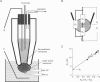
Abstract
1. We have studied the effects on the physiological properties of the Na(+)-K+ pump of both 31- and 40-amino acid N-terminal truncated forms of the alpha-subunit of the Na(+)-K(+)-ATPase. 2. Na(+)-K+ pumps that were moderately ouabain resistant (K1 = 50 microM) were expressed in the Xenopus oocyte by injection of wild-type or truncated variants of the Bufo marinus Na(+)-K(+)-ATPase alpha-subunit cRNA with Bufo beta-subunit cRNA. The function of the Na(+)-K+ pump was studied by electrophysiological methods after Na+ loading and inhibition of the endogenous Xenopus Na(+)-K(+)-ATPase by exposure to a low concentration (0.2 microM) of ouabain. 3. The voltage-dependent potassium activation kinetics of the Na(+)-K+ pump current and the ouabain-sensitive proton-dependent inward current were studied using the two-electrode voltage-clamp technique. A novel technique involving permeabilization of part of the oocyte membrane with digitonin was developed to enable study of the pre-steady-state current following fast voltage perturbation. 4. By comparison with the wild type, the 40-amino acid N-terminal truncation induced a lower level of Na(+)-K+ pump current, a 2- to 3-fold reduction in the apparent external K+ affinity when measured in the presence of extracellular Na+, a relative increase in the proton-dependent inward current, and a reduction in the rate constant of the pre-steady-state current following a voltage step towards a positive membrane potential. The 31-amino acid truncation induced changes that were qualitatively similar but of smaller magnitude. 5. We have analysed these results using a kinetic model of the Na(+)-K+ pump cycle and have shown that all these effects can be explained by the change in a single rate constant in the cycle kinetics, namely a reduction in the rate of the main charge translocating part of the Na(+)-K+ pump cycle, i.e. the forward E1 to E2 conformational change, the deocclusion and release of Na+ to the external side. 6. The highly charged N-terminal segment seems to be directly involved in the mechanism that translocates Na+ ions across the membrane's electrical field.
Full text
PDF



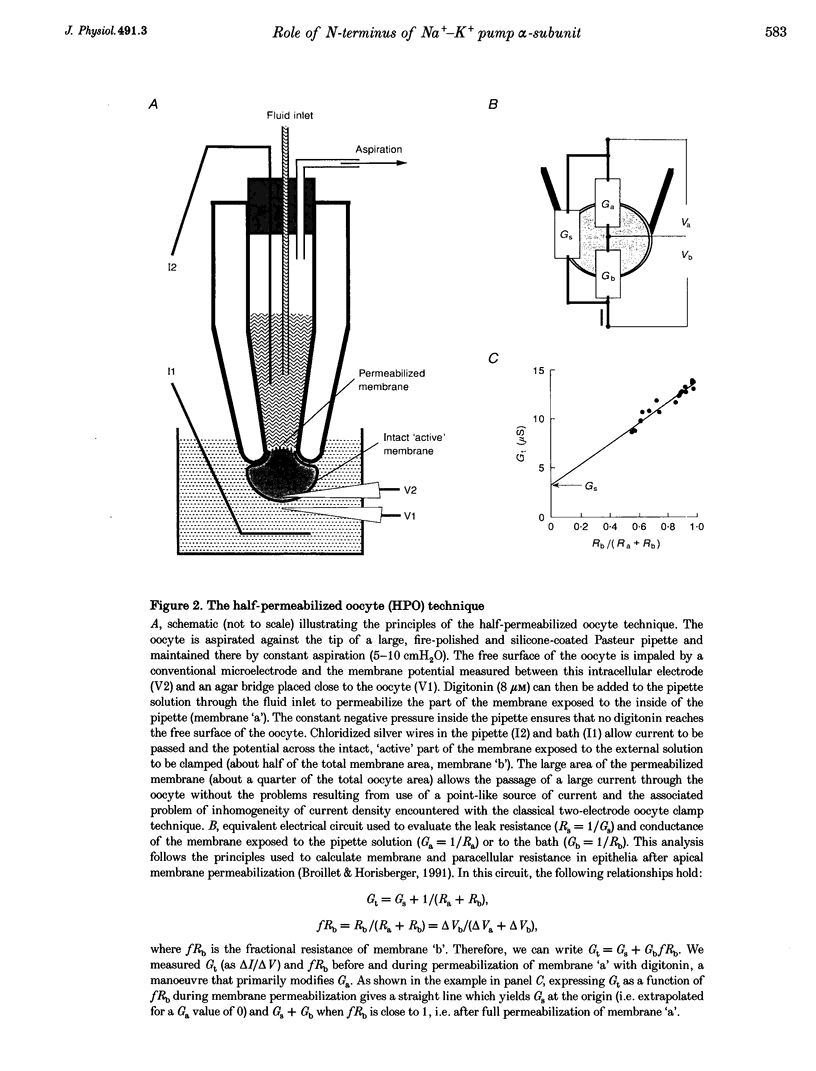
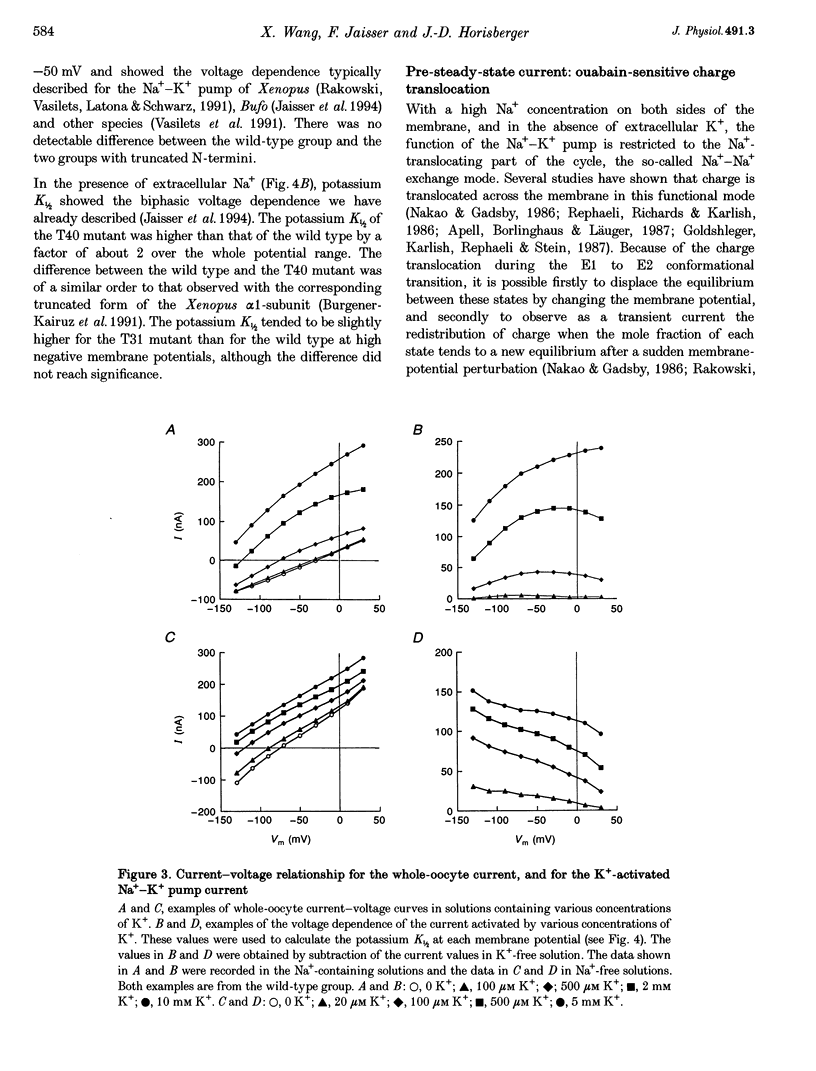
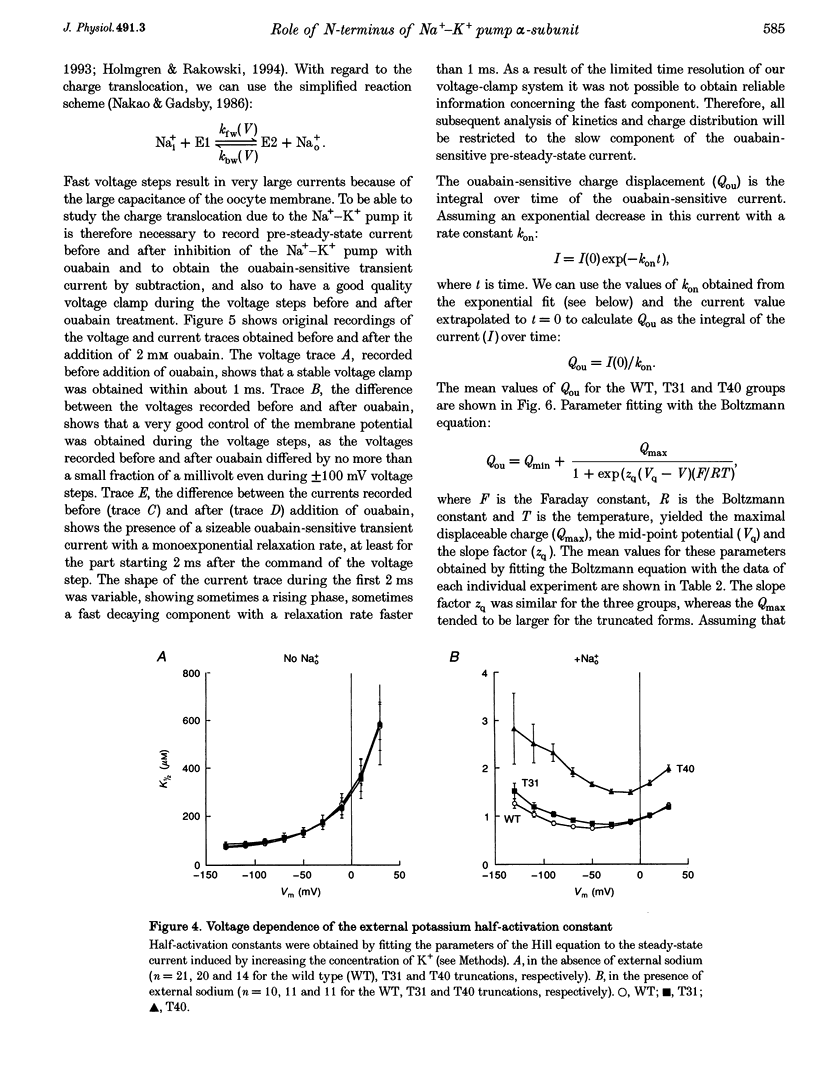
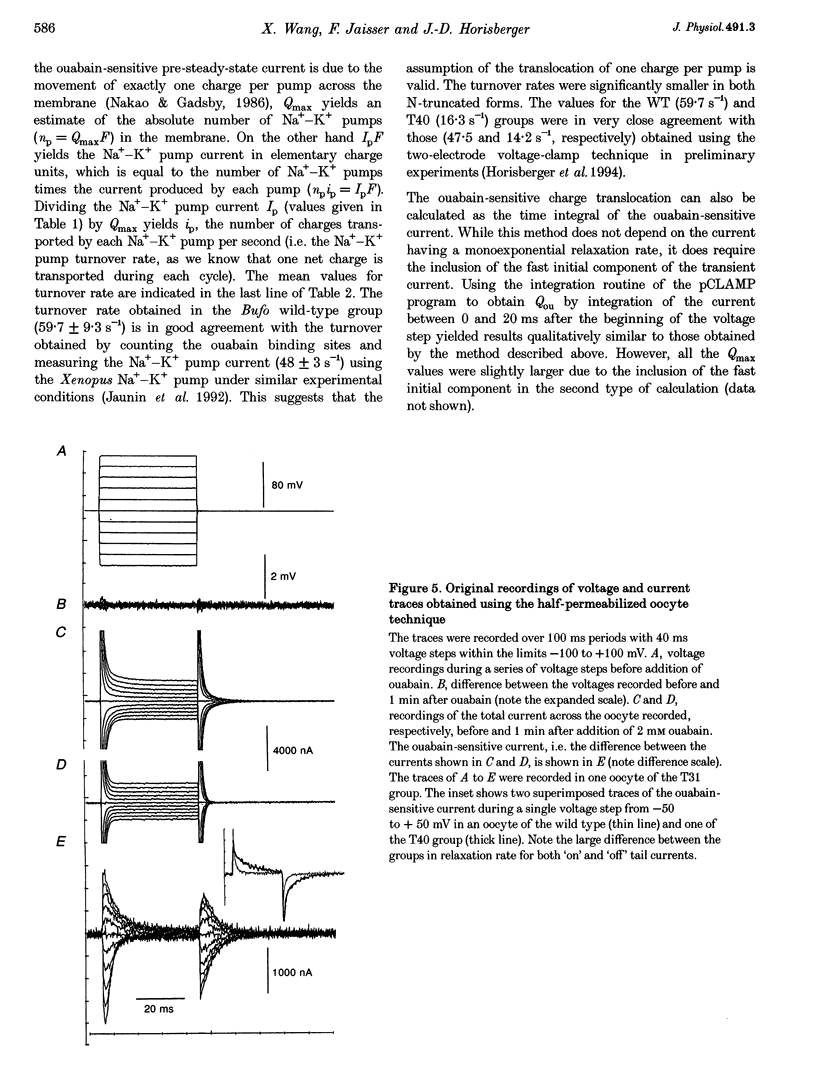
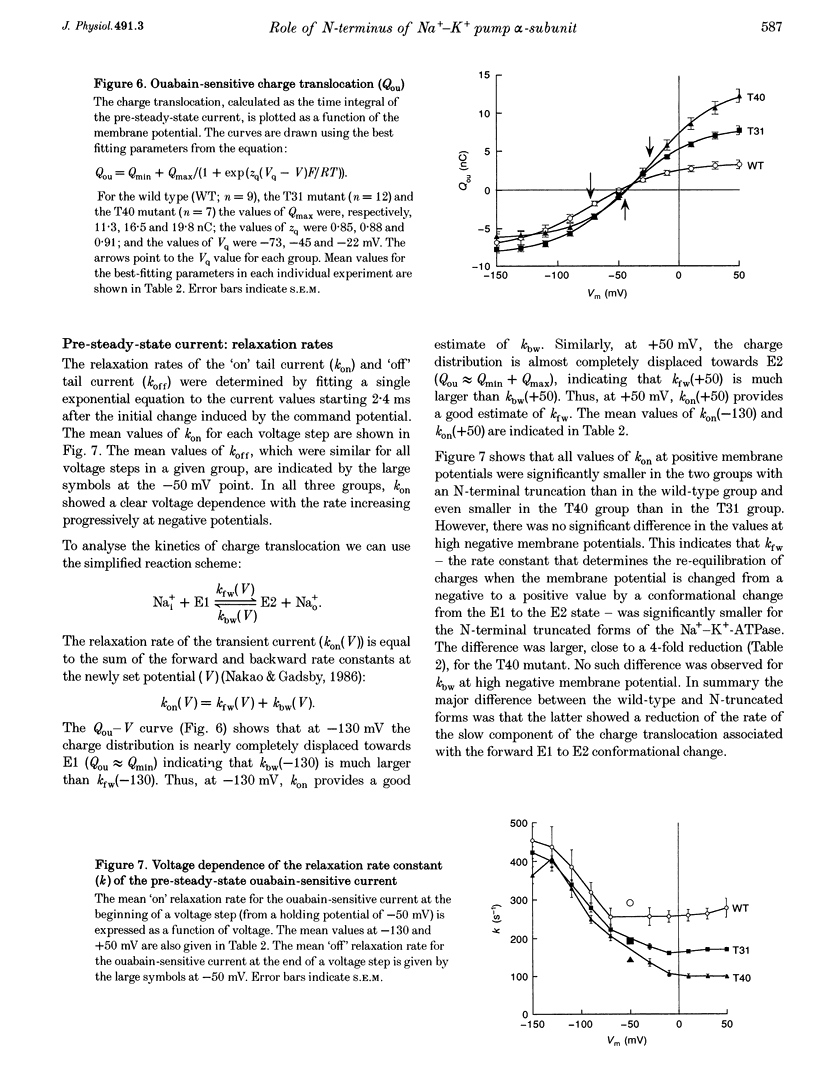
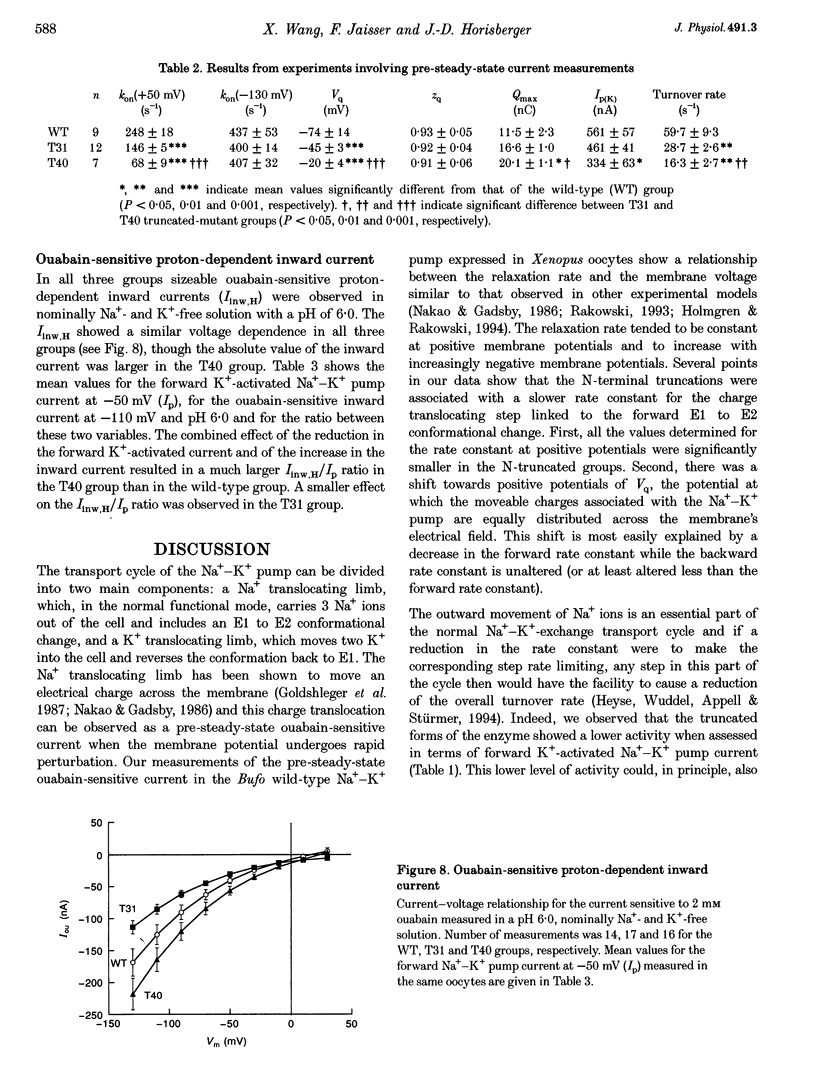

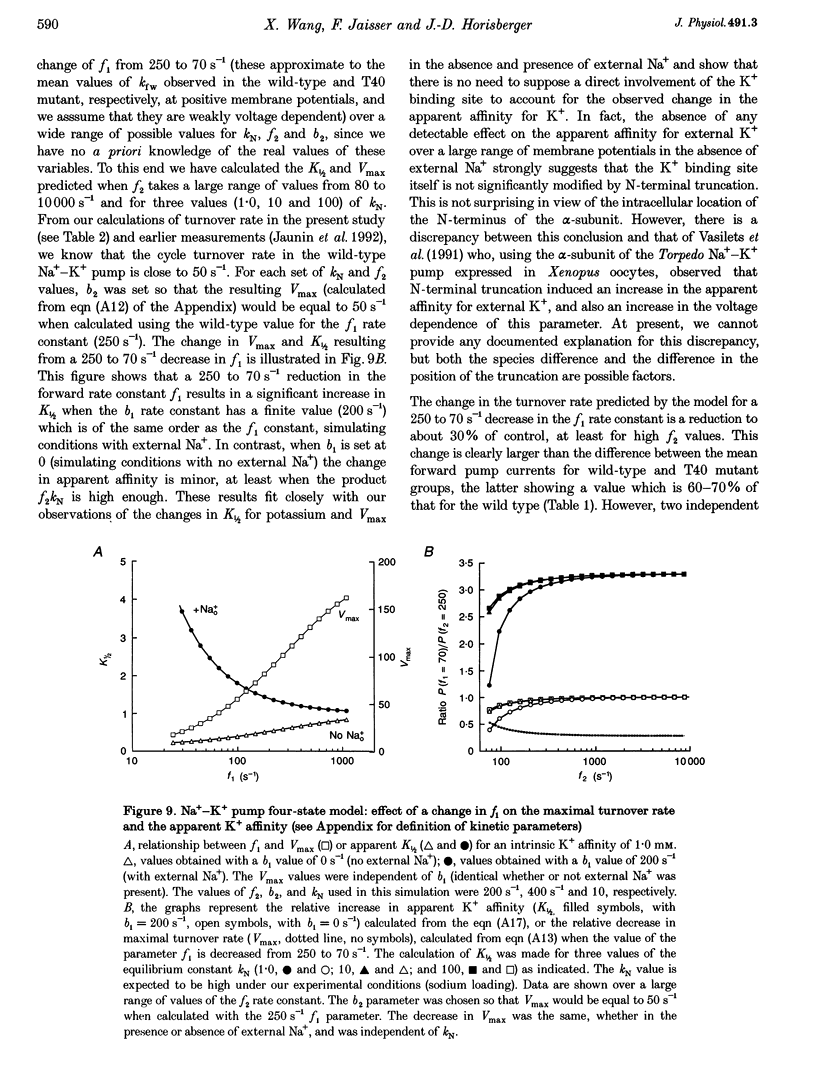




