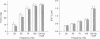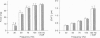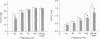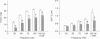Abstract
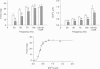
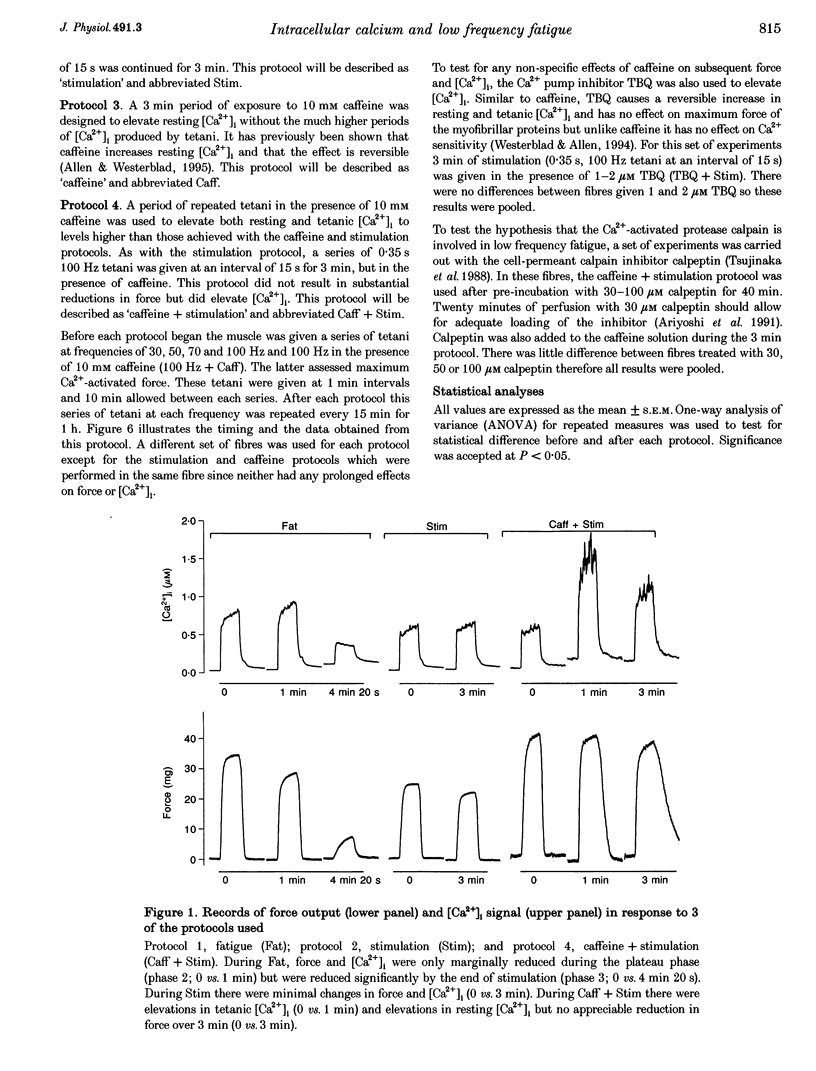
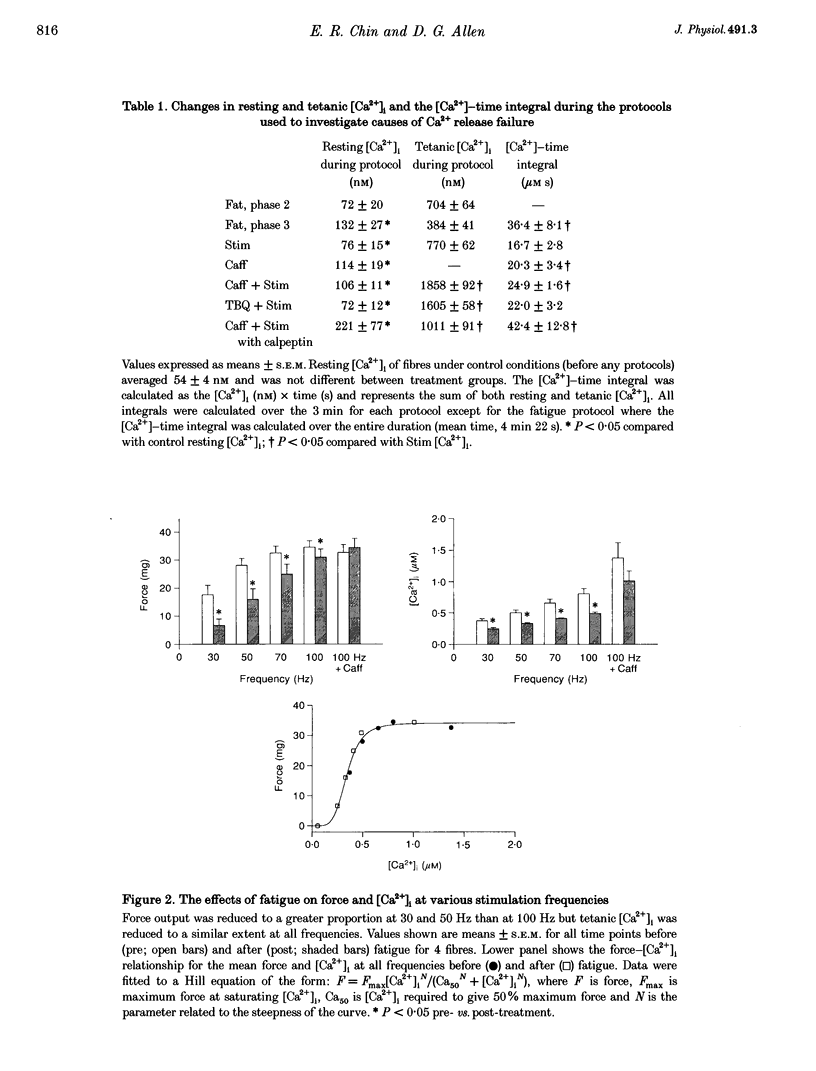
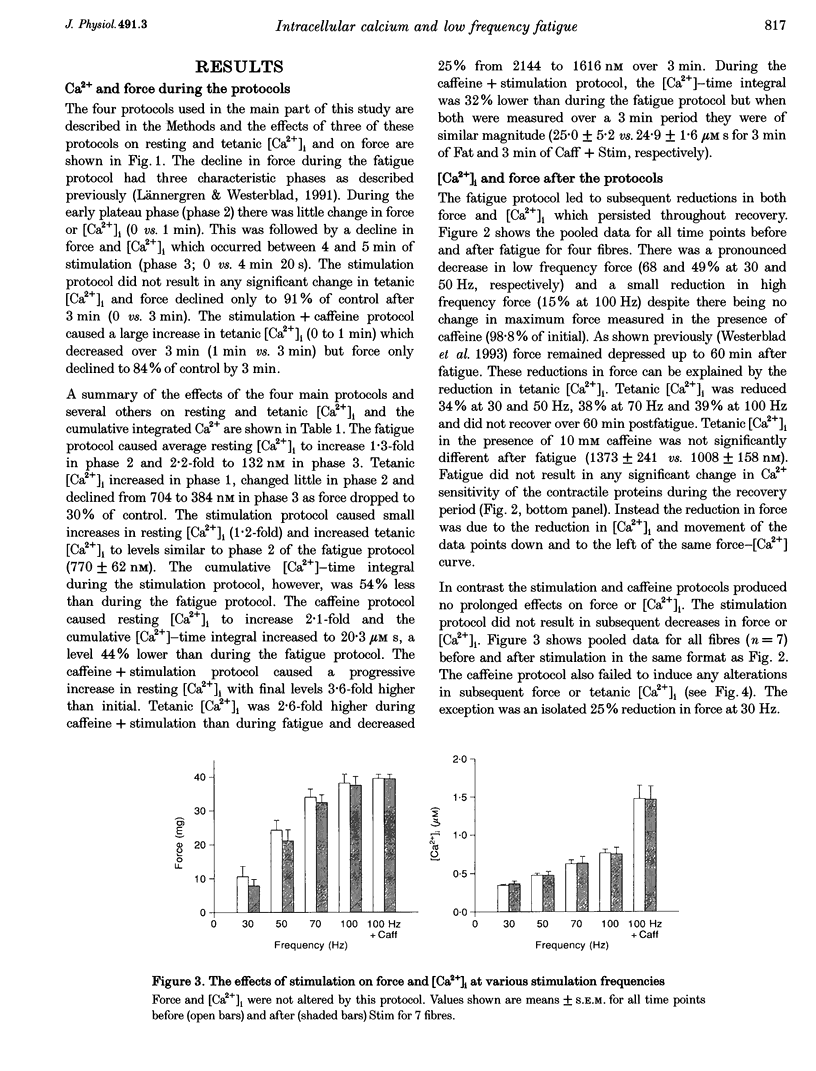
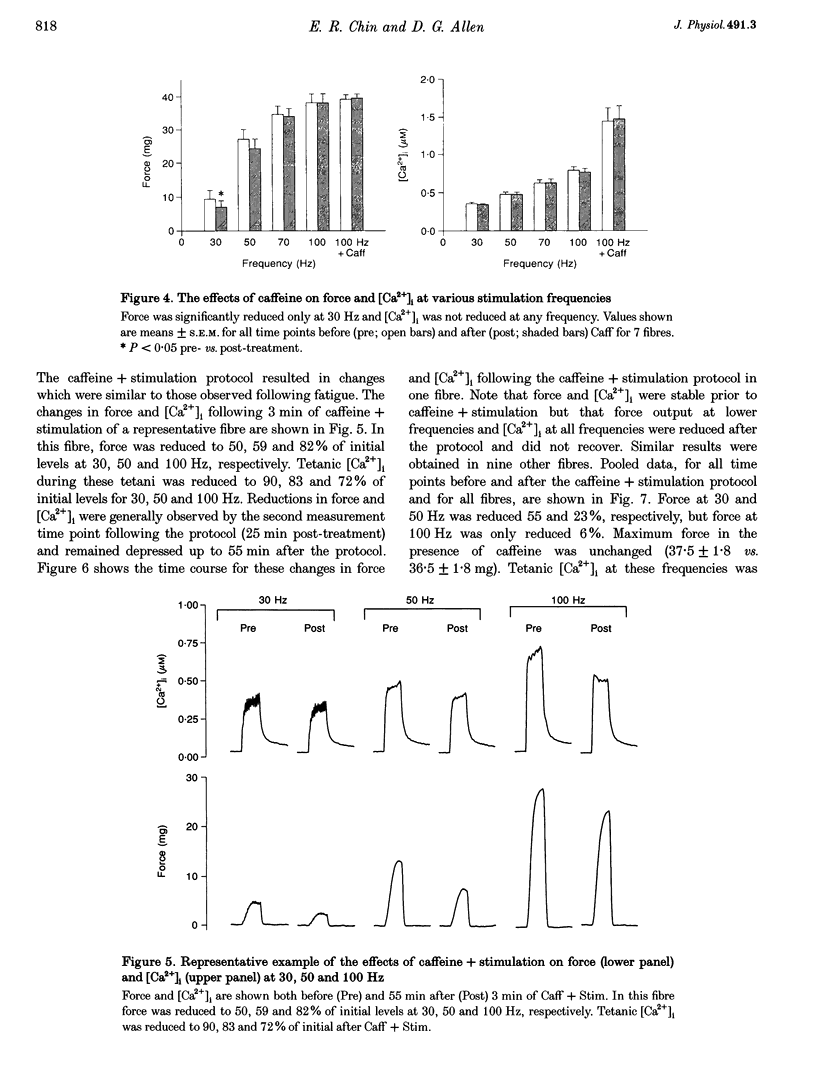
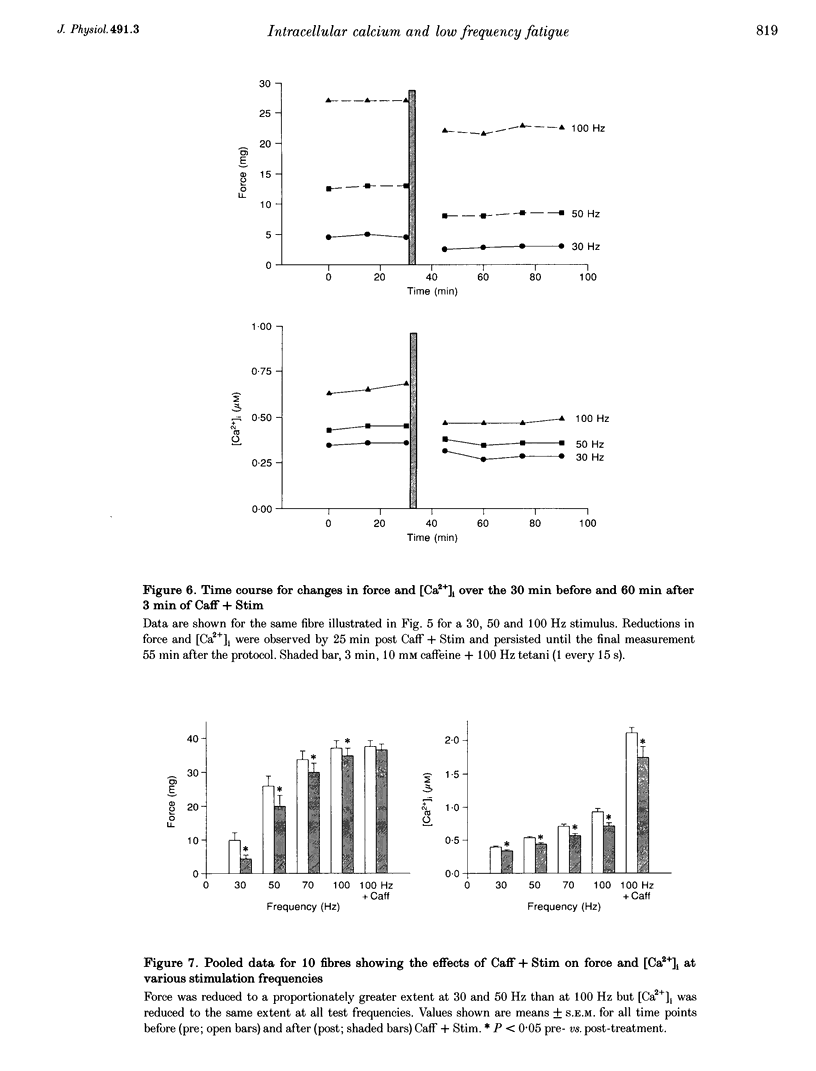
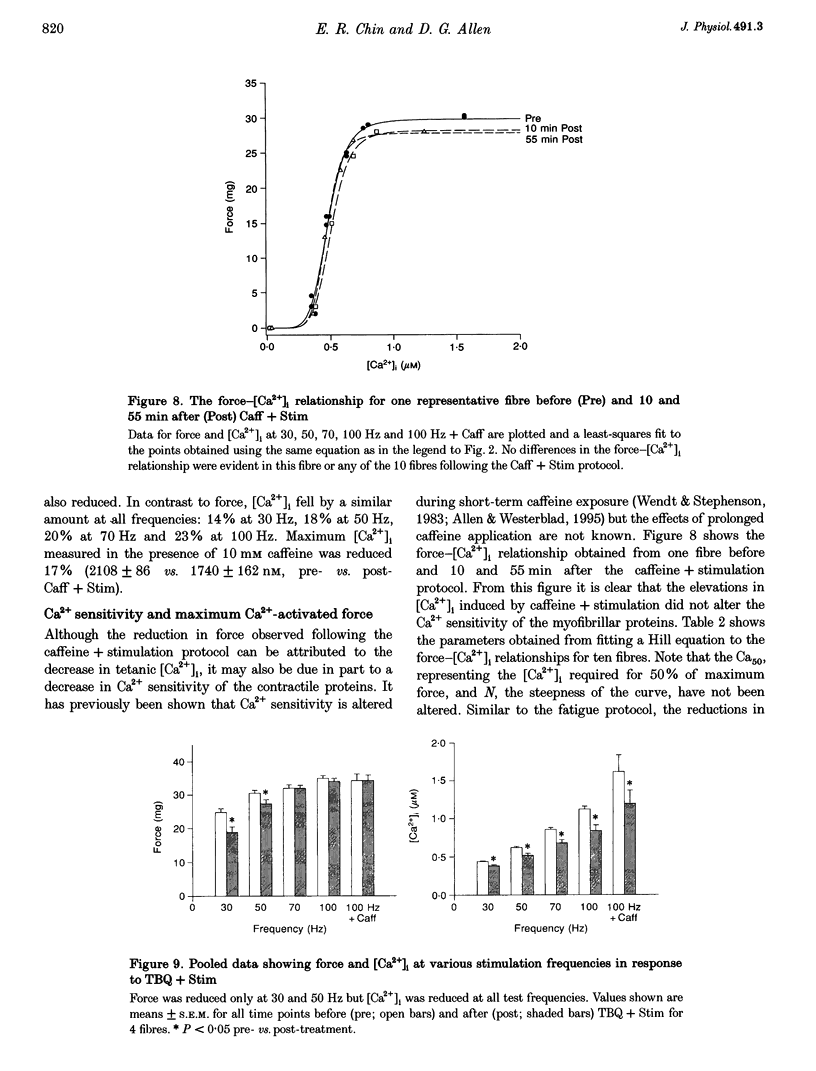
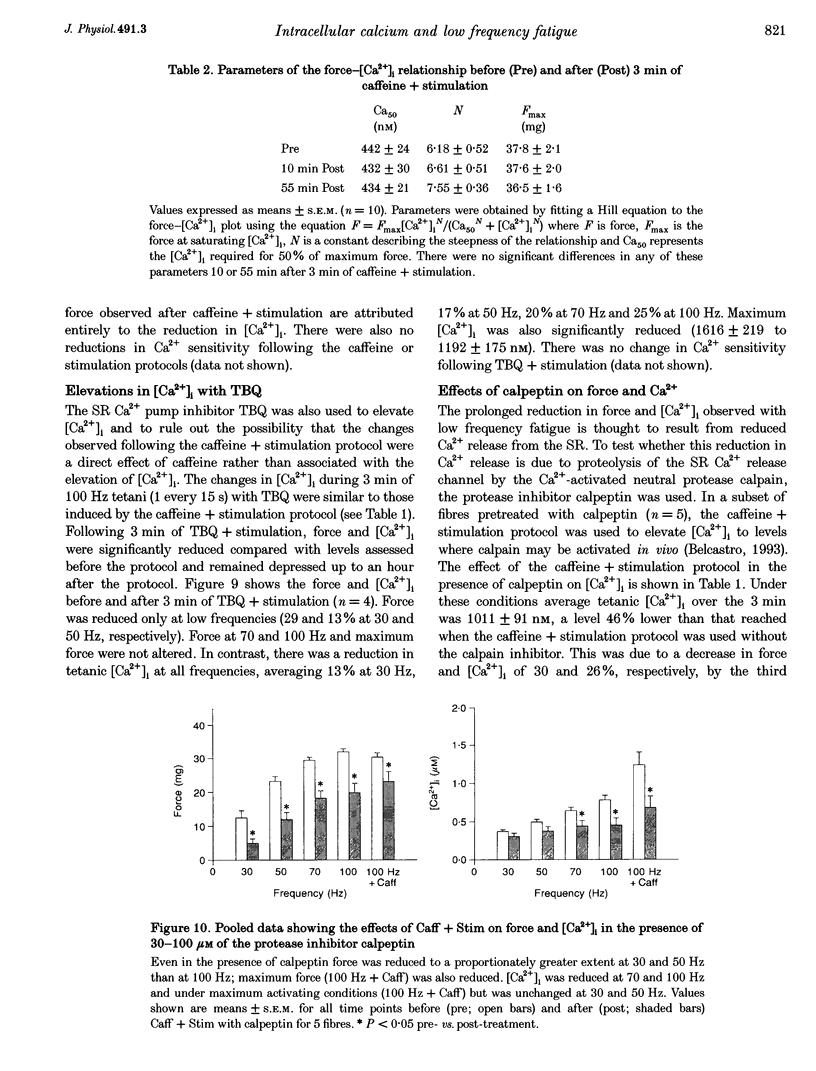
1. Intracellular free calcium concentration ([Ca2+]i) and force were measured in isolated single skeletal muscle fibres from mice. The aim was to determine the extent to which elevations in [Ca2+]i during various stimulation protocols affected subsequent muscle performance. 2. A protocol of repeated tetanic stimulation which elevated [Ca2+]i and caused a large decline in force (fatigue) had a [Ca2+]-time integral of 36.4 +/- 8.1 microM s. A protocol of repeated tetani at a lower duty cycle (stimulation) caused only