Abstract
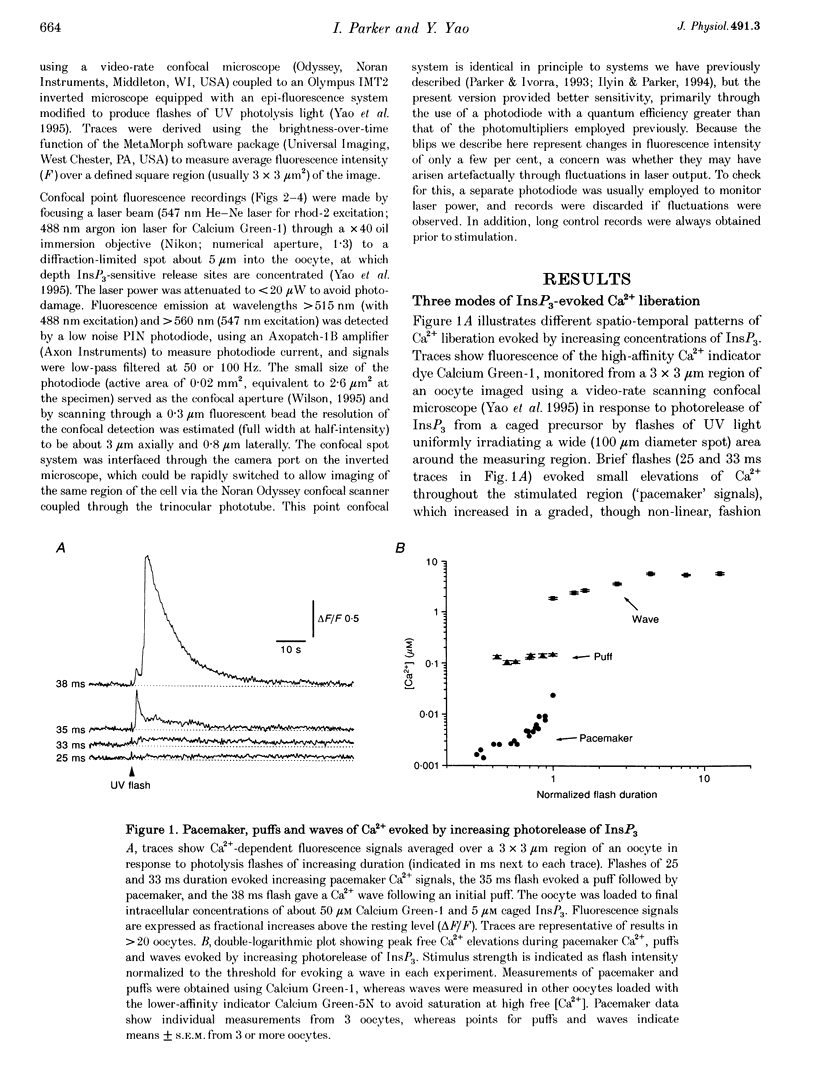
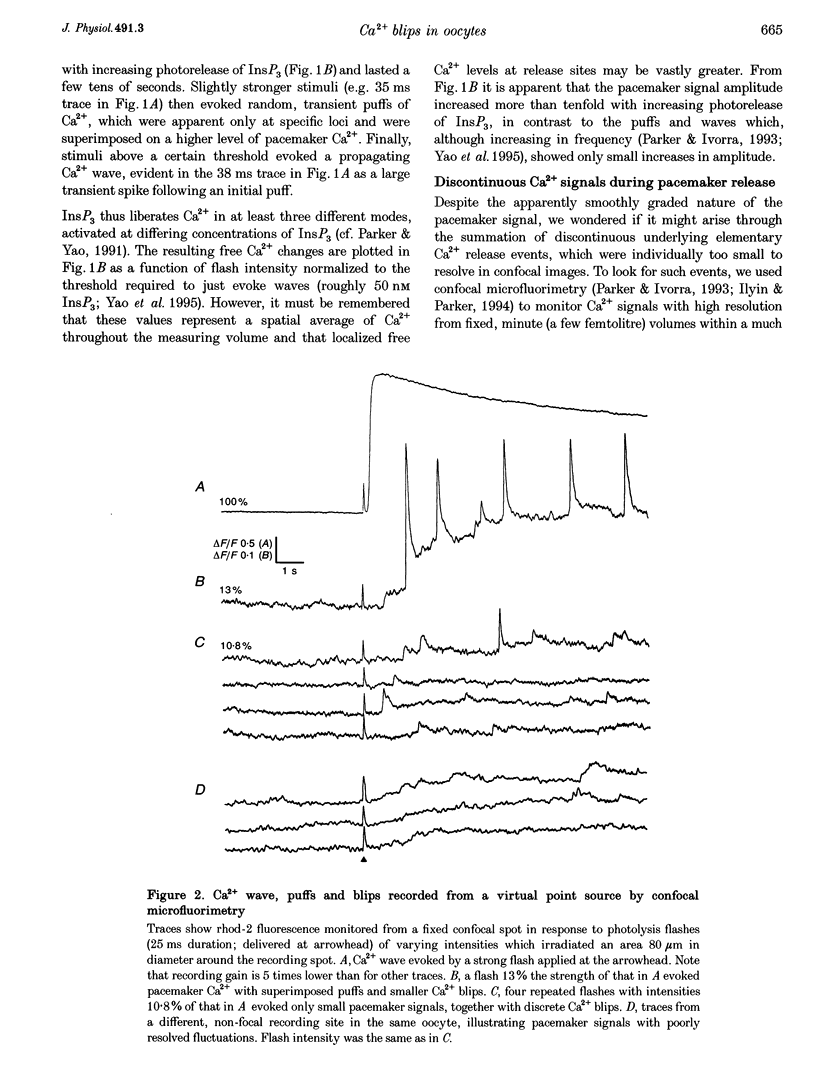
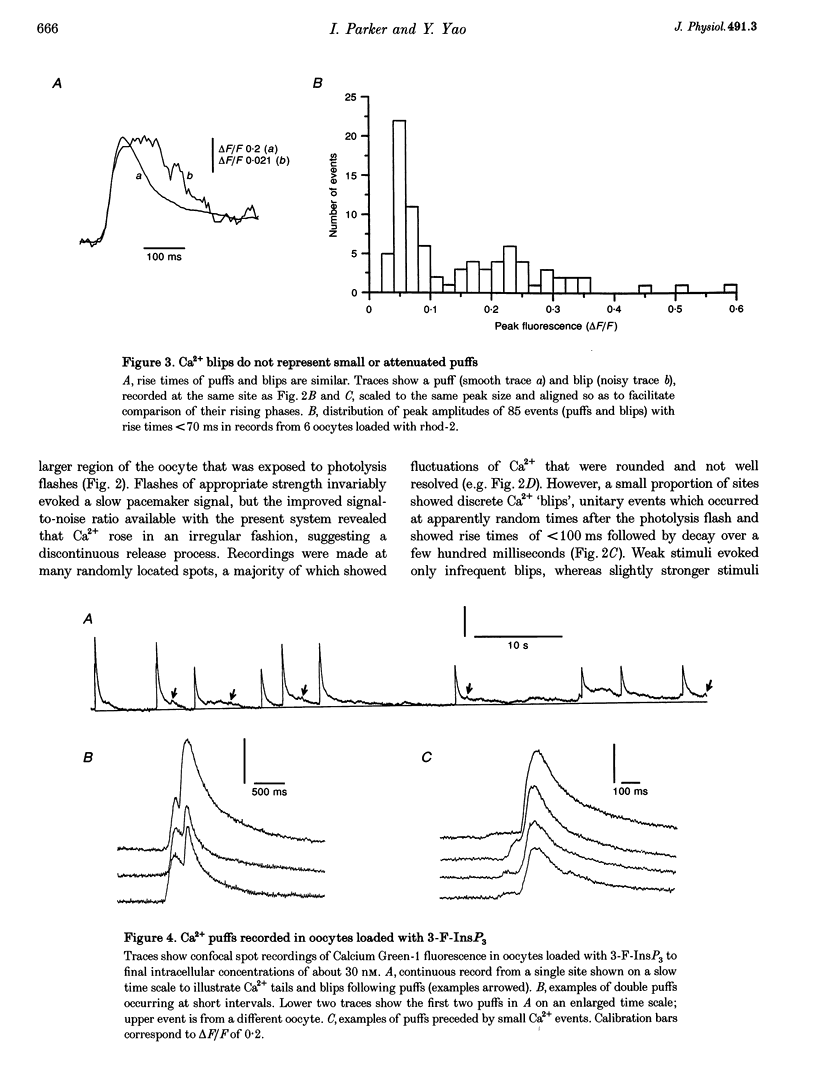
1. The mechanisms underlying inositol 1,4,5-trisphosphate (InsP3)-induced Ca2+ liberation were studied in Xenopus oocytes by using scanning and stationary-point confocal fluorescence microscopy to record Ca2+ signals evoked by photorelease of InsP3 from a caged precursor. 2. Fluorescence measurements from confocal images showed that increasing [InsP3] evoked three distinct modes of Ca2+ liberation: a diffuse 'pacemaker' signal, localized transient puffs, and propagating waves. Peak free Ca2+ concentrations during waves and puffs (respectively, 2-5 microM and 100-200 nM) varied only slightly with [InsP3], whereas the pacemaker amplitude varied over a wider range (at least 1-30 nM Ca2+). 3. The improved resolution provided by confocal point recording revealed discontinuous Ca2+ 'blips' during pacemaker release. These events were resolved only at particular locations and had time courses similar to the puffs (rise, approximately 50 ms; decay, a few hundred milliseconds) but with amplitudes one-fifth or less of puff amplitudes. 