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Borlinghaus R., Läuger P. Fast charge translocations associated with partial reactions of the Na,K-pump: II. Microscopic analysis of transient currents. J Membr Biol. 1987;97(3):179–191. doi: 10.1007/BF01869221. [DOI] [PubMed] [Google Scholar]
- Burgener-Kairuz P., Horisberger J. D., Geering K., Rossier B. C. Functional expression of N-terminal truncated alpha-subunits of Na,K-ATPase in Xenopus laevis oocytes. FEBS Lett. 1991 Sep 23;290(1-2):83–86. doi: 10.1016/0014-5793(91)81231-v. [DOI] [PubMed] [Google Scholar]
- Daly S. E., Lane L. K., Blostein R. Functional consequences of amino-terminal diversity of the catalytic subunit of the Na,K-ATPase. J Biol Chem. 1994 Sep 30;269(39):23944–23948. [PubMed] [Google Scholar]
- Dascal N., Chilcott G., Lester H. A. Intracellular perfusion of Xenopus oocytes. Methods Enzymol. 1992;207:345–352. doi: 10.1016/0076-6879(92)07023-h. [DOI] [PubMed] [Google Scholar]
- Efthymiadis A., Rettinger J., Schwarz W. Inward-directed current generated by the Na+,K+ pump in Na(+)- and K(+)-free medium. Cell Biol Int. 1993 Dec;17(12):1107–1116. doi: 10.1006/cbir.1993.1043. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Rakowski R. F., De Weer P. Extracellular access to the Na,K pump: pathway similar to ion channel. Science. 1993 Apr 2;260(5104):100–103. doi: 10.1126/science.7682009. [DOI] [PubMed] [Google Scholar]