a small decline in force (9-16%) but elevated the [Ca2+]-time integral to 16.7 +/- 2.8 and 24.9 +/- 1.6 microM s in the absence and presence of 10 mM caffeine, respectively. Caffeine alone raised the [Ca2+]-time integral to 20.3 +/- 3.4 microM s. 3. Following the fatigue protocol there was a proportionately greater loss of force at low stimulation frequencies (30 and 50 Hz) compared with high frequencies (100 Hz) which persisted for up to an hour. This pattern of force loss could be attributed to a uniform reduction in [Ca2+]i at all frequencies. Similar effects were observed after elevating [Ca2+]i with the caffeine + stimulation protocol but were not observed after stimulation or caffeine alone. The higher [Ca2+]-time integrals during the fatigue and caffeine + stimulation protocols suggest that some threshold for [Ca2+]i must be reached before these effects are observed. 4. The reductions in low frequency force induced by the fatigue and caffeine + stimulation protocols were not due to decreased Ca2+ sensitivity or to decreases in maximum force-generating capacity of the contractile proteins and therefore are due to a failure of Ca2+ release. 5. The Ca(2+)-activated neutral protease (calpain) inhibitor calpeptin was not effective in preventing the effects of caffeine + stimulation indicating that the reduction in Ca2+ release was not due to calpain-mediated hydrolysis of the Ca2+ release channel. 6. Our findings indicate that low frequency fatigue results from increases in [Ca2+]i during fatigue and that these elevations in [Ca2+]i activate some process which leads to failure of excitation-contraction (E-C) coupling and Ca2+ release.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Westerblad H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. J Physiol. 1995 Sep 1;487(Pt 2):331–342. doi: 10.1113/jphysiol.1995.sp020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyoshi H., Shiba E., Kambayashi J., Sakon M., Tsujinaka T., Uemura Y., Mori T. Characteristics of various synthetic peptide calpain inhibitors and their application for the analysis of platelet reaction. Biochem Int. 1991 Apr;23(6):1019–1033. [PubMed] [Google Scholar]