4. We conclude that blips may arise through opening of single InsP3-gated channels, whereas puffs reflect the concerted opening of several clustered channels due to local regenerative feedback by Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clays K., Hendrickx E., Triest M., Verbiest T., Persoons A., Dehu C., Brédas J. L. Nonlinear optical properties of proteins measured by hyper-rayleigh scattering in solution. Science. 1993 Nov 26;262(5138):1419–1422. doi: 10.1126/science.262.5138.1419. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Ilyin V., Parker I. Role of cytosolic Ca2+ in inhibition of InsP3-evoked Ca2+ release in Xenopus oocytes. J Physiol. 1994 Jun 15;477(Pt 3):503–509. doi: 10.1113/jphysiol.1994.sp020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Li Y. X., Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993 Aug 27;74(4):669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local, stochastic release of Ca2+ in voltage-clamped rat heart cells: visualization with confocal microscopy. J Physiol. 1994 Oct 1;480(Pt 1):21–29. doi: 10.1113/jphysiol.1994.sp020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Confocal microfluorimetry of Ca2+ signals evoked in Xenopus oocytes by photoreleased inositol trisphosphate. J Physiol. 1993 Feb;461:133–165. doi: 10.1113/jphysiol.1993.sp019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 1991 Dec 23;246(1317):269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Lückhoff A., Clapham D. E. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995 Jan;14(1):163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Yagodin S. V., Holtzclaw L., Sheppard C. A., Russell J. T. Nonlinear propagation of agonist-induced cytoplasmic calcium waves in single astrocytes. J Neurobiol. 1994 Mar;25(3):265–280. doi: 10.1002/neu.480250307. [DOI] [PubMed] [Google Scholar]
- Yao Y., Choi J., Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol. 1995 Feb 1;482(Pt 3):533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Parker I. Potentiation of inositol trisphosphate-induced Ca2+ mobilization in Xenopus oocytes by cytosolic Ca2+. J Physiol. 1992 Dec;458:319–338. doi: 10.1113/jphysiol.1992.sp019420. [DOI] [PMC free article] [PubMed] [Google Scholar]