- Geering K., Theulaz I., Verrey F., Häuptle M. T., Rossier B. C. A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am J Physiol. 1989 Nov;257(5 Pt 1):C851–C858. doi: 10.1152/ajpcell.1989.257.5.C851. [DOI] [PubMed] [Google Scholar]
- Heyse S., Wuddel I., Apell H. J., Stürmer W. Partial reactions of the Na,K-ATPase: determination of rate constants. J Gen Physiol. 1994 Aug;104(2):197–240. doi: 10.1085/jgp.104.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M., Rakowski R. F. Pre-steady-state transient currents mediated by the Na/K pump in internally perfused Xenopus oocytes. Biophys J. 1994 Mar;66(3 Pt 1):912–922. doi: 10.1016/s0006-3495(94)80867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger J. D., Jaunin P., Good P. J., Rossier B. C., Geering K. Coexpression of alpha 1 with putative beta 3 subunits results in functional Na+/K+ pumps in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8397–8400. doi: 10.1073/pnas.88.19.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaisser F., Canessa C. M., Horisberger J. D., Rossier B. C. Primary sequence and functional expression of a novel ouabain-resistant Na,K-ATPase. The beta subunit modulates potassium activation of the Na,K-pump. J Biol Chem. 1992 Aug 25;267(24):16895–16903. [PubMed] [Google Scholar]
