Abstract
Candida auris is an emerging multidrug-resistant fungal pathogen that preferentially colonizes and persists in skin tissue, yet the host immune factors that regulate the skin colonization of C. auris in vivo are unknown. In this study, we employed unbiased single-cell transcriptomics of murine skin infected with C. auris to understand the cell type-specific immune response to C. auris. C. auris skin infection results in the accumulation of immune cells such as neutrophils, inflammatory monocytes, macrophages, dendritic cells, T cells, and NK cells at the site of infection. We identified fibroblasts as a major non-immune cell accumulated in the C. auris infected skin tissue. The comprehensive single-cell profiling revealed the transcriptomic signatures in cytokines, chemokines, host receptors (TLRs, C-type lectin receptors, NOD receptors), antimicrobial peptides, and immune signaling pathways in individual immune and non-immune cells during C. auris skin infection. Our analysis revealed that C. auris infection upregulates the expression of the IL-1RN gene (encoding IL-1R antagonist protein) in different cell types. We found IL-1Ra produced by macrophages during C. auris skin infection decreases the killing activity of neutrophils. Furthermore, C. auris uses a unique cell wall mannan outer layer to evade IL-1R-signaling mediated host defense. Collectively, our single-cell RNA seq profiling identified the transcriptomic signatures in immune and non-immune cells during C. auris skin infection. Our results demonstrate the IL-1Ra and IL-1R-mediated immune evasion mechanisms employed by C. auris to persist in the skin. These results enhance our understanding of host defense and immune evasion mechanisms during C. auris skin infection and identify potential targets for novel antifungal therapeutics.
Author summary
C. auris, an emerging fungal pathogen, preferentially colonizes human skin and causes outbreaks of systemic infections. However, the factors that regulate the pathogenesis of C. auris are still unclear. Using unbiased scRNA-seq profiling of murine skin infected with C. auris, we identified the host immune factors that are potentially involved in the regulation of C. auris in skin. Furthermore, our in vivo scRNA-seq reveals that C. auris infection upregulates IL-1Ra in the myeloid cells and limits the neutrophil antifungal activity. In addition, our results suggest that C. auris mannan layer can evade IL-1R signaling mediated skin defense. Collectively, our findings identified the immune and non-immune cells involved in the regulation of C. auris in the skin that could open the door to gain insights into the pathogenesis of this emerging fungal pathogen.
Introduction
C. auris was recently categorized as an urgent threat by the US Centers for Disease Control and Prevention (CDC) and classified in the critical priority fungal pathogens group by the World Health Organization (WHO) [1–3]. Unlike other Candida species, such as Candida albicans, which colonizes the gastrointestinal tract, C. auris preferentially colonizes the human skin, leading to nosocomial transmission and outbreaks of systemic fungal infections [4–6]. Furthermore, unlike skin-tropic fungal pathogens such as Malassezia [7], C. auris not only colonizes the epidermis of the skin but also enters the deeper dermis, a phenomenon that was not observed previously [4]. C. auris can persist in skin tissues for several months and evade routine clinical surveillance [4,8].
Given that the majority of C. auris isolates exhibit resistance to several FDA-approved antifungal drugs, a deeper understanding of C. auris-host interactions is critical to understanding the pathogenesis and developing potential new host-directed therapeutic approaches to prevent and treat this newly emerging skin tropic fungal pathogen. Recent evidence from our laboratory indicates that C. auris skin infection leads to fungal dissemination, suggesting skin infection is a source of invasive fungal infection [9]. Because skin infection is a prerequisite for C. auris transmission and subsequent invasive disease, understanding the immune factors involved in skin defense against C. auris is important to understand the pathogenesis of this skin tropic fungal pathogen. Though antifungal host defense mechanisms against oral, gut, vaginal, and systemic infections of C. albicans are well known [10,11], to date, almost nothing is known regarding the skin immune responses against C. auris.
The fungal cell wall components represent the predominant pathogen-associated molecular patterns (PAMPs) directly interacting with the host to orchestrate the antifungal immune response [12]. Recent evidence indicates that the cell wall of C. auris is structurally and biologically unique compared to other Candida species, including C. albicans [13]. The outer cell wall mannan layer in C. auris is highly enriched in β-1,2-linkages and contains two unique Mα1-phosphate side chains not found in other Candida species [13]. C. auris differentially stimulates cytokine production in peripheral blood mononuclear cells and has a more potent binding to IgG than C. albicans [14,15]. Given that C. auris possesses a unique outer cell wall layer and preferentially colonizes and persists in skin tissue long-term, understanding the skin immune responses during the dynamic pathogen infection in vivo is very important but has not been explored so far. Furthermore, classical population-based gene expression studies using in-vitro differentiated immune cells and mouse models of systemic infection do not completely represent the host defense mechanisms against skin infection in vivo. In addition, understanding the skin immune responses against C. auris is critical, as the murine skin model is widely used to study disease pathogenesis and C. auris-host interactions [4,16–20]. A closer look into cell type-specific host responses requires single-cell resolution to encompass all cell types, including immune and non-immune cells involved in host defense against C. auris skin infection in vivo.
To comprehensively define the transcriptome profiling of mouse skin infected with C. auris in vivo at single-cell resolution, we employed unbiased single-cell RNA sequencing (scRNA-Seq) profiling in skin tissues collected from uninfected and C. auris-infected mice. Our scRNA-Seq analysis identified immune cells such as neutrophils, inflammatory monocytes, macrophages, dendritic cells, T cells, NK cells, and non-immune cells such as fibroblasts accumulated at the site of infection. The scRNA-Seq revealed how skin reprograms genes and signaling pathways in immune and non-immune cell types following C. auris infection. The comprehensive transcriptomic profiling identified the transcriptional changes in genes that encode cytokines, chemokines, host receptors (TLRs, C-type lectin receptors, NOD receptors), antimicrobial peptides, and signaling pathways upregulated in individual myeloid cells, T cells, NK cells, fibroblast, and other non-immune cells. We identified the upregulation of the IL-1RN gene (encoding IL-1R antagonist protein) in different cell types during C. auris skin infection. Subsequently, using mouse models of C. auris skin infection and immune cell depletion studies, we elucidated the role of IL-1Ra in C. auris skin infection. We observed that the IL-1Ra level was significantly increased in the C. auris-infected skin tissue compared with C. albicans. C. auris infection induces IL-1Ra in macrophages and decreases the killing activity of neutrophils. Furthermore, C. auris evades IL-1R-mediated host defense through a unique outer mannan layer to persist the skin tissue.
