Abstract
Permanent cardiac pacing in children with congenital complete atrioventricular block (CCAVB) and/or congenital heart disease (CHD) is challenging. Conduction system pacing (CSP) represents a novel pacing strategy aiming to preserve physiological ventricular activation. Patients with CCAVB or CHD are at high risk of developing pacing-induced cardiomyopathy with chronic conventional right ventricular myocardial pacing. CSP may be a valuable pacing modality in this particular setting because it can preserve ventricular synchrony. In this review, we summarise implantation techniques, the available clinical evidence and future directions related to CSP in CCAVB and CHD.
Keywords: Conduction system pacing, His bundle pacing, left bundle branch area pacing, CHD, congenital complete atrioventricular block
Permanent cardiac pacing in children and young patients is often required for congenital complete atrioventricular block (CCAVB) or atrioventricular (AV) conduction abnormalities related to congenital heart disease (CHD). Decades of chronic myocardial ventricular pacing, especially for those with CHD, may place patients at risk of systemic ventricular dysfunction.[1] Conventional biventricular CRT for these patients can be challenging because coronary sinus (CS) abnormalities are abundant and current tools to access the CS are designed for adults with normal cardiac anatomy. Recently, conduction system pacing (CSP) has emerged as a novel pacing modality, aimed at preserving or restoring ventricular synchrony. Both His bundle pacing (HBP) and left bundle branch area pacing (LBBAP) have been shown to be safe and feasible for a wide range of bradycardia and CRT indications and are very much applicable to children and those with CHD.[2–6] This comprehensive review covers aspects related to the implantation technique, current clinical evidence and future directions for CSP among children and CHD patients.
General Challenges of Pacing in Children and Patients with CHD
Challenges associated with chronic ventricular pacing in children with CCAVB or CHD are multiple. Typical examples include smaller body size, surgical sequelae (septal patches and AV valve abnormalities) and longterm venous patency.[7] Further, somatic growth and patient survival usually surpass the longevity of the pacing systems. Such factors compound the complexity of ventricular pacing in children and CHD, and contribute to a non-negligible risk of adverse outcomes.
Conduction System Pacing in Congenital Complete Atrioventricular Block
Congenital AV block (CAVB) occurs in 1–2% of newborns from mothers with positive anti-SSA/Ro antibodies.[7] Chronic ventricular pacing is required in approximately two-thirds of patients by 1 year of age and pacing-induced cardiomyopathy is observed in 10–15%.[1] Accordingly, CSP represents an attractive option for young patients with CAVB.
There are currently no CSP implant tools designed specifically for children (Supplementary Figure 1). Most experience has been reported using the SelectSecure 3830 lumenless lead (Medtronic) and the Medtronic C315His sheath.[4] For those patients with smaller body size, the C315His sheath tends to direct the lead to a mid to apical septal position. Other sheaths, such as the C315S4 and the C315S5, although initially designed for selectsite atrial pacing, may be more suitable for CSP in children given the shorter shaft (30 versus 43 cm for the C315His) and more appropriate proximal and distal curves. Manually shaping the C315His sheath or using a steerable sheath (Medtronic C304His) are alternatives. Similarly, the use of CS delivery sheaths has been also described alone or in combination with the C315His to achieve additional support in complex cases.[6]
The implant technique for children with CAVB is the same as for the adult population with some specific recommendations related to small body size. For LBBAP, preprocedure assessment of interventricular septum wall thickness, as well as continuous monitoring of fixation beats and unfiltered unipolar electrograms, are essential to avoid perforation into the left ventricle (LV). Once the lead has been implanted, special care should be taken to provide adequate lead slack for future growth (Figure 1).
Figure 1: A 14-year-old Patient with Congenital Heart Block Undergoing Left Bundle Branch Area Pacing.
