Abstract
Since 2011, indocyanine green (ICG) has been increasingly used in surgery as a diagnostic tool. Although allergic reactions to this fluorescent dye are considered rare, they can result in anaphylactic shock. We report the case of a 33-year-old woman who developed anaphylaxis immediately after ICG administration during laparoscopic-assisted high anterior resection. The patient was treated with intravenous adrenaline, and the surgery continued. Elevated plasma histamine and serum tryptase levels immediately after ICG administration and intradermal testing identified ICG as the causative agent. The frequency of ICG use is increasing, and anesthesiologists should recognize ICG as a prevalent perioperative allergen.
Keywords: Allergy, hypersensitivity reaction, indocyanine green, skin test
Introduction
Indocyanine green (ICG) is a water-soluble tricarbocyanine dye that has long been used for assessment of cardiac and liver functions and in ophthalmic surgery. In recent years, it has been increasingly used for intraoperative evaluation of revascularization and organ perfusion.[1,2] Although generally considered safe, ICG can occasionally cause serious adverse reactions[3]; however, more information is needed. We report a case of anaphylaxis following ICG administration under general anesthesia. The anaphylaxis was appropriately treated, and skin testing identified ICG as the causative agent. This case underscores the need for improved recognition of ICG as a potential cause of anaphylaxis.
Case Report
A 33-year-old, 50 kg woman with sigmoid colon cancer underwent laparoscopy-assisted high anterior resection. Despite a childhood history of asthma, she had no known drug or food allergies. Owing to her asthma, corticosteroids were administered before injection of contrast media to prevent allergic reactions during computed tomography. The baseline parameters were normal. Written informed consent was obtained from the patient for publication of this case report.
In the operating room, we inserted an epidural catheter at Th12-L1. General anesthesia was initiated using fentanyl, propofol, and rocuronium and maintained during surgery via continuous infusion of propofol and remifentanil and epidural administration of 0.2% ropivacaine. Tracheal intubation was performed and was monitored via electrocardiography and pulse oximetry and by measuring noninvasive blood pressure and end-tidal CO2 levels. A 22-gauge radial artery catheter was inserted into left radial artery.
At the request of the surgeon, indocyanine green (ICG) (5 mg Diagnogreen; Daiichi Sankyo Co., Ltd, Tokyo, Japan) was administered intraoperatively to assess colonic perfusion. The dye was diluted in 2 mL sterile water and injected via a peripherally inserted central catheter. After the injection, the patient’s blood pressure dropped from 97/61 to 40/27 mmHg, and her heart rate rose from 60 to 111 beats/min. No mucocutaneous signs, changes in SpO2, or wheezing was observed. Despite administration of 10 mg ephedrine and 350 μg phenylephrine, the hypotension persisted. Intravenous adrenaline (100 μg) and adrenaline (0.03 μg/kg/min) and noradrenaline (0.02 μg/kg/min) were then administered, raising the systolic blood pressure from 40 to 95 mmHg. To prevent secondary allergic reactions, 100 mg hydrocortisone, 20 mg famotidine, and 5 mg dexchlorpheniramine were administered. Within 15 minutes, the patient’s blood pressure and heart rate had normalized to 167/90 mmHg and 87 beats/min, respectively [Figure 1]. The surgical procedure continued without further hypotension or the need for vasopressors or inotropes.
Figure 1.
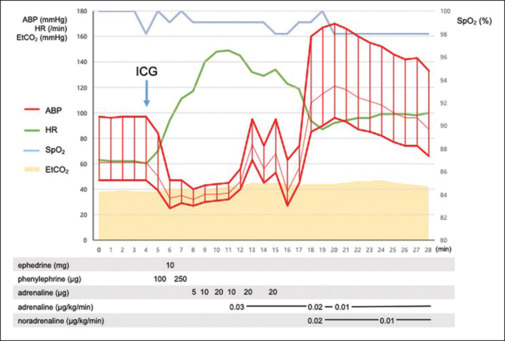
The timeline displays heart rate, arterial pressure, and SpO2 during drug administration. ABP = arterial blood pressure; HR = heart rate; EtCO2 = end-tidal carbon dioxide; SpO2 = percutaneous oxygen saturation; ICG = Indocyanine-green
The tracheal tube was extubated in the operating room, and the patient was transferred to the high-care unit. Ten minutes after ICG administration, the plasma histamine level was 360 nmol/L. The serum tryptase levels were 40.2 μg/L at 2 h and 2.3 μg/L at 24 h after ICG administration.
Six weeks post surgery, skin prick and intradermal tests for ICG, rocuronium, and flomoxef were conducted. The skin prick test showed no positive drug reactions. In the intradermal test, ICG at a 1:10 dilution (0.25 mg/mL) but not a 1:100 or 1:1000 dilution produced a 7 mm wheal and a 27 mm area of erythema [Figure 2]. ICG was considered the possible cause of the intraoperative anaphylactic episode.
Figure 2.