Collectively, this study, for the first time, identified the previously unknown immune and non-immune cell type-specific skin responses and molecular events of skin-C. auris interactions in vivo at single-cell resolution. Furthermore, we demonstrated the IL-1Ra and IL-1R-mediated immune evasion mechanisms employed by C. auris to persist in the skin. This knowledge will be instrumental in understanding the host-pathogen interactions of C. auris. and will form a strong platform for developing novel host and pathogen-directed antifungal therapeutic approaches that potentially target IL-1Ra and fungal mannan, respectively.
Results
Unbiased scRNA seq profiling identified phagocytic cells, dendritic cells, T cells, NK cells, and fibroblast accumulated at the site of C. auris skin infection in vivo
To identify the immune and non-immune cells involved in host defense against C. auris skin infection, we performed unbiased scRNA seq profiling from skin tissues collected from uninfected and C. auris-infected mice. A group of mice was infected intradermally with 1–2 × 106 CFU of C. auris, and another group injected with 100 μl PBS was used as an uninfected control group (Fig 1A). To capture the transcriptome profile of both innate and adaptive immune responses during C. auris skin infection in vivo, we have chosen 12 days after infection for our analysis. After 12 days post-infection (DPI), skin tissues from uninfected and infected groups (3 mice per group) were collected, minced, and digested to make single-cell suspensions as described. The single-cell suspension from infected and uninfected groups was subjected to single-cell partitioning, and RNA was sequenced using the droplet-based 10X Genomics Chromium platform (Fig 1A). After quality filtering by removing noise and batch effects, our data comprised 70,350 cells (S1 Table). The ambient RNA from the data was eliminated, and the expression level of individual genes was quantified based on the number of UMIs (unique molecular indices) detected in each cell (S2 Table). The alignment of the sequencing reads to the mouse reference genome resulted in the overall coverage of 32,285 genes.
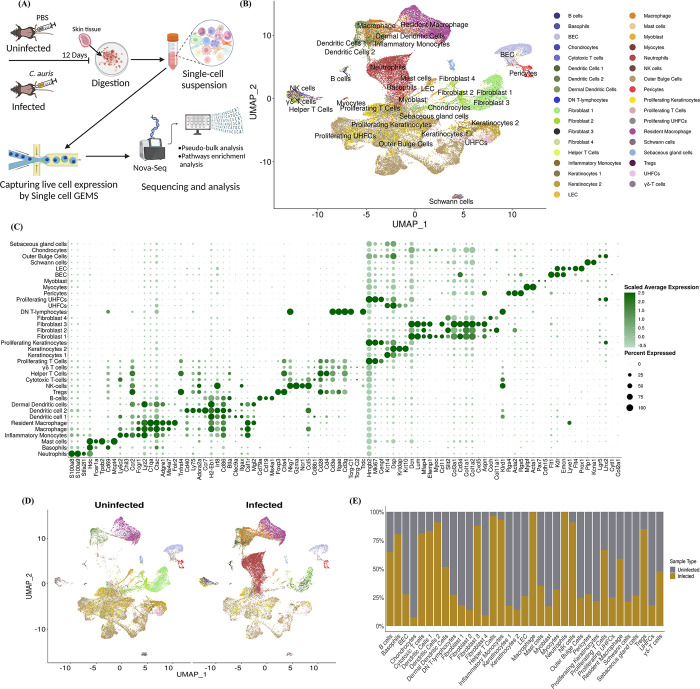
Fig 1. Unbiased scRNA seq profiling identified phagocytic cells, dendritic cells, T cells, NK cells, and fibroblast accumulated at the site of C. auris skin infection in vivo.
A) Schematic representation of the study groups, infection course, sample processing, and single-cell preparation for scRNA sequencing. This illustration was created using Biorender. B) Uniform Manifold Approximation and Projection (UMAP) of identified cell types in the murine skin after clustering. Each cluster was assigned as an individual cell type. After identification, 35 cell types were assigned as individual clusters. C) The dot plot represents the canonical gene marker signatures to classify cell types based on specific identities. A range of 2–7 marker gene expressions were assigned to identify a cell type in the cluster. D) The UMAP of uninfected and C. auris infected murine skin tissue with resident and recruited cell population depicting the cell type heterogenicity between the two groups. E) The composition of the individual cell types in the uninfected and C. auris infected murine skin. Cell type proportions were normalized from the total cells detected in each sample.
After unsupervised graph-based clustering and reference-based annotation from the dataset GSE181720, 30 clusters were identified in our scRNA seq dataset, and cell-type identity was assigned to all the cell populations in the clusters (Fig 1B). To identify each cell lineage from 35 distinct cell types in our scRNA seq dataset, cell-type specific identity was assigned based on the canonical marker expression described elsewhere [21–23] (Fig 1C). After cell-type annotation, we identified 16 cell types as immune cells and the rest 19 as non-immune cells. The UMAP of C. auris infected samples indicated the recruitment of major immune cell types and resident cell populations (Fig 1D). Our analysis identified the percentage of immune and non-immune cells accumulated at the site of infection; (neutrophils– 99.9%, basophils– 80.5%, inflammatory monocytes– 93%, macrophages– 99.6%, dendritic cell 1–83.3%, dendritic cell 2–90.9%, helper T cell– 96.4%, cytotoxic T cells—81%, Tregs—84.9%, NK cells– 90.9%, B-cells—64.7%, proliferating T cells– 66.5%, resident macrophages– 58.9%, dermal dendritic cells– 51.8%, and γδ T cells—47.8%). Surprisingly, we identified fibroblast 3 cell types (87.9%) as major non-immune cells that showed increased accumulation in the skin tissue of infected groups (Fig 1D and 1E) (S1 Table). The recruitment of neutrophils, inflammatory monocytes, macrophages, dendritic cells, and T cell subsets following C. auris skin infection was validated by flow cytometry (S1 Fig). The proportion of cell types between the C. auris infected and uninfected groups varies drastically (Fig 1E) and displays cellular heterogeneity of the resident and recruited cell population. Collectively, our scRNA seq identified the accumulation of various innate and adaptive immune cells at the site of infection. In addition to phagocytic cells, DCs, and T cells, which are known to play a critical role in antifungal defense, our analysis identified an accumulation of NK cells and fibroblast during C. auris skin infection.