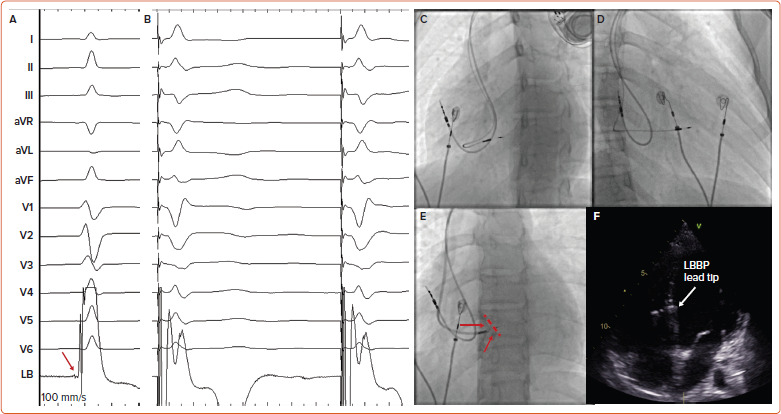
A: 12-lead ECG during spontaneous rhythm showing a left bundle potential at the final lead position (red arrow). B: 12-lead ECG during non-selective left bundle branch capture. C, D: Final lead position in left anterior oblique (C) and right anterior oblique (D) projections showing extra lead slack for both right atrial and left bundle branch area pacing leads. E: Contrast through the sheath showing lead depth in the left anterior oblique projection. The red dashed line indicates the interventricular septum position. Red arrows show the contrast through the sheath. F: Transthoracic echocardiogram showing lead position in the subendocardium of the left ventricular mid-septum. LBBP = left bundle branch pacing.
The need for a backup ventricular lead for paediatric CAVB after HBP is unknown. In the largest paediatric series to date, backup leads were not described and no significant complications were reported despite the well-known increase in chronic pacing threshold associated with HBP.[8] It is reasonable to consider that the more reliable underlying escape rhythm of CCAVB mitigates the issue. For LBBAP, the backup leads are generally not required given stable pacing electrical parameters during follow-up.
Clinical Outcomes of Conduction System Pacing for Complete Atrioventricular Block
The outcomes of CSP for patients with CCAVB appear favourable.[9–13] Dandamudi et al. reported the multicentre outcomes of HBP among a cohort of 17 patients with CCAVB.[14] Overall, New York Heart Association class improved significantly and, for three patients with baseline pacing-induced cardiomyopathy, the LV ejection fraction nearly normalised (from a mean [±SD] of 26.3 ± 14.8% to 48.3 ± 2.9%).
Limitations of Single-site Pacing in CHD
For CHD, anatomical diversity confers profound clinical implications on electromechanical dyssynchrony and the CRT approach. Patients are broadly characterised by either a ‘systemic LV’ or ‘systemic right ventricle’ (RV) to distinguish the dominant ventricular morphology supporting the systemic circulation. With chronic single-site transvenous pacing, electromechanical dyssynchrony can adversely impact the dominant (systemic) ventricle, leading to myocardial dysfunction and failure.
Among those with CHD, systemic LV patients represent the largest group referred for CRT (˜65% of patients).[15,16] Chronic RV pacing is required for 1–3% of patients undergoing intracardiac repairs due to postoperative AV block.[7] Epicardial pacing (usually the anterior RV most accessible to the cardiac surgeon) is pursued for infants and small children (<10–15 kg), whereas transvenous RV apical pacing is pursued for older children and young adults.[17] Regardless of the approach, electromechanical dyssynchrony with inefficient myocardial contraction with wasted energy at late-activated (post-systolic) regions can develop with chronic RV pacing and result in cardiomyopathy in 13–56% of patients.[18–21]
For systemic RV patients, spontaneous AV block is most common for congenitally corrected transposition of the great arteries (cc-TGA; rate of 2% per year).[22] The risk of AV block increases with additional cardiac operations, and it is estimated that approximately 5–10% of patients with systemic RV anatomy will require CRT.[23] Pacing-related RV failure is further compounded by abnormal septal position and progressive tricuspid regurgitation.[24] Accordingly, studies of chronic ventricular pacing for systemic RV patients suggest a very high risk of clinical deterioration.[24,25]
Limitations of Conventional Cardiac Resynchronisation Therapy in CHD
For patient with systemic LV morphology, biventricular CRT using CS leads may be challenging. Enlargement of right-sided cardiac structures with acute angulation at the CS origin, surgical patch material and prior rightsided valve replacement can hinder access to the CS ostium (Supplementary Figure 2). Left superior vena cava anatomy with a dilated CS, diminutive tributaries and unroofing to the left atrium may be present.[26]
Even greater challenges exist for those with systemic RV morphology. For patients with cc-TGA, coronary venous anatomy is highly variable and associated with ectopic or atretic CS ostia (Figure 2).[27,28] Likewise, for patients with dextro (d-) TGA who have undergone atrial baffle operations, the CS is usually directed to the pulmonary venous circulation.[29] As such, a hybrid surgical or entirely epicardial approach may be required.[30–32]
Figure 2: Coronary Sinus Anomalies for Systemic Right Ventricular Anatomy (Congenitally Corrected Transposition).
