Abstract
1. Membrane potentials (Em) and currents (Im) were measured using whole-cell patch clamp techniques in inspiratory (Insp, types I-III), preinspiratory (Pre-I) and tonic expiratory (Exp) neurons of the ventral respiratory group (VRG) in the isolated brainstem-spinal cord preparation of 0- to 4-day-old rats. 2. After blocking on-going synaptic activity with 0.2-0.5 microM tetrodotoxin (TTX), Ca(2+)-dependent responses were analysed using patch pipettes containing 120 mM Cs+ and 20 mM tetraethylammonium (TEA) to block K+ conductances. 3. In all cells studied, all-or-none high voltage-activated (HVA) Ca2+ spikes with an activation threshold of -33 +/- 6.9 mV (n = 37) were evoked by depolarizing current pulses. 4. In less than 15% of Insp and Pre-I cells and in 20% of Exp neurons, termination of hyperpolarizing pulses led to low voltage-activated (LVA) Ca2+ spikes with a threshold potential of between -70 and -60 mV (n = 7). 5. In more than 50% of Insp III and Pre-I neurons, depolarizing pulses evoked graded 'plateau' potentials with an amplitude of 5-20 mV. Slow voltage ramp commands revealed that this type of Ca2+ response was due to an inward current with a mean activation threshold of -42 +/- 2.1 mV (n = 5). These intermediate voltage-activated (IVA) plateau potentials persisted for several seconds after termination of depolarizing current pulses and decreased in amplitude at more negative holding potentials. 6. The HVA and LVA Ca2+ spikes as well as the IVA plateau potentials and the underlying inward current were potentiated after extracellular addition of 2 mM Ba2+ whereas 1-2 mM Co2+ led to blockade of these responses. 7. Nifedipine (10 microM) selectively suppressed HVA Ca2+ potentials whereas 0.2-0.4 microM omega-agatoxin-IVA reduced the IVA response without major effects on HVA Ca2+ spikes. omega-Conotoxin-GVIA (2 microM) led to a partial blockade of both IVA and HVA potentials. 8. After extracellular application of TTX, Ba2+ and/or TEA, HVA and LVA Ca2+ spikes as well as IVA plateau potentials were also revealed using patch pipettes containing K+ instead of Cs+ and TEA. 9. The results indicate that neonatal respiratory neurons have a complex set of Ca(2+)-dependent membrane conductances. The relevance of these conductances for initiation and maintenance of respiratory bursts is discussed.
Full text
PDF

















Selected References
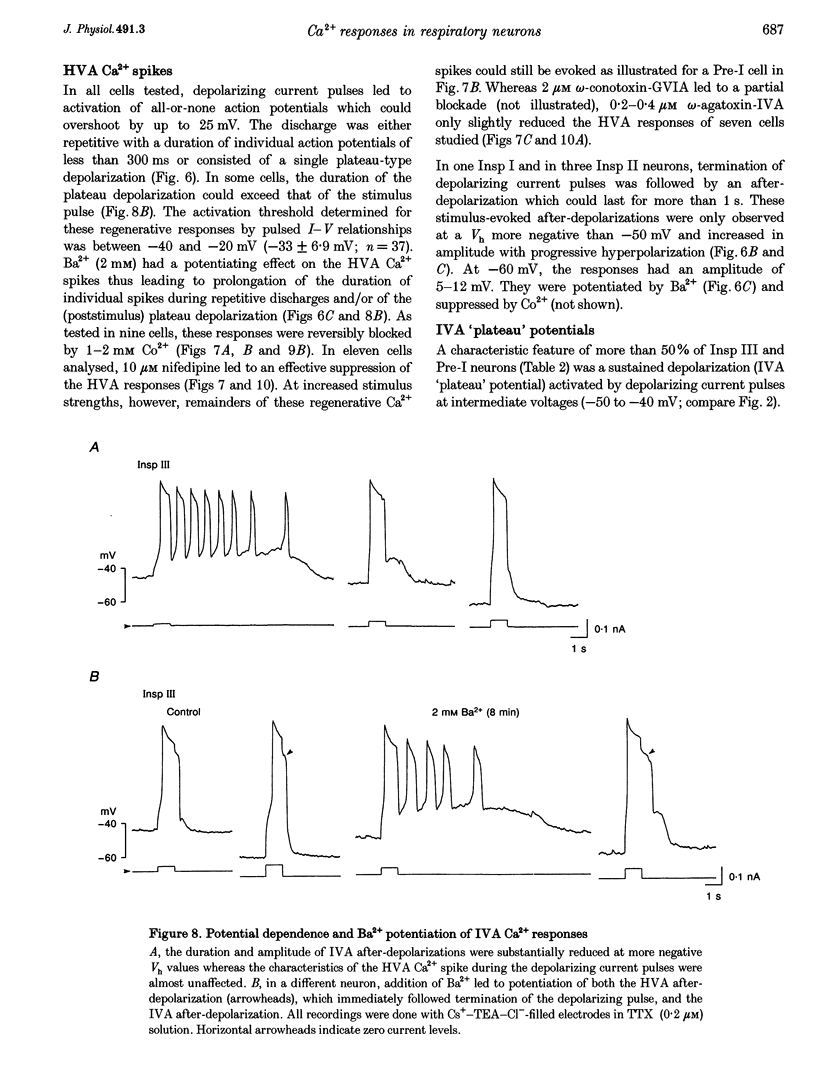
These references are in PubMed. This may not be the complete list of references from this article.
- Arata A., Onimaru H., Homma I. Respiration-related neurons in the ventral medulla of newborn rats in vitro. Brain Res Bull. 1990 Apr;24(4):599–604. doi: 10.1016/0361-9230(90)90165-v. [DOI] [PubMed] [Google Scholar]
- Ballanyi K., Völker A., Richter D. W. Anoxia induced functional inactivation of neonatal respiratory neurones in vitro. Neuroreport. 1994 Dec 30;6(1):165–168. doi: 10.1097/00001756-199412300-00042. [DOI] [PubMed] [Google Scholar]
