Abstract
Clear cell renal cell carcinoma (ccRCC) is a challenging malignancy characterized by intricate biology and clinical characteristics. Despite advancements in treatment strategies, the molecular mechanisms underlying ccRCC initiation, progression, and therapeutic resistance remain elusive. Inflammasomes, multi-protein complexes involved in innate immunity and inflammation, have emerged as potential regulators in cancers. However, their involvement and mechanisms in ccRCC remain poorly understood. In this study, we conducted a systematic investigation into the expression patterns and clinical significance of inflammasome complexes in ccRCC. We found the perturbation of inflammasome complexes genes was related to patient’s prognosis and other clinical characteristics. By developing an Inflammasome Complexes (IFC) score and identifying IFC subtypes with distinct clinical characteristics and oncogenic roles, our study suggested that inflammasome activation could impact tumorigenesis and modulate the tumor immune landscape, particularly its positive correlations with immunosuppressive macrophages. Furthermore, our study revealed the potential of inflammasome complex genes as predictive markers for patient responses to various anti-tumor drugs, including Osimertinib, Ulixertinib, Telomerase Inhibitor IX, and GSK2578215A. These findings have significant clinical implications and offer opportunities for guiding treatment strategies and improving patient outcomes of ccRCC.
Keywords: clear cell renal cell carcinoma, inflammasome complexes, prognosis, immune microenvironment, cancer therapy
1. Introduction
Clear cell renal cell carcinoma (ccRCC) is one of the most prevalent malignant renal tumors, posing a significant challenge due to its intricate biology and clinical characteristics [1,2]. Given its complex nature and global clinical impact, ccRCC has become a critical focal point for researchers and clinicians [3]. The ccRCC exhibits distinct histological traits that underlie its propensity for high metastatic potential, aggressiveness, and inherent resistance to conventional therapeutic interventions [4,5]. Despite the advancement of clinical treatment strategies, a comprehensive understanding of the precise molecular mechanisms governing ccRCC initiation, progression, and the development of therapeutic resistance remains elusive.
The inflammasome, a multifaceted multi-protein complex [6], stands as a key player in the coordination of inflammation and innate immunity, serving as a vital component in host defense against pathogenic microorganisms. Upon activation, these complexes drive the maturation and subsequent secretion of pro-inflammatory cytokines [7,8]. This activation process not only facilitates a robust immune response but also contributes to the resolution of infections [9]. Intriguingly, inflammasome complexes extend beyond immune cells and are detected in various non-immune cell types, including cancer cells [10,11]. Recent years have unveiled compelling evidence showcasing the connection between inflammasome signaling dysregulation and the genesis of multiple malignant tumors [12,13]. However, the involvement and mechanism of the inflammasome in clear cell renal cell carcinoma remains unclear.
Tumor immune microenvironment (TIME) has gained popularity recently as a critical factor influencing cancer progression and treatment response [14]. In this complex environment, inflammatory complexes exert various significant effects on the TIME [15,16]. The activation of these inflammasome complexes within tumor cells stimulates the release of pro-inflammatory cytokines and manipulates the modulation of the immune landscape, thus having a widespread impact on several aspects of tumorigenesis [17,18]. The efficacy of immunotherapy has achieved remarkable success across certain cancers [19,20]. Inflammasome complex participation is a particularly interesting topic in the realm of cancer therapies [21]. Moreover, research efforts have focused on the interplay between inflammatory signaling and the response to chemotherapy and target therapy [16]. Certain anti-tumor drugs have demonstrated the ability to incite inflammasome activation within tumor cells [22]. This activation of the inflammasome might subsequently increase tumor susceptibility to specific drugs. Elucidating the mechanisms by which the inflammasome complex regulates the interaction between the tumor and immune microenvironment, as well as any possible implications for therapeutic responses, is critical to the development of effective treatments for ccRCC.
In this study, we provided a comprehensive analysis of the expression patterns and clinical implications of inflammasome complexes in ccRCC. By generating an Inflammasome Complex (IFC) score and identifying IFC subtypes, we gained insights into the immune microenvironment of ccRCC, particularly its associations with immunosuppressive macrophages. Furthermore, our findings revealed the potential of IFC genes as predictive markers for patients’ responses to immunotherapy, chemotherapy, and target-therapy. These results helped to clarify the role of inflammasome complexes in ccRCC, and have the potential to direct therapeutic approaches and enhance patient outcomes of ccRCC.
2. Materials and methods
2.1. Data collection and integration
A total of 15 IFC genes were collected from a previous study [23], including NLRP1, NLRP3, CASP4, CASP5, NLRC5, NLRP6, NLRP12, NLRP7, NAIP, NLRC4, AIM2, IFI16, MEFV, NLRP2, and PYCARD. FPKM-UQ normalized RNA-Seq expression profile of The Cancer Genome Atlas (TCGA) kidney renal clear cell carcinoma patients was downloaded from UCSC Xena (https://xenabrowser.net/) platform [24], including 535 tumorous samples and 72 adjacent non-tumorous tissues. The expression value of genes was log-transformed for further analysis. Clinical information of patients, including age, gender, overall survival (OS), progression-free interval (PFI), histological grade, and pathological stages, was also obtained from the Xena platform.
2.2. Immunohistochemistry
In order to evaluate the expression of inflammatory complex in ccRCC, we selected NLRP family members, NLRP1, NLRP3, and NLRP, for experiments, and detected the expression of these three proteins in ccRCC and adjacent tissues. A total of 30 patients with ccRCC were collected from the urological department of the Fourth Hospital of Hebei Medical University from January 2016 to June 2017. Meanwhile, adjacent tissues were routinely retained 2 cm away from the tumor margin. All tissues were routinely embedded in paraffin wax and confirmed by pathology. Five white slices of each paraffin-embedded tissue block were cut continuously with a thickness of 4 μm, and immunohistochemical staining was performed. NLRP1 and NLRP3 (Abcam, Cambridge, UK), both in 1:100 dilution. NLRP6 (Millipore), diluted in 1:1,000. Each group of slices was incubated with diluted NLRP1/NLRP3/NLRP6 at 4°C overnight. Sections were coupled with horseradish peroxidase-coupled antibodies (Abcam) at 1:500 dilution at room temperature for 2 h. Then 3,3-diaminobenzidine is added. The staining was observed under optical microscope (ZEISS). Immunohistochemical scores were determined by two pathologists with diagnostic experience. The scoring method was the combination of staining intensity and proportion of positive staining area. The scoring criteria of staining intensity were negative score 0; weak positive marks 1 point; medium positive score 2 points; strong positive score 3 points. The number of stained cells was scored according to the proportion of positive cells in each section, and the percentage of positively stained cells in the total number of cells was taken as the average number of each field score: the score of no positive staining was 0; positive staining of 0–20% was l; positive staining of 20–75% was 2; positive staining greater than 75% was 3. Total score = positive percentage score × staining intensity score. A score of 0–4 was low expression, and a score of 5–9 was high expression.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
2.3. Mutation analysis
The mutation annotation format files of somatic mutation data of ccRCC patients were obtained from the TCGA MC3 project [25]. We used the R package “maftools” to calculate the tumor mutation burden (TMB) for ccRCC samples [26].