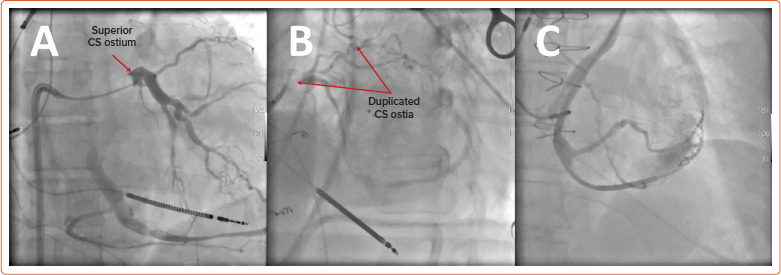
A: Superior coronary sinus (CS) ostium with atresia of the native connection at the posteroseptal right atrium. B: Venogram at the superior ostium showing duplicated CS ostia. Note the absence of venous tributaries for the typical inferior coronary sinus. C: A vein of Marshall connection to the CS system is shown, eventually used for CS lead placement in a patient with congenitally corrected transposition of the great arteries.
Conduction System Pacing in Moderate–Complex CHD
Moderate–complex CHD comprises a wide spectrum of anatomical conditions often requiring surgical intervention. This may be associated with unusual anatomical disposition of the conduction system, either naturally or secondary to fibrosis and prosthetic materials. CSP in this setting should be considered an extremely complex procedure and should be performed by specialised teams with wide experience in both CSP and CHD.
For the patient with a systemic LV, the disposition of the conduction system is comparable to the non-CHD population but with important variations that require consideration (Table 1). For example, among patients with AV septal defect, the AV node and the His bundle are posteriorly displaced. In patients with tetralogy of Fallot, the presence of a perimembranous ventricular septal defect is usually associated with a longer non-branching His bundle.
Table 1: Commonly Encountered Conduction System Anatomical Variants for Dominant Left and Right Ventricular Morphology.
| Congenital Heart Lesion | Compact AV node | Non-branching AV (His) Bundle | Bundle Branches |
|---|---|---|---|
| Systemic Left Ventricle | |||
| Primum ASD | Inferiorly displaced (‘nodal triangle’) | ||
| AVSD | Inferiorly displaced (‘nodal triangle’) | Superficial, ventricular septal crest | Early branching/superiorly directed LBB |
| VSD Perimembranous Muscular |
Normal Normal |
Posteroinferior to VSD Posteroinferior to outlet VSD, anterosuperior to inlet VSD |
Normal Normal |
| Ebstein’s anomaly | Often inferior (anatomical distortion) | Inferiorly displaced | RBB often fibrotic |
| Tetralogy of Fallot | Normal | Posteroinferior to VSD | Frequent proximal interruption of RBB |
| Heterotaxy syndromes | Twin AV nodes possible | Anterior and posterior | Continuous conduction sling |
| Systemic Right Ventricle | |||
| cc-TGA | Anterior (pulmonary–mitral continuity) | Courses within LVOT to septum | Inverted |
| d-TGA – Mustard/Senning | Normal (pulmonary venous atrium) | Normal (subpulmonary LV) | Normal (LBB in subpulmonary LV) |
ASD = atrial septal defect; AV = atrioventricular; AVSD = AV septal defect; cc-TGA = congenitally corrected transposition of the great arteries; d-TGA = dextro transposition of the great arteries; LBB = left bundle branch; LV = left ventricle; LVOT = LV outflow tract; RBB = right bundle branch; VSD = ventricular septal defect.
For patients with a systemic RV, greater variations in the disposition of the conduction system are present (Table 1; Figure 3). In patients with a cc-TGA there is ventricular inversion and complete reversal of the bundle branches, each associated with the corresponding anatomical ventricle.[33,34] For these patients, a hypoplastic posterior AV node (without connection to the AV bundle) may be present, and the anterior AV node usually gives rise to the penetrating His bundle within the immediate subpulmonary area. When the AV bundle reaches the interventricular septum, it assumes an anterior direction until the left bundle arises on the right side of the septum and the right bundle arises on the left side of the septum. Conversely, for patients with a d-TGA who have undergone an atrial switch repair, the AV node remains in the pulmonary venous atrium and is not readily accessible from the transvenous approach. Lead placement for these patients occurs via the superior vena cava baffle to the systemic venous atrium and then the subpulmonary LV. As a result, only the left conduction system is generally accessible.
