Abstract
Research on the evolution of dog foraging and diet has largely focused on scavenging during their initial domestication and genetic adaptations to starch-rich food environments following the advent of agriculture. The Siberian archaeological record evidences other critical shifts in dog foraging and diet that likely characterize Holocene dogs globally. By the Middle Holocene, body size reconstruction for Siberia dogs indicates that most were far smaller than Pleistocene wolves. This contributed to dogs’ tendencies to scavenge, feed on small prey, and reduce social foraging. Stable carbon and nitrogen isotope analysis of Siberian dogs reveals that their diets were more diverse than those of Pleistocene wolves. This included habitual consumption of marine and freshwater foods by the Middle Holocene and reliance on C4 foods by the Late Holocene. Feeding on such foods and anthropogenic waste increased dogs’ exposure to microbes, affected their gut microbiomes, and shaped long-term dog population history.
Since their divergence from wolves, dogs have decreased in size, adapted to diverse foods, and become dietarily reliant on humans.
INTRODUCTION
Around 700 million dogs inhabit Earth, living virtually everywhere humans have settled (1). Dogs have a substantial ecological impact, and much of this is tied to their dietary needs. Globally, commercial pet food requires the use of 40.7 to 57.6 million hectares annually or roughly twice the area of the United Kingdom (2). Such dog foods are now quite diverse. Even the best-selling brands have highly variable protein, carbohydrate, and fat contents deriving from plant and animal sources (2). Most of the world’s dogs are not fed commercial foods but not only live as free-ranging animals that feed on anthropogenic waste but also hunt small wildlife, prey upon livestock, and scavenge (3, 4). Modern dog dietary patterns are wide ranging and have a long-term history tied to their domestication and daily lives in human-dominated environments.
The domestication of dogs began in Eurasia as early as ~40,000 years ago, when they diverged from a now-extinct lineage(s) of gray wolves (5–7). Research on the history of dog diets and foraging has focused on two main issues. First, the most widely cited model of initial dog domestication proposes that stress tolerant or friendly wolves began feeding on human foragers’ meaty leftovers (8–10). That is, dogs’ early domestication history was characterized by a shift to scavenging in human-dominated places. Presently, however, we are unaware of any direct archaeological evidence in support of this model. Second, some dogs evolved the capacity to better digest starches following the emergence of agriculture (6, 9, 10). This adaptation is marked by increased copy numbers of the AMY2B gene, which codes for pancreatic amylase that aids in starch digestion. Dogs with high copy numbers of AMY2B first appear ~7000 years ago. However, low copy numbers persisted in some prehistoric agricultural and forager communities and in some modern dogs (6).
While these dietary transitions in dogs were notable, other critical adaptations also have occurred in these animals over the course of their domestication. Here, we describe three little-discussed but important dietary and foraging challenges experienced by dogs, focusing on archaeological evidence from Siberia. First, by the Early to Middle Holocene, many dogs were significantly smaller than wolves. This likely contributed to dogs developing a greater reliance on small-bodied prey and scavenging and to a decrease in intraspecies social hunting. Both are behaviors widely seen in modern dogs. Second, even in the context of Siberia’s Holocene human foraging communities, dog diets underwent significant diversification as those communities’ subsistence practices became more locally specialized. Third, throughout their domestication, dogs have eaten anthropogenic waste, increasing their exposure to new pathogens and parasites. This affected their gut microbiomes, overall health, and even behavior. Recognition of these patterns provides an increasingly complex picture of dogs’ long-term relationships with humans and the niches they have created.
RESULTS AND DISCUSSION
Body size, prey size, and social hunting
During the latter portion of the Pleistocene, gray wolves occupied much of Eurasia, but knowledge of their variation is incomplete and uneven (11–13). Most wolf skeletal remains from this period originate in Europe and Beringia (Yakutia, Chukotka, Alaska, and Yukon), while those from lower latitudes are scarce. Which specific Pleistocene wolf population(s) gave rise to modern dogs is unclear, but genomic research suggests that they potentially inhabited Siberia (7).
Body mass estimates for gray wolves from the latter portion of the Pleistocene indicate that they were relatively large-bodied animals, with nearly all adults being more than 30 kg (Table 1). We are aware of only two individuals from this period with estimated body masses below this range, and both have been argued to be early domestic dogs (14, 15). Pleistocene wolves from more southerly regions of Asia may have been smaller than the European and Beringian groups, but these were likely still relatively heavy animals. Body mass variation in modern Asian gray wolves provides some support for this. Modern adult wolves inhabiting the southern forest steppe, taiga, and arctic tundra of Siberia all average above 31 kg (16). In contrast, the lightest extant wolves in mainland Asia are found in India, which have a mean body mass of ~25 kg (17).
Table 1. Body mass estimates for Eurasian Pleistocene wolves and putative early dogs and Holocene dogs from Siberia and Western Europe.
With the exception of the Western European Holocene dogs, the n values represent distinct individuals. The Holocene European dog dataset (27) is organized by element not by individual, meaning that single individuals are potentially represented multiple times in the table.
| Location | Age range | Group | n | Mean (kg) | SD | Source |
| Britain | MIS 3, 5a, 7 | Canis lupus | 48 | 36.8 | 1.60 | (71) |
| Grotta Mora Cavorso, Italy | MIS 3 | C. lupus | 7 | 36.9 | – | (72) |
| Aven de l’Arquet, France | End of MIS 3 | C. lupus | 9 | 31.8 | – | (73) |
| Jaurens Cave, France | ~32,630 to 29,300 | C. lupus | 2 | 39.4 | – | (73) |
| Maldidier Cave, France | Aurignacian and Gravettian | C. lupus | 2 | 36.7 | – | (73) |
| Igue du Gral, France | ~42,400 to 10,440 | C. lupus | 7 | 36.2 | – | (73) |
| Various European | Late Pleistocene | C. lupus | 14 | 41.6 | 4.35 | (69) |
| Předmostí, Czech Republic | Late Pleistocene | C. lupus/early dogs? | 25 | 36.6 | 6.05 | (69) |
| Various European and Siberian | Late Pleistocene | C. lupus | 10 | 37.9 | 4.67 | (14) |
| Alaska, USA | Late Pleistocene | C. lupus | 34 | 38.2 | 8.90 | (68), this study |
| Ulakhan Sular, Yakutia, Russia | Late Pleistocene | C. lupus/early dog? | 1 | 21.8 | – | (14) |
| Razboinchya Cave, Altai, Russia | Late Pleistocene | C. lupus/early dog? | 1 | 26.0 | – | (14, 15) |
| Various Siberian | Holocene | Dogs | 199 | 16.4 | 4.64 | (26) |
| Various Western European | Holocene | Dogs | 356 | 12.9 | 5.12 | (27), this study |
Understanding past canid body mass variation is useful because this biometric is an important predictor of prey preference and social hunting among extant carnivores. While there are exceptions, smaller carnivore species, namely, those below 21.5 kg, feed mostly on prey at least 45% lighter than themselves (18). Those with body masses above 21.5 kg typically prefer prey 45% or larger than their own body masses. Maintaining a larger body through habitual predation on markedly smaller fauna is difficult because of time and energy constraints. The energy returned per successful hunt is relatively small compared to that provided by much larger prey (18). Body size alone, of course, does not dictate prey preference, and observations based on extant carnivore behavior should account not only for biases introduced by human activity but also for issues related to competition and diachronic dietary changes. Regardless, unlike felids, canids lack grasping forelimbs, and as a result, social hunting is often required to bring down prey larger than themselves (19). Predation on much smaller fauna can alleviate the need for this social predation in canids, as such prey can be effectively killed and transported by a single animal. However, reliance on smaller prey often requires higher intake rates and, for many extant carnivores, also correlates with a broader diet focusing on a combination of vertebrate and invertebrate prey and, in some cases, omnivory (18).
Size reduction is characteristic of many animals under domestication, but the selective forces responsible for generating these changes are debated (20, 21). Human intentionality likely drives some of these size changes, but a suite of unintentional actions and nonhuman factors is also surely at work. In Siberia, no Pleistocene canid remains are widely accepted as dogs, but dogs are well documented in Siberian forager contexts by the early Holocene (~9000 years ago) and are widespread in southern Siberian pastoralist and agriculturalist societies by the Late Holocene (~3000 years ago) (6, 22–25). Examination of skeletal remains from 199 dogs from 28 Siberian archaeological sites shows a gradual decrease in body mass through the Holocene, with the overall mean being just 16.4 ± 4.64 kg (Table 1) (26). Only 23 of the 199 dogs (11.6%) had body mass estimates greater than 21.5 kg. Dogs below the proposed body mass threshold of 21.5 kg appear in Siberia shortly after 8000 calibrated years before the present. Holocene size reduction in dogs is not limited to Siberia. For example, dog long bone measurements for Late Mesolithic through Early Bronze Age sites in northwestern and central Europe (27), stemming from 29 sites, produced an average body mass estimate of 12.85 ± 5.12 kg (Table 1 and data S3). In this European sample, only 18 (11.5%) of the 356 skeletal element dimensions generated body mass estimates above the threshold of 21.5 kg. In portions of western North America, dog body masses are larger than in the Siberian and European samples, but mean body mass estimates still tend to be below 21.5 kg (28). Briefly, most Holocene dog populations had to adapt to far smaller bodies than their Pleistocene predecessors and those of Holocene wolves.
