Abstract
Background and Objectives
Polyneuropathy associated with an immunoglobulin M (IgM) monoclonal gammopathy is characterized by slowly progressive, predominantly distal sensorimotor deficits, sensory ataxia, and electrophysiologic features of demyelination. IgM antibodies against myelin-associated glycoprotein (MAG) are present in serum from most patients. Nerve damage most likely results from the concerted action of binding of anti-MAG antibodies to nerves, followed by complement activation. The interaction of anti-MAG antibodies with complement activation and their relation to clinical characteristics have not been studied in detail. We studied the correlation among anti-MAG antibody titers, complement activation, and IgM-associated polyneuropathy disease severity.
Methods
We used serum samples from 101 patients with IgM-associated polyneuropathy to assess IgM anti-MAG titers by ELISA and antibody-mediated complement deposition using both an ELISA-based system and a cell-based system of primate peripheral nerve slides. We studied correlations of complement activation with anti-MAG ELISA titers and clinical characteristics.
Results
IgM anti-MAG titers varied from negative to strongly positive. Complement deposition in the ELISA-based system correlated significantly with anti-MAG antibody titer (Spearman rho 0.80; p < 0.0001) despite large variability between serum samples with comparable anti-MAG titers. This variability was even larger in the cell-based assay, which also showed complement deposition in IgM anti-MAG negative patients, indicating the presence of autoantibodies directed against epitopes other than MAG in a subset of patients with IgM-associated polyneuropathy. Clinical characteristics did not correlate with anti-MAG titers or complement activation.
Discussion
Anti-MAG antibody titers correlate with the level of complement activation in both ELISA and cell-based systems. However, clinical characteristics of IgM-associated polyneuropathy do not or only weakly correlate with titers or the level of complement deposition. The lack of clear correlations between complement activation and clinical characteristics does, at this stage, not support the use of complement inhibitors in the treatment of IgM-associated polyneuropathy.
Introduction
Polyneuropathy associated with immunoglobulin M (IgM) monoclonal gammopathy1 is characterized by slowly progressive, predominantly distal sensorimotor deficits, sensory ataxia, and (postural) tremor2-5 and can lead to substantial disability.3,6 Lack of efficacious treatment strategies that do not pose a considerable burden to patients represents a clear unmet medical need.1,3,7,8 The IgM monoclonal protein (M protein) in patients with an associated polyneuropathy usually targets a specific component of the peripheral nerve.
In most of the patients,9 the M protein targets the myelin-associated glycoprotein (MAG).10 The antigenic region of MAG, the human natural killer 1 (HNK-1) carbohydrate epitope,11,12 is shared with other components of the myelin sheath such as myelin protein zero (P0),13 peripheral myelin protein 22,14 sulfated glucuronyl paragloboside (SGPG),15 and sulfated glucuronyl lactosaminyl paragloboside.15,16
MAG is situated in the periaxonal Schwann cell membranes and on opposing myelin membranes in noncompact myelin compartments such as the Schmidt-Lanterman incisures and the paranodal loops17 and binds to ligands on the axolemma, thus anchoring the myelin sheath to the axon. There is a large body of evidence to support the pathogenic role of anti-MAG antibodies in IgM-associated polyneuropathy. When bound to an antigen, IgM can trigger activation of the complement system, because of its multiple C1q binding sites.18 Activation of the complement cascade ultimately results in direct and indirect tissue damage through deposition of the complement membrane attack complex (MAC) and the cell-activating and chemotactic properties of complement components.19 Although systemic biomarkers of complement activation in patients with IgM anti-MAG polyneuropathy are not increased compared with healthy controls (HCs),20 IgM depositions in nerves from these patients colocalize with MAG;21,22 complement factors C1q, C3, C5, and C5-C9 (i.e., MAC);21,23-26 and pathologic changes.26,27 These changes include widening of myelin lamellae, demyelination, axonal atrophy, and decreased neurofilament spacing.26 Human monoclonal IgM anti-MAG serum induced similar morphological changes in passive transfer studies in experimental animals.28,29 Some studies found a correlation between the amount of IgM and complement deposition and the extent of myelin morphological changes.24,26,27
Strategies that reduce anti-MAG titers are successful in a subgroup of patients.8,30 The anti-MAG titer before start of treatment with rituximab correlated with treatment response, i.e., responders had higher baseline anti-MAG titers,30 while patients with low baseline anti-MAG titers (even those with high anti-SGPG titers) did not respond to rituximab. This suggests that anti-MAG and not anti-SGPG antibodies determine treatment response. This is further illustrated by a recent meta-analysis that concluded that anti-MAG titers might predict response to treatment.31
In a randomized controlled trial (RCT) with rituximab, responding patients had both a higher anti-MAG titer and more sensory impairments,30 suggesting a correlation between anti-MAG titer and sensory deficits, while other studies failed to find a clear correlation between anti-MAG titer and disease severity.3,32 The anti-MAG titer and/or the currently used clinical scales may not be sensitive enough to detect small changes and to analyze correlations between the titer and outcome. One other possible explanation is that anti-MAG titers do not properly reflect proinflammatory properties of antibodies, such as complement activation. In the light of recent developments in the treatment of polyneuropathies with complement inhibitors,19 the exploration of complement inhibitors as novel treatment options for IgM-associated polyneuropathies is warranted. Our objectives were, therefore, to study anti-MAG titers in relation to their complement-activating properties as assessed by 2 in vitro systems (one ELISA-based and the other cell-based) and clinical deficits in a large cohort of patients with IgM-associated polyneuropathy.
Methods
Patients and Controls
We enrolled 101 patients who previously gave their consent for participation in the Dutch arm of the international cohort study on IgM-associated polyneuropathy (IMAGiNe).33 We used previously documented clinical characteristics and retrieved serum samples that had been stored at the biobank of the University Medical Center Utrecht (UMCU) between August 2016 and March 2020. The number of patients with IgM M protein–associated polyneuropathy during this period determined the sample size. We obtained additional serum samples from HCs through the in-house healthy donor facility of the UMCU. Serum samples of both HCs and patients were heat-inactivated for 30 minutes at 56°C and stored in aliquots at −80°C until used. After thawing, samples were stored at 4°C.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all study participants before inclusion, and this study was approved by the Medical Ethics Assessment Committee of the UMCU (protocol number 16-177).