Figure 3: Distribution of the Conduction System in Complex Congenital Heart Disease with Systemic Right Ventricle.
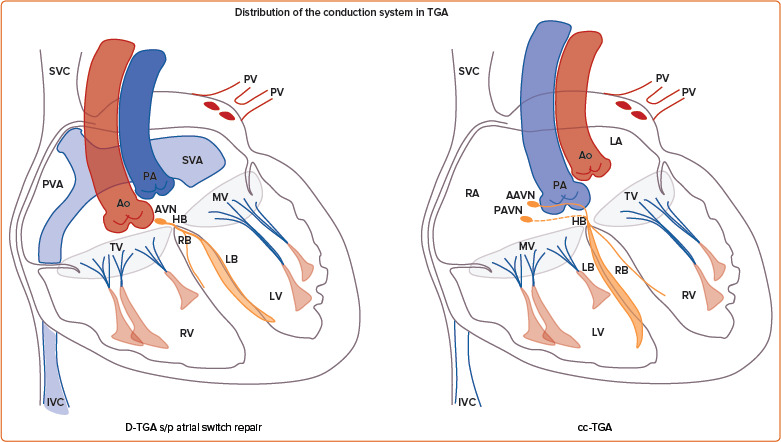
Schematic representation of the conduction system in D-TGA and cc-TGA. AAVN = anterior AV node; Ao = aorta; AVN = atrioventricular node; cc-TGA = congenitally corrected transposition of the great arteries; d-TGA = dextro transposition of the great arteries; HB = His bundle; IVC = inferior vena cava; LA = left atrium; LB = left bundle; LV = left ventricle; MV = mitral valve; PA = pulmonary artery; PAVN = posterior AV node; PV = pulmonary vein; PVA = pulmonary venous atrium; RA = right atrium; RB = right bundle; RV = right ventricle; s/p = status post; SVA = systemic venous atrium; SVC = superior vena cava; TGA = transposition of the great arteries; TV = tricuspid valve.
Pre-CSP procedural evaluation should include imaging techniques such as CT or MRI for procedural planning. Characterisation of the patient’s anatomy and available venous access is mandatory. Although left-sided access is usually employed for the majority of patients, in specific conditions right-side access may represent an advantage. In our experience, in patients with a d-TGA and previous atrial switch repair, access from the right side may facilitate sheath orientation to reach the left-sided His or the left anterior fascicle in the subpulmonary LV (Figure 4). In the same manner, for patients with dextrocardia and a normal AV and ventriculoarterial relationship, right-sided access is advantageous.
Figure 4: A 16-year-old Patient with Atrial Switch Repair and Post-surgical Complete Atrioventricular Block.
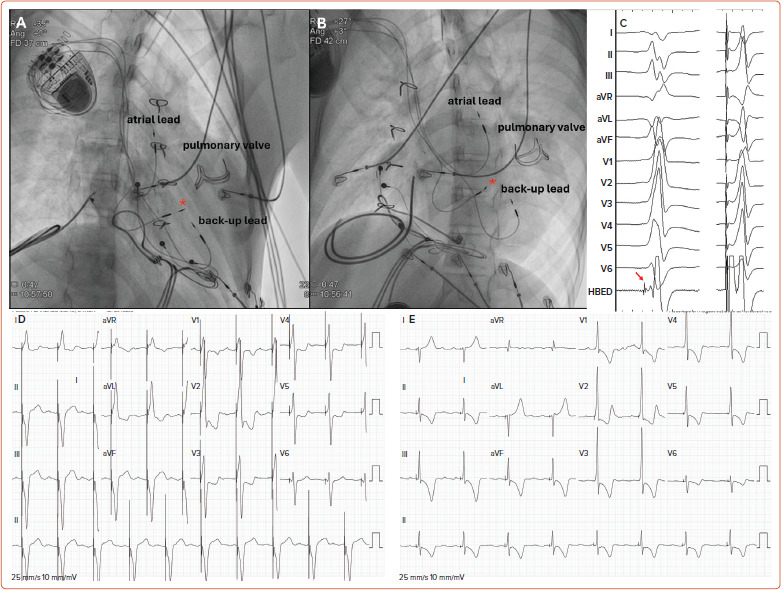
The patient received an epicardial dual-chamber pacemaker but the epicardial ventricular lead was dysfunctioning and was planned for transvenous conduction system pacing. A, B: Right anterior oblique (A) and left anterior oblique (b) fluoroscopic views of the final lead position. The red asterisk indicates the His bundle lead. A second endocardial ventricular lead was implanted as a backup pacing lead. C: Spontaneous rhythm with a His bundle potential recorded from the subpulmonary left ventricle and then non-selective His bundle capture. D: Baseline 12-lead ECG during epicardial ventricular pacing. E: Final paced 12-lead ECG during conduction system pacing. Source: Cano et al. 2021.[36] Reproduced with permission from Wiley.
