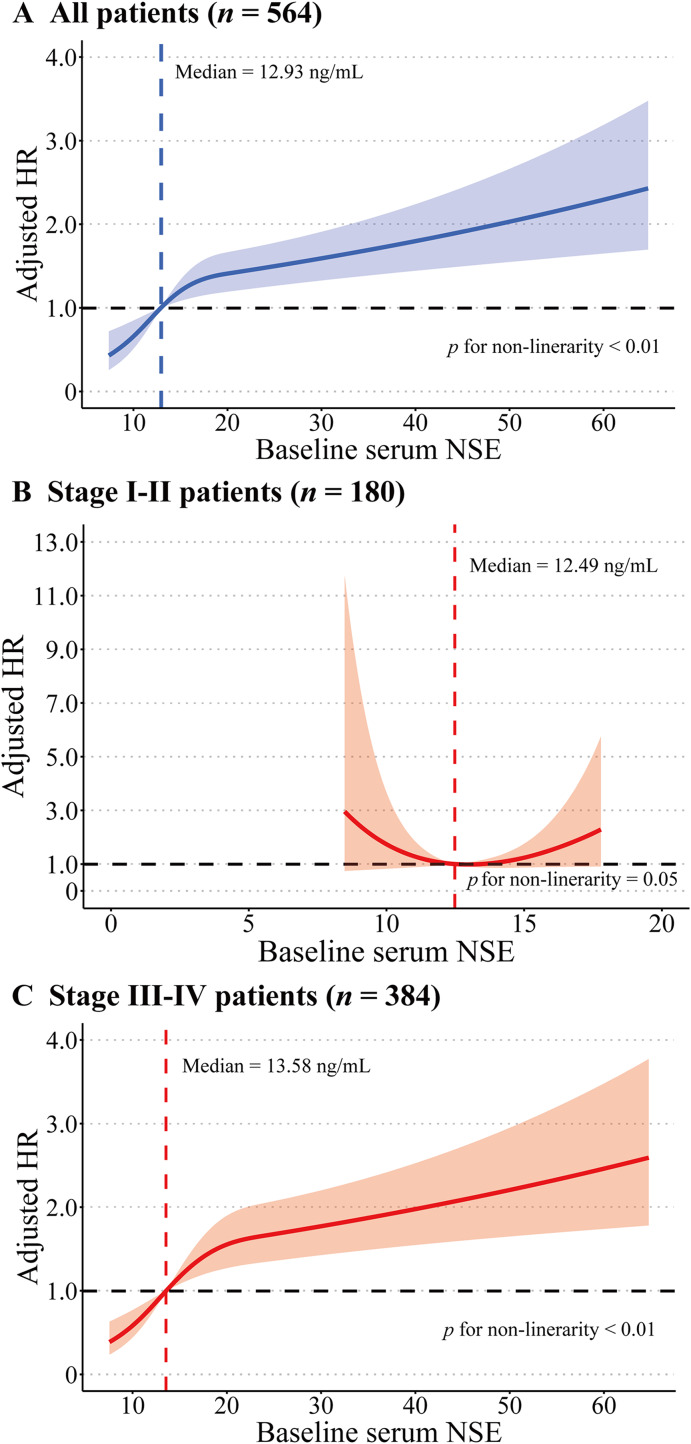
We further delineate the trend of this possible dose-response relationship. RCS curve was used by including NSE in its original quantitative form. The RCS supported a prominent dose-response association in CRAD patients: along with the increase of baseline serum NSE, the adjusted HR of CRAD patients increased gradually, and the adjusted HR rose particularly steeply for baseline RCS of the interval 12.93–18.05 ng/mL (Fig. 5A). Moreover, a similar dose-response relationship for the NSE-OS association existed in stage III–IV CRAD patients, but this association was apparently absent in stage I–II patients (Figs. 5B and 5C).
Figure 5. (A–C) Adjusted HRs with 95% confidence band for the NSE-OS association of CRAD patients by using RCS.
Adjusted by age at diagnosis, BMI, curative operation, NLR, ALB, CEA, CA125, CA19-9. (A) Additionally adjusted for clinical stage.