Correlated with their reduced sizes, Holocene dogs likely had smaller gape sizes and less bite strength than wolves, making handling and processing larger prey and larger food items more difficult (26). Body size is also positively correlated with foraging range size in extant carnivores (but does not necessarily determine it), and this could be used to infer that most Holocene dogs, particularly those living as free-ranging animals, would have had reduced range sizes compared to those of wolves (26). Such changes surely necessitated shifts in how dogs fed—their capabilities, on average, were quite different from their Pleistocene kin. This likely contributing to dogs’ greater dietary reliance on humans and human-dominated places through time. Dogs in most locations, Australia likely being an important exception, likely could not easily shift to a full-time focus on small prey in response to this rapid decline in body size. Long-standing competition for such prey existed from various other small carnivores, including other canids such as foxes, coyotes, jackals, and dholes. For dogs, maintaining the foraging patterns of their Pleistocene wolf ancestors also seems unlikely. In much of Eurasia and North America, Holocene wolves continued to offer competition for larger-bodied prey, and most had size and strength advantages over their smaller-bodied dog kin. However, dogs had an emerging adaptation, the social ability to successfully cohabitate with humans, which allowed them to exploit human-dominated places. Over the long term, these same niches proved to be largely hostile to most other carnivores, an exception being the domestic cat, which developed similar social abilities to live among humans. That is, the presence of other carnivores in human-dominated niches was limited. The main competitors for domestic dogs in most locations were other dogs, perhaps contributing to cooperation in foraging becoming less common as their domestication unfolded.
This complex process likely began during the Pleistocene, when the initial selective forces of domestication began to permit the increased persistence of smaller body sizes (21). At that time, human subsistence was based on foraging, and mobility was relatively high. In the Holocene, human-dominated dietary niches increased in abundance, scale, and type, even among foraging societies, providing greater quantities and types of accessible food items to dogs, either as intentionally provided food or as leftovers from human meals. This process was further expanded and accelerated with the advent of food production. Holocene dogs potentially became entangled in a feedback loop not only where domestication contributed to an overall decrease in average dog body size but also where dietary niches that favored such body sizes were expanding and diversifying. Dogs would have progressively relied less on intraspecies foraging as the food items they were increasingly accessing were scraps and handouts. Individual dogs could successfully obtain these food items, with other dogs merely being competition for them. Most of the world’s dogs today are free-ranging animals, and they fit this pattern well, predominantly subsisting on anthropogenic waste and through predation on small fauna, with low reliance on intraspecies social hunting (1). This foraging pattern was enhanced by global urbanization, but its roots can likely be traced back through the Holocene to early foraging and food-producing societies.
Emergence of dietary diversification
Human subsistence economies in Siberia diversified during the Holocene and elsewhere, contributing to new dietary adaptations in dogs. For example, riverine and lakeshore that adapted foraging groups are well evidenced around Lake Baikal by at least 8000 years ago (29). Marine-based subsistence economies appear just over 6000 years ago in southern Primorye (30). Pastoralism emerges in southwest Siberia ~4000 years ago, with clear signs of millet consumption by 3400 years ago (31). Cultivated millet was present in Primorye on the Pacific Coast by ~5500 years ago, and horses, cattle, and sheep were present in Primorye on the Pacific Coast by ~3000 years ago (32). Reindeer keeping was emerging at least in Northwest Siberia by ~2200 years ago (33).
Stable carbon (δ13C) and nitrogen (δ15N) isotope compositions of bone collagen are widely used as a proxy for examining dietary patterns among past humans and animals. In general, δ13C reflects the relative contribution of carbon to the diet from terrestrial and/or aquatic sources. For example, marine carbon sources have higher δ13C values than those of temperate terrestrial sources (34), and C4 crops such as maize and millet have higher values than C3 crops such as wheat and rice (35). Nitrogen isotope compositions (δ15N) provide an indicator of the trophic level and the source of dietary protein (36). The longer food webs of freshwater and marine environments, for instance, result in higher δ15N values than in terrestrial environments (37). Interpretation of these dietary patterns requires consideration of potential aquatic and terrestrial isotopic baseline variations arising from natural and human impacts on carbon and nitrogen cycles at local and regional scales (38, 39). Turnover rates in bone collagen vary not only by skeletal element but also by factors such as biological age and level of activity (40). In general, canid bone collagen stable carbon and nitrogen isotope compositions represent diet over an average of multiple years before death. Dog stable isotope compositions often track those of the human communities they inhabit, albeit not perfectly (41).
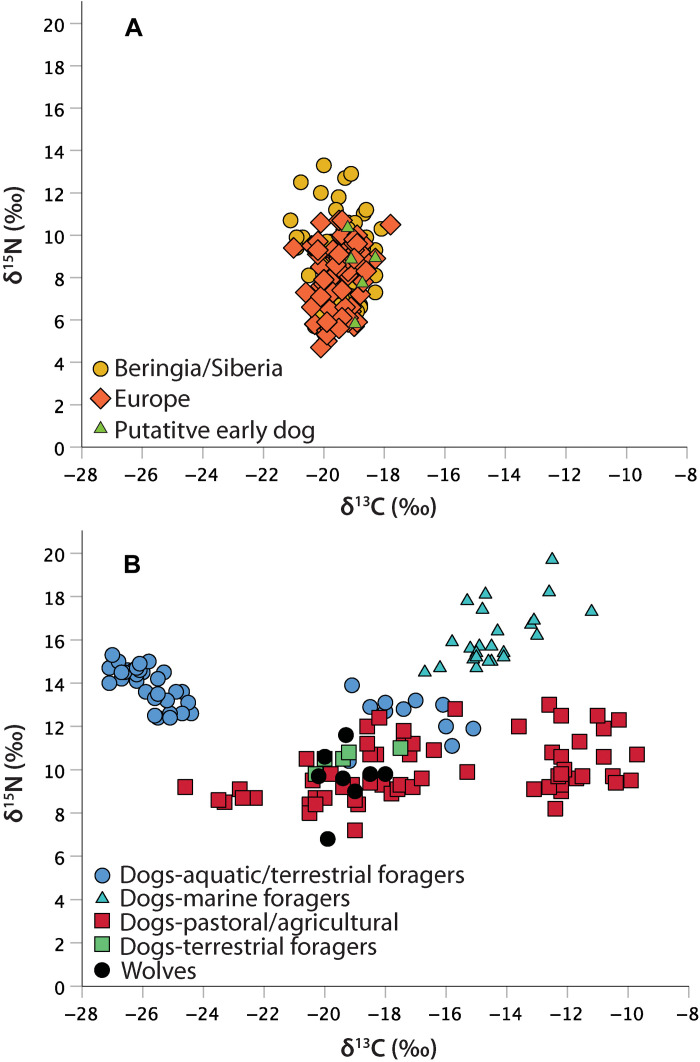
Comparisons of wolf and dog stable isotope values reveal significant dietary diversification and localization in Holocene dogs. Stable carbon and nitrogen isotope compositions were compiled for 138 Pleistocene canids (Figs. 1 and 2A and data S1). Five putative early dogs are included in the group. For this dataset, δ13C values range from −21.1 to −17.8 per mil (‰), with a mean of −19.4 ± 0.6‰, and the δ15N values range from +4.7 to +13.3‰, with a mean of +8.5 ± 1.8‰ (Fig. 2A). Interpretations of these data suggest that Pleistocene wolves and putative early dogs had dietary foci on various combinations of megaherbivores and ungulates, with some temporal and geographic variability—they were generalists (42–47). We are aware of only one Pleistocene canid suggested to have a significant aquatic component in their diet, a wolf from southwest France that consumed some marine resources (48). Much similar to modern wolves (49), Pleistocene wolves preferentially fed on large-bodied terrestrial prey, as expected given their larger body sizes.
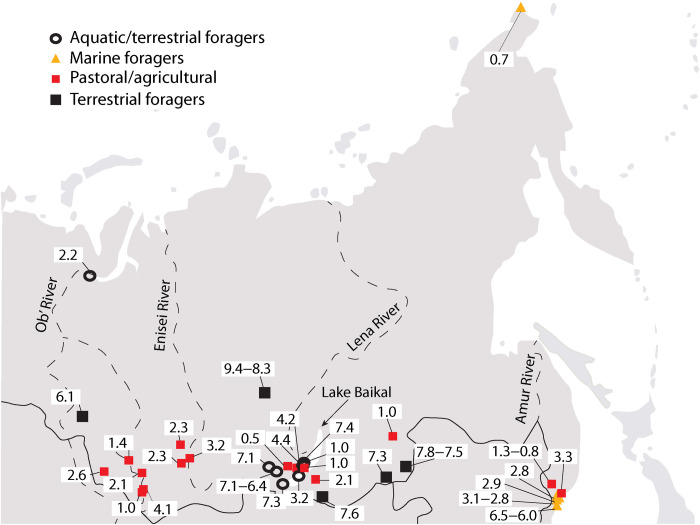
Fig. 1. Map of archaeological sites in Siberia (n = 36) with bone collagen stable carbon (δ13C) and nitrogen (δ15N) isotope composition data for Holocene dogs and wolves.
The colored symbols indicate the human subsistence economy types evidenced at the sites. The numbers provided for each site represent the calibrated age for the canid remains in thousands of years before present.
Fig. 2. Stable carbon (δ13C) and nitrogen (δ15N) isotope compositions for Eurasian Pleistocene wolves and Siberian Holocene wolves and dogs.
Stable isotope data, site name and age, and site coordinates are provided in data S1 and S2. (A) Pleistocene wolf stable isotope composition data by region. The Beringian and Siberian dataset (n = 66) includes two putative early dogs. The European dataset (n = 72) contains three putative early dogs. (B) Holocene Siberian dog and wolf stable isotope composition data. The sample includes isotope values for 144 dogs and 8 wolves. The colored symbols indicate the human subsistence economy types evidenced at the sites.