The use of electroanatomical 3D mapping to delineate anatomy and localise the conduction system is advisable for CSP in complex anatomies, especially cc-TGA and d-TGA after atrial switch repair. Although electroanatomical 3D mapping in these cases can proceed via the femoral venous approach, we have pursued a superior approach through the axillary vein to simplify the workflow (Supplementary Figure 3 and Supplementary Video 1). Using this strategy, the ventricular lead can be connected to the electroanatomic 3D mapping system to accurately guide lead implantation. Other intraprocedural imaging techniques, such as ventriculography through the sheath, can delineate the subpulmonary ventricle anatomy and help determine the target area for both HBP and LBBAP. The summit of the tricuspid (or mitral, depending on the anatomy) valve is an anatomical reference for His bundle location. The use of contrast through the sheath allows clear visualisation of the AV valve, septal area and outflow tract. This can be especially useful in the presence of significant subpulmonary ventricular enlargement, as seen in patients with tetralogy of Fallot (Supplementary Figure 4 and Supplementary Video 2).
Although no implant tools have been designed specifically for CHD, conventional sheaths or preshaped conventional sheaths are usually employed. In the presence of a dilated subpulmonary RV, the primary curve of the C315His sheath can be manually increased with the dilator in place to provide greater access into the RV. Alternatively, the steerable C304His sheath can reach further into the RV. For patients with significant atrial enlargement, placing the C315His sheath inside a multipurpose CS guide sheath after removing the proximal 12 cm has been described to achieve additional support.[6] Device programming and follow-up of CSP devices differs from patients with conventional RV pacing, although there are no specific recommendations in CHD patients undergoing CSP.[35]
Outcomes of Conduction System Pacing for Moderate to Complex CHD
Given the limitations associated with conventional myocardial pacing and biventricular CRT, CSP is increasingly used for CHD. Initial case reports have given way to larger multicentre descriptions that better reflect real-world outcomes associated with this approach.
In general, excellent outcomes of CSP have been reported for patients with systemic LV anatomy. Mixed cohorts, including 65–92% patients with systemic LV anatomies, have demonstrated excellent outcomes at short-to mid-term follow-up for this subgroup (Supplementary Figure 5).[8,36,37] Radiation exposure, acute success and electrical pacing parameters in CHD patients are similar to those in patients with acquired heart disease, suggesting that this strategy is equally feasible for patients with CHD and a systemic LV.[37] In a multicentre study of 65 CHD patients with systemic LV undergoing CSP, the most common strategy was left bundle branch or LV septal pacing in 48 (74%) patients, with His bundle pacing in the remainder.[38] The pacing threshold was higher for HBP versus LBBP at the 8-month follow-up, which was attributed, in part, to the high prevalence of septal scar and related patch closure in this population. Importantly, CSP met the a priori non-inferiority outcome measure and was statistically superior to conventional biventricular pacing in terms of postimplant improvement in LV systolic function and QRS duration.[38]
Reports of CSP for systemic RV anatomy also demonstrate reasonably good outcomes. Especially for cc-TGA, case reports and, more recently, a multicentre series have confirmed the safety and efficacy of CSP (Supplementary Figure 6).[39–42] Significant QRS narrowing for patients with prior chronic LV pacing and stable QRS duration for those with pre-existing junctional escape rhythm have been described and, importantly, New York Heart Association class was improved for 5 of 15 (33%) patients.[42] After the Mustard or Senning (atrial baffle) operation, CSP is less commonly described (only five unique cases are available to date) but holds major promise (Figure 4).[8,36–38,43] Techniques to traverse the superior venous baffle and yet affix the pacing lead against the LV septum are associated with increased procedural complexity.[33–35,40] Due to the inherent challenges associated with this approach, lead instability is of concern, and consideration of a backup lead for patients with severe bradycardia has been advised.[36]
Conclusion
CSP appears to be the ideal pacing modality for children with CCAVB or CHD because it aims to restore normal ventricular activation through the conduction system and preserve ventricular synchrony. However, current implant tools have not been designed for the smaller body size and anatomical variations usually present in this special population. Further development of specific delivery tools and leads is desirable to increase the rate of successful implantation in this complex scenario. Further, integration of implant tools (both sheaths and leads) with electroanatomical 3D mapping systems could greatly facilitate implant procedures for complex CHD. Long-term data related to lead performance and complications are lacking and should be collected to corroborate excellent short- and mid-term results associated with CSP in CCAVB and CHD. Finally, CSP is currently a valid complement or alternative for CRT in CHD and should be considered for every patient with CCAVB or CHB needing chronic ventricular pacing.