Clinical Data
Age at onset was defined as the age at which a patient first noticed symptoms of IgM-associated polyneuropathy. Muscle strength was assessed with the Rasch-transformed Medical Research Council (MRC) sum score of 15 muscles, with a maximum total score of 90.34 Sensation was assessed with the modified inflammatory neuropathy cause and treatment (INCAT) sensory sum score, with a maximum sum score of 66.35 Ataxia was assessed using a face/content validity preselected list of items originating from the Modified International Cooperative Ataxia Rating Scale and Scale for the Assessment and Rating of Ataxia, with a maximum sum score of 94.36,37 Disability was assessed with the Rasch-built Overall Disability Scale (iRODS) for immune-mediated peripheral neuropathies, with a maximum centile sum score of 100.38 Walking ability was assessed with the 10-meter walk test. In case of sum scores, we only used data of patients without missing data.
Nerve conduction studies were performed in 94 of 101 patients using a standardized local protocol. Measured values were available for 93 patients. We used the European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of paraproteinemic demyelinating neuropathies to determine the presence of (distal) demyelinating features.2 We compared measured compound motor action potentials and sensory nerve action potentials with lower limits of normal (LLN) and used the percentage of action potentials below LLN as a measure of axonal damage. We stratified patients for axonal damage into tertiles (0–33%, 34%–66%, and 67%–100% of action potential amplitudes below the lower limit of normal).
IgM Anti-MAG Titer Determination
We measured anti-MAG titers using the Bühlmann ELISA (Bühlmann Laboratories AG) according to the manufacturer's instructions. We assessed the optical density (OD) of ELISA plate wells at 450 nm (OD450) using a SpectraMax M3 (Molecular Devices) and used these values to calculate Bühlmann titer units (BTU) using calibrators included in the ELISA kit. We stratified patients for IgM anti-MAG positivity as follows: <1:1,000 negative; 1:1,000–1:10,000 weak positive; 1:10,000–1:70,000 positive; >70,000 strong positive.
IgM Anti-MAG–Mediated Complement Fixation by ELISA
To investigate complement activation, we adapted the Bühlmann ELISA by adding IgG/IgM-depleted serum as a source of complement after the opsonization step with patient serum samples. For each opsonization sample, a negative EDTA control was included to correct for background complement activation and complement activation was depicted as the ratio between the ODC3 and the ODEDTA as ratioC3/EDTA.
We prediluted heat-inactivated anti-MAG patient serum samples 1:10,000 in incubation buffer and incubated the samples for 1 hour at room temperature (RT). We washed microtiter plates precoated with MAG (4 times using 300 µL of cold wash buffer) and performed all subsequent washing steps using the same procedure. Next, we added 100 µL of prediluted patient serum samples to the plate and incubated the plate for 1 hour at RT while shaking (350 rpm). We used IgM/IgG-depleted complement-active serum (Pel-Freez Biologicals) as the source of complement. As an additional negative control, we supplemented the serum with a final concentration of 10 mM EDTA (Lonza). After washing, we added 100 µL of this serum (i.e., complement source), 80 times diluted in veronal buffer (VB, Lonza) with 0.1% Tween20 (Riedel de Haen), to the plate and incubated the plate for 30 minutes at 37°C. Next, we washed the samples and incubated them with 100 µL of biotin-labeled polyclonal anti-C3, C3c (MyBioSource), diluted 1:32,000 in incubation buffer for 30 minutes at 37°C, followed by a washing step and incubation with 100 µL of streptavidin-POD (Roche), and diluted 1:1,000 in incubation buffer, for 30 minutes at RT while shaking (350 rpm). Finally, we washed and incubated the samples with 100 µL of the 3,3',5,5'-tetramethylbenzidine substrate (Invitrogen) for 2 minutes. This reaction was stopped using 100 µL of HCl (ThermoFischer). We measured OD450 using a SpectraMax M3. To correct for patient-to-patient background activation, complement activation is expressed as the ratio between OD450 of C3 fixation and the OD450 of the respective negative EDTA control.
Ex Vivo Model to Study IgM Anti-MAG–Mediated Complement Activation
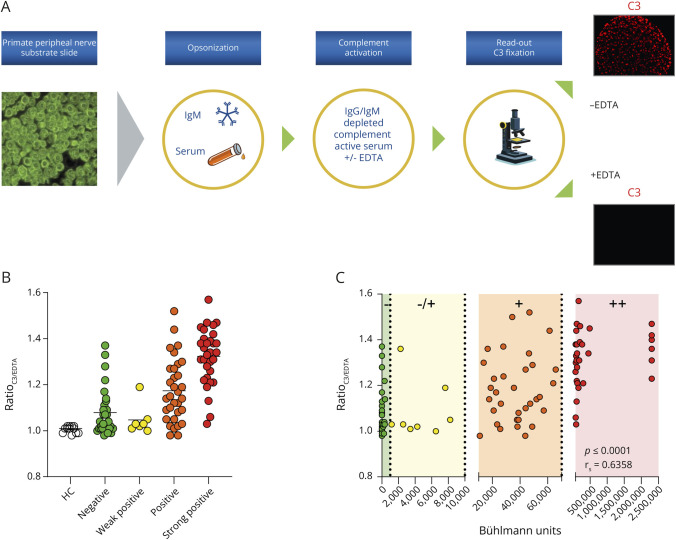
To investigate complement activation in a biologically more relevant model, we assessed C3 fixation using primate peripheral nerve slides. Again, for each patient sample, a negative EDTA control was analyzed in parallel (Figure 1A shows a graphical representation of the experimental procedure). In short, we adapted the ImmuGLo Anti-Myelin Associated Antibody immunofluorescence assay (IFA) kit (Immco Diagnostics) by introducing a serum incubation step (i.e., the addition of a complement source) after the opsonization with heat-inactivated serum of a patient with IgM-associated polyneuropathy. Primate peripheral nerve slides were adapted to RT for at least 10 minutes. Next, we added 50 µL of the heat-inactivated patient serum sample (100 times diluted in the buffered diluent) to the slide for 30 minutes of incubation at RT. Subsequently, we added 50 µL of 15% IgM/IgG-depleted complement-activate serum (diluted in VB), either or not preincubated for 15 minutes at RT with 10 mM EDTA, to the slides for 30 minutes at RT. Next, we added 50 µL of goat anti-human C3 (MyBioSource, 1:2,000 diluted in assay buffer) and Cholera Toxin Subunit B Alexa FluorTM 488 as counterstain (ThermoFischer, 1:500 diluted in assay buffer) to the slide for 30 minutes at RT. Finally, slides were incubated with 50 µL of streptavidin-allophycocyanin (eBiosciences, 1:1,000 diluted in assay buffer) for 30 minutes at RT. After staining, slides were mounted on a coverslip using 3 drops of the evenly spaced mounting medium. We performed all incubation steps in a humidity chamber, and after each of the abovementioned steps, slides were washed in phosphate-buffered saline (PBS) for 10 minutes by submerging in a Coplin jar. We then blotted the slides against tissue paper to remove excess PBS. We analyzed the slides using a Zeiss Z1 microscope with Colibri LEDs with the following settings: ×20 magnification, ∼1.75 V, 10 ms transmitted light differential interference contrast, 25% LED, 100 ms for the allophycocyanin channel. Results were measured by taking 5 pictures throughout the nerve area. For image quantification and normalization, we calculated the mean gray value (MGV) of the C3 (red) channel using ImageJ FIJI analysis software. To correct for patient-to-patient background activation, we measured an EDTA control after opsonization with the respective IgM anti-MAG patient serum. Complement activation is expressed as the ratio between the MGV of C3 fixation and the MGV of the respective negative EDTA control.