scRNA seq revealed the host immune transcriptomic signatures in individual myeloid cells during C. auris skin infection
To identify the host immune genes regulated in individual myeloid cells identified during C. auris skin infection in vivo, we performed pseudo-bulk analysis by normalizing UMI counts for the target genes and identifying differentially expressed genes (DEGs) between the infected and uninfected groups. For the downstream analysis, we filtered DEGs with FDR < 5%, and the log2 fold change ≥ 2 was considered upregulated, and ≤ -2 was considered downregulated. In neutrophils, 1107 genes (1105 upregulated and 2 downregulated), inflammatory monocytes, 299 genes (217 upregulated and 82 downregulated), macrophages, 1340 genes (1322 upregulated and 18 downregulated), resident macrophages, 357 genes (143 upregulated and 214 downregulated), dendritic cell 1, 454 genes (260 upregulated and 194 downregulated), dendritic cell 2, 265 genes (244 upregulated and 21 downregulated) and dermal dendritic cells 262 (91 upregulated and 171 downregulates) were differentially expressed in the C. auris infected groups. The top 10 upregulated and downregulated genes in the myeloid subsets were highlighted in the volcano plot (Fig 2A). Among the top 10 DEGs in myeloid cells, chemokine genes such as Ccl4 and Cxcl3 in neutrophils, Cxcl9 and Ccl5 in inflammatory monocytes, and Cxcr2 in dermal dendritic cells were significantly upregulated in the C. auris infected group (Fig 2A). In macrophages, resident macrophages, and dermal dendritic cells, Nos2 and Arg1 involved in nitric oxide metabolism were upregulated genes after infection. Arg1 was also significantly upregulated in dermal dendritic cell 1 (Fig 2A). In addition, Inhba, slpi, and aw112020 genes known to shift the nitric oxide metabolism were upregulated in macrophages (Fig 2A). Furthermore, the gene encoding for serum amyloid A3 protein (Saa3) was significantly upregulated in all the phagocytic cells (S2A Fig) (S10 Table). We examined the genes that were upregulated in all phagocytic cells. Spp1, Egln3, Slpi, Upp1, Inhba, F10, Acod1, AA467197, Ppp1r3b, Nos2, Slc2a1, Tarm1, Cd300lf, and Ly6a were significantly upregulated in all the phagocytic cells during C. auris infection (S2A Fig) (S10 Table). We identified AA467197, Plac8, Arg1, Ccl17, Slpi, and AW112010 were significantly upregulated in dendritic cell 1, dendritic cell 2, and dermal dendritic cells (S2B Fig) (S10 Table).
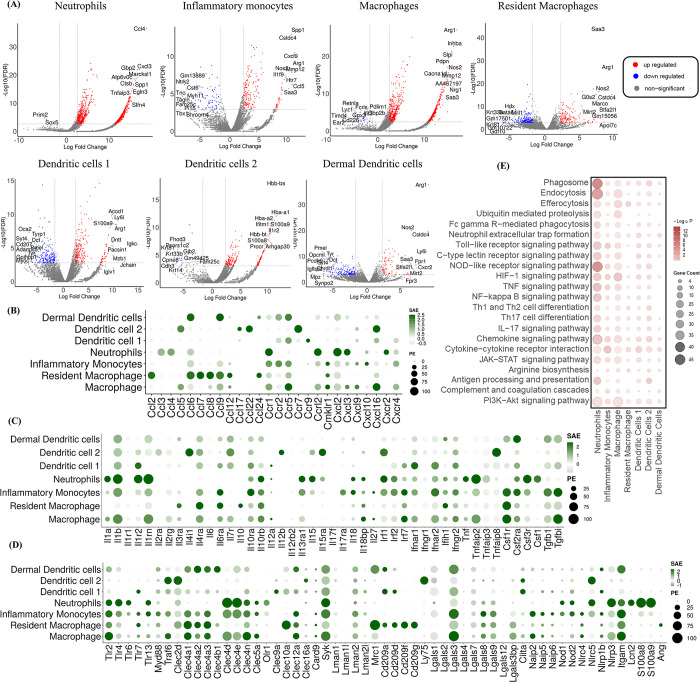
Fig 2. scRNA seq revealed the host immune transcriptomic signatures in individual myeloid cells during C. auris skin infection.
A) Volcano plots indicating significant differentially expressed genes (DEGs) in the infected and uninfected neutrophils, monocytes, macrophages, resident macrophages, dendritic cell 1, dendritic cell 2 and dermal dendritic cells highlighting the top 10 upregulated and downregulated genes; The Dot plot represents the expression of selected B) chemokines and its receptors, C) cytokines and its receptors, D) fungal recognizing receptors and antimicrobial peptides (AMPs) in the myeloid subsets (Y-axis). The dot size represents the percentage of cells with expressions, and the color indicates the scaled average expression calculated from the 3 uninfected and 3 infected samples. E) The bubble plot represents the KEGG pathway of the enriched upregulated genes of the myeloid subsets (X-axis). DEGs from pseudo-bulk analysis with threshold Log 2-fold change ± 2 and FDR > 5% were considered as significant DEGs, and Log 2-fold change ≥ 2 and FDR > 5% as upregulated genes.
Next, we compared the expression of chemokines, cytokines, pattern-recognizing receptors (PRRs), and antimicrobial peptides (AMPs) among different myeloid subsets [11, 24]. The gene list from the mouse genome database was used to explore the expression of the host immune genes [25]. Among the chemokines and chemokine receptors, Ccr1, Ccrl2, Cxcl2, Cxcl3, and Cxcr2 were highly expressed in neutrophils. Ccr1, Ccr2, Ccr5, and Cxcr4 were highly expressed in inflammatory monocytes, whereas Ccr1, Ccrl2, Ccr5, Ccl5, Ccl9, Cmklr1, Cxcl16 and Cxcr4 were highly expressed in the inflammatory macrophage and Ccl2, Ccl6, Ccl7, Ccl8, Ccl9, Ccl12, and Ccl24 were highly expressed in the resident macrophages. Dendritic cell 1 showed increased expression of Ccr5, Ccr9, and Ccl16, whereas Ccl5, ccl17, Ccl22, Ccr7, and Cxcl16 were highly expressed in dendritic cell 2. Ccl6, Ccl9, Ccr2, and Ccr5 were highly expressed in the dermal dendritic cells (Fig 2B). Among the cytokines and cytokine receptors, Il1a, Il1b, Il1r2, and Il1rn were highly expressed in neutrophils. Il1a, Il1b, and Il1rn were highly expressed in macrophages, whereas Il1b and Il1rn were highly expressed in monocytes and dendritic cells 1. We identified increased expression of Il10 in the resident macrophages, Tnf and Csf1 were increased in neutrophils, and Tgfbi expression was upregulated in monocytes and macrophages (Fig 2C).
