Abstract
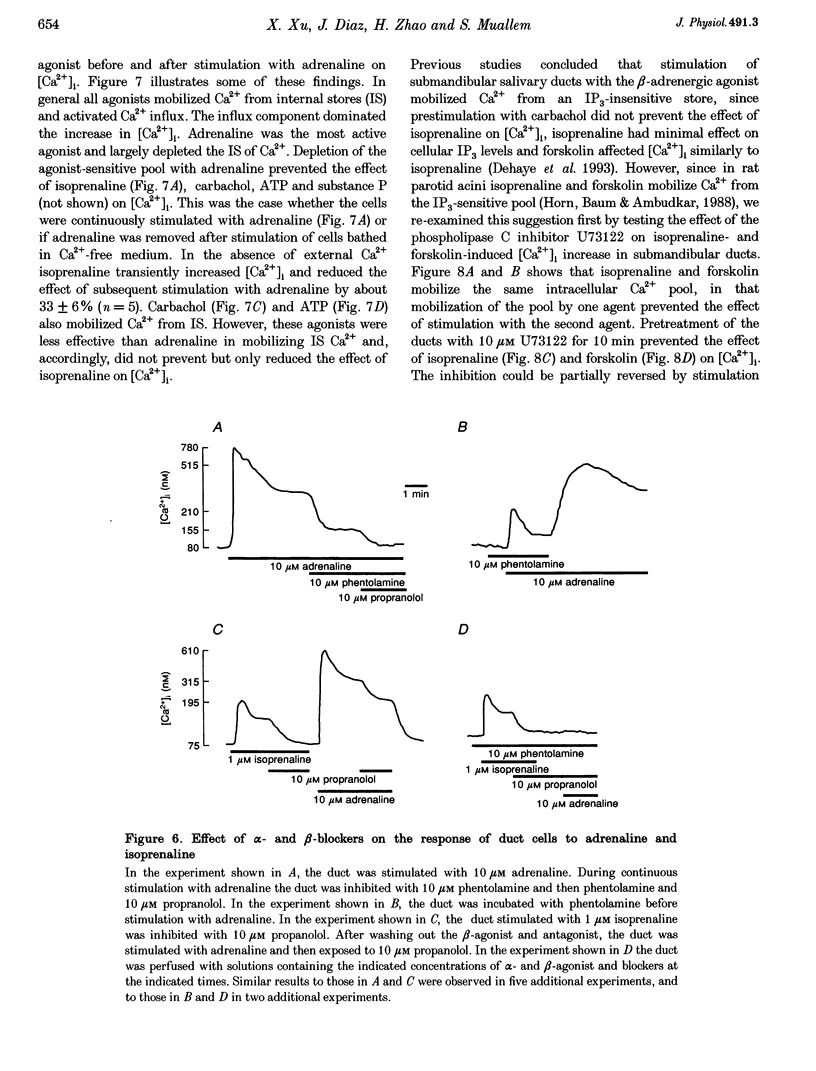
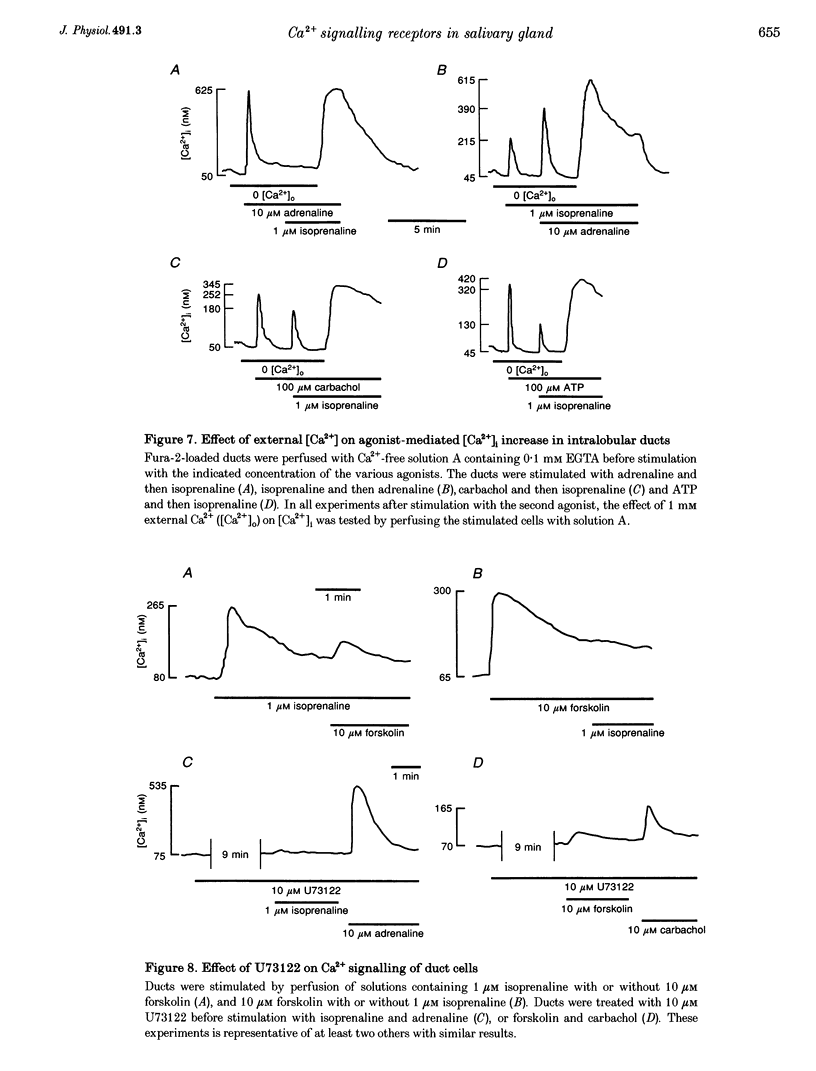
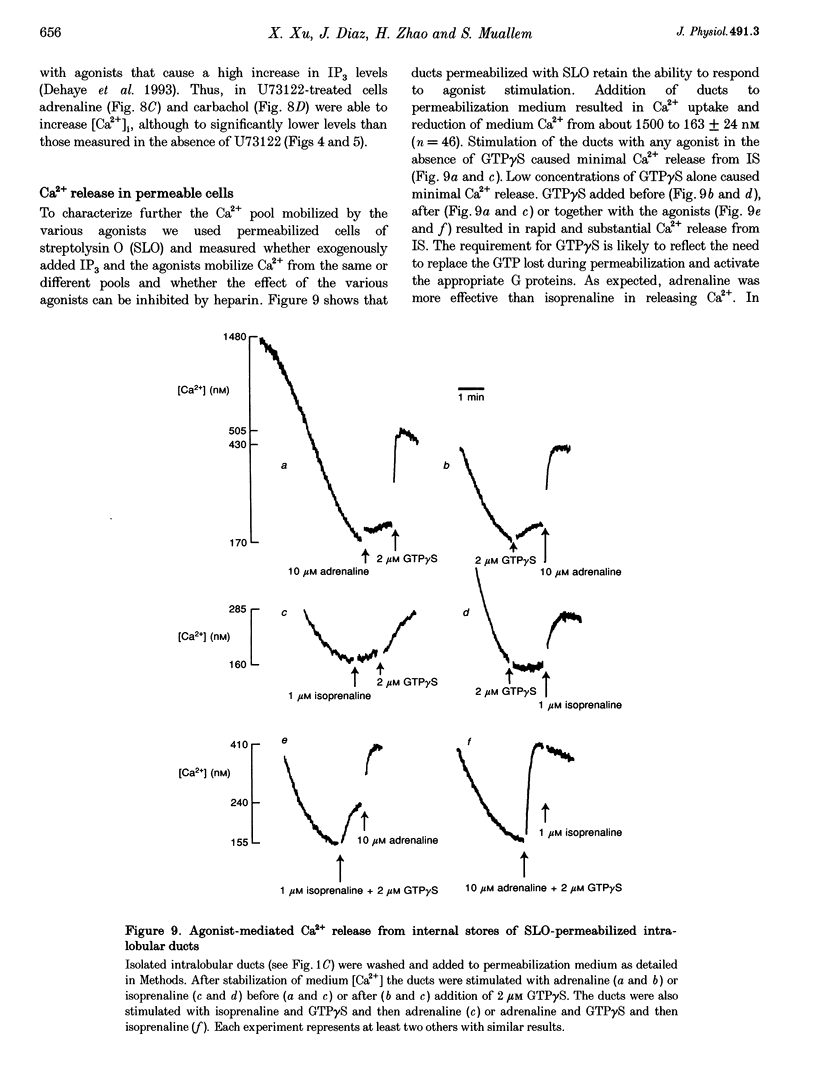
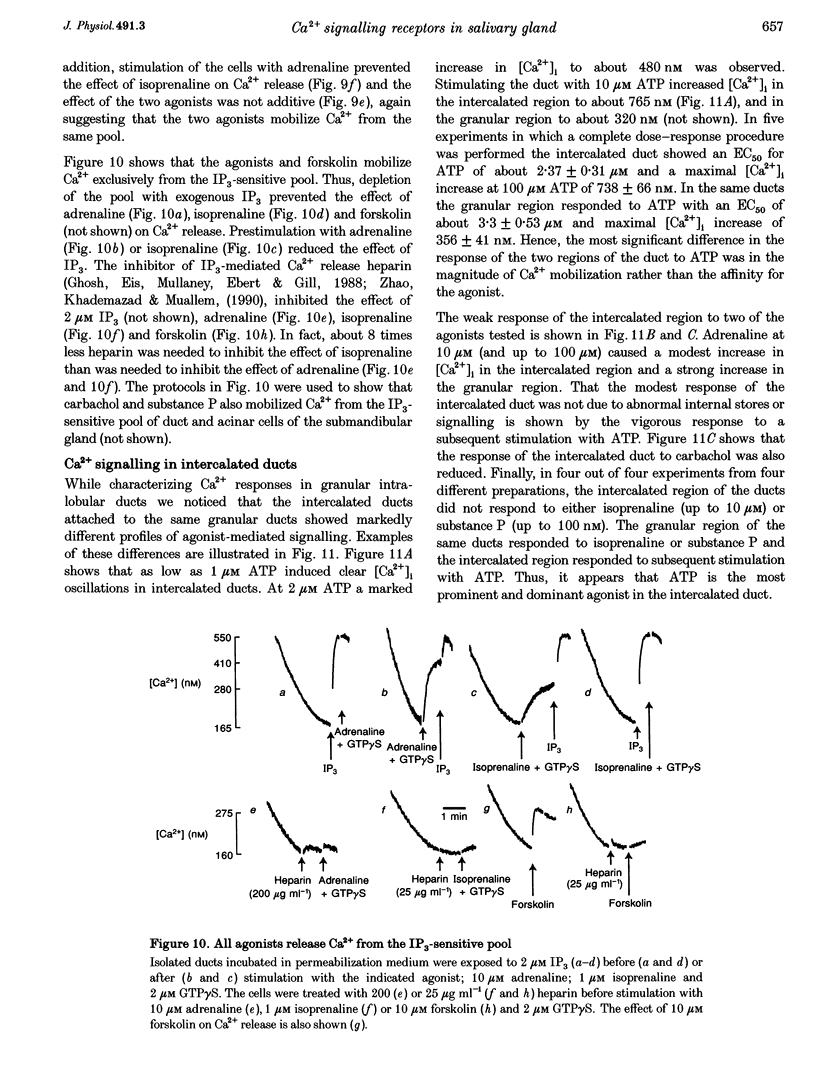
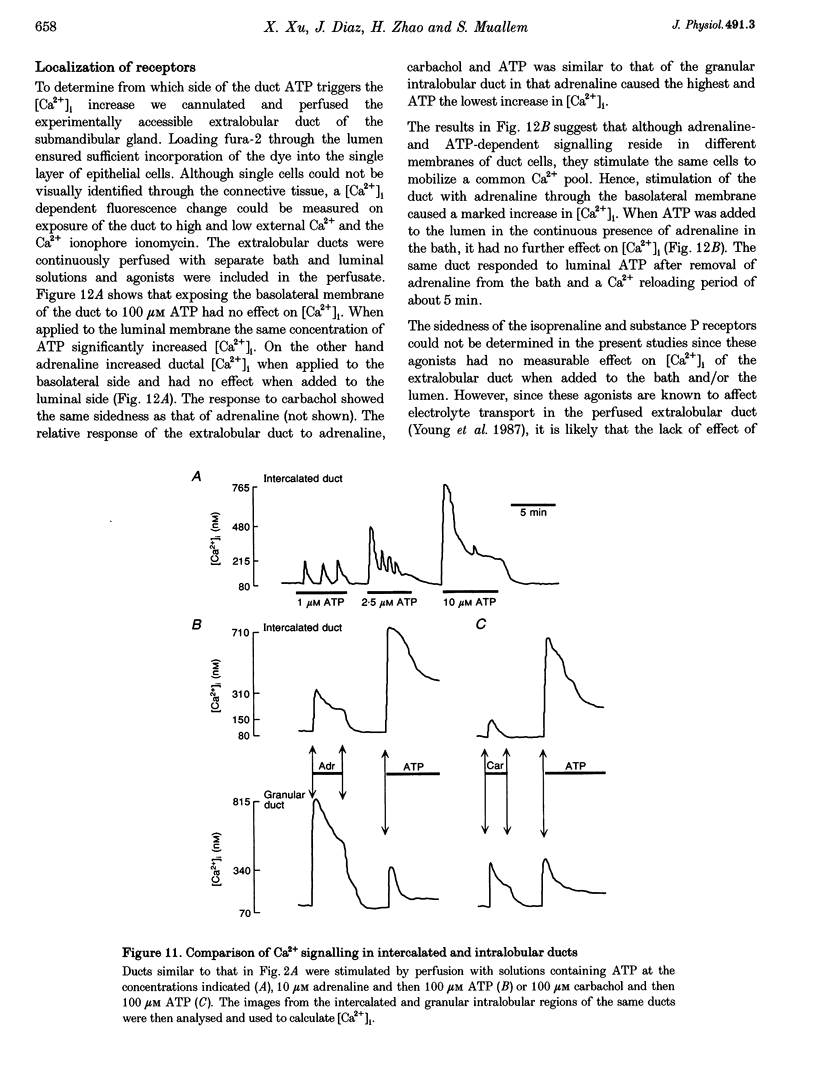
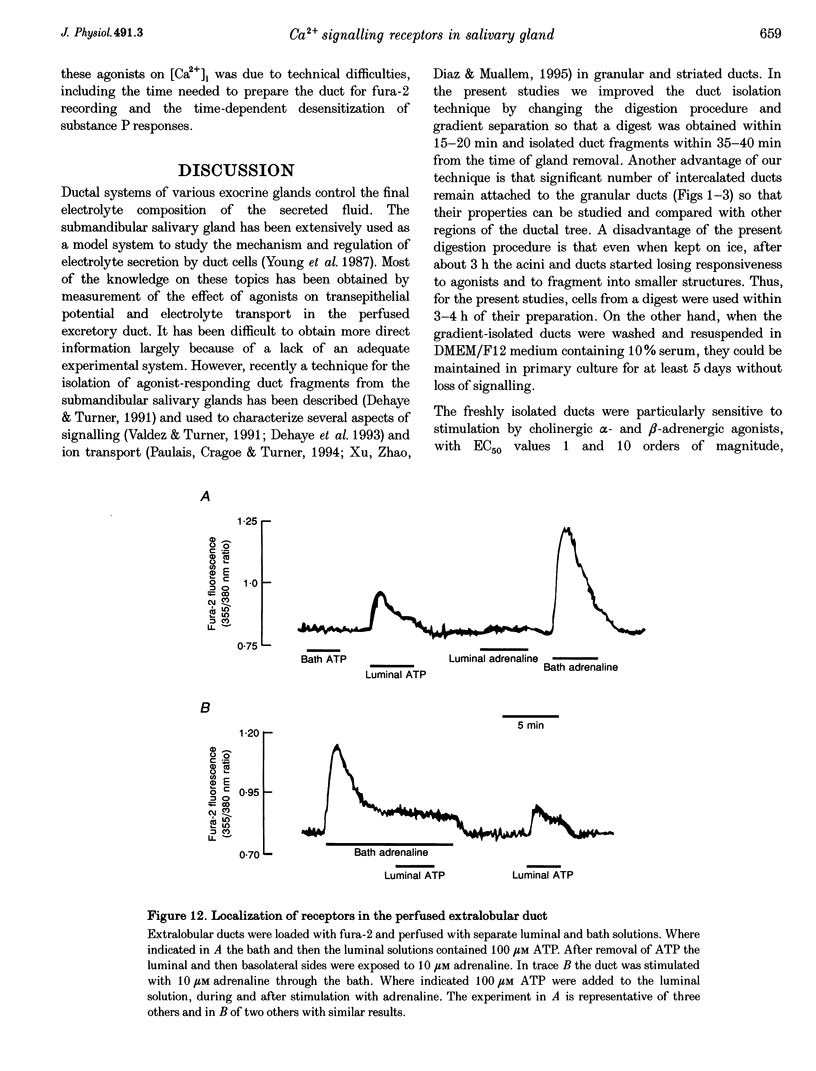
1. To characterize [Ca2+]i signalling in salivary duct cells a procedure was developed for the rapid preparation and isolation of intralobular ducts, some of which had attached intercalated ducts. The isolated ducts retained agonist-induced Ca2+ signalling after permeabilization with streptolysin O (SLO). 2. The improved cell preparation technique was reflected in the repertoire and intensity of agonist responsiveness of the cells. Measurements of [Ca2+]i in intact cells showed that all agonists previously reported to affect electrolyte transport by the submandibular salivary gland (adrenaline, carbachol, isoprenaline and forskolin) mobilized Ca2+ from internal stores and increased Ca2+ influx across the plasma membrane. 