Figure 1. Cell-Based Assay to Study IgM Anti-MAG-Mediated Complement Activation.
Statistical Analysis
We used GraphPad Prism 9 for data analysis and visualization of experimental data. We used R version 4.2.2 for data analysis of clinical data. For descriptive statistics, we used medians with range and interquartile range because outcomes were not normally distributed. In cases where the specific outcome is not observed in every patient, percentages are presented as the ratio of the outcome data that is available. We compared continuous data between groups with the Mann-Whitney U test. Correlations between continuous data were analyzed using a Spearman rank correlation test. We confined analyses with compound outcome measures (INCAT sensory sum score, MRC distal sum score, and the ataxia score), to patients without missing data. Because of the exploratory character of the analysis, we did not correct p values for multiple testing.
Data Availability
Anonymized data not published within this article will be shared on request from qualified investigators and completion of data and material transfer agreements.
Results
Patient Characteristics and IgM Anti-MAG Antibody Titers
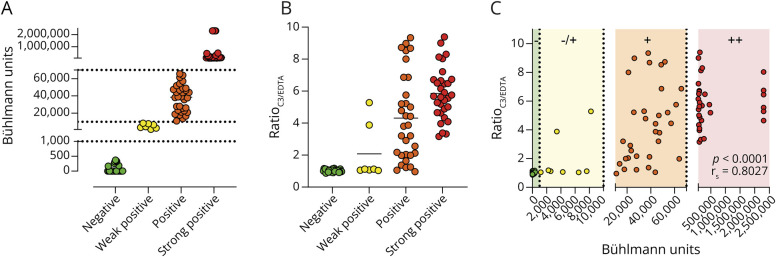
Because the IMAGiNe cohort consists of patients who fulfill the international criteria for IgM monoclonal gammopathy–associated peripheral neuropathy, with or without anti-MAG antibodies,33 we first sought to determine IgM anti-MAG antibody titers in 101 IMAGiNe patient serum samples. Titers were obtained using the Bühlmann anti-MAG ELISA and expressed as BTU. A total of 31 of 101 patients presented with BTU <1,000 (green) and were designated IgM anti-MAG negative. 7 patients were found to be weakly positive (yellow, between 1,000 and 10,000 BTU), 32 patients were positive (orange, between 10,000 and 70,000 BTU), and 31 patients were strongly positive (red, >70,000 BTU). Figure 2A presents a graphical representation of the titer units and patient distribution. All values > 70,000 were extrapolated from the calibration curve and capped at a max of 2,303,333 BTU. Clinical characteristics are summarized in Table 1.
Figure 2. ELISA-Based Essay to Study IgM Anti-MAG-Mediated Complement Activation.
Table 1.
Patient Characteristics
| Patients (n = 101) | |
| Age at onset (y) | 58 (101; 36–85; 17) |
| Age at inclusion (y) | 69 (101; 44–86; 10) |
| Disease duration at inclusion (y) | 4 (100; 0–25; 7.25) |
| Sex, male | 76 (75.2) |
| Anti-MAG antibodies (titer ≥10,000 BTU) | 63 (62.4) |
| Anti-ganglioside antibodies (GM1, GM2, GQ1b or GD1a) | 8 (8.2) |
| M-protein level ≥1 g/L | 32 (32.7) |
| IgM kappa/IgM lambda | 64 (75.3)/13 (15.3) |
| IgM kappa + IgM lambda | 8 (9.4) |
| Nerve conduction studies available | 93 (92.1) |
| Demyelination (nerve conduction studies) | 63 (67.0) |
| Previous IVIG treatment | 27 (26.7) |
| Previous rituximab treatment | 55 (54.5) |
| Previous cytostatic treatment (e.g., cyclophosphamide) | 17 (16.8) |
| Tremor | 55 (60.4) |
| Ataxia sum score | 18 (85; 0–63; 20) |
| Rasch-transformed MRC sum score | 84 (101; 34–90; 8) |
| INCAT-modified sensory sum score | 14 (73; 0–42; 10) |
| iRODS centile sum score | 69 (91; 27–100; 23) |
| 10-meter walk test | 7.8 (91; 4,4–21,0; 3,3) |
Abbreviations: BTU = Bühlmann titer units; CMAP = compound motor action potential; INCAT = inflammatory neuropathy cause and treatment; IVIG = IV immunoglobulin; LLN = lower limit of normal; MAG = myelin-associated glycoprotein; MRC = Medical Research Council; SNAP = sensory nerve action potential.
Data are median (n; minimum-maximum; interquartile range) or number (%).
Complement Activation in Relation to IgM Anti-MAG Antibody Titers
We found a clear increase in complement activation when patients were stratified according to IgM anti-MAG titer positivity. No increase in the ratioC3/EDTA was detected for the IgM anti-MAG–negative patients using the anti-MAG ELISA setup while the strongest increase was observed for the strong positive IgM anti-MAG titer group (Figure 2B). Overall, we observed a strong correlation between anti-MAG units and complement activation (rs = 0.8007, p < 0.0001 Figure 2C).
In the cell-based method, we observed no increase in complement activation after opsonization with HC serum samples. Both negative and weakly positive IgM anti-MAG patients showed an increase in the mean ratioC3/EDTA compared with 12 HC serum samples. Of interest, despite being IgM anti-MAG negative, a strong increase in complement activation was observed for selected patients, indicating that autoantibodies directed against other antigens than MAG could contribute to overall complement activation (Figure 1B). Using this cell-based method, we found even more pronounced variability in complement activation within anti-MAG titer groups, in comparison with the ELISA-based detection method. Consequently, the correlation (Spearman rank correlation test) between IgM anti-MAG titers and complement activation was weaker for the cell-based assay (rs = 0.6579, p < 0.0001) than for the ELISA-based system (rs = 0.8007, p < 0.0001) (Figure 1C).