Discussion
In the present retrospective study, as expected, we found a prominent association between baseline serum NSE and the OS of CRAD patients: after controlled for possible confounding variables, CRAD patients with higher level of baseline NSE were observed inferior OS compared with CRAD patients with lower level of baseline NSE, the elevated baseline NSE had been associated with a HR of 1.82, with apparent dose-response trend. These primary findings suggest that serum NSE at diagnosis could be a meaningful prognostic marker for CRAD patients.
To the best of our knowledge, no existing studies had exclusively investigated the association between serum NSE and survival outcomes for CRC patients. However, this positive association had been reported in other malignant tumors. For instance, Bremnes et al. (2003) revealed that increased pretreatment serum NSE was associated with worse disease-specific survival (DSS) for SCLC patients. Another prospective study of 621 NSCLC patients by Pujol et al. (2001) showed that patients with lower baseline serum NSE had better survival. In a recently published study by Yan et al. (2021), the authors found that in NSCLC patients, higher level of preoperative serum NSE predicted worse progression-free survival (PFS), with similar results in subsequent subgroup analysis of lung adenocarcinoma patients. Our study results were generally in agreement with these previous studies in supporting the detrimental role of baseline serum NSE in cancer prognosis.
Several mechanisms might be involved in the association between serum NSE and the OS of CRAD. First, NSE is a critical enzyme in aerobic glycolysis. Tumor cells with high NSE expression could accelerate glycolysis and thereby proliferate more quickly (Isgrò, Bottoni & Scatena, 2015; Vizin & Kos, 2015). Second, like SCLC tumor cells, CRAD tumor cells may secrete serum NSE, which correlates with tumor mass extension (Carney et al., 1982), therefore higher level of serum NSE may indicate aggravated tumor burden of the patients. This hypothesis had been partly justified in this study, as most patients with higher level of serum NSE were at advanced stage (Stage III–IV). However, our stratified analysis results suggest that, even controlled for tumor stage, the independent association between NSE and OS of CRAD patients was still significant. Third, it has been found that NSE was overexpressed in CRC, which implies that CRC cells could be more aggressive. In addition, NSE was obviously upregulated in metastatic colon cancer cell lines, indicating that NSE is associated with metastatic in vitro and in vivo, higher level of NSE could be more prone to metastasis (Pan et al., 2020).
Another important finding of the current study is that clinical stage presented as a prominent effect modifier in NSE-OS association, as the association was statistically significant only for advanced CRAD patients. This stage-specific NSE-OS association probably suggests that, for advanced CRAD patients, a simple blood test of serum NSE should be simultaneously considered for identifying individuals of less optimistic survival outcome. In prior studies, investigators had identified significant associations between serum NSE and clinical stage and tumor size in patients with tumors, with higher serum NSE commonly observed in patients with advanced stage and larger tumor size (Georgantzi et al., 2018; Luo et al., 2020). This might be due to elevated serum NSE occurring concurrently with malignant proliferation in cancer patients, which can directly reflect a patient’s tumor burden (Isgrò, Bottoni & Scatena, 2015). One thing to be noticed is that, although for early stage (Stage I–II) patients, we failed in observing a significant NSE-OS association, considering that only limited number of patients (N = 180) were in this group, which undoubtedly hampers statistical power, and increases the possibility of false negative inference, future studies for early stage CRAD patients of larger sample sizes are warranted.
The major findings of our study emphasize a promising role of serum NSE in prognosis of CRAD. Monitoring changes of serum NSE could be meaningful in tracing tumor progression and therapeutic response for CRAD patients. A latest study showed that for elderly CRC patients who underwent laparoscopic radical surgery, those who accepted intraoperative intravenous dexmedetomidine showed lower level of postoperative serum NSE than patients who accepted intraoperative intravenous sodium chloride (Tang et al., 2022). Dexmedetomidine might be beneficial for the proliferation and differentiation of neurons and peripheral cells in CRC patients, thereby could alleviate postoperative neurological damage (Tang et al., 2022). Another study observed that cinobufacin combined with chemotherapy can effectively decrease serum NSE and chemotherapy toxicity in NSCLC patients (Zeng et al., 2021). In addition, an animal study found that lung cancer progression was greatly inhibited by oral vanillic acid to reduce serum NSE in mice (Velli et al., 2019). Further studies on these drugs may support new treatment options for CRAD patients.