Clinical Perspective
Conduction system pacing is a physiological pacing modality aimed at preserving or restoring ventricular synchrony.
Children with congenital complete atrioventricular block and/or congenital heart disease may need chronic ventricular pacing and are at high risk of developing pacing-induced cardiomyopathy after conventional right ventricular myocardial pacing.
Conduction system pacing may be challenging in this setting due to anatomical variations and a lack of dedicated implant tools.
Despite the technical challenges, children and congenital heart disease patients may obtain benefit from physiological pacing modalities.
Supplementary Material
References
- 1.Moore BM, Medi C, McGuire MA et al. Pacing-associated cardiomyopathy in adult congenital heart disease. Open Heart. 2020;7:e001374. doi: 10.1136/openhrt-2020-001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelrahman M, Subzposh FA, Beer D et al. Clinical outcomes of His bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. 2018;71:2319–30. doi: 10.1016/j.jacc.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 3.Vinther M, Risum N, Svendsen JH et al. A randomized trial of his pacing versus biventricular pacing in symptomatic HF patients with left bundle branch block (His-alternative). JACC Clin Electrophysiol. 2021;7:1422–32. doi: 10.1016/j.jacep.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Jastrzębski M, Kiełbasa G, Cano O et al. Left bundle branch area pacing outcomes: The multicentre European MELOS study. Eur Heart J. 2022;43:4161–73. doi: 10.1093/eurheartj/ehac445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayaraman P, Sharma PS, Cano Ó et al. Comparison of left bundle branch area pacing and biventricular pacing in candidates for resynchronization therapy. J Am Coll Cardiol. 2023;82:228–41. doi: 10.1016/j.jacc.2023.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Diaz JC, Duque M, Aristizabal J et al. The emerging role of left bundle branch area pacing for cardiac resynchronisation therapy. Arrhythm Electrophysiol Rev. 2023;12:e29. doi: 10.15420/aer.2023.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang PM, Carter C, Bar-Cohen Y. Indications for permanent pacing, device and lead selection. Cardiac Pacing and Defibrillation in Pediatric and Congenital Heart Disease. In: Shah M, Rhodes L, Kaltman J, editors. West Sussex, UK: John Wiley & Sons Ltd, 2017;37–61.: [DOI] [Google Scholar]
- 8.Gordon A, Jimenez E, Cortez D. Conduction system pacing in pediatrics and congenital heart disease, a single center series of 24 patients. Pediatr Cardiol. 2024;45:1165–71. doi: 10.1007/s00246-022-02942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehwinkel AE, Muller JG, Vanburen PC, Lustgarten DL. Ventricular resynchronization by implementation of direct His bundle pacing in a patient with congenital complete AV block and newly diagnosed cardiomyopathy. J Cardiovasc Electrophysiol. 2011;22:818–21. doi: 10.1111/j.1540-8167.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 10.Piemontese GP, Ziacchi M, Statuto G et al. His bundle pacing for congenital complete AV block: an attempt to fix a broken heart? Ann Noninvasive Electrocardiol. 2022;27:e12895. doi: 10.1111/anec.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez E, Zaban N, Sharma N et al. His bundle and left bundle pacing in pediatrics and congenital heart disease: a single center experience. Pediatr Cardiol. 2020;41:1425–31. doi: 10.1007/s00246-020-02398-9. [DOI] [PubMed] [Google Scholar]