- Jaisser F., Jaunin P., Geering K., Rossier B. C., Horisberger J. D. Modulation of the Na,K-pump function by beta subunit isoforms. J Gen Physiol. 1994 Apr;103(4):605–623. doi: 10.1085/jgp.103.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaunin P., Horisberger J. D., Richter K., Good P. J., Rossier B. C., Geering K. Processing, intracellular transport, and functional expression of endogenous and exogenous alpha-beta 3 Na,K-ATPase complexes in Xenopus oocytes. J Biol Chem. 1992 Jan 5;267(1):577–585. [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ + K+)-ATPase. VI. Differential tryptic modification of catalytic functions of the purified enzyme in presence of NaCl and KCl. Biochim Biophys Acta. 1977 Apr 1;466(1):97–108. doi: 10.1016/0005-2736(77)90211-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+, K+)-ATPase. V. Conformational changes in the enzyme Transitions between the Na-form and the K-form studied with tryptic digestion as a tool. Biochim Biophys Acta. 1975 Sep 2;401(3):399–415. doi: 10.1016/0005-2736(75)90239-4. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Collins J. H. Tryptic and chymotryptic cleavage sites in sequence of alpha-subunit of (Na+ + K+)-ATPase from outer medulla of mammalian kidney. Biochim Biophys Acta. 1986 Sep 11;860(3):570–576. doi: 10.1016/0005-2736(86)90555-9. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Karlish S. J. Defective conformational response in a selectively trypsinized (Na+ + K+)-ATPase studied with tryptophan fluorescence. Biochim Biophys Acta. 1980 Apr 10;597(2):305–317. doi: 10.1016/0005-2736(80)90108-x. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Klodos I. Purification and characterization of (Na+ + K+)-ATPase. VII. Tryptic degradation of the Na-form of the enzyme protein resulting in selective modification of dephosphorylation reactions of the (Na+ + K+)-ATPase. Biochim Biophys Acta. 1978 Feb 2;507(1):8–16. doi: 10.1016/0005-2736(78)90369-3. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986 Oct 16;323(6089):628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- Ohta T., Noguchi S., Nakanishi M., Mutoh Y., Hirata H., Kagawa Y., Kawamura M. The 'lysine cluster' in the N-terminal region of Na+/K(+)-ATPase alpha-subunit is not involved in ATPase activity. Biochim Biophys Acta. 1991 Aug 23;1059(2):157–164. doi: 10.1016/s0005-2728(05)80200-2. [DOI] [PubMed] [Google Scholar]