2.4. Survival analysis
We used R package “survival” and “survminer” to apply uni- and multi-variate Cox regression models based on the expression level of IFC genes. Kaplan–Meier (KM) curves were used to compare the survival differences between distinct patient groups.
2.5. Calculation of the IFC score and identification of IFC groups
Single-sample GSEA (ssGSEA) algorithm was applied using R package “GSVA” [27] to calculate the IFC score in each ccRCC sample based on the expression level of 15 IFC genes. Next, all samples were divided into two groups (high and low) based on the median value of IFC scores.
2.6. Differential expression analysis of protein-coding genes
The annotation of protein-coding genes was obtained from GENCODE v22 [28] (https://www.gencodegenes.org/). We used the R package “limma” to identify dysregulated genes between distinct sample groups [29]. Genes with empty expression values in more than 50% of samples were filtered out for further analysis. Genes with Benjamini and Hochberg (BH) generated false discovery rate (FDR) values less than 0.01 and were identified as differentially expressed.
2.7. Functional enrichment analysis
Ten oncogenic pathways and ten cancer hallmark gene sets were collected from previous studies [30,31]. We downloaded the hallmark gene sets representing various biological processes from MSigDB [32] (https://www.gsea-msigdb.org/gsea/msigdb). The ssGSEA algorithm was applied to calculate the enrichment level of each oncogenic pathway and hallmark gene set across ccRCC samples. Metascape platform (https://metascape.org/) was used to perform Gene ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway, and Reactome gene set enrichment analysis [33].
2.8. Identification of the IFC-based subtypes of ccRCC patients
Using the expression of 15 IFC genes, we performed unsupervised non-negative matrix factorization (NMF) [34] clustering on ccRCC patients. R package “NMF” was used to stratify ccRCC samples into two subtypes according to the cophenetic correlation coefficient [35]. Other parameters were set as follows: method = “brunet”, cluster number k = 2:10, nrun = 50.
2.9. The tumor immune microenvironment patterns of ccRCC
We applied Estimation of Stromal and Immune cells in Malignant Tumor tissues using the Expression data (ESTIMATE) method using the R package “estimate” to evaluate the immune cell abundance, stromal cell abundance, tumor purity, and overall tumor immune microenvironment of ccRCC samples [36]. A previous study defined 29 gene signatures representing tumor microenvironment. We collected these signatures and calculated the enrichment level of these signatures using the ssGSEA algorithm. Unsupervised clustering was performed on ccRCC by TIME signatures to define distinct immune clusters. Immune cell infiltration estimation of TCGA ccRCC samples generated by various methods [37,38,39] was downloaded from TIMER 2.0 [40] (http://timer.cistrome.org/). The marker genes of various immune cell types were collected from CellMarker 2.0 [41] (http://bio-bigdata.hrbmu.edu.cn/CellMarker).
2.10. Prediction of immunotherapy, chemotherapy, and target-therapy responses
Tumor Immune Dysfunction and Exclusion (TIDE, http://tide.dfci.harvard.edu/) defined “Dysfunction scores” and “Exclusion scores” representing the dysfunction of T cell and prevention of T cell infiltration, respectively [42]. These two scores could evaluate primary mechanisms of tumor immune evasion, thus representing the possibility of immunotherapy response. The gene expression profile of ccRCC cell lines and drug sensitivity data of chemotherapy were downloaded from the Genomics of Drug Sensitivity in Cancer [43] (https://www.cancerrxgene.org/) database. Based on the median value of ln(IC50) value of each drug, we divided ccRCC cells into two groups (sensitive and resistant), and then we identified dysregulated IFC genes between two groups and considered them as potential drug targets.
2.11. Statistical analysis
The statistical tests employed to analyze data in this study include the Mann–Whitney U test was utilized to compare the non-normally distributed variables between two groups. The Kruskal–Wallis test was employed to assess the association between the IFC score and different pathological stages of ccRCC. Pearson’s correlation coefficient and Spearman’s correlation coefficient were computed to explore the potential relationships between two variables. Fisher’s exact test was employed to perform overlap analysis. All statistical analyses were performed using R statistical software.
3. Results
3.1. Dysregulated IFC genes were correlated with different clinical outcomes in ccRCC
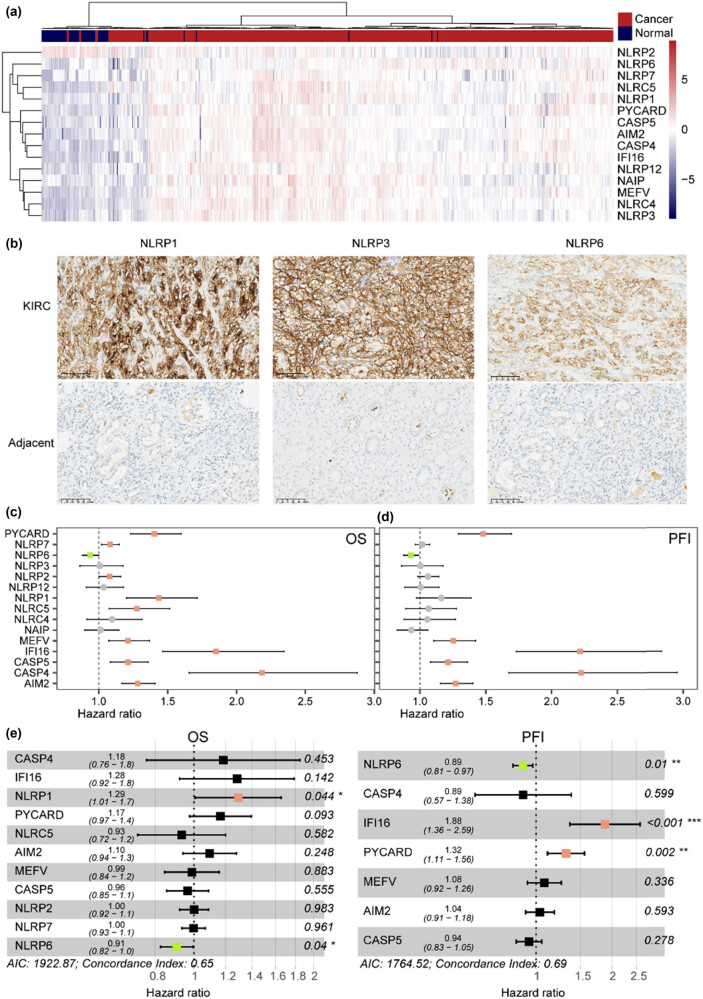
All of the IFC genes were found to be differentially expressed in tumor samples (Figure 1a). All IFC genes, except for NLRP2, showed significant elevated levels in tumor samples than in normal samples (Mann–Whitney U test p < 0.05) (Figure S1a). A clear separation was found between ccRCC and normal samples based on the expression levels of IFC genes, suggesting a potential perturbated expression pattern during tumorigenesis. The results from IHC showed that the expression rate of NLRP1, NLRP3, and NLRP6 in ccRCC tissues was significantly higher than that in adjacent non-tumorous tissues (Figure 1b, Table S1). Next, survival analysis revealed significant correlations between IFC gene expression and ccRCC clinical outcomes. Higher level of NLRP6 expression was associated with a prolonged OS. However, higher level of PYCARD, NLRP7, NLRP2, NLRP1, NLRP5, MEFV, IFI16, CASP5, CASP4, and AIM2 might lead to worse OS (Figure 1c). Additionally, some of these IFC genes were also found to have an impact on patients’ PFI (Figure 1d). In order to identify independent prognostic factors, we also performed a multi-Cox regression analysis. Results showed that NLRP6 could serve as a protective factor for both OS and PFI, whereas NLRP1, IFI16, and PYCARD served as risky factors for OS and PFI, respectively (Figure 1e). In summary, our results revealed significant dysregulation of inflammasome complexes genes, suggesting their potential roles in ccRCC development and patient prognosis.