- Bernath S. Calcium-independent release of amino acid neurotransmitters: fact or artifact? Prog Neurobiol. 1992;38(1):57–91. doi: 10.1016/0301-0082(92)90035-d. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Jacquin T., Richter D. W. Voltage-dependent currents in neurones of the nuclei of the solitary tract of rat brainstem slices. Pflugers Arch. 1986 Apr;406(4):372–379. doi: 10.1007/BF00590939. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Richter D. W. The roles of K+ conductance in expiratory pattern generation in anaesthetized cats. J Physiol. 1994 Aug 15;479(Pt 1):127–138. doi: 10.1113/jphysiol.1994.sp020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J. L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991 Dec;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Smith J. C., Liu G. Respiratory pattern generation in mammals: in vitro en bloc analyses. Curr Opin Neurobiol. 1991 Dec;1(4):590–594. doi: 10.1016/s0959-4388(05)80033-9. [DOI] [PubMed] [Google Scholar]
- Funk G. D., Smith J. C., Feldman J. L. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993 Oct;70(4):1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Gottmann K., Dietzel I. D., Lux H. D., Huck S., Rohrer H. Development of inward currents in chick sensory and autonomic neuronal precursor cells in culture. J Neurosci. 1988 Oct;8(10):3722–3732. doi: 10.1523/JNEUROSCI.08-10-03722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn K., Watson T. W., MacVicar B. A. A novel tetrodotoxin-insensitive, slow sodium current in striatal and hippocampal neurons. Neuron. 1993 Mar;10(3):543–552. doi: 10.1016/0896-6273(93)90341-n. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Kiehn O. Transmitter-controlled properties of alpha-motoneurones causing long-lasting motor discharge to brief excitatory inputs. Prog Brain Res. 1986;64:39–49. doi: 10.1016/S0079-6123(08)63398-1. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Smith J. C., Funk G. D., Feldman J. L. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J Neurophysiol. 1994 Dec;72(6):2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M., Onimaru H., Homma I. Correlation analysis of respiratory neuron activity in ventrolateral medulla of brainstem-spinal cord preparation isolated from newborn rat. Exp Brain Res. 1993;95(2):277–290. doi: 10.1007/BF00229786. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Huguenard J. R. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992 Oct;68(4):1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Miljanich G. P., Ramachandran J., Adams M. E. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Arata A., Homma I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflugers Arch. 1990 Dec;417(4):425–432. doi: 10.1007/BF00370663. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Arata A., Homma I. Intrinsic burst generation of preinspiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Exp Brain Res. 1995;106(1):57–68. doi: 10.1007/BF00241356. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Arata A., Homma I. Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1988 Apr 5;445(2):314–324. doi: 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987 Feb 17;403(2):380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Onimaru H. Studies of the respiratory center using isolated brainstem-spinal cord preparations. Neurosci Res. 1995 Jan;21(3):183–190. doi: 10.1016/0168-0102(94)00863-b. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Swandulla D. Calcium-activated non-specific cation channels. Trends Neurosci. 1988 Feb;11(2):69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Paton J. F., Ramirez J. M., Richter D. W. Mechanisms of respiratory rhythm generation change profoundly during early life in mice and rats. Neurosci Lett. 1994 Mar 28;170(1):167–170. doi: 10.1016/0304-3940(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O., Champagnat J., Richter D. W. Calcium-dependent conductances control neurones involved in termination of inspiration in cats. Neurosci Lett. 1995 Jan 23;184(2):101–104. doi: 10.1016/0304-3940(94)11179-m. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Ballanyi K., Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1992 Dec;2(6):788–793. doi: 10.1016/0959-4388(92)90135-8. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Camerer H., Meesmann M., Röhrig N. Studies on the synaptic interconnection between bulbar respiratory neurones of cats. Pflugers Arch. 1979 Jul;380(3):245–257. doi: 10.1007/BF00582903. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Champagnat J., Jacquin T., Benacka R. Calcium currents and calcium-dependent potassium currents in mammalian medullary respiratory neurones. J Physiol. 1993 Oct;470:23–33. doi: 10.1113/jphysiol.1993.sp019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Ballanyi K., Richter D. W. Whole-cell patch-clamp recordings from respiratory neurons in neonatal rat brainstem in vitro. Neurosci Lett. 1992 Jan 6;134(2):153–156. doi: 10.1016/0304-3940(92)90504-z. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991 Nov 1;254(5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Greer J. J., Liu G. S., Feldman J. L. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990 Oct;64(4):1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984 Sep;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swandulla D., Carbone E., Lux H. D. Do calcium channel classifications account for neuronal calcium channel diversity? Trends Neurosci. 1991 Feb;14(2):46–51. doi: 10.1016/0166-2236(91)90018-p. [DOI] [PubMed] [Google Scholar]
- Takahashi T. The minimal inhibitory synaptic currents evoked in neonatal rat motoneurones. J Physiol. 1992 May;450:593–611. doi: 10.1113/jphysiol.1992.sp019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda R., Haji A. Mechanisms underlying post-inspiratory depolarization in post-inspiratory neurons of the cat. Neurosci Lett. 1993 Feb 5;150(1):1–4. doi: 10.1016/0304-3940(93)90093-z. [DOI] [PubMed] [Google Scholar]
- Umemiya M., Berger A. J. Single-channel properties of four calcium channel types in rat motoneurons. J Neurosci. 1995 Mar;15(3 Pt 2):2218–2224. doi: 10.1523/JNEUROSCI.15-03-02218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F., Bayliss D. A., Berger A. J. Calcium conductances and their role in the firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993 Jun;69(6):2137–2149. doi: 10.1152/jn.1993.69.6.2137. [DOI] [PubMed] [Google Scholar]
- Ye J. H., Akaike N. Calcium currents in pyramidal neurons acutely dissociated from the rat frontal cortex: a study by the nystatin perforated patch technique. Brain Res. 1993 Mar 19;606(1):111–117. doi: 10.1016/0006-8993(93)91577-f. [DOI] [PubMed] [Google Scholar]