Correlations Between Anti-MAG Titer and Clinical Characteristics
We analyzed whether anti-MAG Bühlmann ELISA titers correlated with clinical characteristics. Only the ataxia sum score showed a weak level of correlation with anti-MAG titers, although this was no longer significant when taking into account the level of axonal damage (Table 2).
Table 2.
Correlations Between Anti-MAG ELISA Titer and Outcome Measures
| Correlation with anti-MAG ELISA titer | |
| INCAT sensory sum score (n = 101) | NS (rs = 0.18, p = 0.13) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = 0.04, p = 0.80) |
| iRODS centile sum score | NS (rs = −0.06, p = 0.58) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = −0.18, p = 0.17) |
| Ataxia sum score | rs = 0.22, p = 0.04 |
| Anti-MAG titer ≥10,000 BTU (n = 63) | rs = 0.34, p = 0.02 |
| MRC sum score (Rasch-transformed) | NS (rs = −0.12, p = 0.22) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = −0.16, p = 0.21) |
| 10-meter walk test | NS (rs = 0.15, p = 0.17) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = −0.05, p = 0.70) |
Abbreviations: BTU = Bühlmann titer units; MAG = myelin-associated glycoprotein; NS = not significant.
Analyses were performed with Spearman rank correlation tests.
Correlations Between Complement Deposition and Clinical Characteristics
Because of the large variation in complement-activating properties of patients with IgM anti-MAG polyneuropathy with similar anti-MAG ELISA titers, we evaluated correlations of complement deposition with clinical characteristics (Table 3). There were no significant correlations. A subanalysis with only distal musculature did not alter the correlation between complement deposition and muscle strength.
Table 3.
Correlations Between C3/EDTA Ratio (IFA and ELISA) and Outcome Measures
| Outcome | Correlation with C3/EDTA ratio (IFA) | Correlation with C3/EDTA ratio (ELISA) |
| INCAT sensory sum score (n = 101) | NS (rs = 0.04, p = 0.71) | NS (rs = 0.12, p = 0.29) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = −0.03, p = 0.81) | NS (rs = 0.01, p = 0.95) |
| Anti-MAG titer <10,000 BTU (n = 38) | NS (rs = −0.15, p = 0.48) | NS (rs = 0.07, p = 0.72) |
| Anti-MAG titer ≥10,000 BTU and distal demyelination (n = 40) | NS (rs = −0.04, p = 0.82) | NS (rs = 0.07, p = 0.70) |
| Anti-MAG titer ≥10,000 BTU and tremor (n = 31) | NS (rs = −0.18, p = 0.41) | NS (rs = −0.02, p = 0.91) |
| Anti-MAG titer ≥10,000 BTU and no tremor (n = 25) | NS (rs = −0.10, p = 0.69) | NS (rs = −0.22, p = 0.37) |
| Anti-MAG titer ≥10,000 BTU and response after IVIG (n = 7) | NS (rs = −0.43, p = 0.42) | NS (rs = −0.71, p = 0.14) |
| Anti-MAG titer ≥10,000 BTU and no response after IVIG (n = 12) | NS (rs = −0.08, p = 0.83) | NS (rs = −0.09, p = 0.81) |
| Anti-MAG titer ≥10,000 BTU and response after rituximab (n = 16) | NS (rs = 0.39, p = 0.24) | NS (rs = −0.01, p = 0.98) |
| Anti-MAG titer ≥10,000 BTU and no response after rituximab (n = 24) | NS (rs = −0.03, p = 0.92) | NS (rs = −0.18, p = 0.50) |
| iRODS centile sum score | NS (rs = −0.02, p = 0.84) | NS (rs = −0.01, p = 0.89) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = −0.06, p = 0.68) | NS (rs = −0.08, p = 0.56) |
| Anti-MAG titer <10,000 BTU (n = 38) | NS (rs = −0.16, p = 0.36) | NS (rs = 0.07, p = 0.68) |
| Anti-MAG titer ≥10,000 BTU and distal demyelination (n = 40) | NS (rs = −0.08, p = 0.65) | NS (rs = −0.01, p = 0.94) |
| Anti-MAG titer ≥10,000 BTU and tremor (n = 31) | NS (rs = −0.15, p = 0.44) | NS (rs = 0.16, p = 0.40) |
| Anti-MAG titer ≥10,000 BTU and no tremor (n = 25) | NS (rs = 0.20, p = 0.38) | NS (rs = −0.26, p = 0.25) |
| Anti-MAG titer ≥10,000 BTU and response after IVIG (n = 7) | NS (rs = −0.11, p = 0.81) | NS (rs = 0.24, p = 0.61) |
| Anti-MAG titer ≥10,000 BTU and no response after IVIG (n = 12) | NS (rs = −0.19, p = 0.56) | NS (rs = 0.14, p = 0.67) |
| Anti-MAG titer ≥10,000 BTU and response after rituximab (n = 16) | NS (rs = 0.01, p = 0.97) | NS (rs = −0.06, p = 0.83) |
| Anti-MAG titer ≥10,000 BTU and no response after rituximab (n = 24) | NS (rs = −0.02, p = 0.91) | NS (rs = −0.01, p = 0.97) |
| Ataxia sum score | NS (rs = 0.10, p = 0.35) | NS (rs = 0.20, p = 0.07) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = 0.15, p = 0.28) | NS (rs = 0.21, p = 0.14) |
| Anti-MAG titer <10,000 BTU (n = 38) | NS (rs = 0.07, p = 0.69) | NS (rs = 0.21, p = 0.14) |
| Anti-MAG titer ≥10,000 BTU and distal demyelination (n = 40) | NS (rs = 0.13, p = 0.47) | NS (rs = 0.27, p = 0.12) |
| Anti-MAG titer ≥10,000 BTU and tremor (n = 31) | NS (rs = 0.14, p = 0.49) | NS (rs = 0.07, p = 0.73) |
| Anti-MAG titer ≥10,000 BTU and no tremor (n = 25) | NS (rs = −0.15, p = 0.54) | NS (rs = 0.20, p = 0.39) |
| Anti-MAG titer ≥10,000 BTU and response after IVIG (n = 7) | NS (rs = −0.16, p = 0.76) | NS (rs = −0.38, p = 0.46) |
| Anti-MAG titer ≥10,000 BTU and no response after IVIG (n = 12) | NS (rs = 0.54, p = 0.13) | NS (rs = −0.07, p = 0.88) |