Figure 1.
Expression pattern and survival relevance of inflammasome complex (IFC) genes. (a) Heatmap illustrates the expression levels of IFC genes in ccRCC samples compared to normal samples. The color scale represents gene expression levels, with red indicating upregulation and blue indicating downregulation. (b) Expression of NLRP1, NLRP3, and NLRP6 in ccRCC and adjacent tissues (IHC 200 times magnification). (c and d) Forest plots of uni-variate Cox regression model for OS and PFI in ccRCC patients. The plot displays hazard ratios and corresponding 95% confidence intervals for each IFC gene. Red bars are associated with worse survival outcomes, while green bars are associated with better survival outcomes. (e) Forest plots of multi-variate Cox regression model.
3.2. Significant correlations were revealed between IFC score and clinical features
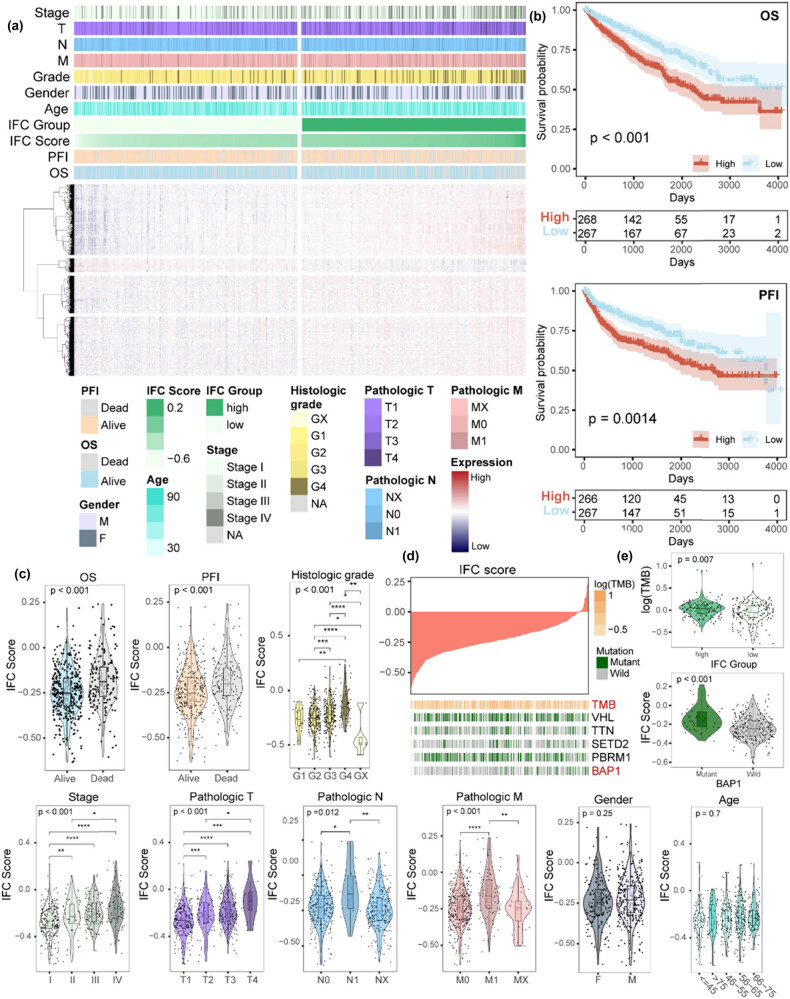
Here, we calculated the IFC score across ccRCC cancer samples using the ssGSEA algorithm based on the expression level of 15 IFC genes. The samples were ranked and divided into high and low IFC groups according to the median value of IFC scores (Figure 2a). KM curves showed that patients from the low IFC group had a prolonged OS and PFI than patients from the high IFC group (Figure 2b). We further explored the clinical associations of distinct IFC groups. Results showed a significant increase in the IFC score as the pathologic stages and grades advanced (Figure 2c). However, no significant differences in the IFC score were observed across different genders and age groups. In addition, we collected genes with a high-frequency somatic mutation in ccRCC and calculated the TMB of the samples (Figure 2d, Figure S1b and c). Subsequently, we investigated the association between the IFC score and gene mutations. We observed an elevated tumor mutational burden in the high IFC group. Moreover, the IFC score was higher in BAP1-mutant samples than in BAP1-wild-type samples (Figure 2e). Previous research has concentrated on the significance of BAP1 mutation status in ccRCC [44,45,46]; our results suggest that inflammasome complexes genes might exert an impact on the interplay between BAP1 and ccRCC development. In summary, our findings revealed a significant correlation between IFC scores, increased TMB, and certain clinical characteristics in ccRCC.
Figure 2.
The clinical features associated with the IFC score in ccRCC patients. (a) Heatmap illustrates the expression patterns of dysregulated protein-coding genes between the high and low IFC groups, along with the corresponding clinical characteristics of the samples. (b) KM survival curves demonstrate the differences in OS and PFI between the high and low IFC groups. (c) Violin plots show the distribution of IFC scores among different clinical groups, including gender, age, survival status, pathological stages, and histologic grades (Mann–Whitney U test p < 0.05; Kruskal–Wallis test p < 0.05). (d) An overview of the association between IFC score and mutation status of the genes with high-frequency somatic mutation and TMB. (e) Violin plots show the TMB differences between high and low IFC groups and the distribution of IFC scores among BAP1 mutant and wild-type samples.