3. The use of the SLO-permeabilized ducts showed that all agonists, including isoprenaline and forskolin, mobilized Ca2+ exclusively from the inositol 1,4,5 trisphosphate (IP3)-sensitive pool. However, in granular ducts only adrenaline mobilized the entire IP3-sensitive pool whereas all other agonists mobilized only part of the pool. 4. All regions of the duct responded to substance P and the luminally secreted agonist ATP. Interestingly, the intercalated duct was most responsive to ATP and demonstrated only a minimal response to all other agonists. The granular region of the same duct and the extralobular duct always responded best to stimulation by adrenaline. 5. The perfused extralobular duct was used to show that adrenaline and carbachol stimulated the duct through the basolateral membrane whereas the receptors for ATP were localized in the luminal membrane of the duct. This suggests the presence of an ATP-dependent positive feedback loop in salivary duct with decreased activity along the ductal tree.
Full text
PDF



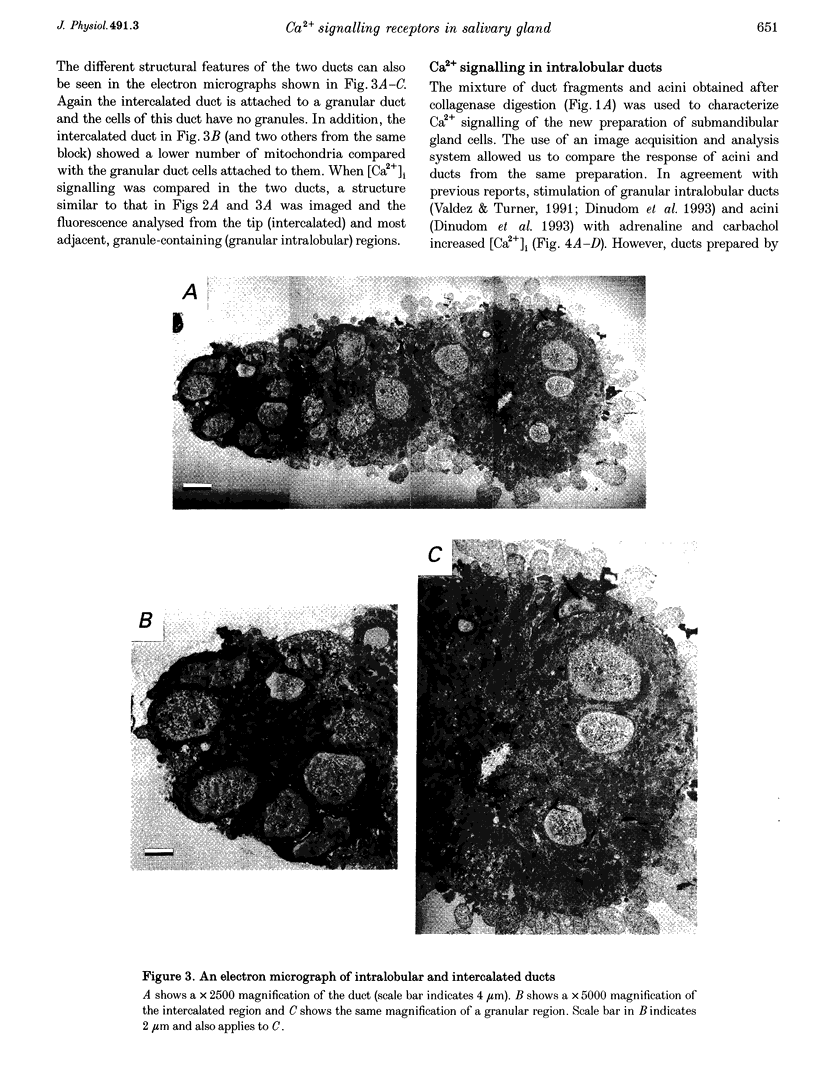
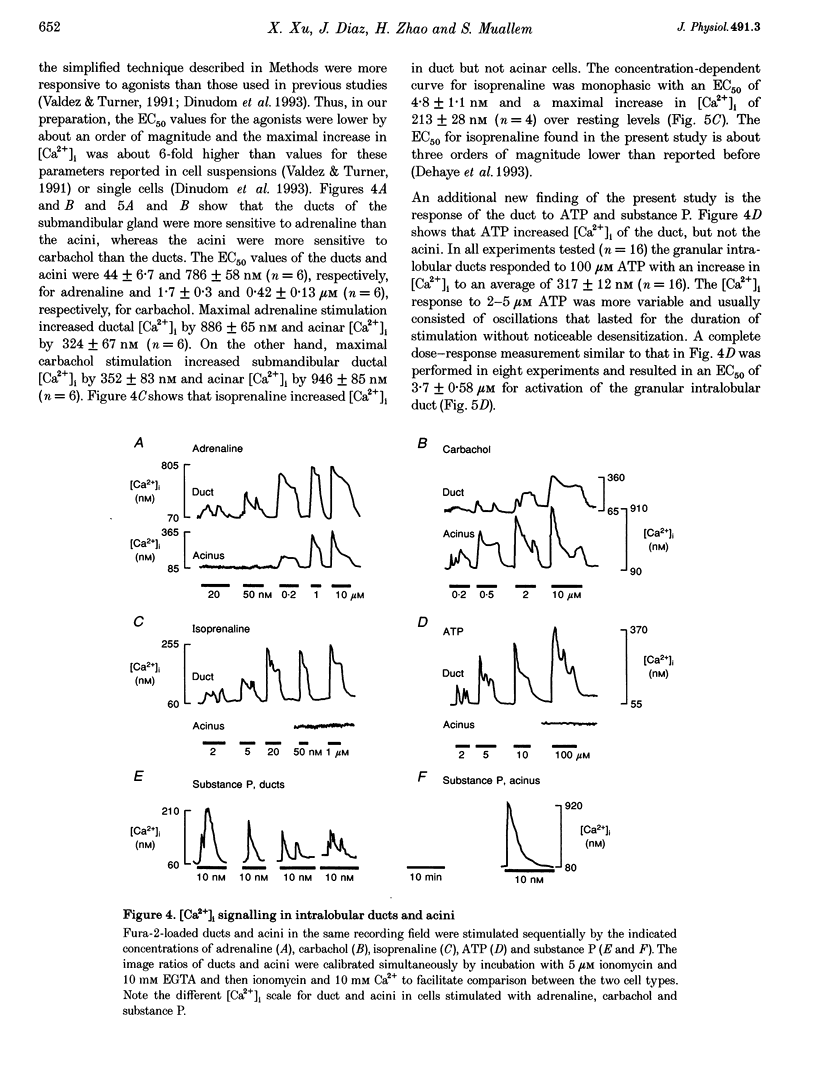
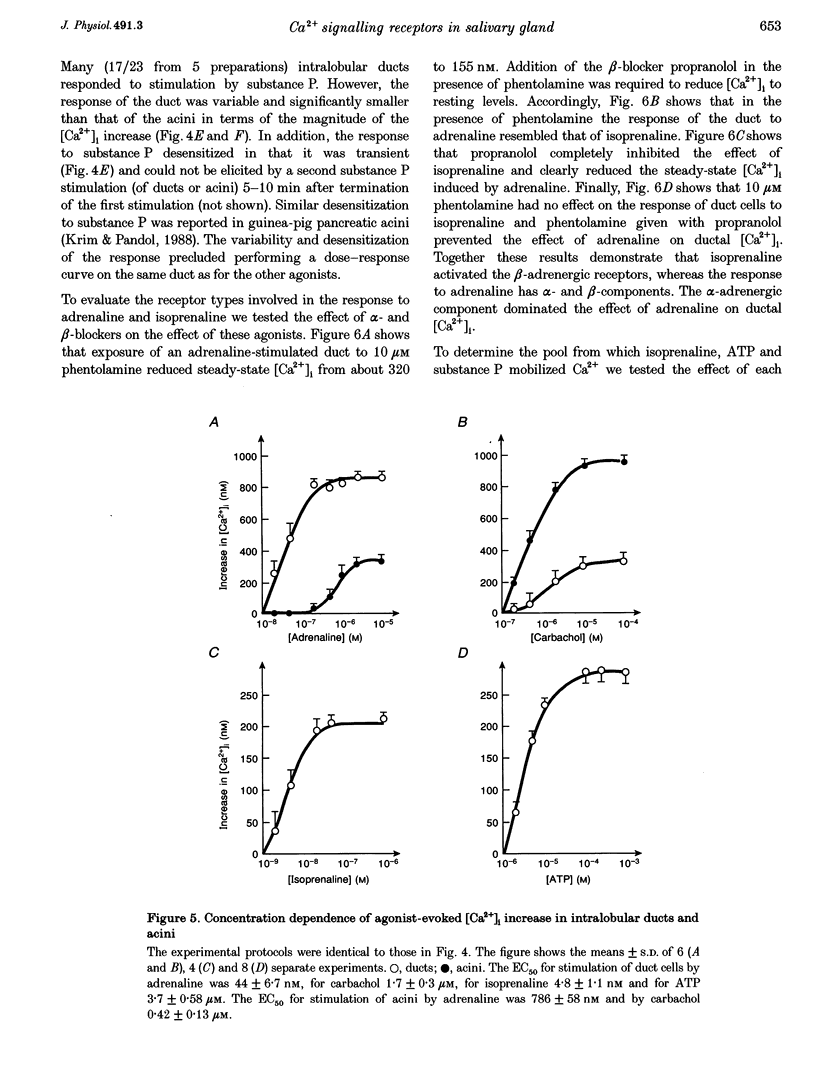



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Case R. M., Howorth A. J., Padfield P. J. Effects of acetylcholine, isoprenaline and forskolin on electrolyte and protein composition of rabbit mandibular saliva. J Physiol. 1988 Dec;406:411–430. doi: 10.1113/jphysiol.1988.sp017388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaye J. P., Turner R. J. Isolation and characterization of rat submandibular intralobular ducts. Am J Physiol. 1991 Sep;261(3 Pt 1):C490–C496. doi: 10.1152/ajpcell.1991.261.3.C490. [DOI] [PubMed] [Google Scholar]
- Denniss A. R., Schneyer L. H., Sucanthapree C., Young J. A. Actions of adrenergic agonists on isolated excretory ducts of submandibular glands. Am J Physiol. 1978 Dec;235(6):F548–F556. doi: 10.1152/ajprenal.1978.235.6.F548. [DOI] [PubMed] [Google Scholar]