Next, we compared the expression of TLRs, C-type lectin receptors, NOD-like receptors (NLRs), and complement receptor 3 among myeloid subsets. Neutrophils showed an increased Tlr2, Tlr4, Tlr6, and Tlr13 expressions. Tlr2, Tlr4, Tlr7, Tlr13, and the adaptor molecules such as MyD88 and Traf6 were highly expressed in the inflammatory monocytes. Tlr2 was highly expressed in macrophages, whereas Tlr2 and Tlr7 were highly expressed in resident macrophages. Tlr7 and Tlr2 were highly expressed in dendritic cell 1 and dermal dendritic cells, respectively. Among the C-type lectin receptors, Clec4d (Dectin-2), Clec4n (Dectin-3), and Clec4e (Mincle) were highly expressed in neutrophils, monocytes, and macrophages. The Syk, an adopter protein involved in Dectin-1 signaling, is highly expressed in all myeloid cells. Card9, the other downstream signaling protein of Dectin-1, is selectively expressed in all the myeloid subsets except neutrophils. The Clec4a1, Clec4a2, and Clec4a3 were highly expressed in monocytes, macrophages, resident macrophages, and dermal dendritic cells, whereas the Clec4b1 was only expressed in the dermal dendritic cells. The mannose-binding receptor Mrc1 (Mannose receptor) was highly expressed in resident macrophages and dermal dendritic cells. The cd209a (DC-SIGN1), cd209d (SIGN-R3), cd209f (SIGN-R8), and cd209g encoding DC-SIGN were highly expressed in resident macrophages. The Lgals3 (Galectin-3) was highly expressed in monocytes and macrophages, followed by dermal dendritic cells and dendritic cell 1. We identified increased expression of intracellular PRR, Nod1, and Nod2 in the neutrophils and monocytes. The Itgam (complement receptor 3) was highly expressed in neutrophils, monocytes, macrophages, resident macrophages, and dermal dendritic cells. We identified increased expression of Nlrp3 in neutrophils. Among the AMPs, the Lcn2, S100a8, and S100a9 were highly expressed in neutrophils, and the Ang was selectively expressed in the resident macrophages (Fig 2D).
To explore the enrichments of DEGs in the myeloid cells, KEGG pathway analysis was performed for significantly upregulated genes (5% FDR, Log2Fc ≥ 2) in neutrophil, monocytes, macrophages, resident macrophages, dendritic cell 1, dendritic cell 2 and dermal dendritic cells 2 to identify the upregulated pathways (Fig 2E). Among myeloid subsets, we identified several pathways involved in pathogen recognition and immune signaling that were highly enriched in neutrophils. The major innate pathways enriched in the neutrophils during C. auris skin infection: 1) phagocytosis (phagosome, endocytosis, efferocytosis, ubiquitin-mediated proteolysis, Fc gamma R-mediated phagocytosis, and neutrophil extracellular trap formation), 2) PRRs (Toll-like receptor signaling pathway, C-type lectin receptor signaling pathway, and NOD-like receptor signaling pathway), 3) inflammasome activation (HIF-1 signaling pathway, TNF signaling pathway, and NF-kappa B signaling pathway), 4) cytokine and chemokine signaling (chemokine signaling, cytokine-cytokine receptor pathway, and JAK-STAT signaling pathway), 5) T-helper cell (Th) differentiation (Th1 and Th2 cell differentiation, Th17 cell differentiation and IL17 signaling pathway). In addition, we identified the enriched upregulated genes in these KEGG pathways (S3 Table). Collectively, our single-cell transcriptomics identified the AMPs, cytokines, chemokines, and fungal recognition receptors upregulated in myeloid subsets during C. auris skin infection. We identified the genes involved in nitric oxide metabolism and acute phase proteins, which were highly upregulated in different myeloid cells. Furthermore, our analysis revealed the enrichment of several pathways involved in fungal recognition, phagocytosis, cytokine and chemokine signaling, and T-cell differentiation in individual myeloid cells during C. auris skin infection in vivo.
C. auris skin infection induces IL-17 and IFNγ signaling pathways in lymphoid cells
To identify the host immune genes and transcription factors (TFs) regulated in T cell subsets and NK cells during C. auris skin infection, we sub-clustered the lymphoid cells at 0.2 resolution to identify the subsets of CD4+ Th cells, CD8+, γδ T cells, Tregs, and NK cells (Fig 3A). Sub-clustering the lymphoid population identified 203 genes in CD4+ Th cells (118 upregulated and 85 downregulated), 41 genes in Tregs (34 upregulated and 7 downregulated), 18 genes in CD8+ cells (14 upregulated and 4 downregulated), 92 genes in γδ T cells (73 upregulated and 19 downregulated) and 16 genes in NK cells (9 upregulated and 7 downregulated) were differentially expressed, and the heatmap shows the significantly upregulated genes (log2 fold change ≥ + 2, FDR < 5% or 1%) in CD4+ Th cells, γδ T cells, Tregs, CD8+ cells, and NK cells (Figs 3D, S3B and S3C). We examined the genes upregulated in lymphoid subsets (S3A Fig). The Nfkbia was upregulated in CD4+ Th cells and CD8+ cells. Epsti1 and Enox2 were significantly upregulated in CD4+ Th cells and NK cells (S3A Fig) (S10 Table).