- Belcastro A. N. Skeletal muscle calcium-activated neutral protease (calpain) with exercise. J Appl Physiol (1985) 1993 Mar;74(3):1381–1386. doi: 10.1152/jappl.1993.74.3.1381. [DOI] [PubMed] [Google Scholar]
- Byrd S. K. Alterations in the sarcoplasmic reticulum: a possible link to exercise-induced muscle damage. Med Sci Sports Exerc. 1992 May;24(5):531–536. [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A., Merton P. A. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977 Nov;272(3):769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts R. H. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994 Jan;74(1):49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Gilchrist J. S., Wang K. K., Katz S., Belcastro A. N. Calcium-activated neutral protease effects upon skeletal muscle sarcoplasmic reticulum protein structure and calcium release. J Biol Chem. 1992 Oct 15;267(29):20857–20865. [PubMed] [Google Scholar]
- Goll D. E., Dayton W. R., Singh I., Robson R. M. Studies of the alpha-actinin/actin interaction in the Z-disk by using calpain. J Biol Chem. 1991 May 5;266(13):8501–8510. [PubMed] [Google Scholar]
- Jones D. A., Jackson M. J., McPhail G., Edwards R. H. Experimental mouse muscle damage: the importance of external calcium. Clin Sci (Lond) 1984 Mar;66(3):317–322. doi: 10.1042/cs0660317. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Westerblad H., Allen D. G. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol. 1991 Feb;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol. 1991 Mar;434:307–322. doi: 10.1113/jphysiol.1991.sp018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann H. P., Waxman L., Goldberg A. L. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem. 1982 Aug 10;257(15):8716–8723. [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T., Kajiwara Y., Kambayashi J., Sakon M., Higuchi N., Tanaka T., Mori T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin). Biochem Biophys Res Commun. 1988 Jun 30;153(3):1201–1208. doi: 10.1016/s0006-291x(88)81355-x. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991 Sep;98(3):615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993 Jul;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. J Physiol. 1994 Jan 15;474(2):291–301. doi: 10.1113/jphysiol.1994.sp020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Duty S., Allen D. G. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol (1985) 1993 Jul;75(1):382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lee J. A., Lännergren J., Allen D. G. Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol. 1991 Aug;261(2 Pt 1):C195–C209. doi: 10.1152/ajpcell.1991.261.2.C195. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Cheung W. Y. Calmodulin stimulates human platelet phospholipase A2. Biochem Biophys Res Commun. 1979 Sep 27;90(2):473–480. doi: 10.1016/0006-291x(79)91259-2. [DOI] [PubMed] [Google Scholar]