Stable isotope compositions are available for 143 dogs and 8 wolves from 35 Holocene Siberian archaeological sites, most from the same sites where dogs were analyzed for body size (Figs. 1 and 2B and data S2). These canids date from ~9000 to 500 years ago. The human communities these dogs participated in included terrestrial foragers, aquatic (lake and river) and terrestrial foragers, marine coastal foragers, and pastoral agriculturalists. The eight wolves had δ13C values ranging from −20.2 to −18.0‰, with a mean of −19.3 ± 0.8‰, and δ15N values ranging from +6.8 to +11.6‰, with a mean of +9.6 ± 1.4‰ (Fig. 2B). The mean values for the Holocene wolves are similar to those from the Pleistocene but with a slightly higher average δ15N value. These dog isotopic compositions are far more variable than those of either set of wolves, with δ13C values ranging from −27.1 to −9.7‰, with a mean of −18.3 ± 5.1‰, and the δ15N values ranging from +7.2 to +19.7‰, with a mean of +12.3 ± 2.7‰.
Dogs from coastal forager settings tend to have high δ13C and δ15N values, consistent with dietary reliance on marine foods (Fig. 2B). These include four individuals from the Boisman II site in Primorye dating to ~6000 years ago (30). Dogs from aquatic and terrestrial forager settings cluster in two groups, one falling between the marine forager dogs and the wolves. These individuals likely had significant freshwater protein in their diets and include multiple specimens dating to 7400 to 6300 years ago, all from the Lake Baikal area (22). The second cluster has high δ15N and low δ13C values and consists of dogs from a ~2200-year-old site on the lower Ob’ River where diets also have been assessed as rich in freshwater foods, most likely fish (23, 50). Dogs from terrestrial forager settings, which are few in number, have isotopic compositions somewhat overlapping those of the Holocene wolves, indicating diets predominantly consisting of terrestrial protein. Last, isotopic compositions of dogs from agricultural and pastoralist settings are wide ranging, likely reflecting human dietary diversity in these communities. Within this subsistence economy group is a cluster of dogs with high δ13C values (above −14‰), all from the Cherepakha 13 and Cherniatino II sites in Primorye, both interpreted as having a C4 component in the diets, most likely millet; the earliest dates to ~3360 years ago (51, 52).
Comparing the Pleistocene wolf and Holocene Siberian dog stable isotope values by necessity involves comparing different regions and ecologies. This is unavoidable given that Pleistocene wolf remains are few in number (n = 4) in Siberia outside of Beringia, and widely accepted Pleistocene dogs are rare everywhere. The isotopic data presented here indicate that Siberian dogs adapted to new dietary niches, including in forager communities, as early as 7400 years ago with the emergence of freshwater resource diets. Evidence for marine-based diets appears ~1400 years later. Such aquatic resource diets are uncharacteristic of Pleistocene wolves in Eurasia, and even modern wolves that seasonally forage on marine and freshwater resources remain reliant on ungulates (53). Further, our Holocene wolf stable isotope data, which derives from various regions of Siberia, not just Beringia, also provide no indication of long-term consumption of aquatic foods by wolves. This is expected given that freshwater and marine water bodies in Siberia are frozen much of the year. Dogs and wolves would have been unable to access most aquatic food resources during such cold seasons. Isotopic data showing habitual consumption of aquatic foods in Siberia suggest that humans fed these foods to dogs or at least that dogs habitually scavenged on the remains of aquatic foods eaten by people. That is, there was some level of dietary dependence on humans by at least 7400 years ago, and this occurred in several areas of Siberia long before the arrival of food production.
Adaptation to such aquatic foods had long-term consequences, with food environments playing a major role in shaping dog population sizes and dogs’ roles in society. For example, outside of pastoral and agricultural settings in southern Siberia, dogs historically were most abundant where freshwater fish and marine foods were readily obtainable (54). In such settings, dogs were often also the dominant animals involved in sled pulling. These aquatic resources often could be efficiently procured and used as dog food, especially compared to the intermittent success achieved in ungulate hunting. In areas of Siberia where such foods were limited, dog populations were lower, and domestic reindeer often were the dominant animal in transport not dogs (54). If such patterns held throughout the Holocene, then it seems that dog sledding most likely became common and sustainable in settings where human subsistence was already aquatically focused, not among societies where terrestrial mammals were the primary prey. Food production, beyond just making carbohydrates accessible to dogs, likely had an even greater long-term effect. More calories were more consistently available, likely in the form of human food waste, meaning that a greater number of dogs could be economically accommodated. Dogs from food-producing societies likely became abundant enough that they eventually supplanted those in most foraging societies, although some introgression also clearly occurred (6). This could have occurred through human-dog colonization of regions occupied by foragers, long-term uncontrolled dog dispersals to such regions, or even sustained trade in dogs between societies. A recent genomic study clearly identifies increasing European or Near East ancestry in Siberian dogs over the past 2000 years, including even in some arctic dogs in Northwest Siberia (25).
Consuming anthropogenic waste and human feces
Feeding in human-dominated places also presented dogs with significant dietary challenges. In past small-scale societies, facilities to restrain the movements of dogs were probably limited, suggesting that most dogs may have been free ranging, as are ~75% of the world’s dogs today (1). Free-ranging dogs mostly obtain sustenance by consuming anthropogenic waste, often as spoiled and discarded human food and unused portions of slaughtered livestock (3, 55, 56). In addition, many past communities likely lacked latrines. In modern settings lacking latrines, human feces is commonly eaten by free-ranging dogs (3, 55). Most modern dogs are probably more accurately classified as scavengers rather than predators.
Dietary reliance on anthropogenic waste had important outcomes for dogs. First, such waste is often small in size and immobile (3, 56). As argued earlier, feeding on such items did not require social foraging, and the small body sizes of many Holocene dogs were well suited to the utilization of small food packages. Second, such resources became more abundant and concentrated on the broader Holocene landscape, providing environments where reliance on nonsocial foraging was increasingly feasible. Holocene foraging societies were often more sedentary than their Pleistocene predecessors, particularly where they focused on spatially and temporally restricted resources such as runs of fish and concentrations of sea mammals. As mentioned, recurrent use of such resources in Siberia occurs early in the Middle Holocene, by which time dogs are well evidenced in the Arctic, coastal Primorye, and near Lake Baikal (22, 24, 57). Later pastoral and agricultural societies were larger in scale in Siberia and elsewhere, potentially producing yet more waste.
Third, feeding on anthropogenic waste permitted ready transmission of microbes from humans and their food items to dogs, subjecting them to increasing health problems and microbiome alterations. Study of modern rural and urban coyotes illustrates such challenges (58). Urban coyotes consume more carbohydrate-rich food waste, have lower body fat reserves, exhibit greater immune system stress, and more often carry the zoonotic parasite Echinococcus multilocularis (58). Further, their gut microbiomes are more diverse and have increased abundances of lactic acid bacteria, the latter being an adaptation to increased carbohydrate consumption. Such changes in the gut microbiomes of dogs are linked to aggression and health issues (59, 60). These are not just modern phenomena. Similar dog microbiome adaptations were recently documented in a Bronze Age agricultural settlement in Italy (61). These dogs lacked increased copy numbers of the AMY2B gene, but their gut microbiome metagenomes exhibit adaptation to a carbohydrate-rich diet. Such adaptations also likely characterized some ancient Siberian dogs, particularly with the Late Holocene emergence of agriculture, which provided more access to carbohydrates than foraging. The best candidates in our dataset for such adaptations are the Primorye dogs with high δ13C values consistent with millet intake. However, given that dogs’ diets were diversifying among early foraging societies in Siberia and involved some level of dependence on humans, gut microbiome adaptations were likely occurring throughout the Holocene wherever dogs were present and perhaps even into the Pleistocene as part of their early domestication history.
Further, evidence for parasitic infections in dogs through their interactions with humans is evident in the deep past, including in Siberia. For example, many Late Holocene humans in Northwest Siberia became infected with Opisthorchis felineus (cat liver fluke) and Diphyllobothrium sp. (tapeworm) by eating uncooked fish (62). Dog coprolites containing large numbers of O. felineus eggs were found at Iarte VI, a ~900-year-old forager site in this same region (63). Just to the south, 17th and 18th century Russian sites produced dog coprolites with O. felineus and Diphyobothrium sp. (63). The earliest such parasitic infections in Eurasian dogs appear ~9000 to 7000 years ago at the Zamostie II site near Moscow (64). All of these dogs likely became infected by being fed fish or by scavenging on their discarded remains. In southwest Siberia, dog coprolites at the ~2400-year-old pastoral Marai I site contained Opisthorchis sp. eggs and larva from Strongyloides papillosus and Strongyloides westeri, both of which are species of threadworm (65). These parasites likely infected dogs via consumption of hide and viscera of ungulates, probably cattle and horses (65, 66). Wolves can be infected with many of these same parasites (66), but dogs’ far better access to fish and livestock increased their parasitic exposure millennia ago.
While the ability to feed on anthropogenic foods benefitted dogs in the long run, allowing these animals to become the most abundant carnivorous mammal on Earth, this niche also posed some hazards. The increased exposure to some microbes from inhabiting this niche likely initially increased dog mortality rates, providing some limitations on population growth. The extent of this impact is largely unknown, in part due to the dearth of research on ancient microbes and trends in dog population sizes. Over time, some dog populations surely developed a level of resistance to these microbes. As these populations dispersed to new regions, newly encountered dog populations would have been adversely affected. The European colonization of the Americas may be the best example of this, but this remains to be tested in any way. Dogs over time, of course, also became vectors for microbes to humans and wildlife, affecting their evolution. Feeding in the human niche posed other threats to dogs, with human violence being perhaps the most obvious. Further, increasing reliance on humans and their niches rendered dogs susceptible to marked declines in human population resulting from the interrelated effects of pandemics, colonization, and warfare. Their long-term fates were increasingly tied to those of humans, for better or worse.