- Denniss A. R., Young J. A. Modification of salivary duct electrolyte transport in rat and rabbit by physalaemin, VIP, GIP and other enterohormones. Pflugers Arch. 1978 Aug 25;376(1):73–80. doi: 10.1007/BF00585250. [DOI] [PubMed] [Google Scholar]
- Dinudom A., Poronnik P., Allen D. G., Young J. A., Cook D. I. Control of intracellular Ca2+ by adrenergic and muscarinic agonists in mouse mandibular ducts and end-pieces. Cell Calcium. 1993 Oct;14(9):631–638. doi: 10.1016/0143-4160(93)90088-n. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Lau K. R., Case R. M. Structural and functional characterization of striated ducts isolated from the rabbit mandibular salivary gland. Exp Physiol. 1993 Jan;78(1):49–64. doi: 10.1113/expphysiol.1993.sp003670. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Horn V. J., Baum B. J., Ambudkar I. S. Beta-adrenergic receptor stimulation induces inositol trisphosphate production and Ca2+ mobilization in rat parotid acinar cells. J Biol Chem. 1988 Sep 5;263(25):12454–12460. [PubMed] [Google Scholar]
- Martin C. J., Frömter E., Gebler B., Knauf H., Young J. A. The effects of carbachol on water and electrolyte fluxes and transepithelial electrical potential differences of the rabbit submaxillary main duct perfused in vitro. Pflugers Arch. 1973 Jun 26;341(2):131–142. doi: 10.1007/BF00587320. [DOI] [PubMed] [Google Scholar]
- Martin C. J., Young J. A. A microperfusion investigation of the effects of a sympathomimetic and a parasympathomimetic drug on water and electrolyte fluxes in the main duct of the rat submaxillary gland. Pflugers Arch. 1971;327(4):303–323. doi: 10.1007/BF00588450. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Simon M. I. G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem. 1995 Jun 23;270(25):15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- Paulais M., Cragoe E. J., Jr, Turner R. J. Ion transport mechanisms in rat parotid intralobular striated ducts. Am J Physiol. 1994 Jun;266(6 Pt 1):C1594–C1602. doi: 10.1152/ajpcell.1994.266.6.C1594. [DOI] [PubMed] [Google Scholar]