| Anti-MAG titer ≥10,000 BTU and response after rituximab (n = 16) | NS (rs = 0.01, p = 0.97) | NS (rs = 0.62, p = 0.03) |
| Anti-MAG titer ≥10,000 BTU and no response after rituximab (n = 24) | NS (rs = 0.42, p = 0.07) | NS (rs = −0.17, p = 0.49) |
| MRC sum score (Rasch-transformed) | NS (rs = −0.09, p = 0.36) | NS (rs = −0.05, p = 0.60) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = −0.14, p = 0.26) | NS (rs = −0.08, p = 0.55) |
| Anti-MAG titer <10,000 BTU (n = 38) | NS (rs = −0.05, p = 0.76) | NS (rs = 0.10, p = 0.53) |
| Anti-MAG titer ≥10,000 BTU and distal demyelination (n = 40) | NS (rs = −0.14, p = 0.41) | NS (rs = −0.15, p = 0.35) |
| Anti-MAG titer ≥10,000 BTU and tremor (n = 31) | NS (rs = −0.25, p = 0.17) | NS (rs = 0.11, p = 0.56) |
| Anti-MAG titer ≥10,000 BTU and no tremor (n = 25) | NS (rs = 0.12, p = 0.58) | NS (rs = −0.17, p = 0.42) |
| Anti-MAG titer ≥10,000 BTU and response after IVIG (n = 7) | NS (rs = −0.13, p = 0.78) | NS (rs = 0.56, p = 0.19) |
| Anti-MAG titer ≥10,000 BTU and no response after IVIG (n = 12) | NS (rs = 0.02, p = 0.96) | NS (rs = −0.30, p = 0.34) |
| Anti-MAG titer ≥10,000 BTU and response after rituximab (n = 16) | NS (rs = −0.36, p = 0.17) | NS (rs = −0.39, p = 0.13) |
| Anti-MAG titer ≥10,000 BTU and no response after rituximab (n = 24) | NS (rs = 0.04, p = 0.85) | NS (rs = −0.02, p = 0.93) |
| 10-meter walk test | NS (rs = 0.04, p = 0.69) | NS (rs = 0.14, p = 0.17) |
| Anti-MAG titer ≥10,000 BTU (n = 63) | NS (rs = −0.05, p = 0.70) | NS (rs = 0.05, p = 0.69) |
| Anti-MAG titer <10,000 BTU (n = 38) | NS (rs = 0.02, p = 0.92) | NS (rs = 0.24, p = 0.18) |
| Anti-MAG titer ≥10,000 BTU and distal demyelination (n = 40) | NS (rs = 0.00, p = 0.99) | NS (rs = 0.00, p = 0.99) |
| Anti-MAG titer ≥10,000 BTU and tremor (n = 31) | NS (rs = −0.11, p = 0.57) | NS (rs = 0.03, p = 0.89) |
| Anti-MAG titer ≥10,000 BTU and no tremor (n = 25) | NS (rs = −0.14, p = 0.19) | NS (rs = 0.01, p = 0.98) |
| Anti-MAG titer ≥10,000 BTU and response after IVIG (n = 7) | NS (rs = 0.06, p = 0.89) | NS (rs = −0.09, p = 0.85) |
| Anti-MAG titer ≥10,000 BTU and no response after IVIG (n = 12) | NS (rs = 0.48, p = 0.16) | NS (rs = 0.28, p = 0.43) |
| Anti-MAG titer ≥10,000 BTU and response after rituximab (n = 16) | NS (rs = 0.30, p = 0.26) | NS (rs = 0.33, p = 0.21) |
| Anti-MAG titer ≥10,000 BTU and no response after rituximab (n = 24) | NS (rs = −0.18, p = 0.44) | NS (rs = −0.08, p = 0.75) |
Abbreviations: BTU = Bühlmann titer units; IFA = immunofluorescence assay; MAG = myelin-associated glycoprotein; NS = not significant.
Analyses were performed with Spearman rank correlation tests.
We did not find correlations between complement deposition and clinical outcomes of the 40 patients with anti-MAG antibodies (titer ≥10,000 BTU) and nerve conduction studies that showed predominantly distal demyelination, the presence of postural tremor, or the response to treatment with IVIg or rituximab (Table 3). The levels of axonal damage in anti-MAG patients did not influence these correlations.
The group of 13 patients without anti-MAG antibodies (BTU <10,000 BTU) but with complement deposition (IFA C3/EDTA ratio ≥1.1) did not differ in clinical outcomes from the group of 9 patients with anti-MAG antibodies (BTU ≥10,000 BTU) but without complement deposition (IFA C3/EDTA ratio <1.1), as summarized in Table 4.
Table 4.
Mann-Whitney U Test Between Anti-MAGneg/IFAC3/EDTA ≥1.1 (n = 13) and Anti-MAGpos/IFAC3/EDTA <1.1 (n = 9)
| Outcome | Mann-Whitney U test between anti-MAGneg/IFAC3/EDTA ≥1.1 and anti-MAGpos/IFAC3/EDTA <1.1 |
| INCAT sensory sum score | p = 0.86 |
| iRODS centile sum score | p = 0.99 |
| Ataxia sum score | p = 0.49 |
| MRC sum score (Rasch-transformed) | p = 0.92 |
| 10-meter walk test | p = 0.50 |
Abbreviations: IFA = immunofluorescence assay; INCAT = inflammatory neuropathy cause and treatment; MAG = myelin-associated glycoprotein; MRC = Medical Research Council.
Discussion
In this study, we further investigated the immunopathogenesis of IgM-associated polyneuropathy, in particular the relation between MAG antibody titers and complement deposition using 2 in vitro assays in a large patient cohort. Moreover, we explored the correlation between these immunologic tests and clinical characteristics. Our findings demonstrate that anti-MAG titers and complement activation correlate in both in vitro systems, but with large variability among patients with similar antibody titers. This variability was greater in the cell-based compared with the ELISA-based system, which may reflect the higher biological complexity of the cell-based system, including the expression of antigens other than MAG, but relevant in IgM-associated polyneuropathy. Other antigens sharing the HNK-1 epitope with MAG, such as SGPG, may serve as targets for complement-activating antibodies in patients without detectable MAG antibodies but with high complement activation.