Our study is among the first to investigate the prognostic role of serum NSE in CRAD patients. Multivariate model and the subsequent dose-response analyses all reached congruent results, which further corroborates the robustness of the positive association between serum NSE and the OS of CRAD. Nonetheless, some limitations should not be ignored. First, our study was retrospective with risk of information bias. Second, patients were screened out from a single institution, so the results should be extrapolated with caution. Third, only 564 from 3,217 CRAD patients with complete data were analyzed, which may further introduce selection bias. Finally, due to data limitations, our study was unavailable to obtain information for other possible confounders or potential indicators of study interest, such as resection margin status, presence of positive regional lymph nodes, patient NSE immunohistochemical staining samples, progression-free survival outcomes, post-operative NSE measurements, and baseline genomic characteristics regarding to CRAD patients of different NSE levels, future studies should be done to address these deficiencies.
Conclusions
In conclusion, elevated serum NSE measured at diagnosis was associated with poorer OS in CRAD patients, especially for patients of advanced stage. Baseline serum NSE might be a meaningful prognostic marker for CRAD. Future studies of multiple data sources and prospective design should be done to corroborate our major findings.
Supplemental Information
Funding Statement
This study was supported by the Basic Research Program of Yunnan (202201AT070200), First-Class Discipline Team of Kunming Medical University (2024XKTDTS16), and the Scientific Research Fund Project of Yunnan Provincial Department of Education (2023Y0798; 2024Y212). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yunchao Huang, Email: huangych2001@aliyun.com.
Yuanyuan Xiao, Email: 33225647@qq.com.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Junwei Peng performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Jie Ma performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Jian Lu performed the experiments, prepared figures and/or tables, and approved the final draft.
Hailiang Ran performed the experiments, prepared figures and/or tables, and approved the final draft.
Zhongqin Yuan performed the experiments, prepared figures and/or tables, and approved the final draft.
Hai Zhou performed the experiments, prepared figures and/or tables, and approved the final draft.
Yunchao Huang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Yuanyuan Xiao conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Ethics Review Board of Kunming Medical University.
Data Availability
The following information was supplied regarding data availability:
The raw data for analysis of this manuscript is available in the Supplemental File.
References
- Arnold et al. (2017).Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- Atasoy et al. (2003).Atasoy P, Ensari A, Demirci S, Kurşun N. Neuroendocrine differentiation in colorectal carcinomas: assessing its prognostic significance. Tumori Journal. 2003;89(1):49–53. doi: 10.1177/030089160308900111. [DOI] [PubMed] [Google Scholar]
- Baudin et al. (1998).Baudin E, Gigliotti A, Ducreux M, Ropers J, Comoy E, Sabourin JC, Bidart JM, Cailleux AF, Bonacci R, Ruffié P, Schlumberger M. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. British Journal of Cancer. 1998;78(8):1102–1107. doi: 10.1038/bjc.1998.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremnes et al. (2003).Bremnes RM, Sundstrom S, Aasebø U, Kaasa S, Hatlevoll R, Aamdal S, Norweigian Lung Cancer Study Group The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer. 2003;39(3):303–313. doi: 10.1016/S0169-5002(02)00508-1. [DOI] [PubMed] [Google Scholar]
- Carney et al. (1982).Carney DN, Marangos PJ, Ihde DC, Bunn PA, Cohen MH, Jr, Minna JD, Gazdar AF. Serum neuron-specific enolase: a marker for disease extent and response to therapy of small-cell lung cancer. Lancet. 1982;1(8272):583–585. doi: 10.1016/S0140-6736(82)91748-2. [DOI] [PubMed] [Google Scholar]