3.3. Dysregulated genes between high and low IFC groups were involved in distinct biological functions
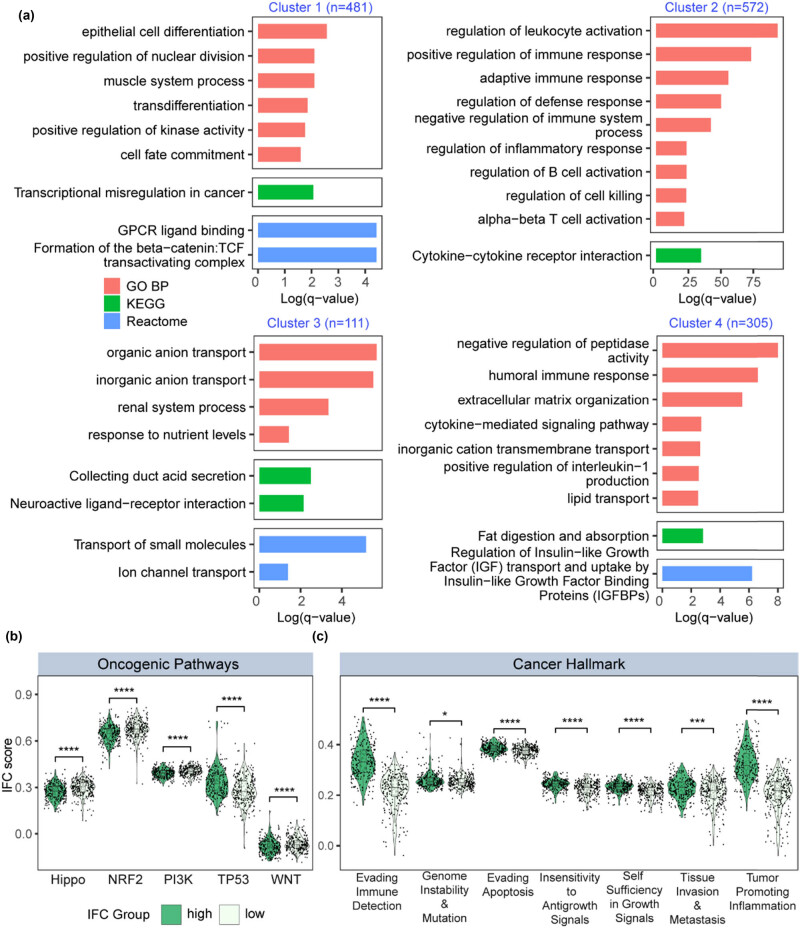
We performed differential expression analysis and identified dysregulated protein-coding genes (110 down-regulated genes and 1361 up-regulated genes, fold change > 2 and FDR < 0.01) between high and low IFC groups (Figure 2a). Dysregulated genes were divided into four clusters by an unsupervised hierarchical clustering method. Then, we performed functional enrichment analysis on the four gene clusters, respectively (Figure 3a). Cluster 1 genes were enriched in tumor invasion, metastasis, and proliferation-related process, including epithelial cell differentiation, positive regulation of nuclear division, and GPCR ligand binding. Cluster 2 genes were involved in biological processes regarding tumor immune response, immune regulation, and formation of tumor microenvironment. Cluster 3 genes were related to abnormal renal function and metabolic regulation. Cluster 4 genes were enriched in terms that were related to various aspects, including renal dysfunction, metabolic regulation, tumor biological behavior, and immune regulation. The results from functional enrichment analysis implied the potential relationship among inflammasome signaling, immune landscape, renal development and tumorigenesis of ccRCC. In addition, we collected ten oncogenic pathways and ten cancer hallmark gene sets and quantified the enrichment level of these gene sets. As a result, we found that Hippo, NRF2, PI3K, TP53, and WNT pathways showed a dysregulated pattern between high and low IFC groups (Figure 3b). Seven cancer hallmark gene sets were significantly up-regulated in the high IFC group (Figure 3c), further suggesting the oncogenic roles of inflammasome signaling. In summary, our finding suggested a potential involvement of the inflammasome complex genes in the pathogenesis and progression of ccRCC.
Figure 3.
Functional enrichment analysis of high and low IFC groups. (a) Barplot illustrates the results of enrichment analysis for GO, KEGG, and Reactome gene sets. (b) Violin plots demonstrate the differences in oncogenic pathway activation levels between the high and low IFC groups (Mann–Whitney U test p < 0.05). (c) Violin plots depict the differential expression of cancer hallmark gene sets between the high and low IFC groups (Mann–Whitney U test p < 0.05; Kruskal–Wallis test p < 0.05).
3.4. Patients’ stratification revealed correlations with distinct clinical features and immunotherapy response
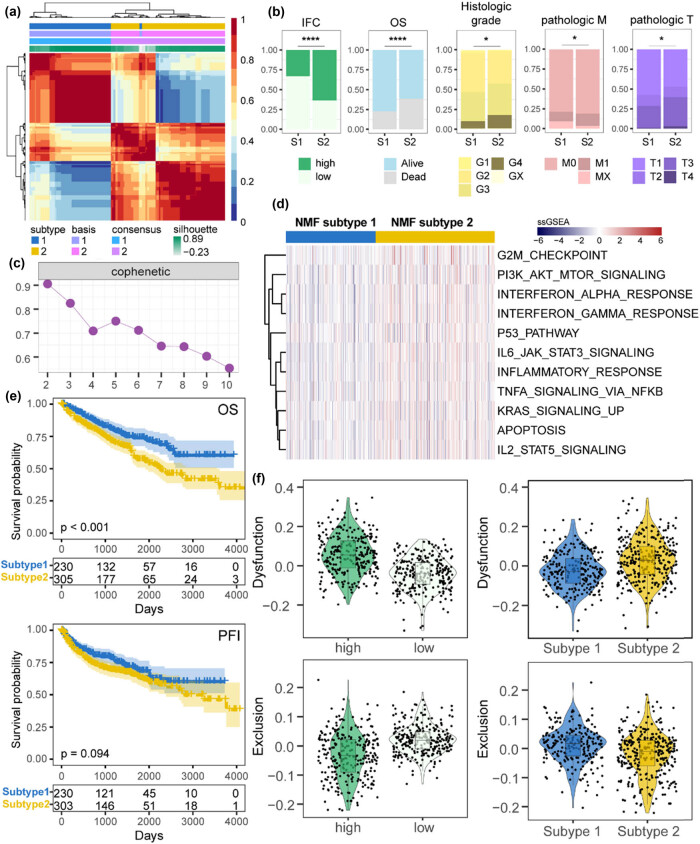
Here, we sought to stratify ccRCC samples into clinically relevant molecular subtypes based on IFC genes. Applying NMF clustering, two IFC subtypes were identified through iterative analysis, including subtype 1 (n = 230) and subtype 2 (n = 305) (Figure 4a and c, Figure S1d). Patients from subtype 1 had better OS and PFI than patients from subtype 2 (Figure 4e). Moreover, we found that subtype 1 patients had relatively lower IFC scores than subtype 2 patients (Figure 4b). Then, we performed an overlap analysis to explore the clinical associations of distinct IFC subtypes. Results showed that patients with advanced pathologic stages and histologic grade were significantly enriched in subtype 2 (Figure 4b), suggesting a potential association between subtype 2 and more aggressive disease characteristics. To further characterize the subtypes, we performed differential expression analysis and identified 839 dysregulated genes, including 10 up-regulated and 829 down-regulated genes (fold change > 2 and FDR < 0.01) (Figure S1e). Next, we quantified various functional gene sets and compared the expression levels between two subtypes (Figure 4d). As a result, oncogenic and immune pathways showed significant up-regulation in subtype 2. Considering the strong associations between IFC subtypes, high/low IFC groups, and tumor immunity, we hypothesized that different patients may exhibit varying responses to immunotherapy. Thus, we calculated the Dysfunction score and Exclusion score of ccRCC samples using TIDE. Results showed that patients from subtype 1 and patients with lower IFC scores exhibited lower Dysfunction levels of score and higher levels of Exclusion scores, suggesting a worse chance to respond to immunotherapy (Figure 4f). In summary, our results demonstrate a strong correlation between IFC-based subtypes and the tumor immune characteristics.
