Abstract
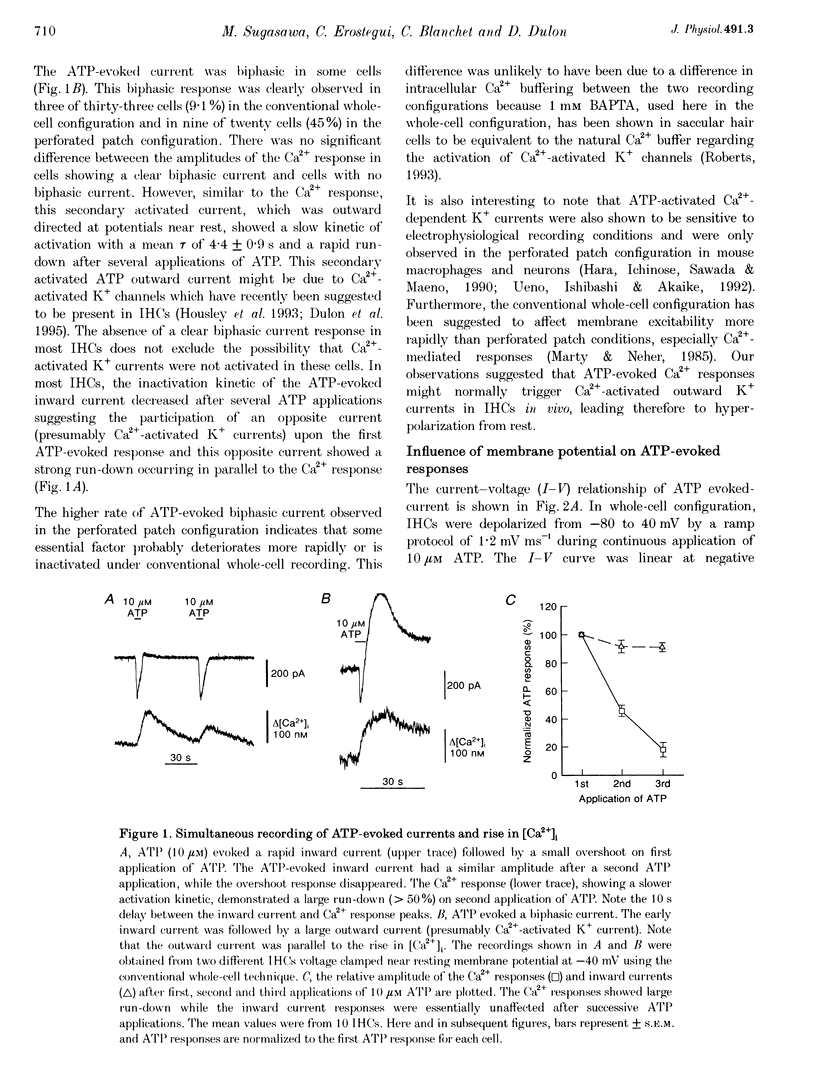
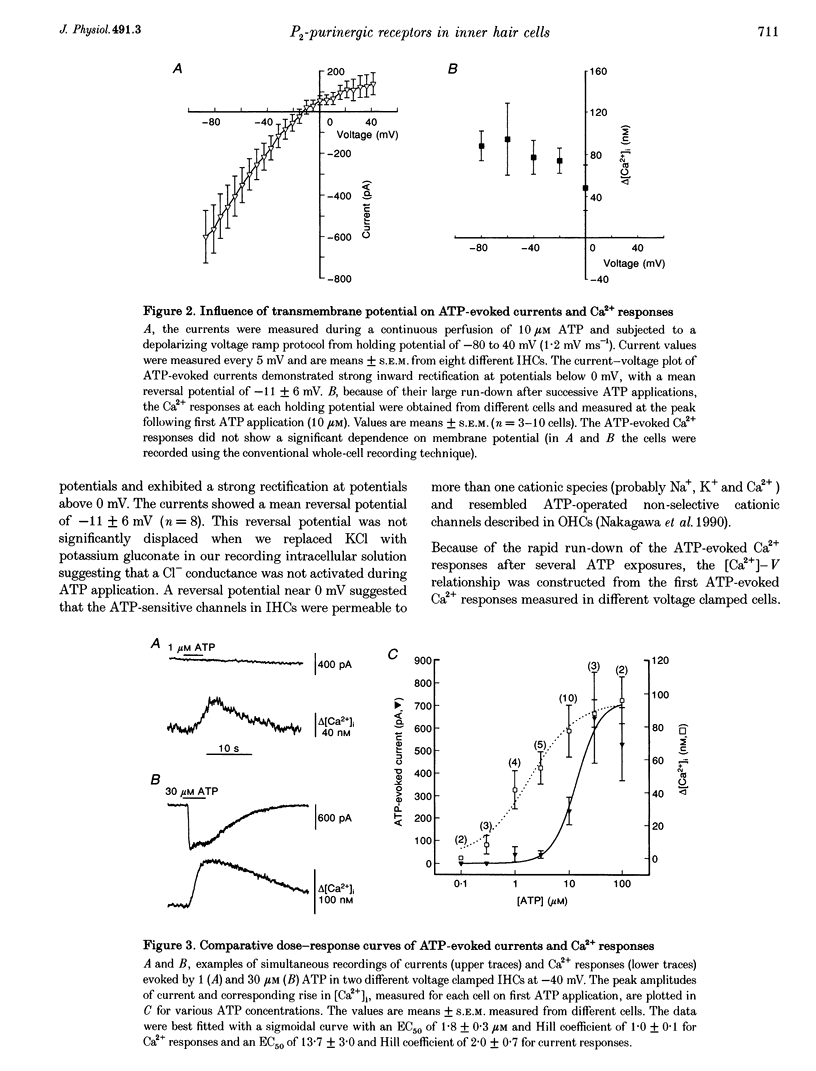
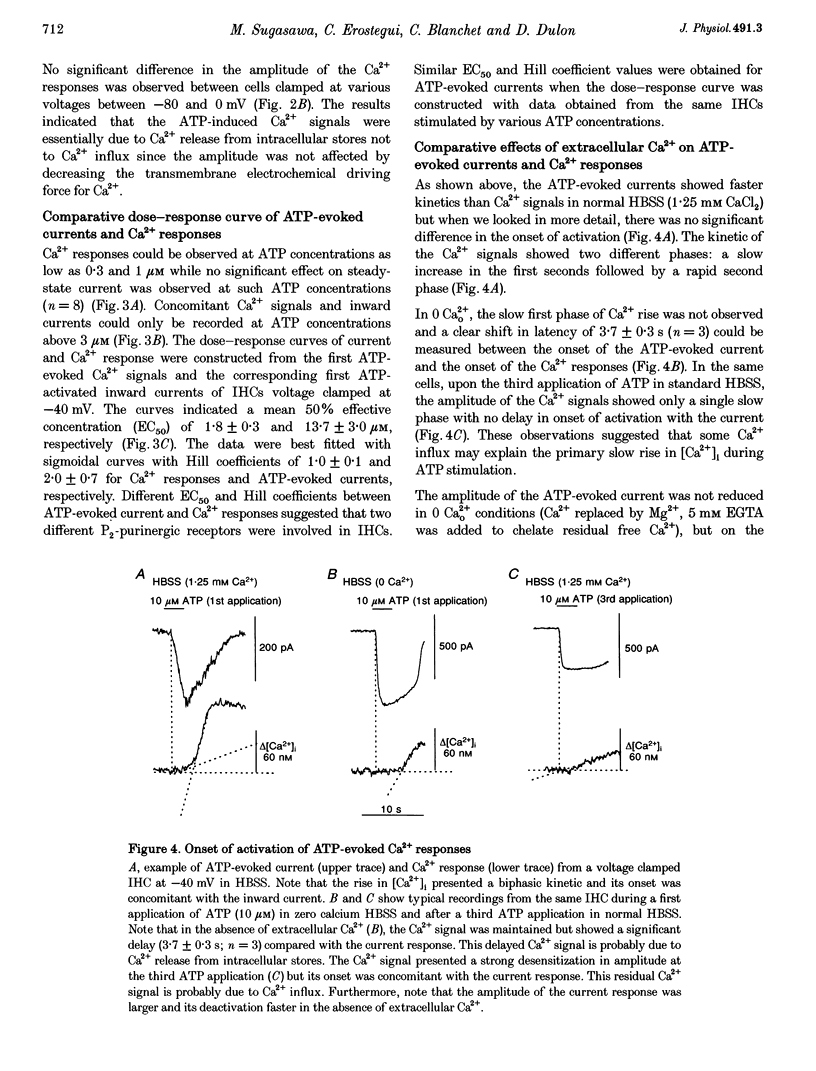
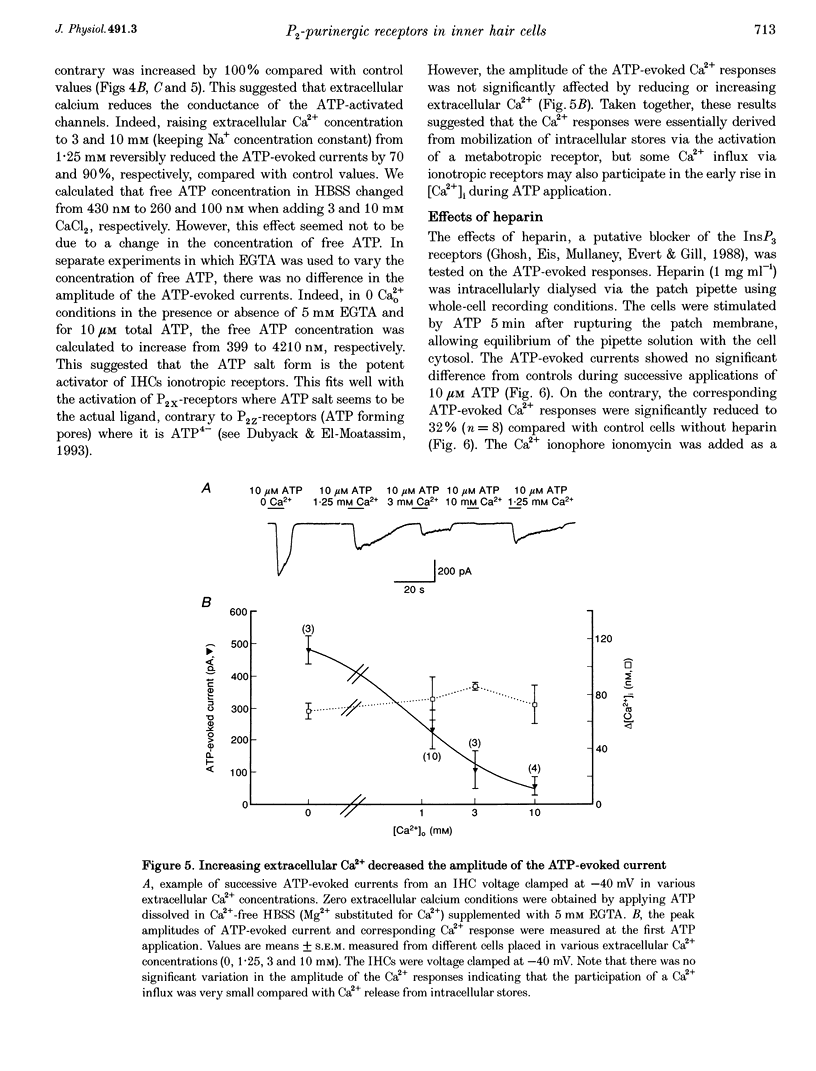
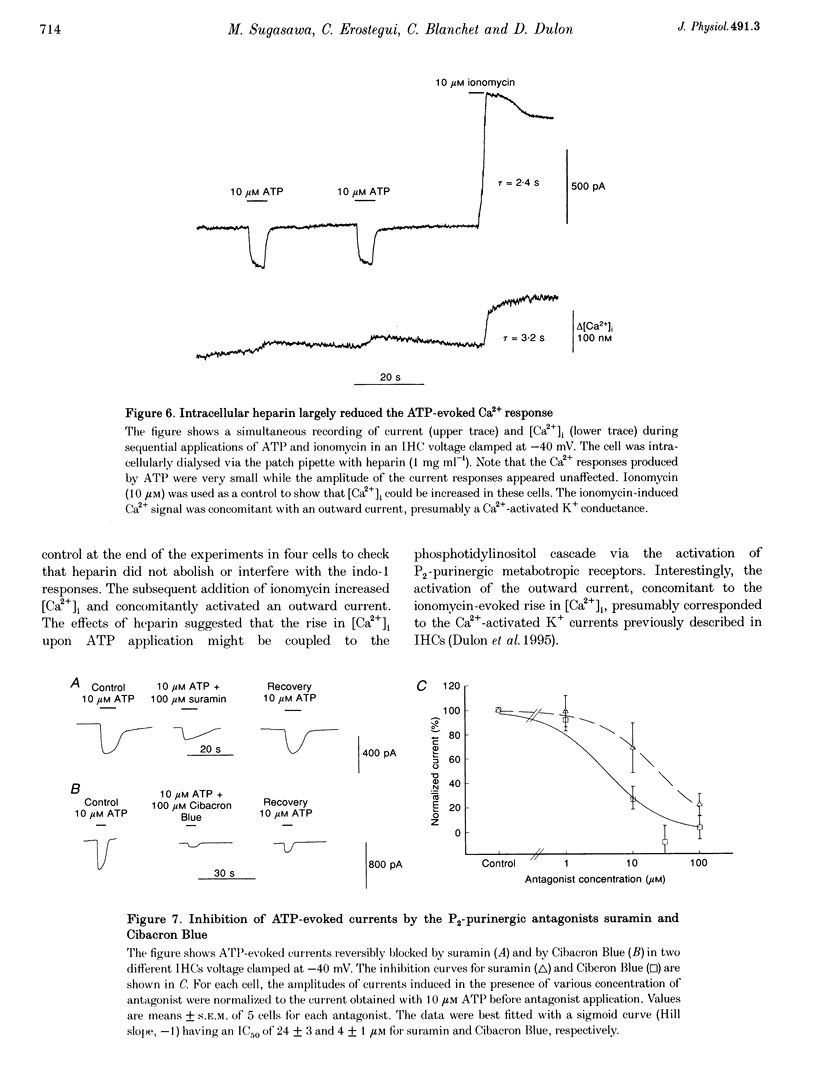
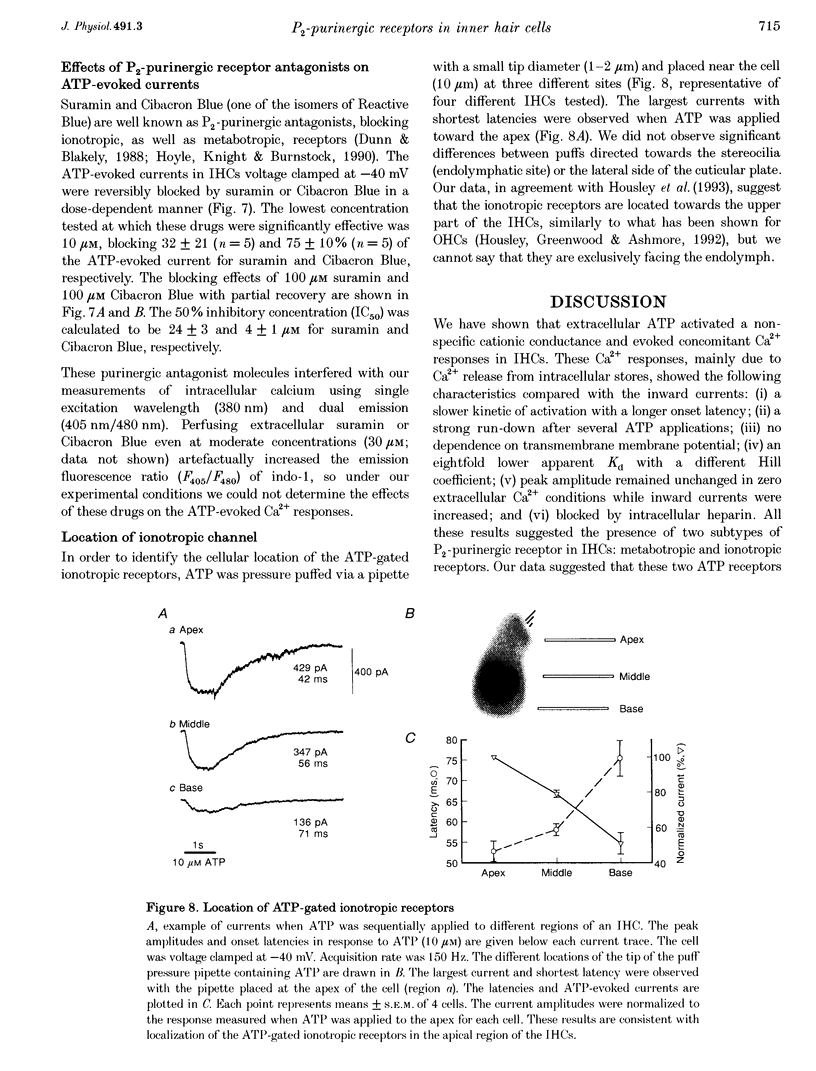
1. ATP-evoked currents and Ca2+ signals were simultaneously recorded in isolated inner hair cells (IHC) of guinea-pig cochlea by combining conventional whole-cell or perforated patch clamp recording with indo-1 dual emission microfluorometry. 2. In most IHCs, voltage clamped near resting membrane potential (-40 mV), extracellular ATP evoked a rapid inward current (time constant, 150 ms). This current was concomitant with a slow rise in [Ca2+]i (time constant, 5 s). The ATP-evoked inward currents could be repeated several times with only a small run-down in amplitude (< 10%), while the ATP-evoked Ca2+ responses showed a rapid run-down (> 80% at the third ATP application). 3. The current-voltage relationship of ATP-evoked currents showed a reversal potential at -11 +/- 6 mV (n = 8), suggesting that ATP essentially activated a non-specific cationic conductance. On the contrary, the amplitude of the ATP-evoked Ca2+ responses did not show significant dependence on holding membrane potential. 