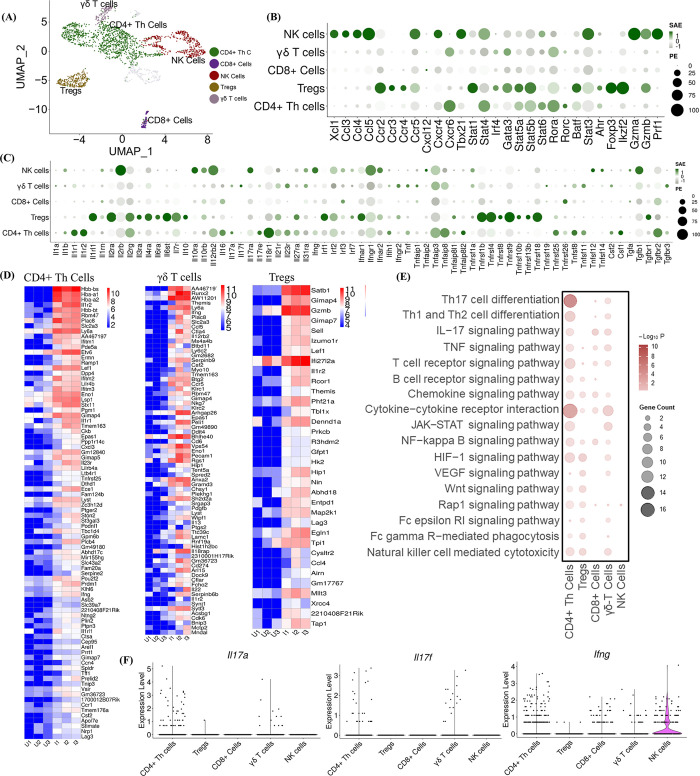
Fig 3. Single-cell profiling of lymphoid subsets.
A) Uniform Manifold Approximation and Projection (UMAP) represents the sub-clustering analysis of lymphoid subsets at 0.2 resolution. The CD4+ Th cells, Tregs, CD8+ cells, γδ T cells, and NK cells were identified in the subpopulation. The Dot plot represents the expression of selected B) chemokines and their receptors, transcription factors (TFs), and cytolytic enzymes, and C) cytokines and their receptors in the lymphoid subsets (Y-axis). The dot size represents the percentage of cells with expressions, and the color indicates the scaled average expression calculated from the 3 uninfected and 3 infected samples. D) The heatmap represents the expression of the significantly upregulated genes in CD4+ Th cells, γδ T cells, and Tregs in uninfected and infected groups. The normalized gene counts were plotted in the heatmap, and the scale indicates red for high, blue for low, and white for moderate expression in the samples. Each column represents a different sample. E) The bubble plot represents the KEGG pathway enrichment analysis of significantly upregulated genes in the lymphoid subsets (X-axis). F) Violin plots depict the scaled count of Il17a, Il17f, and Ifng in CD4+ Th cells, Tregs, CD8+ cells, γδ T cells, and NK cells in both uninfected and infected samples. The scaled count were normalized using SCTransform method. DEGs from pseudo-bulk analysis with threshold Log 2-fold change ≥ 1 and FDR > 5% were considered as significant upregulated genes for KEGG pathways. Upregulated genes with Log 2-fold change ≥ 2 and FDR > 1% for CD4+ Th cells and FDR > 5% for Tregs, and γδ T cells were represented for the heatmap.
Next, we compared the expression of chemokines, cytokines, and TFs among lymphoid subsets. Cxcr6 was highly expressed in CD4+ Th cells and γδ T cells. We identified increased expression of Xcl1, Ccl3, Ccl4, Ccl5, Ccr5, and Cxcr4 in NK cells, and Ccr2, Ccr3, and Ccr4 in the Tregs. TFs such as Stat4, Stat5a, Stat5b, Stat6, Rora, Rorc, and Ahr were highly expressed in the CD4+ Th cells. Tregs showed increased expression of Stat1, Gata3, Stat5a, Stat5b, Batf, Foxp3, Ikzf2, Irf4 and Ahr. We identified the increased Tbx21, Stat4, Stat6, and Stat3 expression in NK cells. Gzma, Gzmb, and prf1 in NK cells and Gzmb were highly expressed in the Tregs (Fig 3B). Il16, Csf1, Il1b, Il17a, Tnf, and Csf2 were highly expressed in the CD4+ Th cells. Tregs showed a higher expression of Il10. Il17a and Il17f were selectively expressed in γδ T cells. We identified increased expression of tgfb1 and ifng in the NK cells (Fig 3C).
The upregulated pathways enriched in CD4+ Th cells, CD8+ cells, γδ T cells, Tregs, and NK cells were identified from KEGG pathway analysis of the upregulated genes with Log2Fc ≥ 1 and 5% FDR threshold (Fig 3E). We identified pathways involved in Th17 cell differentiation, cytokine-cytokine receptor interaction, Th1, and Th2 cell differentiation, and HIF-1 signaling pathway were highly enriched in CD4+ Th cells during C. auris skin infection (Fig 3E). Other pathways involved in TNF, JAK-STAT, NF-kappa B and HIF-1 signaling were enriched in CD4+ Th cells, Tregs and γδ T cells (Fig 3E). In addition, we examined the upregulated DEGs enriched in these KEGG pathways (S4 Table). Next, we used violin plots to identify the cell type level expression of Il17a, Il17f, and Ifng in CD4+ Th cells, CD8+ cells, γδ T cells, Tregs, and NK cells (Fig 3F). Our analysis revealed that Il17a and Il17f were mainly expressed in CD4+ Th cells and γδ T cells during C. auris skin infection. Our analysis identified that NK cells showed an increased expression of Ifng, followed by CD4+ Th cells, CD8+ cells, and γδ T cells (Fig 3F). IL-17 signaling pathway, which is known to play a critical role in antifungal defense, is upregulated in CD4+ Th cells and γδ T cells. Taken together, our scRNA analysis identified the expression of cytokines, chemokines, and TFs upregulated in T cells and NK cells.
scRNA seq revealed the transcriptomic signatures in fibroblast and other non-immune cells during C. auris skin infection
Our unbiased scRNA profiling identified fibroblast as a major non-immune cell accumulated at the site of skin infection. To understand the transcriptomic signatures of fibroblast during C. auris skin infection, the gene expression profiling of fibroblast sub-clusters, fibroblast 1, fibroblast 2, fibroblast 3, and fibroblast 4, were explored. The DEGs from the pseudo-bulk analysis with a threshold of FDR < 5% and the log2 fold change ≥ 2 were used for further analysis. Log2 fold change of ≤ -2 were considered downregulated for the volcano plots. Among the DEGs in fibroblast subsets, 370 genes in fibroblast 1 (150 upregulated and 220 downregulated), 68 genes in fibroblast 2 (12 upregulated and 56 downregulated), 389 genes in fibroblast 3 (294 upregulated and 95 downregulated) and 9 genes in fibroblast 4 (1 upregulated and 8 downregulated) were regulated upon C. auris infection. The top 10 upregulated and downregulated genes in fibroblast subsets were denoted in the volcano plots (Fig 4A). The antimicrobial peptide S100a9 is the top upregulated genes in fibroblast 1, fibroblast 2 and fibroblast 4. The S100a8, which forms a complex with S100a9 as calprotectin, is among the top 10 upregulated genes in fibroblast 2. The serum amyloid protein Saa3 is the highest upregulated gene in fibroblast 3, and the Saa1 and Saa3 were highly upregulated in fibroblast 1 (Fig 4A). Further, the antimicrobial peptides NOS2 and Lcn2 are highly upregulated in fibroblast 1 and fibroblast 3 (Fig 4A) (S5A Fig).