Figure 4.
Identification and characterization of NMF-defined IFC subtypes. (a) Heatmap displays the results of NMF clustering (k = 2), which defined distinct IFC subtypes based on gene expression patterns. (b) The barplot illustrates the differences in clinical characteristics between the two IFC subtypes (Fisher’s exact test p < 0.05). (c) Cophenetic plot visualizing the quality of the NMF clustering results. (d) Heatmap shows the expression perturbations of functional gene sets between the two IFC subtypes. (e) KM curves demonstrate the differences in OS and PFI between the two IFC subtypes. (f) Violin plots display the differences in Exclusion score and Dysfunction score between the high and low IFC groups, as well as between the two IFC subtypes (Mann–Whitney U test p < 0.05).
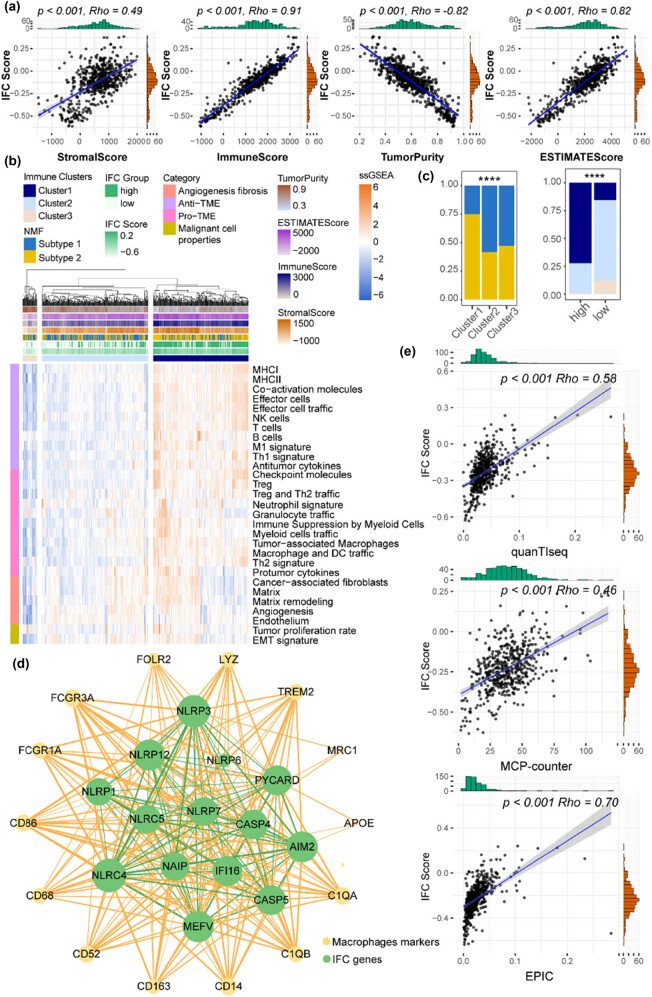
3.5. IFC scores were positively correlated with immunosuppressive macrophages
Here, we sought to further explore the relationship between IFC and TIME patterns. First, we evaluated ccRCC TIME using the ESTIMATE algorithm, and results showed that IFC scores were positively correlated with ESTIMATE, immune, and stromal scores, whereas they negatively correlated with tumor purity, suggesting increased infiltration levels of immune cells in high IFC score samples (Figure 5a). Next, we collected 29 immune signatures from a previous study and quantified their expression levels across ccRCC samples. ccRCC samples were divided into three immune clusters (high-immunity Cluster 1 n = 236, medium-immunity Cluster 2 n = 263, low-immunity Cluster 3 n = 36) by unsupervised hierarchical clustering method (Figure 5b). As shown in Figure 5c, Cluster 1 samples were enriched in NMF subtype 2 and high IFC Score group, whereas Cluster 3 samples showed a significant overlap with NMF subtype 1 and low IFC score group, suggesting a distinct immune landscape. In addition, we explored the relationship between IFC scores and various immune cell abundance. The strongest correlation was found between IFC scores and macrophage abundances (Figure 5e), suggesting a potential immunosuppressive microenvironment in ccRCC patients with high IFC scores. Thus, we constructed a co-expression network and found intense interplay between macrophage marker genes and IFC genes (Figure 5d), suggesting a potential functional relationship and shared regulatory mechanisms between macrophages and the inflammasome signaling in ccRCC. The relationship between changes in macrophage abundance and the immunotherapy response of patients might be explained by our findings from the standpoint of inflammatory complexes genes.
Figure 5.
IFC scores were positively correlated with macrophage abundance. (a) Correlation curves display the correlation between the ESTIMATE score, immune score, stromal score, tumor purity, and IFC score. (b) TIME signatures defined three immune clusters. The heatmap displays the expression levels of 29 signatures across ccRCC samples. (c) Barplots illustrate the overlap among the three immune clusters, the high and low IFC groups, and the two IFC subtypes (Fisher’s exact test p < 0.05). (d) Co-expression network between IFC genes and macrophage marker genes. The network visualizes the co-expression relationships within IFC genes (shown in green edges), as well as between IFC and macrophage marker genes (shown in yellow edges). (e) Correlation curves show the correlation between the IFC score and the abundance of macrophages derived from three different data sources.