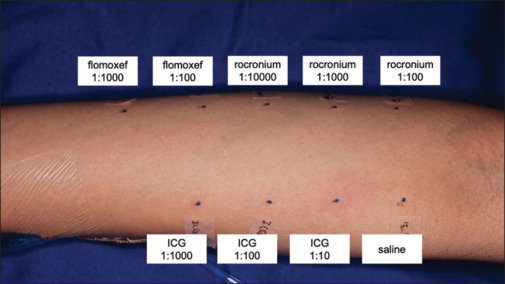
Intradermal tests yielded a positive reaction (a 27 mm erythema) for ICG at a 1:10 dilution (0.25 mg/mL) and negative results for other drugs. The stock solutions for rocuronium, flomoxef, and ICG were 10, 10, and 2.5 mg/mL, respectively
Discussion
Recognizing anaphylaxis in the perioperative period is challenging owing to factors such as concealed skin changes under sterile drapes and the inability of anesthetized patients to relate symptoms.[4] In our case, the patient exhibited no symptoms other than severe hypotension, which occurred shortly after ICG injection, leading to suspicion of anaphylaxis. The perioperative immediate hypersensitivity reaction score was 25.[5] Cases with scores greater than 21 are considered as almost certain to be an immediate hypersensitivity reaction, thus supporting our diagnosis. The elevation of the tryptase level greater than [2+ (1.2 × baseline tryptase)] and the plasma histamine level greater than 6.35 nmol/L supported the degranulation of mast cells and basophil.[5,6]
Intradermal testing identified ICG as the cause of the anaphylaxis. However, the interpretation of the results of this procedure is controversial. The possibility of false positive results cannot be excluded as the appropriate concentration of ICG for skin testing has not been established. Although all pharmaceutical agents used before the anaphylactic event should be tested,[7] this was not done in our case, leaving the possibility that drugs in addition to ICG may have contributed to the event. Basophil activation or nonspecific IgE tests could have provided a more definitive diagnosis[7]; however, our institution does not perform these tests.
The incidence of anaphylaxis induced by muscle relaxants and antibiotics, well-known causes of perioperative allergies, is approximately 0.02% and 0.01–0.05%, respectively, and is comparable to that of ICG.[8,9] However, anesthesiologists are less aware of anaphylaxis due to ICG than to muscle relaxants, antibiotics, and antiseptics. Since 2011,[1] the use of ICG has increased, and a corresponding increase in the frequency of ICG-associated anaphylaxis is anticipated. According to the Japanese Adverse Drug Reaction Report database maintained by the Pharmaceuticals and Medical Devices Agency, the number of anaphylaxis cases involving ICG has increased from 2 (2004–2010) to 20 (2011–2023).[10]
Our case of intraoperative anaphylaxis, later confirmed as ICG-induced through skin testing, urges anesthesiologists to be vigilant about potential ICG allergies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Zelken JA, Tufaro AP. Current trends and emerging future of indocyanine green usage in surgery and oncology: An update. Ann Surg Oncol. 2015;22(Suppl 3):S1271–83. doi: 10.1245/s10434-015-4743-5. [DOI] [PubMed] [Google Scholar]
- 2.Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjerregaard J, Pandia MP, Jaffe RA. Occurrence of severe hypotension after indocyanine green injection during the intraoperative period. A A Case Rep. 2013;1:26–30. doi: 10.1097/ACC.0b013e3182933c12. [DOI] [PubMed] [Google Scholar]
- 4.Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381–95. doi: 10.1213/01.ANE.0000082993.84883.7D. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins PM, Cooke PJ, Clarke RC, Guttormsen AB, Platt PR, Dewachter P, et al. Consensus clinical scoring for suspected perioperative immediate hypersensitivity reactions. Br J Anaesth. 2019;123:e29–37. doi: 10.1016/j.bja.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Laroche D, Gomis P, Gallimidi E, Malinovsky JM, Mertes PM. Diagnostic value of histamine and tryptase concentrations in severe anaphylaxis with shock or cardiac arrest during anesthesia. Anesthesiology. 2014;121:272–9. doi: 10.1097/ALN.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 7.Garvey LH, Dewachter P, Hepner DL, Mertes PM, Voltolini S, Clarke R, et al. Management of suspected immediate perioperative allergic reactions: An international overview and consensus recommendations. Br J Anaesth. 2019;123:e50–64. doi: 10.1016/j.bja.2019.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Tacquard C, Iba T, Levy JH. Perioperative anaphylaxis. Anesthesiology. 2023;138:100–10. doi: 10.1097/ALN.0000000000004419. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey A. Penicillin allergy and perioperative anaphylaxis. Front Allergy. 2022;3:903161. doi: 10.3389/falgy.2022.903161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Review reports, prescription drug. Pharmaceuticals and Medical Devices Agency. Available from https://www.info.pmda.go.jp/fsearchnew/jsp/menu_fukusayou_base.jsp . (In Japanese) [Last accessed on 2024 Apr 24] [Google Scholar]