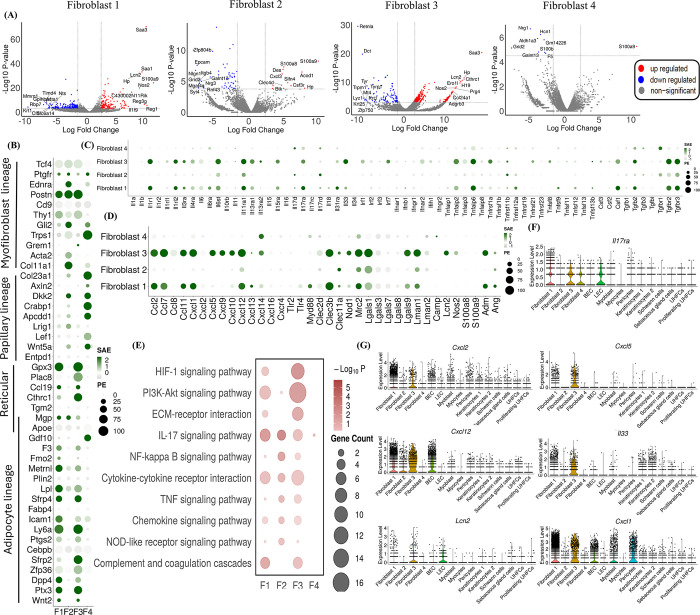
Fig 4. scRNA seq revealed the transcriptomic signatures in fibroblast and other non-immune cells during C. auris skin infection.
A) The Volcano plots indicate significant differentially expressed genes (DEGs) in the infected and uninfected fibroblast subsets, highlighting the top 10 upregulated and downregulated genes. The Dot plot represents the expression of selected B) fibroblast lineage markers, C) cytokines and their receptors, and D) chemokines and their receptors, fungal recognizing receptors, and antimicrobial peptides (AMPs) in the fibroblast subsets (Y-axis). The dot size represents the percentage of cells with expressions, and the color indicates the scaled average expression calculated from the 3 uninfected and 3 infected samples. E) The bubble plot represents the KEGG pathway enrichment of the upregulated genes of fibroblast subsets (X-axis).; Violin plots depict the scaled count of F) IL17ra and G) Cxcl1, Cxcl2, Cxcl5, Cxcl12, Lcn2, and IL33 in fibroblast subsets and other non-immune cells in both uninfected and infected samples. The scaled count were normalized using SCTransform method; DEGs from pseudo-bulk analysis with threshold Log 2-fold change ± 2 and FDR > 5% were considered as significant DEGs and Log 2-fold change ≥ 2 and FDR > 5% as upregulated genes.
We classified the fibroblast subsets based on their lineage-specific marker expression. Fibroblasts 1 and 3 highly expressed most adipocyte lineage markers, and fibroblast 4 substantially expressed papillary lineage markers (Fig 4B). Next, we compared the expression of chemokines, cytokines, fungal-recognizing receptors, and AMP among four fibroblast subsets (Fig 4C and 4D). Il33, Csf1, and Tgfb2 were highly expressed in fibroblast 3 and fibroblast 1, whereas Il17d was selectively expressed in fibroblast 2 and fibroblast 4. Among the cytokine receptors, Il1r1, Il1rl2, Il6st, Il11ra1, Il17ra, Tgfbr2, and Tgfbr3 were highly expressed in fibroblast 3 and fibroblast 1, whereas Il11ra1 and Tgfbr3 were highly expressed in fibroblast 2 (Fig 4C). Among the chemokines, Ccl2, Ccl7, Ccl11, Cxcl1, and Cxcl12 were highly expressed in fibroblast 1 and fibroblast 3. Cxcl5, Cxcl9, Cxcl10, and Cxcl14 were highly expressed in fibroblast 3. Among the fungal recognizing receptors, Clec3b, Mrc2, Lgals1, Lgals9, Lman1, and Clec11a were highly in fibroblast 3 and fibroblast 1 (Fig 4D). We identified increased expressions of Clec11a, Mrc2, Lgals1, and Lman1 in fibroblast 2 and selective expression of Nod2 in fibroblast 3. Among the AMPs, fibroblast 3 showed increased expression of Lcn2, Nos2, and Amd, whereas Amd and Ang were highly expressed in fibroblast 1 (Fig 4D).
To identify the upregulated pathways in fibroblast subsets during C. auris skin infection, KEGG pathway enrichment was performed for upregulated genes with Log2Fc ≥ 2 and 5% FDR in fibroblast 1, fibroblast 2, fibroblast 3, and fibroblast 4 (Fig 4E). We identified pathways involved in HIF-1 signaling, PI3K-Akt signaling, cytokine-chemokine receptor interaction, and complement coagulation cascade pathways that were highly enriched in fibroblast 3 and fibroblast 1. ECM-receptor interaction pathway was enriched in fibroblast 3. The IL-17 signaling pathway was enriched in all three fibroblast subtypes except fibroblast 4 (Fig 4E). Next, we analyzed other non-immune cells, such as BEC, outer bulge cells, and pericytes, that showed DEGs during C. auris skin infection (S4 Fig). We examined the upregulated DEGs enriched in these KEGG pathways (S5 Table). KEGG pathways, such as Th17 cell differentiation and cytokine-cytokine receptor interactions, were highly enriched in BEC, pericytes, and outer bulge cells (S5B–S5D Fig). To understand if fibroblast and other non-immune skin cells play a role in the recruitment of immune cells during C. auris infection, we analyzed the expression of genes such as IL-17ra and chemokines involved in recruiting immune cells such as neutrophils. Among non-immune cells, fibroblast subsets and LEC showed increased expression of the IL-17ra gene (Fig 4E). The chemoattractants such as Cxcl1, Cxcl2, Cxcl5, Cxcl12, Lcn2, and Il33 involved in the recruitment of neutrophils were highly expressed in different non-immune cells, but fibroblast subsets showed relatively higher expression (Fig 4G). Taken together, our unbiased scRNA seq revealed the increased expression of cytokines, chemokines, PRRs, and AMPs in fibroblasts and other non-immune cells that could either directly (or) indirectly contribute to the host defense against C. auris skin infection.