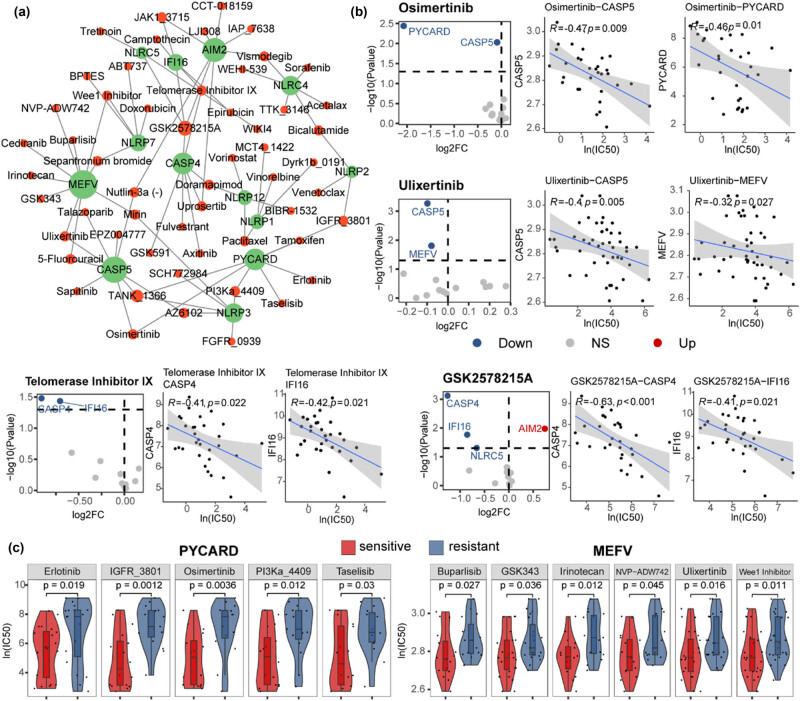
3.6. Increased expression of IFC genes could sensitize ccRCC patients to anti-tumor drugs
Here, we aimed to predict potential drug targets for ccRCC treatment by IFC genes. Therefore, we explored the correlation between drug sensitivity information and the expression of IFC genes in ccRCC cell lines, constructing a network of drug–target interactions (Figure 6). Through this investigation, we found that the expression perturbation of IFC genes could lead to the resistance of several drugs which have been used for renal cell carcinoma treatment in clinical trials including targeted therapy drugs Axitinib [47,48,49,50], Sorafenib [51], Pazopanib [52,53], Sunitinib [54], and immunotherapy drugs Bevacizumab [55,56], Interleukin-2 [57,58], Pembrolizumab [47], Nivolumab [59,60]. Additionally, we identified several potential anti-tumor drugs for ccRCC treatment, including Osimertinib, Ulixertinib, Telomerase Inhibitor IX, and GSK2578215A (Figure 6a and b). Osimertinib is a chemotherapy drug which has been widely used for metastatic EGFR-mutant non-small-cell lung cancer treatment [61]. Ulixertinib is an ERK1/2 inhibitor that has been clinically tested effective for patients with advanced solid tumors [62,63]. Telomerase Inhibitor IX has the potential to inhibit the proliferation of cancer cells because of the abnormally high telomerase activity in cancer cells [64]. GSK2578215A is an LRRK2 inhibitor that could affect the sensitivity of ovarian cancer treatment by suppressing homologous recombination [65]. Our results suggested the potential of these anti-tumor drugs in ccRCC treatment by targeting inflammasome signaling. Among IFC genes, we found that MEFV and PYCARD were predicted to be the targets of most drugs in the network. The increased expression level of these two IFC genes could sensitize ccRCC cells to various therapy methods (Figure 6c). In summary, our results indicated a potential role of the inflammasome pathway in modulating ccRCC patients’ response to these specific drugs.
Figure 6.
Increased expression of IFC genes could sensitize ccRCC patients to anti-tumor drugs. (a) Network shows the interactions between anti-tumor drugs and predicted target IFC genes. (b) Volcano plots show the expression variation of IFC genes between sensitive and resistant groups of each drug. Red nodes represent IFC genes that were up-regulated in drug-sensitive groups, while blue nodes represent down-regulated. Correlation curves depicting the relationship between the expression levels of IFC genes and drug sensitivity IC50 value in cancer cell lines. (Spearman’s correlation analysis, p < 0.05, |Rho| > 0.3). (c) Violin plots of gene expression between sensitive and resistant groups (Mann–Whitney U test p < 0.05).
4. Discussion
ccRCC is a multifaceted cancer with complicated biological and clinical features. As a key hallmark of cancer, inflammation has been shown to be closely related to the progression and prognosis of various tumors. In some cases, inflammation can promote the occurrence and development of tumors, which supports the therapeutic potential of targeting inflammation for cancer [66]. As the core of inflammation, the inflammasome, a multiprotein complex within cells, plays a vital role in initiating and regulating inflammatory responses, and the precise regulation of its function is significant for maintaining the body’s immune homeostasis [67]. The activation of inflammasome complexes is an important regulatory node in the inflammatory response. It can sense a variety of abnormal signals and regulate the intensity and duration of the inflammatory response through downstream signaling pathways [68]. In this study, we comprehensively investigated the expression patterns and clinical significance of inflammasome complexes in ccRCC, providing insight into their role in tumorigenesis, prognosis, and therapeutic response.
We found that most inflammasome complexes genes showed a significantly up-regulated pattern in ccRCC samples, which was further validated by immunohistochemistry (Figure 1b). Previous studies have established a positive correlation between high levels of inflammasome complexes and the development of cancer; the overactivation of inflammasome signaling in some cancer types is linked to the persistence of inflammatory responses, thereby promoting tumor formation and progression [10,11]. Our results further suggested the role of inflammasome complexes during the tumorigenesis of ccRCC. Moreover, our results showed that perturbations in inflammasome complex genes were associated with patient prognosis and other clinical characteristics by developing an IFC score and identifying IFC subtypes. Therefore, we provided evidence of inflammasome complexes genes’ clinical relevance in ccRCC and highlighted the potential of inflammasome complexes as prognostic markers for patient outcomes.
In addition, we found that there was a positive correlation between inflammasome signaling and immunosuppressive macrophages (Figure 5), indicating that inflammasome activation might contribute to an immunosuppressive tumor microenvironment, thereby facilitating tumor immune evasion and progression. These findings further validated the hypothesis that patients with lower IFC scores would be more likely to respond to immunotherapy (Figure 4f). Our study provided insights into the interplay between inflammasome complexes and the immune response in ccRCC. Significantly, our research also revealed the potential of inflammasome complex genes as predictive markers for patient responses to different anti-tumor drugs, such as GSK2578215A, Ulixertinib, Osimertinib, and Telomerase Inhibitor IX (Figure 6). This suggested that inflammasome activation may have implications for treatment selection and personalized medicine in ccRCC, which also implied the potential mechanisms by which inflammasome complexes modulate therapeutic response.