To conclude, our results and review of the Siberian archaeological literature indicate that dogs in this region experienced several previously little-discussed foraging and dietary transitions since their divergence from wolves, and these transitions likely characterize Holocene dogs across much of the globe. These transitions include decreased social hunting and greater reliance on smaller food packages, dietary diversification to include aquatic resources and anthropogenic waste, and increased exposure to select microbes introduced through feeding in human-dominated places. Some of these transitions and adaptations helped dog populations increase and develop into the hunting, herding, and sled pulling roles that they are historically known for in Siberia and elsewhere. Others were detrimental to long-term population health and size. Recognition of such transitions provides new directions for research into early dog gut microbiome adaptation, particularly in foraging societies, as well as the spread and impacts of zoonotic disease on human and canid health. Further, these transitions also affected dog life histories, and some should be evident in dog skeletal remains if greater attention is given to features such as dental wear and pathological and traumatic lesions. Diachronic trends in dog body size are poorly documented in most areas of the globe, and future studies could readily assess whether the trends documented across the vast expanse of Siberia are present elsewhere. Further, this study demonstrates the necessity of further analyzing dog stable isotope values for better understanding the life histories and evolution of dogs. Overall, dogs have and continue to evolve within rapidly changing human niches, and our research highlights several important ways their lives and long-term histories have been shaped by cohabiting with humans.
MATERIALS AND METHODS
Body masses were estimated for Pleistocene and Holocene canids in several ways. For the Siberian dogs (Table 1 and data S2) (26), body masses were calculated using skeletal element dimensions that were entered into regression formulas (67, 68). These regression formulas were built using modern dogs (n = 36 for the cranium and mandible and 47 for postcranial elements) of known body mass at death; those for estimating wolf body sizes were built using North American gray wolves (n = 108 for the cranium and mandible; n = 40 for postcranial elements). In addition, the dog and wolf cranium and mandible data were combined to create a third set of Canis regression equations for calculating body mass in large canids of uncertain taxonomic status (67). To apply such equations, the length of a dog cranium, for example, is taken in millimeters using a spreading or sliding caliper, in our example from akrokranium to prosthion. For this specific dimension of the dog cranium, the value is entered in the following formula (67): log(y) = a + β log(x). Here, β is the regression coefficient, which, for cranial length in dogs, is 3.140, and a is the constant, which, for this formula, is −5.883. For each individual analyzed, the regression equation with the lowest percent prediction error was chosen, meaning that only one body mass estimation was calculated per individual. These same procedures and formulas were applied to the Alaskan Pleistocene wolf data (68) in this study (Table 1 and data S1). The Pleistocene wolf body mass estimates in Table 1 from (14, 69) also were calculated in this manner. Note that the data presented in Table 1 from (69) for putative early dogs used the generalized Canis regression equations (66). For these formulae, percent prediction errors range from ~9 to 15%.
Estimation of the body masses of the European Middle Holocene dogs required a slightly different approach (Table 1 and data S2). The database for these specimens (70) includes dimensions for postcranial elements only and is organized by element not by individual. That is, it is impossible to determine whether elements from a given site in the database come from discrete individuals or whether several derive from a single individual. In this case, we chose a single dimension for each skeletal element and estimated the body mass for that specimen using the regression formulae from (67). This not only creates the possibility of individuals’ body masses being estimated multiple times but also ensures that all individuals are represented in the analysis.
For the British, Italian, and French Pleistocene wolves, body mass estimates were taken from the source literature (71–73), as some publications do not provide raw measurements, and others appear not to have taken skeletal element dimensions in the same manner as (73, 74). For the British Pleistocene wolves (71), carnassial length measurements were used in regression formulas constructed with multiple extant carnivorous mammals. For the Italian wolves (72), lengths of the mandible, radius, and tibia were used, along with the length and width of the mandibular carnassial, also with regression formula built using multiple extant carnivorous mammals (74). Last, for the French wolves (73), body mass estimates were made using the dimensions of multiple skeletal elements and using regression formulas constructed using 12 modern wolves from Portugal.
Methods used for canid stable isotope analyses conducted through this, and other projects are based on protocols initially developed by Longin (75). Methods used in other studies can be found in the literature cited in data S1 and S2. Stable isotope data obtained during the current project were primarily generated at the University of Alberta (Edmonton, Canada) and Leiden University (Leiden, The Netherlands), with methods described below. Additional canid stable isotope data were generated by this project through radiocarbon dating conducted at the Ångström Laboratory at Uppsala University (Uppsala, Sweden) and Beta Analytic Inc. (Miami, USA).
Canid samples prepared for collagen stable isotope analyses at the University of Alberta Department of Anthropology’s archaeological laboratory used a modified version of the Oxford sample preparation method (76). Samples weighing ~1 g were sawn from larger specimens with a Dremel multitool. All samples were surface-cleaned with a soft brush and distilled water (dH2O), and ~1 mm of outer bone surfaces was burred off with a Dremel sandpaper cone attachment. Samples were then sonicated for 10 min in three changes of double-distilled water at room temperature and allowed to air dry for at least 48 hours. Following cleaning, samples were ground to powder in a Spex Certiprep liquid nitrogen mill. Approximately 500 mg from each sample was placed in a polyethylene or polyphenylene vial with 12 ml of 1% hydrochloric acid (HCl), shaken, and allowed to demineralize. The 1% HCl solution was changed every 2 to 3 days during the demineralization process.
After demineralization, samples were centrifuged and rinsed in double-distilled water until they reached neutrality as determined by EMD Millipore colorpHast pH testing strips. Upon reaching neutrality, the samples were drained of water. After the demineralization process, 12 ml of 0.1 M sodium hydroxide (NaOH) solution was added to each sample to remove humates. The vials were shaken and allowed to react at room temperature for 20 hours. Samples were then centrifuged and rinsed in changes of double-distilled water until they reached neutrality and were drained of water. Immediately following this step, another 12 ml of 1% HCl was added to sample vials. Vials were shaken and left to react for 2 hours at room temperature. After this time had elapsed, samples were centrifuged and rinsed with double-distilled water until neutrality and then drained.
Six milliliters of acidulated water with a pH of 3 was added to each vial and shaken. The samples were then placed in a 75°C water bath and left undisturbed to allow the collagen to gelatinize into solution. After 20 hours, the samples were removed from the bath. The supernatant from each sample was filtered through a FisherBrand glass fiber filter paper using a Nalgene 40-mm Büchner filter and a 125-ml sidearm/filtering flask. Approximately 6 ml of filtrate from each sample was poured into a dual-chambered Vivaspin 30-μl ultrafiltration vial (prerinsed) and centrifuged until 1 ml remained in the upper chamber. This amount was pipetted into a preweighed centrifuge vial, frozen, lyophilized, and then analyzed at the University of Alberta’s Biogeochemical Analytical Services Laboratory.
Samples were analyzed for δ13C and δ15N ratios using a EuroVector EuroEA3028-HT elemental analyzer coupled to a GV Instruments IsoPrime continuous-flow isotope ratio mass spectrometer. National Institute of Standards and Technology (NIST) 8415 whole egg powder standard reference material was run as an in-house δ15N (+6.89 ± 0.2‰) and δ13C (−23.99 ± 0.01‰) quality assurance/quality control (QC) check every 20 samples throughout analyses. Analytical precision based on the repeated NIST 8415 measurements was ±0.2‰ for δ15N and ±0.01‰ for δ13C. Measurement calibration for δ15N was done using a three-point calibration curve with certified reference materials USGS34 (potassium nitrate, δ15N = −1.8‰), IAEA-NO-3 (potassium nitrate, δ15N = +4.7‰), and IAEA-N-2 (ammonium sulfate, δ15N = +20.3‰) relative to atmospheric nitrogen. Measurement calibration for δ13C was done using a three-point calibration curve with certified reference materials IAEA-CH-7 (polyethylene foil, δ13C = −31.8‰), IAEA-CH-3 (cellulose, δ13C = −24.5‰), and IAEA-CH-6 (sucrose, δ13C = −10.4‰) relative to Vienna Pee Dee Belemite.
For canid remains processed at the Faculty of Archaeology, Leiden University, all bone samples were cleaned manually and then placed in dH2O and repeatedly washed ultrasonically until all sediment was removed. Dry bone samples were demineralized in a dilute 1% HCl solution, changed every 24 to 48 hours until complete. Collagen pseudomorphs were then rinsed in dH2O until neutrality was reached and then transferred into a 0.1 M solution of NaOH for 20 hours to remove humic acids. Last, samples were again rinsed in dH2O to neutrality and then freeze-dried. After freeze drying, collagen yields were calculated with the total dry bone weight expressed as a percentage of the starting weight.
Measurement of δ13C and δ15N occurred on a continuous flow Delta V plus isotope ratio mass spectrometer, paired with a Thermo Fisher Scientific Flash 2000 organic elemental analyzer at Department of Earth Sciences, Cluster Geology and Geochemistry, Vrije Universiteit, Amsterdam. Measurement calibration was performed using USGS40 (mean for all analytical sessions: δ13C, −26.4 ± 0.05‰; δ15N, −4.5 ± 0.1‰) and USGS41 (mean for all analytical sessions: δ13C, 37.6 ± −0.1‰; δ15N, 47.6 ± 0.1‰). The precision of the mass spectrometer based on repeat measurements of glycine (USGS64) is 0.1‰ for δ13C and 0.2‰ for δ15N. The average 2 SDs (~95% range) of percentages C and N in all standards (USGS40, USGS41, and USGS64), measured via the elemental analyzer, are 1.6 and 1.1%, respectively.
Where possible, we applied the same QC criteria to data presented in this study and data collected from the literature. This included atomic C/N ratio (77) (liberal criteria used), collagen yield, which should minimally be higher than 1.0%, and elemental concentrations, which are expected to be >13.0 and >4.8% for carbon and nitrogen, respectively (78, 79).