- 12.Vinocur JM. Fortuitous left bundle branch area pacing in a small child. JACC Case Rep. 2021;3:1730–5. doi: 10.1016/j.jaccas.2021.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramdat Misier NL, Jessen ME, Fares M et al. Left bundle branch pacing after His bundle lead dysfunction due to physical activity in a pediatric patient. HeartRhythm Case Rep. 2023;9:715–9. doi: 10.1016/j.hrcr.2023.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dandamudi G, Simon J, Cano O et al. Permanent His bundle pacing in patients with congenital complete heart block: a multicenter experience. JACC Clin Electrophysiol. 2021;7:522–9. doi: 10.1016/j.jacep.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Dubin AM, Janousek J, Rhee E et al. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol. 2005;46:2277–83. doi: 10.1016/j.jacc.2005.05.096. [DOI] [PubMed] [Google Scholar]
- 16.Cecchin F, Frangini PA, Brown DW et al. Cardiac resynchronization therapy (and multisite pacing) in pediatrics and congenital heart disease: five years experience in a single institution. J Cardiovasc Electrophysiol. 2009;20:58–65. doi: 10.1111/j.1540-8167.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 17.Silvetti MS, Drago F, Di Carlo D et al. Cardiac pacing in paediatric patients with congenital heart defects: transvenous or epicardial? Europace. 2013;15:1280–6. doi: 10.1093/europace/eut029. [DOI] [PubMed] [Google Scholar]
- 18.Friedberg MK, Mertens L. Echocardiographic assessment of ventricular synchrony in congenital and acquired heart disease in children. Echocardiography. 2013;30:460–71. doi: 10.1111/echo.12110. [DOI] [PubMed] [Google Scholar]
- 19.Balaji S, Sreeram N. The development of pacing induced ventricular dysfunction is influenced by the underlying structural heart defect in children with congenital heart disease. Indian Heart J. 2017;69:240–3. doi: 10.1016/j.ihj.2016.11.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebauer RA, Tomek V, Salameh A et al. Predictors of left ventricular remodelling and failure in right ventricular pacing in the young. Eur Heart J. 2009;30:1097–104. doi: 10.1093/eurheartj/ehp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard TS, Niu MC, Morris SA et al. Right ventricular pacing after ventricular septal defect closure is associated with development of cardiac dysfunction. JACC Clin Electrophysiol. 2020;6:348–50. doi: 10.1016/j.jacep.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Huhta JC, Maloney JD, Ritter DG et al. Complete atrioventricular block in patients with atrioventricular discordance. Circulation. 1983;67:1374–7. doi: 10.1161/01.cir.67.6.1374. [DOI] [PubMed] [Google Scholar]
- 23.Diller GP, Okonko D, Uebing A et al. Cardiac resynchronization therapy for adult congenital heart disease patients with a systemic right ventricle: analysis of feasibility and review of early experience. Europace. 2006;8:267–72. doi: 10.1093/europace/euj048. [DOI] [PubMed] [Google Scholar]
- 24.Yeo WT, Jarman JWE, Li W et al. Adverse impact of chronic subpulmonary left ventricular pacing on systemic right ventricular function in patients with congenitally corrected transposition of the great arteries. Int J Cardiol. 2014;171:184–91. doi: 10.1016/j.ijcard.2013.11.128. [DOI] [PubMed] [Google Scholar]
- 25.Hofferberth SC, Alexander ME, Mah DY et al. Impact of pacing on systemic ventricular function in L-transposition of the great arteries. J Thorac Cardiovasc Surg. 2016;151:131–8. doi: 10.1016/j.jtcvs.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 26.Manchanda M, McLeod CJ, Killu A, Asirvatham SJ. Cardiac resynchronization therapy for patients with congenital heart disease: technical challenges. J Interv Card Electrophysiol. 2013;36:71–9. doi: 10.1007/s10840-012-9726-x. [DOI] [PubMed] [Google Scholar]
- 27.Bottega NA, Kapa S, Edwards WD et al. The cardiac veins in congenitally corrected transposition of the great arteries: delivery options for cardiac devices. Heart Rhythm. 2009;6:1450–6. doi: 10.1016/j.hrthm.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Moore JP, Cho D, Lin JP et al. Implantation techniques and outcomes after cardiac resynchronization therapy for congenitally corrected transposition of the great arteries. Heart Rhythm. 2018;15:1808–15. doi: 10.1016/j.hrthm.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Zartner PA, Wiebe W, Volkmer M et al. Transvenous cardiac resynchronization therapy in complex congenital heart diseases: dextrocardia with transposition of the great arteries after Mustard operation. Europace. 2009;11:530–2. doi: 10.1093/europace/eun388. [DOI] [PubMed] [Google Scholar]