C. auris induces IL-1Ra in macrophages to decrease the neutrophil function in the skin
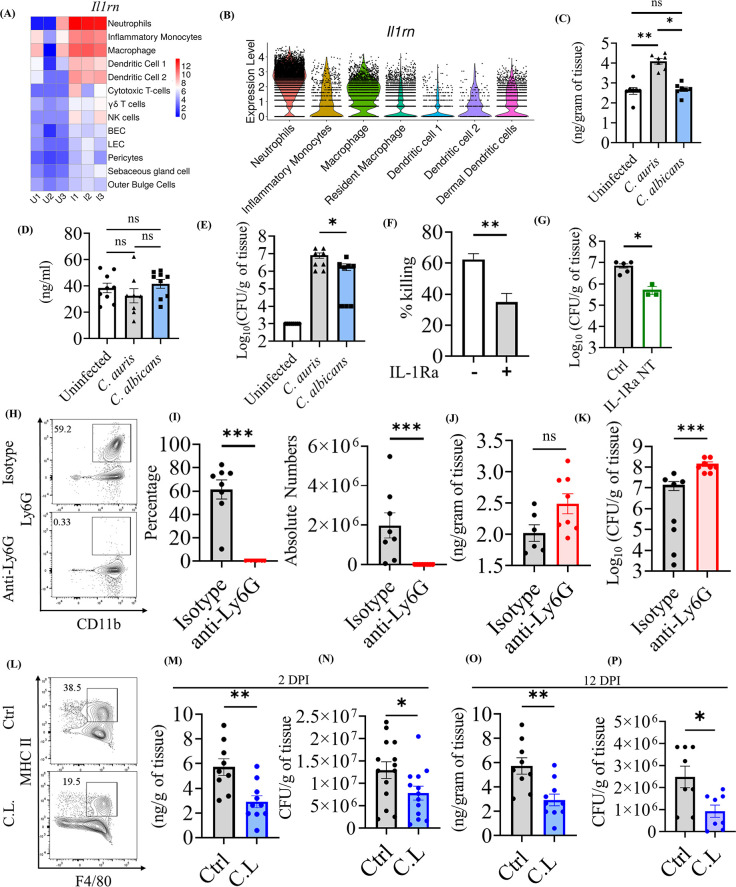
Our scRNA seq identified that C. auris skin infection upregulated the expression of the Il1rn gene in different cell types, especially in myeloid and lymphoid cells (Fig 5A). The Il1rn gene encodes four isoforms of IL-1R antagonist (IL-1Ra), which regulates the inflammatory signaling of the IL-1 pathway by competing with IL-1α and IL-1β in IL-1R activation [26,27]. We analyzed the expression of Il1rn in the cell types identified in our analysis. In our dataset, the Il1rn was expressed only in hematopoietic cells in the C. auris infected samples (Fig 5A) (S6A Fig). However, in the non-hematopoietic cells, such as fibroblasts and keratinocytes, that are known to express intracellular isoforms of IL-1Ra [28,29], the C. auris infection did not considerably altered Il1rn expression (S6A Fig).
Fig 5. C. auris induces IL-1Ra in macrophages to decrease the neutrophil function in the skin.
(A) Heatmap represents the expression of Il1rn in the cell types (y-axis) between the infected and uninfected groups. The normalized gene counts were plotted in the heatmap, and the scale indicates red for high, blue for low, and white for moderate expression in the samples. Each column represents a different sample. (B) The violin plot depicts the scaled count of Il1rn in myeloid subsets from both uninfected and infected samples. The scaled count were normalized using SCTransform method. IL-1Ra levels in the (C) skin and (D) serum of mice after 3 DPI of C. auris AR0387 or C. albicans SC5314 (n = 8–10 mice/group). Uninfected mice received 100 μL of 1X PBS on the day of infection. (E) Fungal burden in the skin tissue of indicated mice on 3 DPI of C. auris AR0387 or C. albicans SC5314 (n = 8–10 mice/group). Uninfected mice received 100 μL of 1X PBS on the day of infection. (F) The bar graph represents the neutrophil-killing activity of C. auris AR0387 in the presence and absence of recombinant IL-1Ra. (G) Skin fungal burden of the mice groups injected with neutralizing anti-mouse IL-1Ra monoclonal antibody or 100 μl of 1X PBS after 3 DPI of C. auris AR0387 in the murine skin. (n = 3–5 mice/group) (H-K) Quantification of IL-1Ra level and fungal burden in mice injected with anti-Ly6G antibody and Rat IgG2a isotype control (Isotype) for neutrophil depletion after 2 DPI of C. auris AR0387 (n = 6–8 mice/group). H) Representative flow plots and I) percentage and absolute number of CD11b+ Ly6G+ neutrophils in the infected skin tissue of mice injected with anti-Ly6G antibody or Rat IgG2a isotype control (Isotype) antibody. (J) IL-1Ra level and (K) CFU were determined from the homogenate of infected skin tissue. (L-O) Measurement of IL-1Ra production and fungal burden in clodrosome (C.L) and 1X PBS (Ctrl) injected mice groups for macrophage depletion after 2 DPI of C. auris AR0387 (n = 9–10 mice/group). (L) Representative flow plots of F4/80+ MHCII+ macrophage in the infected skin tissue of mice injected with clodrosome (C.L) or 1X PBS (Ctrl) injected mice groups. (M) IL-1Ra level and (N) CFU determined from the homogenate of infected skin tissue from 2 DPI of C. auris 0387 (n = 12 mice/group).(O) IL-1Ra level and (P) CFU determined from the homogenate of infected skin tissue from 12 DPI of C. auris 0387 (n = 8–10 mice/group). Error bars represent mean ± SEM. * p < 0.05, ** p <0.01, *** p <0.001, **** p <0.0001, NS, non-significant. Statistical significances were calculated using Mann–Whitney U. Abbreviations–DPI, days post-infection; C.L, clodrosome.