The colocalization of MAG, IgM, and complement in peripheral nerves of patients with IgM-associated polyneuropathy, along with the association of antibody-complement deposits and the extent of morphological changes, strongly suggests a pathogenesis where antibody-complement interaction is involved. Consequently, complement-activating properties of anti-MAG IgM, rather than the titer level, may be most important in the pathology of IgM-associated polyneuropathy. However, unlike in multifocal motor neuropathy (MMN), where IgM anti-GM1 titers and their complement-activating properties correlate with patient weakness,39,40 we found no correlations between complement activation and clinical characteristics in IgM-associated polyneuropathy. This suggests that complement activation may not directly influence clinical characteristics in IgM-associated polyneuropathy. The lack of convincing long-term effects from IV immunoglobulin treatment in IgM anti-MAG polyneuropathy,41 which inhibits complement,42 compared with its effectiveness in MMN,43 further supports this hypothesis. The absence of correlations between complement activation and clinical characteristics may also be attributed to other factors: the cross-sectional study design, the variable presence of concomitant axonal damage, or the potential insensitivity of currently used clinical scales for IgM-associated polyneuropathy.
The anti-MAG titer may correlate with the level of sensory impairment in a prospective controlled study,30 although this correlation was not observed in another study.32 Correlations between anti-MAG and other clinical characteristics seem to be absent,32 with no clear association between titer level and disease severity in retrospective studies.3,4 In our study, we also found no significant correlations between anti-MAG titer levels and clinical characteristics, with the possible exception of the ataxia sum score. One possible explanation for these conflicting results is that current anti-MAG ELISAs may identify a range of antibodies predominated by ones that do not contribute to IgM-associated polyneuropathy pathogenesis. Antibodies specifically targeting the HNK-1 epitope might be more relevant because HNK-1 antibody titers have shown correlations with multiple clinical characteristics in a study with a small patient sample.32 This suggests that a more sensitive antibody marker than anti-MAG is needed to distinguish relevant patient subgroups. Given the strong correlation between complement activation and anti-MAG titer and the absence of correlations between anti-MAG titer and clinical characteristics in our study, the lack of correlation between complement activation and clinical characteristics might also stem from the uncertain correlation between anti-MAG titer and clinical characteristics. Therefore, a follow-up study investigating correlations between anti-HNK1 and other epitopes such as anti-SGPG is needed to determine whether similar results with a lack of correlation are observed with these antibodies.
Our results do not support the application of anticomplement treatments in IgM–associated polyneuropathies. Although the lack of correlations might stem from inadequate sensitivity of clinical scales, RCTs with rituximab30,44 did demonstrate a treatment effect on some clinical scales. While B-cell targeting therapies have demonstrated effectiveness in a subset of IgM anti-MAG patients, there is no information on the potential effectiveness of complement inhibitors in IgM-associated polyneuropathies. Provided with the immunologic evidence of complement involvement, this knowledge gap should be bridged by prospective treatment data. A clinical trial comparing the efficacy of rituximab and complement inhibition in decreasing IgM anti-MAG related disability would probably help to fill this gap.
We acknowledge that this study has limitations, primarily its cross-sectional rather than longitudinal design combined with a wide variation in disease duration (median of 4 years, ranging up to 25 years). However, this is an inherent challenge in studying rare diseases. Although subgroup analyses with different levels of axonal damage had no influence on the correlations, electrophysiologic data were limited with only cross-sectional data availability and heterogeneous durations between nerve conduction studies and serum sampling. The progressive nerve damage and associated concomitant axonal loss could complicate or even preclude the detection of meaningful correlations. The strengths of this study include its relatively large sample size, systematic and extensive patient characterization, and detailed immunologic analyses.
Anti-MAG antibody titers correlate with the level of complement deposition in 2 in vitro systems, but with large variability among patients. We found no significant correlations between complement deposition and outcome measures. We also found no significant correlations between anti-MAG titer and outcome measures. Our results do not support the application of complement inhibitors in the treatment of IgM-associated polyneuropathy. With immunologic evidence of complement involvement, clinical applications of complement inhibitors should be investigated prospectively, ideally with trial designs to compare efficacy with B-cell targeting therapies.
Acknowledgment
The consortium recognizes and thanks the Foundation for Peripheral Neuropathy (FPN) and Louis T. Mazawey for their valuable support and contributions to the study. In addition, the authors thank the study participants for volunteering their medical information for scientific use and all involved physicians and support personnel at the consortia sites for making this research possible.
Glossary
- BTU
Bühlmann titer units
- HC
healthy control
- HNK-1
human natural killer 1
- IFA
immunofluorescence assay
- IgM
immunoglobulin M
- INCAT
inflammatory neuropathy cause and treatment
- LLN
lower limits of normal
- MAC
membrane attack complex
- MAG
myelin-associated glycoprotein
- MGV
mean gray value
- MMN
multifocal motor neuropathy
- MRC
Medical Research Council
- PBS
phosphate-buffered saline
- RCT
randomized controlled trial
- RT
room temperature
- SGPG
sulfated glucuronyl paragloboside
- UMCU
University Medical Center Utrecht
- VB
veronal buffer
Appendix 1. Authors
| Name | Location | Contribution |
| Johannes P.M. van de Mortel, MD | Department of Neurology and Neurosurgery, UMC Utrecht Brain Center, University Medical Center Utrecht, The Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Kevin Budding, PhD | Center for Translational Immunology, University Medical Center Utrecht, The Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Kim Dijkxhoorn, BSc | Center for Translational Immunology, University Medical Center Utrecht, The Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Monique C. Minnema, MD, PhD | Department of Hematology, University Medical Center Utrecht, Utrecht University, The Netherlands | Drafting/revision of the manuscript for content, including medical writing for content |
| Alexander F.J.E. Vrancken, MD, PhD | Department of Neurology and Neurosurgery, UMC Utrecht Brain Center, University Medical Center Utrecht, The Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Nicolette C. Notermans, MD, PhD | Department of Neurology and Neurosurgery, UMC Utrecht Brain Center, University Medical Center Utrecht, The Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| W. Ludo van der Pol, MD, PhD | Department of Neurology and Neurosurgery, UMC Utrecht Brain Center, University Medical Center Utrecht, The Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Appendix 2. Coinvestigators
| Coinvestigators are listed at Neurology.org/NN. |
Contributor Information
Collaborators: Shahram Attarian, Ivana Basta, Peter Y.K. van den Bergh, Mariangela Bianco, Chiara Briani, Cécile Cauquil, Aisling S. Carr, David R. Cornblath, Claudia Cutellè, Shirley D'Sa, Emilien Delmont, Perry T.C. van Doormaal, Chris Doughty, Nicolas Dubuisson, Andoni EchanizLaguna, Bakri H. Elsheikh, Catharina G. Faber, H. Stephan Goedee, Tatiana Hamadeh, Thomas Harbo, Ahmet Hoke, Lionel Karlin, Stephen Keddie, Mohammad Khoshnoodi, Céline Labeyrie, Michael P. Lunn, Manuele Marasca, Devan Mair, Wilson Marques, Jr, Ingemar S.J. Merkies, Hélène Merle, Osvaldo J.M. Nascimento, Eduardo NobileOrazio, Aleksa Palibrk, Elba Pascual-Goñi, Stojan Peric, Luis Querol, Hans-Werner Rausch, Alessandro Salvalaggio, Lucas Schirmer, Amro M. Stino, Juliette Svahn, Clara Tejada, and Simone Thomas
Study Funding
The authors report no targeted funding.