- 30.Moore JP, Gallotti RG, Shannon KM, Biniwale R. A minimally invasive hybrid approach for cardiac resynchronization of the systemic right ventricle. Pacing Clin Electrophysiol. 2019;42:171–7. doi: 10.1111/pace.13568. [DOI] [PubMed] [Google Scholar]
- 31.Kharbanda RK, Moore JP, Lloyd MS et al. Cardiac resynchronization therapy for adult patients with a failing systemic right ventricle: a multicenter study. J Am Heart Assoc. 2022;11:e025121. doi: 10.1161/JAHA.121.025121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacquemart E, Combes N, Duthoit G et al. Cardiac resynchronization therapy in patients with congenital heart disease and systemic right ventricle. Heart Rhythm. 2022;19:658–66. doi: 10.1016/j.hrthm.2021.11.032. [DOI] [PubMed] [Google Scholar]
- 33.Baruteau AE, Abrams DJ, Ho SY et al. Cardiac conduction system in congenitally corrected transposition of the great arteries and its clinical relevance. J Am Heart Assoc. 2017;6:e007759. doi: 10.1161/jaha.117.007759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bharati S, Lev M. The conduction system in simple, regular (D-), complete transposition with ventricular septal defect. J Thorac Cardiovasc Surg. 1976;72:194–201. doi: 10.1016/S0022-5223(19)40436-4. [DOI] [PubMed] [Google Scholar]
- 35.Bakelants E, Burri H. Troubleshooting programming of conduction system pacing. Arrhythm Electrophysiol Rev. 2021;10:85–90. doi: 10.15420/aer.2021.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cano Ó, Dandamudi G, Schaller RD et al. Safety and feasibility of conduction system pacing in patients with congenital heart disease. J Cardiovasc Electrophysiol. 2021;32:2692–703. doi: 10.1111/jce.15213. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor M, Riad O, Shi R et al. Left bundle branch area pacing in congenital heart disease. Europace. 2023;25:561–70. doi: 10.1093/europace/euac175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore JP, de Groot NMS, O’Connor M et al. Conduction system pacing versus conventional cardiac resynchronization therapy in congenital heart disease. JACC Clin Electrophysiol. 2023;9:385–93. doi: 10.1016/j.jacep.2022.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Nam MCY, Grigg L, Stevenson I. Left bundle branch area pacing in congenitally corrected transposition of great arteries – the obvious choice? J Electrocardiol. 2021;66:77–8. doi: 10.1016/j.jelectrocard.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Whitaker J, Kreidieh O, Singh M et al. Radiation-free nonselective His pacing lead implantation in a pregnant patient with congenitally corrected transposition of the great arteries. HeartRhythm Case Rep. 2021;7:323–7. doi: 10.1016/j.hrcr.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Namboodiri N, Kakarla S, Mohanan Nair KK et al. Threedimensional electroanatomical mapping guided right bundle branch pacing in congenitally corrected transposition of great arteries. Europace. 2023;25:1110–5. doi: 10.1093/europace/euac239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore JP, Gallotti R, Shannon KM Permanent conduction system pacing for congenitally corrected transposition of the great arteries: a Pediatric and Congenital Electrophysiology Society (PACES)/International Society for Adult Congenital Heart Disease (ISACHD) collaborative study. Heart Rhythm. 2020. [DOI] [PubMed]
- 43.O’Connor M, Ho SY, McCarthy KP et al. Left bundle pacing in transposition of the great arteries with previous atrial redirection operation. HeartRhythm Case Rep. 2022;8:176–9. doi: 10.1016/j.hrcr.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