- Rakowski R. F. Charge movement by the Na/K pump in Xenopus oocytes. J Gen Physiol. 1993 Jan;101(1):117–144. doi: 10.1085/jgp.101.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski R. F., Vasilets L. A., LaTona J., Schwarz W. A negative slope in the current-voltage relationship of the Na+/K+ pump in Xenopus oocytes produced by reduction of external [K+]. J Membr Biol. 1991 Apr;121(2):177–187. doi: 10.1007/BF01870531. [DOI] [PubMed] [Google Scholar]
- Rephaeli A., Richards D. E., Karlish S. J. Electrical potential accelerates the E1P(Na)----E2P conformational transition of (Na,K)-ATPase in reconstituted vesicles. J Biol Chem. 1986 Sep 25;261(27):12437–12440. [PubMed] [Google Scholar]
- Sagar A., Rakowski R. F. Access channel model for the voltage dependence of the forward-running Na+/K+ pump. J Gen Physiol. 1994 May;103(5):869–893. doi: 10.1085/jgp.103.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbaky N. M., Pressley T. A. Mammalian alpha 1-subunit of Na(+)-K(+)-ATPase does not need its amino terminus to maintain cell viability. Am J Physiol. 1994 Aug;267(2 Pt 1):C590–C597. doi: 10.1152/ajpcell.1994.267.2.C590. [DOI] [PubMed] [Google Scholar]
- Stürmer W., Bühler R., Apell H. J., Läuger P. Charge translocation by the Na,K-pump: II. Ion binding and release at the extracellular face. J Membr Biol. 1991 Apr;121(2):163–176. doi: 10.1007/BF01870530. [DOI] [PubMed] [Google Scholar]