- Chiang et al. (2017).Chiang JM, Chang CJ, Jiang SF, Yeh CY, You JF, Hsieh PS, Huang HY. Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. European Journal of Cancer Care. 2017;26(2):e12403. doi: 10.1111/ecc.12403. [DOI] [PubMed] [Google Scholar]
- Fan et al. (2017).Fan L, Wang Y, Chi C, Pan J, Xun S, Xin Z, Hu J, Zhou L, Dong B, Xue W. Chromogranin A and neurone-specific enolase variations during the first 3 months of abiraterone therapy predict outcomes in patients with metastatic castration-resistant prostate cancer. BJU International. 2017;120(2):226–232. doi: 10.1111/bju.13781. [DOI] [PubMed] [Google Scholar]
- Fedirko et al. (2011).Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islam F, Negri E, Straif K, Romieu I, La Vecchia C, Boffetta P, Jenab M. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Annals of Oncology. 2011;22(9):1958–1972. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- Fleming et al. (2012).Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: pathologic aspects. Journal of Gastrointestinal Oncology. 2012;3(3):153–173. doi: 10.3978/j.issn.2078-6891.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgantzi et al. (2018).Georgantzi K, Sköldenberg EG, Stridsberg M, Kogner P, Jakobson Å, Janson ET, Christofferson RHB. Chromogranin A and neuron-specific enolase in neuroblastoma: correlation to stage and prognostic factors. Pediatric Hematology and Oncology. 2018;35(2):156–165. doi: 10.1080/08880018.2018.1464087. [DOI] [PubMed] [Google Scholar]
- Gulubova & Vlaykova (2008).Gulubova M, Vlaykova T. Chromogranin A-, serotonin-, synaptophysin- and vascular endothelial growth factor-positive endocrine cells and the prognosis of colorectal cancer: an immunohistochemical and ultrastructural study. Journal of Gastroenterology and Hepatology. 2008;23(10):1574–1585. doi: 10.1111/j.1440-1746.2008.05560.x. [DOI] [PubMed] [Google Scholar]
- Harrell (2015).Harrell FE. General aspects of fitting regression models. In: Harrell FE, editor. Regression Modeling Strategies. Second Edition. Cham: Springer; 2015. pp. 13–44. [Google Scholar]
- Isgrò, Bottoni & Scatena (2015).Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Advances in Experimental Medicine and Biology. 2015;867:125–143. doi: 10.1007/978-94-017-7215-0. [DOI] [PubMed] [Google Scholar]
- Jasperson et al. (2010).Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahalios et al. (2016).Karahalios A, Simpson JA, Baglietto L, MacInnis RJ, Hodge AM, Giles GG, English DR. Change in weight and waist circumference and risk of colorectal cancer: results from the Melbourne collaborative cohort study. BMC Cancer. 2016;16:157. doi: 10.1186/s12885-016-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike et al. (2008).Koike Y, Miki C, Okugawa Y, Yokoe T, Toiyama Y, Tanaka K, Inoue Y, Kusunoki M. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. Journal of Surgical Oncology. 2008;98(7):540–544. doi: 10.1002/jso.21154. [DOI] [PubMed] [Google Scholar]
- Lai et al. (2011).Lai CC, You JF, Yeh CY, Chen JS, Tang R, Wang JY, Chin CC. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. International Journal of Colorectal Disease. 2011;26(4):473–481. doi: 10.1007/s00384-010-1113-4. [DOI] [PubMed] [Google Scholar]
- Li et al. (2023).Li C, Zhao K, Zhang D, Pang X, Pu H, Lei M, Fan B, Lv J, You D, Li Z, Zhang T. Prediction models of colorectal cancer prognosis incorporating perioperative longitudinal serum tumor markers: a retrospective longitudinal cohort study. BMC Medicine. 2023;21:63. doi: 10.1186/s12916-023-02773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo et al. (2020).Luo H, Shen K, Li B, Li R, Wang Z, Xie Z. Clinical significance and diagnostic value of serum NSE, CEA, CA19-9, CA125 and CA242 levels in colorectal cancer. Oncology Letters. 2020;20(1):742–750. doi: 10.3892/ol.2020.11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangos, Zomzely-Neurath & York (1976).Marangos PJ, Zomzely-Neurath C, York C. Determination and characterization of neuron specific protein (NSP) associated enolase activity. Biochemical and Biophysical Research Communications. 1976;68(4):1309–1316. doi: 10.1016/0006-291X(76)90339-9. [DOI] [PubMed] [Google Scholar]