4. The Ca2+ response showed an apparent Kd for ATP (EC50, 1.8 +/- 0.3 microM; Hill coefficient, 1.0 +/- 0.1) eightfold smaller than for the evoked currents (EC50, 13.7 +/- 3.0 microM; Hill coefficient, 2.0 +/- 0.7). 5. Perfusion with high extracellular Ca2+ solution (10 mM CaCl2) reduced the amplitude of the ATP-evoked currents by 90%, while perfusion with zero Ca2+ solution increased it by more than 100%. However, similar variations in external Ca2+ concentration did not change the amplitude of the ATP-evoked Ca2+ responses. Furthermore, intracellular heparin (1 mg mL-1), a potent inhibitor of InsP3 receptors, did not significantly change the amplitude of ATP-evoked currents but reduced the ATP-evoked Ca2+ response, suggesting again that the latter is related to Ca2+ release from intracellular stores. 6. The results suggested that two types of P2-purinergic receptor are expressed in IHCs: ATP-gated ion channels and ATP-activated metabotropic receptors. At submicromolar ATP concentrations, the metabotropic receptors raising intracellular [Ca2+] would hyperpolarize IHCs via Ca(2+)-sensitive K+ channels. The ATP-gated ion channels activated at higher ATP concentrations would mainly have a depolarizing effect on IHCs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Ohmori H. Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol. 1990 Sep;428:109–131. doi: 10.1113/jphysiol.1990.sp018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Dulon D., Moataz R., Mollard P. Characterization of Ca2+ signals generated by extracellular nucleotides in supporting cells of the organ of Corti. Cell Calcium. 1993 Mar;14(3):245–254. doi: 10.1016/0143-4160(93)90071-d. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa N. P., Hudspeth A. J. Clustering of Ca2+ channels and Ca(2+)-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7578–7582. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang N. Y., Rho J. M., Northrop C. C., Liberman M. C., Ryugo D. K. Hair-cell innervation by spiral ganglion cells in adult cats. Science. 1982 Jul 9;217(4555):175–177. doi: 10.1126/science.7089553. [DOI] [PubMed] [Google Scholar]