- Vasilets L. A., Ohta T., Noguchi S., Kawamura M., Schwarz W. Voltage-dependent inhibition of the sodium pump by external sodium: species differences and possible role of the N-terminus of the alpha-subunit. Eur Biophys J. 1993;21(6):433–443. doi: 10.1007/BF00185871. [DOI] [PubMed] [Google Scholar]
- Vasilets L. A., Omay H. S., Ohta T., Noguchi S., Kawamura M., Schwarz W. Stimulation of the Na+/K+ pump by external [K+] is regulated by voltage-dependent gating. J Biol Chem. 1991 Sep 5;266(25):16285–16288. [PubMed] [Google Scholar]
- Wang X., Horisberger J. D. A conformation of Na(+)-K+ pump is permeable to proton. Am J Physiol. 1995 Mar;268(3 Pt 1):C590–C595. doi: 10.1152/ajpcell.1995.268.3.C590. [DOI] [PubMed] [Google Scholar]
- Wierzbicki W., Blostein R. The amino-terminal segment of the catalytic subunit of kidney Na,K-ATPase regulates the potassium deocclusion pathway of the reaction cycle. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):70–74. doi: 10.1073/pnas.90.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. L., Guidotti G. Studies on the membrane topology of the (Na,K)-ATPase. J Biol Chem. 1994 Nov 11;269(45):28249–28258. [PubMed] [Google Scholar]