Disclosure
J. van de Mortel reports no disclosures relevant to the manuscript; K. Budding and K. Dijkxhoorn are employees of the UMC Utrecht on a research collaboration agreement with argenx BVBA. M. Minnema, A. Vrancken, and N. Notermans report no disclosures relevant to the manuscript; W. van der Pol serves on the scientific advisory board for SMA Europe, provides ad hoc consultancy for argenx, Biogen, Roche, and Novartis, is the local PI of the ARDA and ARDA + trials for MMN, and receives research support from the Prinses Beatrix Spierfonds, Vriendenloterij, and Stichting Spieren voor Spieren. Go to Neurology.org/NN for full disclosures.
References
- 1.D'Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol. 2017;176(5):728-742. doi: 10.1111/bjh.14492 [DOI] [PubMed] [Google Scholar]
- 2.Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of paraproteinemic demyelinating neuropathies. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15(3):185-195. doi: 10.1111/j.1529-8027.2010.00278.x [DOI] [PubMed] [Google Scholar]
- 3.Svahn J, Petiot P, Antoine JC, et al.; Francophone anti-MAG cohort Group. Anti-MAG antibodies in 202 patients: clinicopathological and therapeutic features. J Neurol Neurosurg Psychiatry. 2018;89(5):499-505. doi: 10.1136/jnnp-2017-316715 [DOI] [PubMed] [Google Scholar]
- 4.Magy L, Kaboré R, Mathis S, et al. Heterogeneity of polyneuropathy associated with anti-MAG antibodies. J Immunol Res. 2015;2015:450391. doi: 10.1155/2015/450391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardel B, Molinier-Frenkel V, Le Bras F, et al. Revisiting the spectrum of IgM-related neuropathies in a large cohort of IgM monoclonal gammopathy. J Neurol. 2022;269(9):4955-4960. doi: 10.1007/s00415-022-11139-2 [DOI] [PubMed] [Google Scholar]
- 6.Nobile-Orazio E, Meucci N, Baldini L, Di Troia A, Scarlato G. Long-term prognosis of neuropathy associated with anti-MAG IgM M-proteins and its relationship to immune therapies. Brain. 2000;123(pt 4):710-717. doi: 10.1093/brain/123.4.710 [DOI] [PubMed] [Google Scholar]
- 7.Niermeijer JM, Eurelings M, Lokhorst HL, et al. Rituximab for polyneuropathy with IgM monoclonal gammopathy. J Neurol Neurosurg Psychiatry. 2009;80(9):1036-1039. doi: 10.1136/jnnp.2008.155325 [DOI] [PubMed] [Google Scholar]
- 8.Lunn MP, Nobile-Orazio E. Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies. Cochrane Database Syst Rev. 2016;10(10):Cd002827. doi: 10.1002/14651858.CD002827.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuijf ML, Eurelings M, Tio-Gillen AP, et al. Detection of anti-MAG antibodies in polyneuropathy associated with IgM monoclonal gammopathy. Neurology. 2009;73(9):688-695. doi: 10.1212/WNL.0b013e3181b59a80 [DOI] [PubMed] [Google Scholar]
- 10.Latov N, Sherman WH, Nemni R, et al. Plasma-cell dyscrasia and peripheral neuropathy with a monoclonal antibody to peripheral-nerve myelin. N Engl J Med. 1980;303(11):618-621. doi: 10.1056/NEJM198009113031105 [DOI] [PubMed] [Google Scholar]
- 11.Ilyas AA, Quarles RH, MacIntosh TD, et al. IgM in a human neuropathy related to paraproteinemia binds to a carbohydrate determinant in the myelin-associated glycoprotein and to a ganglioside. Proc Natl Acad Sci U S A. 1984;81(4):1225-1229. doi: 10.1073/pnas.81.4.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGarry RC, Helfand SL, Quarles RH, Roder JC. Recognition of myelin-associated glycoprotein by the monoclonal antibody HNK-1. Nature. 1983;306(5941):376-378. doi: 10.1038/306376a0 [DOI] [PubMed] [Google Scholar]
- 13.Bollensen E, Steck AJ, Schachner M. Reactivity with the peripheral myelin glycoprotein P0 in serum from patients with monoclonal IgM gammopathy and polyneuropathy. Neurology. 1988;38(8):1266-1270. doi: 10.1212/wnl.38.8.1266 [DOI] [PubMed] [Google Scholar]
- 14.Snipes GJ, Suter U, Shooter EM. Human peripheral myelin protein-22 carries the L2/HNK-1 carbohydrate adhesion epitope. J Neurochem. 1993;61(5):1961-1964. doi: 10.1111/j.1471-4159.1993.tb09840.x [DOI] [PubMed] [Google Scholar]
- 15.Ilyas AA, Dalakas MC, Brady RO, Quarles RH. Sulfated glucuronyl glycolipids reacting with anti-myelin-associated glycoprotein monoclonal antibodies including IgM paraproteins in neuropathy: species distribution and partial characterization of epitopes. Brain Res. 1986;385(1):1-9. doi: 10.1016/0006-8993(86)91540-4 [DOI] [PubMed] [Google Scholar]
- 16.Ariga T, Kohriyama T, Freddo L, et al. Characterization of sulfated glucuronic acid containing glycolipids reacting with IgM M-proteins in patients with neuropathy. J Biol Chem. 1987;262(2):848-853. doi: 10.1016/s0021-9258(19)75864-5 [DOI] [PubMed] [Google Scholar]
- 17.Trapp BD, Quarles RH. Presence of the myelin-associated glycoprotein correlates with alterations in the periodicity of peripheral myelin. J Cell Biol. 1982;92(3):877-882. doi: 10.1083/jcb.92.3.877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system Part I—molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalakas MC, Alexopoulos H, Spaeth PJ. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol. 2020;16(11):601-617. doi: 10.1038/s41582-020-0400-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaador K, Wieske L, Koel-Simmelink MJA, et al. Serum neurofilament light chain, contactin-1 and complement activation in anti-MAG IgM paraprotein-related peripheral neuropathy. J Neurol. 2022;269(7):3700-3705. doi: 10.1007/s00415-022-10993-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lombardi R, Erne B, Lauria G, et al. IgM deposits on skin nerves in anti-myelin-associated glycoprotein neuropathy. Ann Neurol. 2005;57(2):180-187. doi: 10.1002/ana.20364 [DOI] [PubMed] [Google Scholar]