- Kros C. J., Crawford A. C. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol. 1990 Feb;421:263–291. doi: 10.1113/jphysiol.1990.sp017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G., Erostegui C., Fallon M., Crist J., Bobbin R. P. Effects of adenosine 5'-triphosphate and related agonists on cochlear function. Hear Res. 1994 Jun 1;76(1-2):87–100. doi: 10.1016/0378-5955(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett B. G., Housley G. D., Thorne P. R. Fluorescence imaging of extracellular purinergic receptor sites and putative ecto-ATPase sites on isolated cochlear hair cells. J Neurosci. 1994 Nov;14(11 Pt 2):6992–7007. doi: 10.1523/JNEUROSCI.14-11-06992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Ryu P. D., Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989 Aug 14;103(1):56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Akaike N., Kimitsuki T., Komune S., Arima T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol. 1990 May;63(5):1068–1074. doi: 10.1152/jn.1990.63.5.1068. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. An ATP-activated conductance in pheochromocytoma cells and its suppression by extracellular calcium. J Physiol. 1990 Sep;428:257–272. doi: 10.1113/jphysiol.1990.sp018211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilles R., Järlebark L., Zenner H. P., Heilbronn E. ATP-induced cytoplasmic [Ca2+] increases in isolated cochlear outer hair cells. Involved receptor and channel mechanisms. Hear Res. 1994 Feb;73(1):27–34. doi: 10.1016/0378-5955(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Roberts W. M. Spatial calcium buffering in saccular hair cells. Nature. 1993 May 6;363(6424):74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Ueno S., Ishibashi H., Akaike N. Perforated-patch method reveals extracellular ATP-induced K+ conductance in dissociated rat nucleus solitarii neurons. Brain Res. 1992 Nov 27;597(1):176–179. doi: 10.1016/0006-8993(92)91523-h. [DOI] [PubMed] [Google Scholar]
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994 Oct 6;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Westfall D. P., Sedaa K. O., Shinozuka K., Bjur R. A., Buxton I. L. ATP as a cotransmitter. Ann N Y Acad Sci. 1990;603:300–310. doi: 10.1111/j.1749-6632.1990.tb37681.x. [DOI] [PubMed] [Google Scholar]