- Schneyer L. H. Parasympathetic control of Na, K transport in perfused submaxillary duct of the rat. Am J Physiol. 1977 Jul;233(1):F22–F28. doi: 10.1152/ajprenal.1977.233.1.F22. [DOI] [PubMed] [Google Scholar]
- Schneyer L. H. Sympathetic control of Na, K transport in perfused submaxillary main duct of rat. Am J Physiol. 1976 Feb;230(2):341–345. doi: 10.1152/ajplegacy.1976.230.2.341. [DOI] [PubMed] [Google Scholar]
- Stauffer P. L., Zhao H., Luby-Phelps K., Moss R. L., Star R. A., Muallem S. Gap junction communication modulates [Ca2+]i oscillations and enzyme secretion in pancreatic acini. J Biol Chem. 1993 Sep 15;268(26):19769–19775. [PubMed] [Google Scholar]
- THAYSEN J. H., THORN N. A., SCHWARTZ I. L. Excretion of sodium, potassium, chloride and carbon dioxide in human parotid saliva. Am J Physiol. 1954 Jul;178(1):155–159. doi: 10.1152/ajplegacy.1954.178.1.155. [DOI] [PubMed] [Google Scholar]
- Tortorici G., Zhang B. X., Xu X., Muallem S. Compartmentalization of Ca2+ signaling and Ca2+ pools in pancreatic acini. Implications for the quantal behavior of Ca2+ release. J Biol Chem. 1994 Nov 25;269(47):29621–29628. [PubMed] [Google Scholar]
- Valdez I. H., Turner R. J. Effects of secretagogues on cytosolic Ca2+ levels in rat submandibular granular ducts and acini. Am J Physiol. 1991 Aug;261(2 Pt 1):G359–G363. doi: 10.1152/ajpgi.1991.261.2.G359. [DOI] [PubMed] [Google Scholar]
- Xu X., Zhao H., Diaz J., Muallem S. Regulation of [Na+]i in resting and stimulated submandibular salivary ducts. J Biol Chem. 1995 Aug 18;270(33):19606–19612. [PubMed] [Google Scholar]
- Young J. A., Martin C. J., Asz M., Weber F. D. A microperfusion investigation of bicarbonate secretion by the rat submaxillary gland. The action of a parasumpathomimetic drug on electrolyte transport. Pflugers Arch. 1970;319(3):185–199. doi: 10.1007/BF00586449. [DOI] [PubMed] [Google Scholar]
- Zhang B. X., Tortorici G., Xu X., Muallem S. Antagonists inactivate the inositol 1,4,5-trisphosphate (Ins-1,4,5-P3)-dependent Ca2+ channel independent of Ins-1,4,5-P3 metabolism. J Biol Chem. 1994 Jun 24;269(25):17132–17135. [PubMed] [Google Scholar]
- Zhao H., Khademazad M., Muallem S. Agonist-mediated Ca2+ release in permeabilized UMR-106-01 cells. Transport properties and generation of inositol 1,4,5-trisphosphate. J Biol Chem. 1990 Sep 5;265(25):14822–14827. [PubMed] [Google Scholar]
- Zhao H., Star R. A., Muallem S. Membrane localization of H+ and HCO3- transporters in the rat pancreatic duct. J Gen Physiol. 1994 Jul;104(1):57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Xu X., Diaz J., Muallem S. Na+, K+, and H+/HCO3- transport in submandibular salivary ducts. Membrane localization of transporters. J Biol Chem. 1995 Aug 18;270(33):19599–19605. [PubMed] [Google Scholar]
- al-Awqati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995 Aug 11;269(5225):805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]