Acknowledgments
We are grateful to the museum of the Institute of Plant and Animal Ecology UB RAS (Ekaterinburg, Russia) for the provision of some samples. R. Schulting, S. Svyatko, A. Kuznetsov, Z. Landry, D. Fraser, and L. Beckel shared data for this paper.
Funding: Funding for this research was provided by the Social Sciences and Humanities Research Council of Canada IG 435-2019-0706 (to R.J.L. and T.N.) and the European Research Council #295458 (to D. Anderson and R.L.).
Author contributions: Conceptualization: R.J.L., T.N., E.G., L.S.F., S.J.G.-L., and A.L.W.-R. Methodology: R.J.L., T.N., E.G., L.S.F., S.J.G.-L., A.L.W.-R., P.S., and M.B. Writing—original draft: R.J.L., T.N., and E.G. Writing—review and editing: R.J.L., T.N., E.G., L.S.F., S.J.G.-L., A.L.W.-R., M.B., P.S., O.P.B., V.I.B., N.E.B., N.G.D., I.V.F., V.V.G., O.I.G., S.P.G., A.V.G., L.G.I., G.L.I., A.A.K., M.V.K., P.A.K., E.V.K., B.L., I.G.N., D.V.P., A.N.P., M.V.S., N.A.S., A.B.S., and A.A.T.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
References
Other Supplementary Material for this manuscript includes the following:
Datasets S1 to S3
REFERENCES AND NOTES
- 1.Hughes J., Macdonald D. W., A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 157, 341–351 (2013). [Google Scholar]
- 2.Alexander P., Berri A., Moran D., Reay D., Rounsevell M. D. A., The global environmental paw print of pet food. Glob. Environ. Change 65, 102153 (2020). [Google Scholar]
- 3.Butler J. R. A., Brown W. Y., Du Toit J. T., Anthropogenic food subsidy to a commensal carnivore: The value and supply of human faeces in the diet of free-ranging dogs. Animals 8, 67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young J. K., Olson K. A., Reading R. P., Amgalanbaatar S., Berger J., Is wildlife going to the dogs? Impacts of feral and free-roaming dogs on wildlife populations. BioScience 61, 125–132 (2011). [Google Scholar]
- 5.Freedman A. H., Gronau I., Schweizer R. M., Vecchyo D. O.-D., Han E., Silva P. M., Galaverni M., Fan Z., Marx P., Lorente-Galdos B., Beale H., Ramirez O., Hormozdiari F., Alkan C., Vilà C., Squire K., Geffen E., Kusak J., Boyko A. R., Parker H. G., Lee C., Tadigotla V., Siepel A., Bustamante C. D., Harkins T. T., Nelson S. F., Ostrander E. A., Marques-Bonet T., Wayne R. K., Novembre J., Genome sequencing highlights the dynamic early history of dogs. PLOS Genet. 10, e1004016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergström A., Frantz L., Schmidt R., Ersmark E., Lebrasseur O., Girdland-Flink L., Lin A. T., Storå J., Sjögren K.-G., Anthony D., Antipina E., Amiri S., Bar-Oz G., Bazaliiskii V. I., Bulatović J., Brown D., Carmagnini A., Davy T., Fedorov S., Fiore I., Fulton D., Germonpré M., Haile J., Irving-Pease E. K., Jamieson A., Janssens L., Kirillova I., Horwitz L. K., Kuzmanovic-Cvetković J., Kuzmin Y., Losey R. J., Dizdar D. L., Mashkour M., Novak M., Onar V., Orton D., Pasarić M., Radivojević M., Rajković D., Roberts B., Ryan H., Sablin M., Shidlovskiy F., Stojanović I., Tagliacozzo A., Trantalidou K., Ullén I., Villaluenga A., Wapnish P., Dobney K., Götherström A., Linderholm A., Dalén L., Pinhasi R., Larson G., Skoglund P., Origins and genetic legacy of prehistoric dogs. Science 370, 557–564 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perri A. R., Feuerborn T. R., Frantz L. A. F., Larson G., Malhi R. S., Meltzer D. J., Witt K. E., Dog domestication and the dual dispersal of people and dogs into the Americas. Proc. Natl. Acad. Sci. U.S.A. 118, e2010083118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.R. Coppinger, L. Coppinger, Dogs-a Startling New Understanding of Canine Origin, Behavior & Evolution (University of Chicago Press, 2001). [Google Scholar]
- 9.Axelsson E., Ratnakumar A., Arendt M.-L., Maqbool K., Webster M. T., Perloski M., Liberg O., Arnemo J. M., Hedhammar Å., Lindblad-Toh K., The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495, 360–364 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Arendt M., Cairns K. M., Ballard J. W. O., Savolainen P., Axelsson E., Diet adaptation in dog reflects spread of prehistoric agriculture. Heredity 117, 301–306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perri A., A wolf in dog’s clothing: Initial dog domestication and Pleistocene wolf variation. J. Archaeol. Sci. 68, 1–4 (2016). [Google Scholar]
- 12.Skoglund P., Ersmark E., Palkopoulou E., Dalén L., Ancient wolf genome reveals an early divergence of domestic dog ancestors and admixture into high-latitude breeds. Curr. Biol. 25 ( 11), 1515–1519 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Ramos-Madrigal J., Sinding M.-H. S., Carøe C., Mak S. S. T., Niemann J., Castruita J. A. S., Fedorov S., Kandyba A., Germonpré M., Bocherens H., Feuerborn T. R., Pitulko V. V., Pavlova E. Y., Nikolskiy P. A., Kasparov A. K., Ivanova V. V., Larson G., Frantz L. A. F., Willerslev E., Meldgaard M., Petersen B., Sicheritz-Ponten T., Bachmann L., Wiig Ø., Hansen A. J., Gilbert M. T. P., Gopalakrishnan S., Genomes of Pleistocene Siberian wolves uncover multiple extinct wolf lineages. Curr. Biol. 31, 198–206.e8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germonpré M., Fedorov S., Danilov P., Galeta P., Jimenez E.-L., Sablin M., Losey R. J., Palaeolithic and prehistoric dogs and Pleistocene wolves from Yakutia: Identification of isolated skulls. J. Archaeol. Sci. 78, 1–19 (2017). [Google Scholar]
- 15.Ovodov N. D., Crockford S. J., Kuzmin Y. V., Higham T. F. G., Hodgins G. W. L., van der Plicht J., A 33,000-year-old incipient dog from the Altai mountains of Siberia: Evidence of the earliest domestication disrupted by the Last Glacial Maximum. PLOS ONE. 6, e22821 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suvorov A. P., Geograficheskaia izmenchivost’ parametrov tela volka Prieniseiskoi Sibiri. Vestnik KrasGAY 7, 119–125 (2017). [Google Scholar]
- 17.Shrotriya S., Lyngdoh S., Habib B., Wolves in Trans-Himalayas: 165 years of taxonomic confusion. Curr. Sci. 103, 885–887 (2012). [Google Scholar]
- 18.Carbone C., Mace G. M., Roberts S. C., Macdonald D. W., Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Van Valkenburgh B., Koepfli K., Cranial and dental adaptations to predation in canids. Symp. Zool. Soc. Lond. 65, 15–37 (1993). [Google Scholar]
- 20.Rimbault M., Beale H. C., Schoenebeck J. J., Hoopes B. C., Allen J. J., Kilroy-Glynn P., Wayne R. K., Sutter N. B., Ostrander E. A., Derived variants at six genes explain nearly half of size reduction in dog breeds. Genome Res. 23, 1985–1995 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plassais J., vonHoldt B. M., Parker H. G., Carmagnini A., Dubos N., Papa I., Bevant K., Derrien T., Hennelly L. M., Whitaker D. T., Harris A. C., Hogan A. N., Huson H. J., Zaibert V. F., Linderholm A., Haile J., Fest T., Habib B., Sacks B. N., Benecke N., Outram A. K., Sablin M. V., Germonpré M., Larson G., Frantz L., Ostrander E. A., Natural and human-driven selection of a single non-coding body size variant in ancient and modern canids. Cur. Biol. 889–897.e9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Losey R. J., Nomokonova T., Fleming L. S., Kharinskii A. V., Kovychev E. V., Konstantinov M. V., Diatchina N. G., Sablin M. V., Iaroslavtseva L. G., Buried, eaten, sacrificed: Archaeological dog remains from Trans-Baikal, Siberia. Archaeol. Res. Asia 16, 58–65 (2018). [Google Scholar]
- 23.Losey R. J., Nomokonova T., Gusev A. V., Bachura O. P., Fedorova N. V., Kosintsev P. A., Sablin M. V., Dogs were domesticated in the Arctic: Culling practices and dog sledding at Ust’-Polui. J. Anthropol. Archaeol. 51, 113–126 (2018). [Google Scholar]
- 24.Pitulko V. V., Kasparov A. K., Archaeological dogs from the early Holocene Zhokhov site in the Eastern Siberian Arctic. J. Archaeol. Sci. Rep. 13, 491–515 (2017). [Google Scholar]
- 25.Feuerborn T. R., Carmagnini A., Losey R. J., Nomokonova T., Askeyev A., Askeyev I., Askeyev O., Antipina E. E., Appelt M., Bachura O. P., Beglane F., Bradley D. G., Daly K. G., Gopalakrishnan S., Gregersen K. M., Guo C., Gusev A. V., Jones C., Kosintsev P. A., Kuzmin Y. V., Mattiangeli V., Perri A. R., Plekhanov A. V., Ramos-Madrigal J., Schmidt A. L., Shaymuratova D., Smith O., Yavorskaya L. V., Zhang G., Willerslev E., Meldgaard M., Gilbert M. T. P., Larson G., Dalén L., Hansen A. J., Sinding M.-H. S., Frantz L., Modern Siberian dog ancestry was shaped by several thousand years of Eurasian-wide trade and human dispersal. Proc. Natl. Acad. Sci. U.S.A. 118, e2100338118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Losey R. J., Nomokonova T., Kosintsev P. A., Bachura O. P., Gusev A. V., Vasyukov D. D., Savinetsky A. B., Tishkin A. A., Grushin S. P., Gorbunov V. V., Papin D. V., Sablin M. V., Popov A. N., Lazin B., Nikitin I. G., Bazaliiskii V. I., Pitulko V. V., Kasparov A. K., Dog body size in Siberia and the Russian Far East and its implications. Quat. Sci. Rev. 241, 106430 (2020). [Google Scholar]
- 27.Manning K., The cultural evolution of Neolithic Europe. EUROEVOL dataset 2: Zooarchaeological data. J. Open Archaeol. Data 5, e3 (2016). [Google Scholar]