- 22.Gabriel J-M, Erne B, Bernasconi L, et al. Confocal microscopic localization of anti-myelin-associated glycoprotein autoantibodies in a patient with peripheral neuropathy initially lacking a detectable IgM gammopathy. Acta Neuropathologica. 1998;95(5):540-546. doi: 10.1007/s004010050835 [DOI] [PubMed] [Google Scholar]
- 23.Takatsu M, Hays AP, Latov N, et al. Immunofluorescence study of patients with neuropathy and IgM M proteins. Ann Neurol. 1985;18(2):173-181. doi: 10.1002/ana.410180203 [DOI] [PubMed] [Google Scholar]
- 24.Monaco S, Bonetti B, Ferrari S, et al. Complement-mediated demyelination in patients with IgM monoclonal gammopathy and polyneuropathy. N Engl J Med. 1990;322(10):649-652. doi: 10.1056/NEJM199003083221002 [DOI] [PubMed] [Google Scholar]
- 25.Hays AP, Lee SSL, Latov N. Immune reactive C3d on the surface of myelin sheaths in neuropathy. J Neuroimmunol. 1988;18(3):231-244. doi: 10.1016/0165-5728(88)90101-4 [DOI] [PubMed] [Google Scholar]
- 26.Kawagashira Y, Koike H, Tomita M, et al. Morphological progression of myelin abnormalities in IgM-monoclonal gammopathy of undetermined significance anti-myelin-associated glycoprotein neuropathy. J Neuropathol Exp Neurol. 2010;69(11):1143-1157. doi: 10.1097/NEN.0b013e3181fa44af [DOI] [PubMed] [Google Scholar]
- 27.Ritz MF, Erne B, Ferracin F, Vital A, Vital C, Steck AJ. Anti-MAG IgM penetration into myelinated fibers correlates with the extent of myelin widening. Muscle Nerve. 1999;22(8):1030-1037. doi: [DOI] [PubMed] [Google Scholar]
- 28.Tatum AH. Experimental paraprotein neuropathy, demyelination by passive transfer of human IgM anti-myelin-associated glycoprotein. Ann Neurol. 1993;33(5):502-506. doi: 10.1002/ana.410330514 [DOI] [PubMed] [Google Scholar]
- 29.Willison HJ, Trapp BD, Bacher JD, Dalakas MC, Griffin JW, Quarles RH. Demyelination induced by intraneural injection of human antimyelin-associated glycoprotein antibodies. Muscle Nerve. 1988;11(11):1169-1176. doi: 10.1002/mus.880111111 [DOI] [PubMed] [Google Scholar]
- 30.Dalakas MC, Rakocevic G, Salajegheh M, et al. Placebo-controlled trial of rituximab in IgM anti–myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009;65(3):286-293. doi: 10.1002/ana.21577 [DOI] [PubMed] [Google Scholar]
- 31.Hänggi P, Aliu B, Martin K, Herrendorff R, Steck AJ. Decrease in serum anti-MAG autoantibodies is associated with therapy response in patients with anti-MAG neuropathy: retrospective study. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1109. doi: 10.1212/NXI.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delmont E, Attarian S, Antoine JC, et al. Relevance of anti-HNK1 antibodies in the management of anti-MAG neuropathies. J Neurol. 2019;266(8):1973-1979. doi: 10.1007/s00415-019-09367-0 [DOI] [PubMed] [Google Scholar]
- 33.Hamadeh T, van Doormaal PTC, Pruppers MHJ, et al.; IMAGiNe Consortium. IgM anti-MAG± peripheral neuropathy (IMAGiNe) study protocol: an international, observational, prospective registry of patients with IgM M-protein peripheral neuropathies. J Peripher Nerv Syst. 2023;28(2):269-275. doi: 10.1111/jns.12547 [DOI] [PubMed] [Google Scholar]
- 34.Vanhoutte EK, Faber CG, van Nes SI, et al.; PeriNomS Study Group. Modifying the Medical Research Council grading system through Rasch analyses. Brain. 2012;135(pt 5):1639-1649. doi: 10.1093/brain/awr318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Draak TH, Vanhoutte EK, van Nes SI, et al.; PeriNomS Study Group. Comparing the NIS vs. MRC and INCAT sensory scale through Rasch analyses. J Peripher Nerv Syst. 2015;20(3):277-288. doi: 10.1111/jns.12127 [DOI] [PubMed] [Google Scholar]
- 36.Schmahmann JD, Gardner R, MacMore J, Vangel MG. Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord. 2009;24(12):1820-1828. doi. 10.1002/mds.22681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717-1720. doi: 10.1212/01.wnl.0000219042.60538.92 [DOI] [PubMed] [Google Scholar]
- 38.van Nes SI, Vanhoutte EK, van Doorn PA, et al. Rasch-built overall disability scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology. 2011;76(4):337-345. doi: 10.1212/WNL.0b013e318208824b [DOI] [PubMed] [Google Scholar]
- 39.Cats EA, Jacobs BC, Yuki N, et al. Multifocal motor neuropathy: association of anti-GM1 IgM antibodies with clinical features. Neurology. 2010;75(22):1961-1967. doi: 10.1212/WNL.0b013e3181ff94c2 [DOI] [PubMed] [Google Scholar]
- 40.Vlam L, Cats EA, Harschnitz O, et al. Complement activity is associated with disease severity in multifocal motor neuropathy. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e119. doi: 10.1212/NXI.0000000000000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalakas MC, Quarles RH, Farrer RG, et al. A controlled study of intravenous immunoglobulin in demyelinating neuropathy with IgM gammopathy. Ann Neurol. 1996;40(5):792-795. doi: 10.1002/ana.410400516 [DOI] [PubMed] [Google Scholar]
- 42.Basta M, Dalakas MC. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest. 1994;94(5):1729-1735. doi: 10.1172/JCI117520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn AF, Beydoun SR, Lawson V, et al.; IVIG in MMN Study Team. A controlled trial of intravenous immunoglobulin in multifocal motor neuropathy. J Peripher Nerv Syst. 2013;18(4):321-330. doi: 10.1111/jns5.12046 [DOI] [PubMed] [Google Scholar]
- 44.Léger JM, Viala K, Nicolas G, et al. ; RIMAG Study Group France and Switzerland. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein neuropathy. Neurology. 2013;80(24):2217-2225. doi: 10.1212/WNL.0b013e318296e92b [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be shared on request from qualified investigators and completion of data and material transfer agreements.