- Naito et al. (2017).Naito A, Taguchi S, Nakagawa T, Matsumoto A, Nagase Y, Tabata M, Miyakawa J, Suzuki M, Nishimatsu H, Enomoto Y, Takahashi S, Okaneya T, Yamada D, Tachikawa T, Minowada S, Fujimura T, Fukuhara H, Kume H, Homma Y. Prognostic significance of serum neuron-specific enolase in small cell carcinoma of the urinary bladder. World Journal of Urology. 2017;35(1):97–103. doi: 10.1007/s00345-016-1846-y. [DOI] [PubMed] [Google Scholar]
- Norat et al. (2017).Norat T, Vieira AR, Abar L, Aune D, Polemiti E, Chan D, Vingeliene S. Continuous update project report: diet, nutrition, physical activity, and colorectal cancer. 2017. https://www.aicr.org/research/the-continuous-update-project/colorectal-cancer/ [10 March 2023]. https://www.aicr.org/research/the-continuous-update-project/colorectal-cancer/
- Olén et al. (2017).Olén O, Askling J, Sachs MC, Frumento P, Neovius M, Smedby KE, Ekbom A, Malmborg P, Ludvigsson JF. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964–2014. BMJ. 2017;358:j3951. doi: 10.1136/bmj.j3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordóñez-Mena et al. (2018).Ordóñez-Mena JM, Walter V, Schöttker B, Jenab M, O’Doherty MG, Kee F, Bueno-de-Mesquita B, Peeters PHM, Stricker BH, Ruiter R, Hofman A, Söderberg S, Jousilahti P, Kuulasmaa K, Freedman ND, Wilsgaard T, Wolk A, Nilsson LM, Tjønneland A, Quirós JR, van Duijnhoven FJB, Siersema PD, Boffetta P, Trichopoulou A, Brenner H. Impact of prediagnostic smoking and smoking cessation on colorectal cancer prognosis: a meta-analysis of individual patient data from cohorts within the CHANCES consortium. Annals of Oncology. 2018;29(2):472–483. doi: 10.1093/annonc/mdx761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. (2020).Pan X, Wu H, Chen G, Li W. Prognostic value of enolase gene family in colon cancer. Medical Science Monitor. 2020;26:e922980. doi: 10.12659/MSM.922980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol et al. (2001).Pujol JL, Boher JM, Grenier J, Quantin X. Cyfra 21-1, neuron specific enolase and prognosis of non-small cell lung cancer: prospective study in 621 patients. Lung Cancer. 2001;31(2–3):221–231. doi: 10.1016/S0169-5002(00)00186-0. [DOI] [PubMed] [Google Scholar]
- Ramphal et al. (2019).Ramphal W, Boeding JRE, van Iwaarden M, Schreinemakers JMJ, Rutten HJT, Crolla RMPH, Gobardhan PD. Serum carcinoembryonic antigen to predict recurrence in the follow-up of patients with colorectal cancer. The International Journal of Biological Markers. 2019;34(1):60–68. doi: 10.1177/1724600818820679. [DOI] [PubMed] [Google Scholar]
- Ran et al. (2022).Ran H, Ma J, Cai L, Zhou H, Yuan Z, Chen Y, Chang W, Huang Y, Xiao Y. Serum cholinesterase may independently predict prognosis in non-small-cell lung cancer. BMC Cancer. 2022;22:93. doi: 10.1186/s12885-022-09212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao et al. (2021).Rao H, Wu H, Huang Q, Yu Z, Zhong Z. Clinical value of serum CEA, CA24-2 and CA19-9 in patients with colorectal cancer. Clinical Laboratory. 2021;67(4):1079–1089. doi: 10.7754/Clin.Lab.2020.200828. [DOI] [PubMed] [Google Scholar]
- R Core Team (2022).R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2022. Version 4.1.3. [Google Scholar]
- Robsahm et al. (2013).Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. European Journal of Cancer prevention. 2013;22(6):492–505. doi: 10.1097/CEJ.0b013e328360f434. [DOI] [PubMed] [Google Scholar]
- Rutherford, Crowther & Lambert (2015).Rutherford MJ, Crowther MJ, Lambert PC. The use of restricted cubic splines to approximate complex hazard functions in the analysis of time-to-event data: a simulation study. Journal of Statistical Computation and Simulation. 2015;85(4):777–793. doi: 10.1080/00949655.2013.845890. [DOI] [Google Scholar]