- 28.Welker M. H., Byers D. A., The birch creek canids and dogs as transport labor in the intermountain west. Am. Antiq. 84, 88–106 (2019). [Google Scholar]
- 29.Nomokonova T., Losey R. J., Goriunova O. I., Novikov A. G., Weber A. W., A 9,000 year history of seal hunting on lake baikal, Siberia: The Zooarchaeology of Sagan-Zaba II. PLOS ONE 10, e0128314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popov A. N., Tabarev A. V., Mikishin Y. A., Neolithization and ancient landscapes in Southern Primorye, Russian Far East. J. World Prehist. 27, 247–261 (2014). [Google Scholar]
- 31.Svyatko S. V., Schulting R. J., Mallory J., Murphy E. M., Reimer P. J., Khartanovich V. I., Chistov Y. K., Sablind M. V., Stable isotope dietary analysis of prehistoric populations from the Minusinsk Basin, Southern Siberia, Russia: A new chronological framework for the introduction of millet to the eastern Eurasian steppe. J. Archaeol. Sci. 40, 3936–3945 (2013). [Google Scholar]
- 32.Li T., Ning C., Zhushchikhovskaya I. S., Hudson M. J., Robbeets M., Millet agriculture dispersed from Northeast China to the Russian Far East: Integrating archaeology, genetics, and linguistics. Archaeol. Res. Asia 22, 100177 (2020). [Google Scholar]
- 33.Losey R. J., Nomokonova T., Arzyutov D. V., Gusev A. V., Plekhanov A. V., Fedorova N. V., Anderson D. G., Domestication as enskilment: Harnessing reindeer in Arctic Siberia. J. Archaeol. Method Theory 28, 197–231 (2021). [Google Scholar]
- 34.Chisholm B. S., Nelson D. E., Schwarcz H. P., Stable-Carbon isotope ratios as a measure of marine versus terrestrial protein in ancient diets. Science 216, 1131–1132 (1982). [DOI] [PubMed] [Google Scholar]
- 35.Smith B. N., Epstein S., Two categories of 13C/12C ratios for higher plants. Plant Physiol. 47, 380–384 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deniro M. J., Epstein S., Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341–351 (1981). [Google Scholar]
- 37.Minagawa M., Wada E., Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 48, 1135–1140 (1984). [Google Scholar]
- 38.Guiry E., Complexities of stable carbon and nitrogen isotope biogeochemistry in ancient freshwater ecosystems: Implications for the study of past subsistence and environmental change. Front. Ecol. Evol. 7, 313 (2019). [Google Scholar]
- 39.Szpak P., Complexities of nitrogen isotope biogeochemistry in plant-soil systems: Implications for the study of ancient agricultural and animal management practices. Front. Plant Sci. 5, 288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hedges R. E. M., Clement J. G., Thomas C. D. L., O’connell T. C., Collagen turnover in the adult femoral mid-shaft: Msodeled from anthropogenic radiocarbon tracer measurements. Am. J. Phys. Anthropol. 133, 808–816 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Guiry E. J., Dogs as analogs in stable isotope-based human paleodietary reconstructions: A review and considerations for future use. J. Archaeol. Method Theory 19, 351–376 (2012). [Google Scholar]
- 42.Fox-Dobbs K., Leonard J. A., Koch P. L., Pleistocene megafauna from eastern Beringia: Paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records. Palaeogeogr. Palaeoclimatol. Palaeoecol. 261, 30–46 (2008). [Google Scholar]
- 43.Germonpré M., Sablin M. V., Stevens R. E., Hedges R. E. M., Hofreiter M., Stiller M., Després V. R., Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: Osteometry, ancient DNA and stable isotopes. J. Archaeol. Sci. 36, 473–490 (2009). [Google Scholar]
- 44.Bocherens H., Isotopic tracking of large carnivore palaeoecology in the mammoth steppe. Quat. Sci. Rev. 117, 42–71 (2015). [Google Scholar]
- 45.Yeakel J. D., Guimarães P. R., Bocherens H., Koch P. L., The impact of climate change on the structure of Pleistocene food webs across the mammoth steppe. Proc Biol Sci. 280, 20130239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumann C., Bocherens H., Drucker D. G., Conard N. J., Fox dietary ecology as a tracer of human impact on Pleistocene ecosystems. PLOS ONE 15, e0235692 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landry Z., Kim S., Trayler R. B., Gilbert M., Zazula G., Southon J., Fraser D., Dietary reconstruction and evidence of prey shifting in Pleistocene and recent gray wolves (Canis lupus) from Yukon Territory. Palaeogeogr. Palaeoclimatol. Palaeoecol. 571, 110368 (2021). [Google Scholar]
- 48.Drucker D., Henry-Gambier D., Determination of the dietary habits of a Magdalenian woman from Saint-Germain-la-Rivière in southwestern France using stable isotopes. J. Hum. Evol. 49, 19–35 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Newsome T. M., Boitani L., Chapron G., Ciucci P., Dickman C. R., Dellinger J. A., López-Bao J. V., Peterson R. O., Shores C. R., Wirsing A. J., Ripple W. J., Food habits of the world’s grey wolves. Mamm. Rev. 46, 255–269 (2016). [Google Scholar]
- 50.Losey R. J., Guiry E., Nomokonova T., Gusev A. V., Szpak P., Storing fish?: A dog’s isotopic biography provides insight into Iron Age food preservation strategies in the Russian Arctic. Archaeol. Anthropol. Sci. 12, 200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuzmin Y. V., Panov V. S., Gasilin V. V., Batarshev S. V., Paleodietary patterns of the Cherepakha 13 site population (Early Iron Age) in Primorye (Maritime) province, Russian Far East, based on stable isotope analysis. Radiocarbon 60, 1611–1620 (2018). [Google Scholar]
- 52.L. S. Fleming, Examination of Ancient Animal Management Practices in Siberia and the Russian Far East through Dietary Stable Isotope Analyses (University of Alberta, 2020). [Google Scholar]
- 53.Szepanski M. M., Ben-David M., Van Ballenberghe V., Assessment of anadromous salmon resources in the diet of the Alexander Archipelago wolf using stable isotope analysis. Oecologia 120, 327–335 (1999). [DOI] [PubMed] [Google Scholar]
- 54.V. Davydov, K. Klokov, “Dogs, reindeer and humans in Siberia: Threefold synergetic in the northern landscape” in Dogs in the North: Stories of Cooperation and Co-domestication, R. J. Losey, R. P. Wishart, J. P. Laurens, Eds. (Routledge, 2018), pp. 45–60. [Google Scholar]
- 55.Atickem A., Bekele A., Williams S. D., Competition between domestic dogs and Ethiopian wolf (Canis simensis) in the Bale Mountains National Park, Ethiopia. Afr. J. Ecol. 48, 401–407 (2010). [Google Scholar]
- 56.Majumder S. S., Bhadra A., Ghosh A., Mitra S., Bhattacharjee D., Chatterjee J., Nandi A. K., Bhadra A., To be or not to be social: Foraging associations of free-ranging dogs in an urban ecosystem. Acta Ethol. 17, 1–8 (2014). [Google Scholar]
- 57.Ameen C., Feuerborn T. R., Brown S. K., Linderholm A., Hulme-Beaman A., Lebrasseur O., Sinding M.-H. S., Lounsberry Z. T., Lin A. T., Appelt M., Bachmann L., Betts M., Britton K., Darwent J., Dietz R., Fredholm M., Gopalakrishnan S., Goriunova O. I., Grønnow B., Haile J., Hallsson J. H., Harrison R., Heide-Jørgensen M. P., Knecht R., Losey R. J., Masson-MacLean E., McGovern T. H., McManus-Fry E., Meldgaard M., Midtdal Å., Moss M. L., Nikitin I. G., Nomokonova T., Pálsdóttir A. H., Perri A., Popov A. N., Rankin L., Reuther J. D., Sablin M., Schmidt A. L., Shirar S., Smiarowski K., Sonne C., Stiner M. C., Vasyukov M., West C. F., Ween G. B., Wennerberg S. E., Wiig Ø., Woollett J., Dalén L., Hansen A. J., Gilbert M. T. P., Sacks B. N., Frantz L., Larson G., Dobney K., Darwent C. M., Evin A., Specialized sledge dogs accompanied Inuit dispersal across the North American Arctic. Proc. Royal Soc. B. 286, 20191929 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugden S., Sanderson D., Ford K., Stein L. Y., Clair C. C. S., An altered microbiome in urban coyotes mediates relationships between anthropogenic diet and poor health. Sci. Rep. 10, 22207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.AlShawaqfeh M. K., Wajid B., Minamoto Y., Markel M., Lidbury J. A., Steiner J. M., Serpedin E., Suchodolski J. S., A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 93, 10.1093/femsec/fix136, (2017). [DOI] [PubMed] [Google Scholar]
- 60.Wernimont S. M., Radosevich J., Jackson M. I., Ephraim E., Badri D. V., MacLeay J. M., Jewell D. E., Suchodolski J. S., The Effects of nutrition on the gastrointestinal microbiome of cats and dogs: Impact on health and disease. Front. Microbiol. 11, 1266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rampelli S., Turroni S., Debandi F., Alberdi A., Schnorr S. L., Hofman C. A., Taddia A., Helg R., Biagi E., Brigidi P., D’Amico F., Cattani M., Candela M., The gut microbiome buffers dietary adaptation in Bronze Age domesticated dogs. iScience 24, 102816 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slepchenko S., Opisthorchis felineus as the basis for the reconstruction of migrations using archaeoparasitological materials. J. Archaeol. Sci. Rep. 33, 102548 (2020). [Google Scholar]
- 63.G. P. Vizgalov, O. V. Kardash, P. V. Kosintsev, T. V. Lobanova, Istoricheskaia Ekologiia Naseleniia Zapadnoi Sibiri (Izd-vo AMB, 2013).