In summary, the results of our study demonstrated the clinical significance of inflammasome complexes genes in ccRCC and their potential as prognostic indicators and therapeutic targets. Our findings highlighted the importance of understanding the molecular mechanisms underlying inflammasome activation and its impact on tumorigenesis, the immune landscape, and treatment response in ccRCC.
Supplementary Material
Footnotes
Funding information: Authors state no funding involved.
Author contributions: A.L.Z. and F.C.Y. designed the study and performed analysis. A.L.Z. supervised research and provided critical advice on the study. F.C.Y. and F.L. collected clinical and online data, developed the methodology, and performed experiments. F.C.Y. wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. 10.3322/caac.21820. [DOI] [PubMed]
- [2].Dizman N, Philip EJ, Pal SK. Genomic profiling in renal cell carcinoma. Nat Rev Nephrol. 2020;16(8):435–51. 10.1038/s41581-020-0301-x. [DOI] [PubMed]
- [3].Stolpa W, Stręk-Cholewińska A, Mizia-Malarz A. Clear cell renal cell carcinoma, diagnostic and therapeutic difficulties, case report and literature review. Medicina (Kaunas). 2022;58(10):1329. 10.3390/medicina58101329. [DOI] [PMC free article] [PubMed]
- [4].Shuch B, Amin A, Armstrong AJ, Eble JN, Ficarra V, Lopez-Beltran A, et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol. 2015;67:85–97. 10.1016/j.eururo.2014.04.029. [DOI] [PubMed]
- [5].Abu-Ghanem Y, Powles T, Capitanio U, Beisland C, Järvinen P, Stewart GD, et al. The impact of histological subtype on the incidence, timing, and patterns of recurrence in patients with renal cell carcinoma after surgery-results from RECUR consortium. Eur Urol Oncol. 2021;4(3):473–82. 10.1016/j.euo.2020.09.005. [DOI] [PubMed]
- [6].Amin J, Boche D, Rakic S. What do we know about the inflammasome in humans? Brain Pathol. 2017;27:192–204. 10.1111/bpa.12479. [DOI] [PMC free article] [PubMed]
- [7].Christgen S, Place DE, Kanneganti TD. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020;30(4):315–27. 10.1038/s41422-020-0295-8. [DOI] [PMC free article] [PubMed]
- [8].Fu J, Schroder K, Wu H. Mechanistic insights from inflammasome structures. Nat Rev Immunol. 2024;24(7):518–35. 10.1038/s41577-024-00995-w. [DOI] [PMC free article] [PubMed]
- [9].Yao J, Sterling K, Wang Z, Zhang Y, Song W. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct Target Ther. 2024;9(1):10. 10.1038/s41392-023-01687-y. [DOI] [PMC free article] [PubMed]
- [10].Sundaram B, Tweedell RE, Prasanth Kumar S, Kanneganti TD. The NLR family of innate immune and cell death sensors. Immunity. 2024;57(4):674–99. 10.1016/j.immuni.2024.03.012. [DOI] [PMC free article] [PubMed]
- [11].Deng Z, Lu L, Li B, Shi X, Jin H, Hu W. The roles of inflammasomes in cancer. Front Immunol. 2023;14:1195572. 10.3389/fimmu.2023.1195572. [DOI] [PMC free article] [PubMed]
- [12].Okin D, Kagan JC. Inflammasomes as regulators of non-infectious disease. Semin Immunol. 2023;69:101815. 10.1016/j.smim.2023.101815. [DOI] [PMC free article] [PubMed]
- [13].Liang Q, Wu J, Zhao X, Shen S, Zhu C, Liu T, et al. Establishment of tumor inflammasome clusters with distinct immunogenomic landscape aids immunotherapy. Theranostics. 2021;11:9884–903. 10.7150/thno.63202. [DOI] [PMC free article] [PubMed]
- [14].Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39(845-65):e7. 10.1016/j.ccell.2021.04.014. [DOI] [PubMed]
- [15].Kantono M, Guo B. Inflammasomes and cancer: The dynamic role of the inflammasome in tumor development. Front Immunol. 2017;8:1132. 10.3389/fimmu.2017.01132. [DOI] [PMC free article] [PubMed]
- [16].Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87. 10.1038/nm.3893. [DOI] [PMC free article] [PubMed]
- [17].Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed]
- [18].Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–503. 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed]
- [19].Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer. 2018;6(1):132. 10.1186/s40425-018-0449-0. [DOI] [PMC free article] [PubMed]
- [20].Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed]
- [21].Karki R, Kanneganti TD. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19:197–214. 10.1038/s41568-019-0123-y. [DOI] [PMC free article] [PubMed]
- [22].Huang Y, Wang H, Hao Y, Lin H, Dong M, Ye J, et al. Myeloid PTEN promotes chemotherapy-induced NLRP3-inflammasome activation and antitumour immunity. Nat Cell Biol. 2020;22:716–27. 10.1038/s41556-020-0510-3. [DOI] [PubMed]
- [23].Li YF, Nanayakkara G, Sun Y, Li X, Wang L, Cueto R, et al. Analyses of caspase-1-regulated transcriptomes in various tissues lead to identification of novel IL-1beta-, IL-18- and sirtuin-1-independent pathways. J Hematol Oncol. 2017;10:40. 10.1186/s13045-017-0406-2. [DOI] [PMC free article] [PubMed]
- [24].Goldman MJ, Craft B, Hastie M, Repecka K, McDade F, Kamath A, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38:675–8. 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed]
- [25].Zhang Y, Kwok-Shing Ng P, Kucherlapati M, Chen F, Liu Y, Tsang YH, et al. A pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell. 2017;31:820–32.e3. 10.1016/j.ccell.2017.04.013. [DOI] [PMC free article] [PubMed]
- [26].Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747–56. 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed]
- [27].Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinforma. 2013;14:7. 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed]
- [28].Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47:D766–73. 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed]
- [29].Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed]
- [30].Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173(321-37):e10. 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed]
- [31].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed]
- [32].Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–25. 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed]
- [33].Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed]
- [34].Brunet JP, Tamayo P, Golub TR, Mesirov JP. Metagenes and molecular pattern discovery using matrix factorization. Proc Natl Acad Sci U S A. 2004;101:4164–9. 10.1073/pnas.0308531101. [DOI] [PMC free article] [PubMed]
- [35].Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinforma. 2010;11:367. 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed]
- [36].Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed]