- Shia et al. (2002).Shia J, Tickoo SK, Guillem JG, Qin J, Nissan A, Hoos A, Stojadinovic A, Ruo L, Wong WD, Paty PB, Weiser MR, Minsky BD, Klimstra DS. Increased endocrine cells in treated rectal adenocarcinomas: a possible reflection of endocrine differentiation in tumor cells induced by chemotherapy and radiotherapy. The American Journal of Surgical Pathology. 2002;26(7):863–872. doi: 10.1097/00000478-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Siegel et al. (2022).Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- Sung et al. (2021).Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tampellini et al. (2015).Tampellini M, Ottone A, Alabiso I, Baratelli C, Forti L, Berruti A, Aroasio E, Scagliotti GV. The prognostic role of baseline CEA and CA 19-9 values and their time-dependent variations in advanced colorectal cancer patients submitted to first-line therapy. Tumor Biology. 2015;36(3):1519–1527. doi: 10.1007/s13277-014-2693-3. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2022).Tang Y, Liu J, Huang X, Ding H, Tan S, Zhu Y. Effect of dexmedetomidine-assisted intravenous inhalation combined anesthesia on cerebral oxygen metabolism and serum Th1/Th2 level in elderly colorectal cancer patients. Frontiers in Surgery. 2022;8:832646. doi: 10.3389/fsurg.2021.832646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton et al. (2014).Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- Tian et al. (2020).Tian Z, Liang C, Zhang Z, Wen H, Feng H, Ma Q, Liu D, Qiang G. Prognostic value of neuron-specific enolase for small cell lung cancer: a systematic review and meta-analysis. World Journal of Surgical Oncology. 2020;18(1):116. doi: 10.1186/s12957-020-01894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velli et al. (2019).Velli SK, Sundaram J, Murugan M, Balaraman G, Thiruvengadam D. Protective effect of vanillic acid against benzo(a)pyrene induced lung cancer in Swiss albino mice. Journal of Biochemical and Molecular Toxicology. 2019;33(10):e22382. doi: 10.1002/jbt.22382. [DOI] [PubMed] [Google Scholar]
- Vizin & Kos (2015).Vizin T, Kos J. Gamma-enolase: a well-known tumour marker, with a less-known role in cancer. Radiology and Oncology. 2015;49(3):217–226. doi: 10.1515/raon-2015-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao et al. (2019).Xiao Y, Yang H, Lu J, Li D, Xu C, Risch HA. Serum gamma-glutamyltransferase and the overall survival of metastatic pancreatic cancer. BMC Cancer. 2019;19:1020. doi: 10.1186/s12885-019-6250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2021).Yan P, Han Y, Tong A, Liu J, Wang X, Liu C. Prognostic value of neuron-specific enolase in patients with advanced and metastatic non-neuroendocrine non-small cell lung cancer. Bioscience Reports. 2021;41(8):BSR20210866. doi: 10.1042/BSR20210866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao et al. (2016).Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Öberg K. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. Journal of Clinical Oncology. 2016;34(32):3906–3913. doi: 10.1200/JCO.2016.68.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng et al. (2018).Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Global Health. 2018;6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- Zeng et al. (2021).Zeng L, Lu Z, Huang X, Li R. Effects of cinobufacin on Th17, Treg cells, related cytokines and tumor markers in the peripheral blood of patients with non-small cell lung cancer. Acta Medica Mediterranea. 2021;37(1):151–155. doi: 10.19193/0393-6384_2021_1_22. [DOI] [Google Scholar]
- Zhang et al. (2020).Zhang J, Yang Y, Fu X, Guo W. Development and validation of nomograms for prediction of overall survival and cancer-specific survival of patients of colorectal cancer. Japanese Journal of Clinical Oncology. 2020;50(3):261–269. doi: 10.1093/jjco/hyz182. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2017).Zhao Z, Feng Q, Yin Z, Shuang J, Bai B, Yu P, Guo M, Zhao Q. Red and processed meat consumption and colorectal cancer risk: a systematic review and meta-analysis. Oncotarget. 2017;8(47):83306–83314. doi: 10.18632/oncotarget.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data for analysis of this manuscript is available in the Supplemental File.