- 64.A. V. Engovatova, A. V. Khrustalev, Issledovanie koprolitov so stoianok kamennogo veka v Podmoskov’e, in Tverskoi Arkheologicheskii Sbornik Vol 2., I.N. Chernykh, Ed. (Tver’ Knizhno-Zhurn. Izd-vo, 1996), pp.148–154. [Google Scholar]
- 65.B. A. Zakh, S. I. Tsembaliuk, A. N. Siben, “Parasity” v zhisni cheloveka: K postanovke problemy, in Ekologia Drevnikh i Sovremennykh Obshchestv, N. P. Matveeva et al., Eds. (IPOS SO RAN, 2011), pp. 107–110. [Google Scholar]
- 66.Schurer J. M., Pawlik M., Huber A., Elkin B., Cluff H. D., Pongracz J. D., Gesy K., Wagner B., Dixon B., Merks H., Bal M. S., Jenkins E. J., Intestinal parasites of gray wolves (Canis lupus) in northern and western Canada. Can. J. Zool. 94, 643–650 (2016). [Google Scholar]
- 67.Losey R. J., Osipov B., Sivakumaran R., Nomokonova T., Kovychev E. V., Diatchina N. G., Estimating body mass in Dogs and wolves using cranial and mandibular dimensions: Application to Siberian canids. Int. J. Osteoarchaeol. 25, 946–959 (2015). [Google Scholar]
- 68.Leonard J. A., Vilà C., Fox-Dobbs K., Koch P. L., Wayne R. K., Van Valkenburgh B., Megafaunal extinctions and the disappearance of a specialized wolf ecomorph. Curr. Biol. 17, 1146–1150 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Germonpré M., Lázničková-Galetová M., Losey R. J., Räikkönen J., Sablin M. V., Large canids at the Gravettian Předmostí site, the Czech Republic: The mandible. Quat. Int. 359, 261–279 (2015). [Google Scholar]
- 70.Germonpré M., Fedorov S., Danilov P., Galeta P., Jimenez E.-L., Sablin M., Losey R. J., Palaeolithic and prehistoric dogs and Pleistocene wolves from Yakutia: Identification of isolated skulls. J. Archaeol. Sci. 78, 1–19 (2017). [Google Scholar]
- 71.Flower L. O. H., New body mass estimates of British Pleistocene wolves: Palaeoenvironmental implications and competitive interactions. Quat. Sci. Rev. 149, 230–247 (2016). [Google Scholar]
- 72.Salari L., Achino K. F., Gatta M., Petronio C., Rolfo M. F., Silvestri L., Pandolfi L., The wolf from Grotta Mora Cavorso (Simbruini mountains, Latium) within the evolution of Canis lupus L., 1758 in the Quaternary of Italy. Palaeogeog. Palaeoclimatol. Palaeoecol. 476, 90–105 (2017). [Google Scholar]
- 73.M. Boudadi-Maligne, Les Canis pléistocènes du Sud de la France: approche biosystématique, évolutive et biochronologique (Université Bordeaux, 2010). [Google Scholar]
- 74.Legendre S., Roth C., Correlation of carnassial tooth size and body weight in recent carnivores (mammalia). Hist. Biol. 1, 85–98 (1988). [Google Scholar]
- 75.Longin R., New method of collagen extraction for radiocarbon dating. Nature 230, 241–242 (1971). [DOI] [PubMed] [Google Scholar]
- 76.Bronk Ramsey C., Higham T., Bowles A., Hedges R., Improvements to the pretreatment of bone at Oxford. Radiocarbon 46, 155–163 (2004). [Google Scholar]
- 77.Guiry E. J., Szpak P., Improved quality control criteria for stable carbon and nitrogen isotope measurements of ancient bone collagen. J. Archaeol. Sci. 132, 105416 (2021). [Google Scholar]
- 78.Ambrose S. H., Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 17, 431–451 (1990). [Google Scholar]
- 79.Van Klinken G. J., Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 26, 687–695 (1999). [Google Scholar]
- 80.Reimer P. J., Austin W. E. N., Bard E., Bayliss A., Blackwell P. G., Ramsey C. B., Butzin M., Cheng H., Edwards R. L., Friedrich M., Grootes P. M., Guilderson T. P., Hajdas I., Heaton T. J., Hogg A. G., Hughen K. A., Kromer B., Manning S. W., Muscheler R., Palmer J. G., Pearson C., van der Plicht J., Reimer R. W., Richards D. A., Scott E. M., Southon J. R., Turney C. S. M., Wacker L., Adolphi F., Büntgen U., Capano M., Fahrni S. M., Fogtmann-Schulz A., Friedrich R., Köhler P., Kudsk S., Miyake F., Olsen J., Reinig F., Sakamoto M., Sookdeo A., Talamo S., The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon. 62, 725–757 (2020). [Google Scholar]
- 81.A. A. Tishkin, V. V. Gorbunov, Issledovanie pamiatnikov rannego zheleznogo veka i srednevekov’ia v lesostepnom i Gornom Altae, in Problemy Arkheologii, Etnografii, Antropologii Sibiri i Sopredel’nykh Territorii, (2002), vol. VIII, pp. 456–461. [Google Scholar]
- 82.Iu. F. Kiriushin, A. M. Maloletko, A. A. Tishkin, Berezovaia Luka – Poselenie Epokhi Bronzy v Aleiskoi Stepi (Izd-vo AGU, 2005), Vol. I. [Google Scholar]
- 83.P. A. Kosintsev, Zhivotnovodtsvo i okhota naseleniia Berezovoi Luki, in Berezovaia Luka – Poselenie Epokhi Bronzy v Aleiskoi Stepi, A. P. Derevianko, Ed. (Izd-vo AGU, 2005), vol. I, pp. 150–164. [Google Scholar]
- 84.P. A. Kosintsev, D. A. Iavsheva, M. M. Deviashin, Kompleks kostnykh ostatkov zhivotnykh iz raskopa # 2 poseleniia Berezovoia Luka, in Berezovaia Luka – Poselenie Epokhi Bronzy v Aleiskoi Stepi, V. I. Molodin, Ed. (Izd-vo AGU, 2011), vol. II, pp. 139–148. [Google Scholar]
- 85.V. V. Gorbunov, Issledovanie kurgannogo mogil’nika Inia-1 v lesostepnom Altae, in Arkheologicheskie Otkrytiia 1998 Goda, V. V. Sedov, N. V. Lopatin, Eds. (Editorial URSS. 2000), pp. 285–286. [Google Scholar]
- 86.Iu. F. Kiriushin, D. V. Papin, A. S. Pilipenko, A. S. Fedoruk, O. A. Fedoruk, Ia. V. Frolov, Pogrebal’nyi Obriad Drevnego Naseleniia Barnaul’skogo Priob’ia: Materialy iz Raskopok 2010–2011 Gruntovogo Mogil’nika Firsovo-XIV. (Izd-vo AGU, 2015). [Google Scholar]
- 87.Iu. F. Kiriushin, P. A. Kosintsev, D. V. Papin, A. S. Fedoruk, Voprosy khoziastvennoi deiatel’nosti naseleniia stepnogo Ob’-Irtysh’ia v epokhu pozdnei bronzy, in Khoziaistvenno-Kul’turnye Traditsii Altaiia v Epokhy Bronzy, Iu. F. Kiruishin, Ed. (Slovo, 2010), pp. 112–127. [Google Scholar]
- 88.Ivanov G. L., Kharinskii A. V., Losey R. J., Nomokonova T., Klementiev A. M., Balin I—Site of the Iron Age in the Valley of River Kuda. Journal of Ancient Technology Laboratory 13, 44–69 (2017). [Google Scholar]
- 89.Losey R. J., Bazaliiskii V. I., Garvie-Lok S., Germonpré M., Leonard J. A., Allen A. L., Anne Katzenberg M., Sablin M. V., Canids as persons: Early Neolithic dog and wolf burials, Cis-Baikal, Siberia. J. Anthropol. Archaeol. 30, 174–189 (2011). [Google Scholar]
- 90.R. J. Losey, S. Garvie-Lok, J. A. Leonard, M. A. Katzenberg, M. Germonpré, T. Nomokonova, M.V. Sablin, O. I. Goriunova, N. E. Berdnikova, M. A. Savel’ev, Burying dogs in ancient Cis-Baikal: Temporal trends and relationships with human diet and subsistence practices. PLOS ONE 8, e63740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.A. Davydova, The Ivolga Archaeological Complex. The Ivolga Fortress (Asiatic Fund, 1995), vol. 1. [Google Scholar]
- 92.Drake A. G., Coquerelle M., Kosintsev P. A., Bachura O. P., Sablin M., Gusev A. V., Fleming L. S., Losey R. J., Three-dimensional geometric morphometric analysis of fossil canid mandibles and skulls. Sci. Rep. 7, 9508 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fleming L. S., Losey R. J., Nomokonova T., Garvie-Lok S., Kharinskii A. A., Kovychev E. V., Medieval animal management practices at Proezzhaia I: Insights from dietary stable isotope analysis. J. Archaeol. Sci. Rep. 22, 45–57 (2018). [Google Scholar]
- 94.Losey R. J., Fleming L. S., Nomokonova T., Gusev A. V., Fedorova N. V., Garvie-Lok S., Bachura O. P., Kosintsev P. A., Sablin M. V., Human and dog consumption of fish on the Lower Ob River of Siberia: Evidence for a major freshwater reservoir effect at the Ust’-Polui Site. Radiocarbon 60, 239–260 (2018). [Google Scholar]