- [37].Finotello F, Mayer C, Plattner C, Laschober G, Rieder D, Hackl H, et al. Molecular and pharmacological modulators of the tumor immune contexture revealed by deconvolution of RNA-seq data. Genome Med. 2019;11:34. 10.1186/s13073-019-0638-6. [DOI] [PMC free article] [PubMed]
- [38].Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17:218. 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed]
- [39].Racle J, de Jonge K, Baumgaertner P, Speiser DE, Gfeller D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. Elife. 2017;6:e26476. 10.7554/eLife.26476. [DOI] [PMC free article] [PubMed]
- [40].Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174. 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed]
- [41].Hu C, Li T, Xu Y, Zhang X, Li F, Bai J, et al. CellMarker 2.0: an updated database of manually curated cell markers in human/mouse and web tools based on scRNA-seq data. Nucleic Acids Res. 2023;51:D870–6. 10.1093/nar/gkac947. [DOI] [PMC free article] [PubMed]
- [42].Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–8. 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed]
- [43].Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, et al. Genomics of drug sensitivity in cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41:D955–61. 10.1093/nar/gks1111. [DOI] [PMC free article] [PubMed]
- [44].Hakimi AA, Ostrovnaya I, Reva B, Schultz N, Chen YB, Gonen M, et al. Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: a report by MSKCC and the KIRC TCGA research network. Clin Cancer Res. 2013;19:3259–67. 10.1158/1078-0432. [DOI] [PMC free article] [PubMed]
- [45].Niersch J, Vega-Rubin-de-Celis S, Bazarna A, Mergener S, Jendrossek V, Siveke JT, et al. A BAP1 synonymous mutation results in exon skipping, loss of function and worse patient prognosis. iScience. 2021;24:102173. 10.1016/j.isci.2021.102173. [DOI] [PMC free article] [PubMed]
- [46].Wu XH, Zhu JM, Lin BH, Qiu QR, Ruan ZT, Wei Y, et al. Semantic computed tomography features for predicting BRCA1-associated protein 1 and/or tumor protein p53 gene mutation status in clear cell renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2023;116:666–75. 10.1016/j.ijrobp.2022.12.023. [DOI] [PubMed]
- [47].Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27. 10.1056/NEJMoa1816714. [DOI] [PubMed]
- [48].Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15. 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed]
- [49].Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–62. 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed]
- [50].Tomita Y, Motzer RJ, Choueiri TK, Rini BI, Miyake H, Uemura H, et al. Efficacy and safety of avelumab plus axitinib in elderly patients with advanced renal cell carcinoma: extended follow-up results from JAVELIN Renal 101. ESMO Open. 2022;7:100450. 10.1016/j.esmoop.2022.100450. [DOI] [PMC free article] [PubMed]
- [51].Rini BI, Pal SK, Escudier BJ, Atkins MB, Hutson TE, Porta C, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21:95–104. 10.1016/S1470-2045(19)30735-1. [DOI] [PubMed]
- [52].Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. 10.1200/JCO.2009.23.9764. [DOI] [PubMed]
- [53].Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. 10.1056/NEJMoa1303989. [DOI] [PubMed]
- [54].Motzer RJ, Powles T, Burotto M, Escudier B, Bourlon MT, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:888–98. 10.1016/S1470-2045(22)00290-X. [DOI] [PMC free article] [PubMed]
- [55].McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–57. 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed]
- [56].Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Alekseev BY, et al. Final overall survival and molecular analysis in IMmotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol. 2022;8:275–80. 10.1001/jamaoncol.2021.5981. [DOI] [PMC free article] [PubMed]
- [57].Raeber ME, Sahin D, Karakus U, Boyman O. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. EBioMedicine. 2023;90:104539. 10.1016/j.ebiom.2023.104539. [DOI] [PMC free article] [PubMed]
- [58].Hannan R, Mohamad O, Diaz de Leon A, Manna S, Pop LM, Zhang Z, et al. Outcome and immune correlates of a phase II trial of high-dose interleukin-2 and stereotactic ablative radiotherapy for metastatic renal cell carcinoma. Clin Cancer Res. 2021;27(24):6716–25. 10.1158/1078-0432. [DOI] [PMC free article] [PubMed]
- [59].Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40:127–37. 10.1200/JCO.21.02229. [DOI] [PMC free article] [PubMed]
- [60].Choueiri TK, Powles T, Albiges L, Burotto M, Szczylik C, Zurawski B, et al. Cabozantinib plus nivolumab and ipilimumab in renal-cell carcinoma. N Engl J Med. 2023;388:1767–78. 10.1056/NEJMoa2212851. [DOI] [PMC free article] [PubMed]
- [61].Remon J, Steuer CE, Ramalingam SS, Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol. 2018;29:i20–7. 10.1093/annonc/mdx704. [DOI] [PubMed]
- [62].Mendzelevski B, Ferber G, Janku F, Li BT, Sullivan RJ, Welsch D, et al. Effect of ulixertinib, a novel ERK1/2 inhibitor, on the QT/QTc interval in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol. 2018;81:1129–41. 10.1007/s00280-018-3564-1. [DOI] [PMC free article] [PubMed]
- [63].Sullivan RJ, Infante JR, Janku F, Wong DJL, Sosman JA, Keedy V, et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: Results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8:184–95. 10.1158/2159-8290. [DOI] [PubMed]
- [64].Li Y, Gu J, Ding Y, Gao H, Li Y, Sun Y, et al. A small molecule compound IX inhibits telomere and attenuates oncogenesis of drug-resistant leukemia cells. FASEB J. 2020;34:8843–57. 10.1096/fj.201902651RR. [DOI] [PubMed]
- [65].Chen L, Hou J, Zeng X, Guo Q, Deng M, Kloeber JA, et al. LRRK2 inhibition potentiates PARP inhibitor cytotoxicity through inhibiting homologous recombination-mediated DNA double strand break repair. Clin Transl Med. 2021;11:e341. 10.1002/ctm2.341. [DOI] [PMC free article] [PubMed]
- [66].Wang M, Chen S, He X, Yuan Y, Wei X. Targeting inflammation as cancer therapy. J Hematol Oncol. 2024;17(1):13. 10.1186/s13045-024-01528-7. [DOI] [PMC free article] [PubMed]
- [67].Zhang WJ, Li KY, Lan Y, Zeng HY, Chen SQ, Wang H. NLRP3 inflammasome: A key contributor to the inflammation formation. Food Chem Toxicol. 2023;174:113683. 10.1016/j.fct.2023.113683. [DOI] [PubMed]
- [68].Chen Y, Ye X, Escames G, Lei W, Zhang X, Li M, et al. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell Mol Biol Lett. 2023;28(1):51. 10.1186/s11658-023-00462-9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.