- 95.A. N. Popov, B. V. Lazin, Arkheologicheskie issledovaniia na ostrove Russkom v g. Vladivostoke v 2010–2011 godakh, in Drevnosti po Obe Storony Velikogo Okeana, D. L. Brodianskii, Ed. (DVGTU, 2011), pp. 118–126. [Google Scholar]
- 96.V. A. Rakov, A. N. Popov, L. E. Vasil’eva, Iu. V. Zavertanova, Iu. A Mikishin, Fauna pribrezhnoi zony proliva Bosfor-Vostochnyi perioda zheleznogo veka (po materialan spasatel’nykh raskopok pamiatnikov Nazimova-1 i Pospelova-1 v g. Vladivostoke), in Ot Mongolii do Primor’ia i Sakhalina, D. L. Brodianskii, Ed. (DVGTU, 2009). pp. 162–212. [Google Scholar]
- 97.Iu. G. Nikitin, S. Chzhun, Li Ch. Chzho, Eds., Arkheologicheskie Issledovaniia na Poselenii Cherniatino 2 v Primor’e v 2007 Gody. (Chunnam Vuekyn, DVGTU, IIAiE DVO RAN, 2008), vol. 1–2. [Google Scholar]
- 98.Sergusheva E. A., Kulturnye rasteniia srednevekovogo naseleniia Primor’ia. Rossiia i ATR 4, 151–158 (2010). [Google Scholar]
- 99.M. M. Bronshtein, K. A. Dneprovskii, Zhilishche morskikh zveroboev Chukotki, in Pamiatniki Kul’tury: Novye Otkrytiia, D. S. Likhachev, Ed. (Nauka, 2001), pp. 587–619. [Google Scholar]
- 100.P. P. Chu, Dietary Variation Among the Prehistoric Asiatic Eskimo (Simon Fraser University, 1998). [Google Scholar]
- 101.Marchenko Z. V., Orlova L. A., Panov V. S., Zubova A. V., Molodin V. I., Pozdnyakova O. A., Grishin A. E., Uslamin E. A., Paleodiet, radiocarbon chronology, and the possibility of Freshwater reservoir effect for Preobrazhenka 6 burial Ground, Western Siberia: Preliminary results. Radiocarbon 57, 595–610 (2015). [Google Scholar]
- 102.S. V. Svyatko, Palaeodietary Analysis of Prehistoric Populations from the Minusinsk Basin, Southern Siberia (Queen’s University Belfast, 2009). [Google Scholar]
- 103.A. M. Kuznetsov, A. M. Khubanova, E. O. Rogovskoi, A. M. Klement’ev, V. B. Khubanov, V. F. Posokhov, Stabil’nye isotopy ugleroda i azota kostnykh ostatkov mlekopitaiushchikh rannego i srednego golotsena stoianki Ostrov Listvenichnyi (Punkt 2). Bulletin of the Irkutsk State University. Geoarchaeology, Ethnology, and Anthropology Series 27, 27–35 (2019). [Google Scholar]
- 104.Iu.F. Kiriushin, P.A. Kosintsev, D.V. Papin, A.S. Fedoruk, “Voprosy khoziastvennoi deiatel’nosti naseleniia stepnogo Ob’-Irtysh’ia v epokhu pozdnei bronzy” in Khoziaistvenno-Kul’turnye Traditsii Altaiia v Epokhy Bronzy, Iu.F. Kiruishin, Ed. (Slovo, 2010), pp. 112–127. [Google Scholar]
- 105.Sinding M.-H. S., Gopalakrishnan S., Ramos-Madrigal J., de Manuel M., Pitulko V. V., Kuderna L., Feuerborn T. R., Frantz L. A. F., Vieira F. G., Niemann J., Castruita J. A. S., Carøe C., Andersen-Ranberg E. U., Jordan P. D., Pavlova E. Y., Nikolskiy P. A., Kasparov A. K., Ivanova V. V., Willerslev E., Skoglund P., Fredholm M., Wennerberg S. E., Heide-Jørgensen M. P., Dietz R., Sonne C., Meldgaard M., Dalén L., Larson G., Petersen B., Sicheritz-Pontén T., Bachmann L., Wiig Ø., Marques-Bonet T., Hansen A. J., Gilbert M. T. P., Arctic-adapted dogs emerged at the Pleistocene-Holocene transition. Science 368, 1495–1499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.N. V. Fedorova, P. A. Kosintsev, W. W. Fitzhugh, “Ushedshie v Kholmy”: Kul’tura Naselenii Poberezhii Severo-Zapadnogo Iamala v Zheleznom Veke (Ekaterinburg, 1998). [Google Scholar]
- 107.V. M. Morozov, S. G. Parkhimovich, Gorodishche Peregrebnoe I, in Zapadnaia Sibir’ v Drevnosti, R. S. Vasilevskii, Ed. (Tiumen’skii Gosudarstvennyi Universitet, 1985), pp. 89–99. [Google Scholar]
- 108.P. N. Butsinksii, Zaselenie Sibiri i Byt ee Pervykh Nasel’nikov. (Izd-vo Iu. Mandryka, 1999), vol. 1. [Google Scholar]
- 109.G. F. Shafranov-Kutsev, Iugoriia. Entsiklopediia Khanty-Mansiiskogo Avtonomnogo Okruga. – Iugry (Tiumenskii GU Sokrat, 2000), vol. 3. [Google Scholar]
- 110.D. Vasyukov, A. Savinetsky, On the history of aboriginal dogs of Chukotka, in Facing the Sea, I. I. Krupnik, Ed. (Ekotsentr Zapovedniki, 2016), pp. 447–473. [Google Scholar]
- 111.Kirillova I. V., Tiunov A. V., Levchenko V. A., Chernova O. F., Yudin V. G., Bertuch F., Shidlovskiy F. K., On the discovery of a cave lion from the Malyi Anyui River (Chukotka, Russia). Quat. Sci. Rev. 117, 135–151 (2015). [Google Scholar]
- 112.Salazar-García D. C., Power R. C., Rudaya N., Kolobova K., Markin S., Krivoshapkin A., Henry A. G., Richards M. P., Viola B., Dietary evidence from Central Asian Neanderthals: A combined isotope and plant microremains approach at Chagyrskaya Cave (Altai, Russia). J. Human Evol. 156, 102985 (2021). [DOI] [PubMed] [Google Scholar]
- 113.Bocherens H., Billiou D., Mariotti A., Patou-Mathis M., Otte M., Bonjean D., Toussaint M., Palaeoenvironmental and palaeodietary implications of isotopic biogeochemistry of last interglacial neanderthal and mammal bones in scladina cave (Belgium). J. Archaeol. Sci. 26, 599–607 (1999). [Google Scholar]
- 114.Bocherens H., Billiou D., Mariotti A., Patou-Mathis M., Otte M., Bonjean D., Toussaint M., Palaeoenvironmental and palaeodietary implications of isotopic biogeochemistry of last interglacial neanderthal and mammal bones in scladina cave (Belgium). J. Archaeol. Sci. 26, 599–607 (1999). [Google Scholar]
- 115.Bocherens H., Drucker D. G., Bonjean D., Bridault A., Conard N. J., Cupillard C., Germonpré M., Höneisen M., Münzel S. C., Napierala H., Patou-Mathis M., Stephan E., Uerpmann H.-P., Ziegler R., Isotopic evidence for dietary ecology of cave lion (Panthera spelaea) in North-Western Europe: Prey choice, competition and implications for extinction. Quat. Int. 245, 249–261 (2011). [Google Scholar]
- 116.Bocherens H., Drucker D. G., Germonpré M., Lázničková-Galetová M., Naito Y. I., Wissing C., Brůžek J., Oliva M., Reconstruction of the Gravettian food-web at Předmostí I using multi-isotopic tracking (13C, 15N, 34S) of bone collagen. Quat. Int. 359-360, 211–228 (2015). [Google Scholar]
- 117.Drucker D. G., Stevens R. E., Germonpré M., Sablin M. V., Péan S., Bocherens H., Collagen stable isotopes provide insights into the end of the mammoth steppe in the central East European plains during the Epigravettian. Quatern. Res. 90, 457–469 (2018). [Google Scholar]
- 118.Richards M. P., Pacher M., Stiller M., Quilès J., Hofreiter M., Constantin S., Zilhão J., Trinkaus E., Isotopic evidence for omnivory among European cave bears: Late Pleistocene Ursus spelaeusfrom the Peştera cu Oase, Romania. Proc. Natl. Acad. Sci. U.S.A. 105, 600–604 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baumann C., Starkovich B. M., Drucker D. G., Münzel S. C., Conard N. J., Bocherens H., Dietary niche partitioning among Magdalenian canids in southwestern Germany and Switzerland. Quat. Sci. Rev. 227, 106032 (2020). [Google Scholar]
- 120.Baumann C., Pfrengle S., Münzel S. C., Molak M., Feuerborn T. R., Breidenstein A., Reiter E., Albrecht G., Kind C.-J., Verjux C., Leduc C., Conard N. J., Drucker D. G., Giemsch L., Thalmann O., Bocherens H., Schuenemann V. J., A refined proposal for the origin of dogs: The case study of Gnirshöhle, a Magdalenian cave site. Sci. Rep. 11, 5137 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
References
Datasets S1 to S3