Our analysis revealed that Il1rn expression was highly expressed in neutrophils, followed by macrophages (Fig 5B). Given that C. auris persists in the skin long-term, the role of IL-1Ra in host defense against C. auris skin infection is unknown. Therefore, we investigated if the increased Il1rn identified in our scRNA analysis plays a role in host defense against C. auris. To confirm our scRNA data, we infected mice intradermally with C. auris and examined the IL-1Ra level by ELISA. In addition, mice intradermally infected with C. albicans was used to compare IL-1Ra levels with C. auris-infected groups. We identified that the skin tissue of C. auris-infected mice had significantly higher IL-1Ra levels compared with the uninfected and C. albicans-infected groups (Fig 5C). We found that all four C. auris clades (AR0381, AR0383, AR0285, and AR0387) induced comparable levels of IL-1Ra in skin tissues (Figs S6B and 6C). On the other hand, no significant difference was observed in the IL-1Ra level in the serum of uninfected, C. auris, and C. albicans-infected groups (Fig 5D). Furthermore, the increased level of IL-1Ra in the skin tissue of C. auris-infected mice corresponds to the increased fungal burden observed in C. auris-infected skin tissue compared with C. albicans infection groups (Fig 5E).
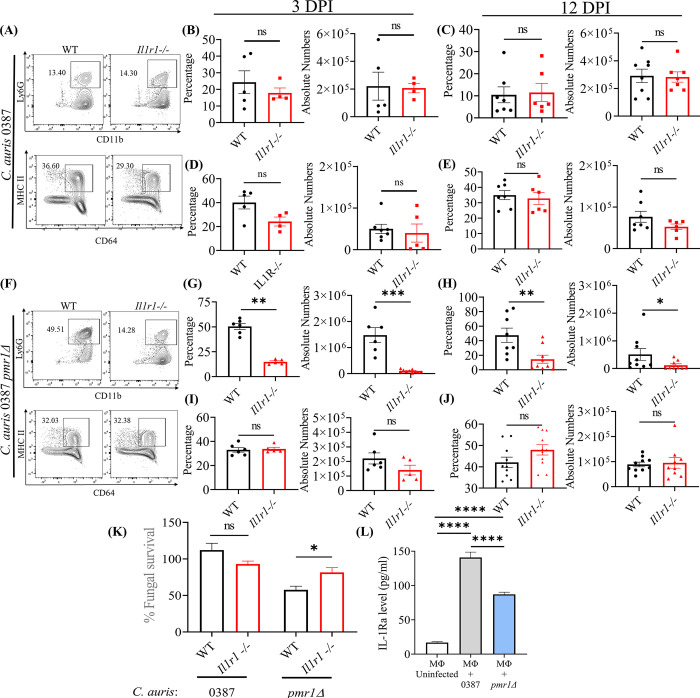
Fig 6. C. auris uses a unique cell wall mannan layer to evade IL-1R signaling mediated host defense.
(A) The representative flow plots represents the percentage of CD11b+ Ly6G+ neutrophils and CD64+ MHCII+ macrophage in the WT and Il1r1-/- mice skin tissue infected with C. auris 0387. The bar graph represents the percentage and absolute number of CD11b+ Ly6G+ neutrophils after (B) 3 DPI and (C) 12 DPI and the CD64+ MHCII+ macrophage after (D) 3 DPI and (E) 12 DPI in the WT and Il1r1-/- mice skin tissue infected with C. auris 0387 (n = 4–8 mice/group). (F) The representative flow plots represents the percentage of CD11b+ Ly6G+ neutrophils and CD64+ MHCII+ macrophage in the WT and Il1r1-/- mice skin tissue infected with 0387 pmr1Δ. The bar graph represents the percentage and absolute number of CD11b+ Ly6G+ neutrophils after (G) 3 DPI and (H) 12 DPI and the CD64+ MHCII+ macrophage after (I) 3 DPI and (J) 12 DPI in the WT and Il1r1-/- mice skin tissue infected with 0387 pmr1Δ (n = 5–10 mice/group). For C. auris 0387 pmr1Δ infection 5 × 106 CFU per mice were used. (K) The bar graph represents the fungal survival of C. auris 0387 and 0387 pmr1Δ primed with neutrophils isolated from bone marrow of WT and Il1r1-/- mice. (n = 6) (L) Measurement of IL-1Ra levels from the culture supernatant of BMDM uninfected or infected with live C. auris 0387 or 0387 pmr1Δ for 16 h. (n = 12). Error bars represent mean ± SEM. * p < 0.05, ** p <0.01, *** p <0.001, **** p <0.0001, NS, non-significant. Abbreviations—DPI, days post-infection; WT, wild type; BMDM, bone marrow-derived macrophages. Statistical significances were calculated using Mann–Whitney U.
IL-1Ra binds with IL-1R [27,30], and IL-1R signaling is critical for neutrophil function [26,31,32]. We investigated if the presence of IL-1Ra modulates the neutrophil killing of C. auris. The neutrophils isolated from the mouse peritoneum were assessed for C. auris-killing activity in the presence and absence of recombinant IL-1Ra. We identified that the neutrophils exhibited significantly decreased killing activity against C. auris in the presence of recombinant IL-1Ra (Fig 5F). Next, we examined the in vivo role of the secreted IL-1Ra in C. auris murine skin infection using neutralizing antibody. We found that C. auris-infected mice treated with the IL-1Ra neutralizing antibody exhibited significantly reduced fungal burdens in the skin tissue compared to untreated groups (Fig 5G). These findings indicate that the secreted IL-1Ra during C. auris skin infection decreases neutrophil function and potentially favors fungal growth in the skin tissue in vivo.
Next, we determined to examine the source of IL-1Ra during C. auris skin infection. Since neutrophils followed by macrophages highly expressed Il1rn, we examined if IL-1Ra produced by neutrophils and macrophages play a role in host defense against C. auris skin infection. We depleted neutrophils using an anti-Ly6G antibody, and as expected, the percentage and absolute number of CD11b+ Ly6G+ neutrophils were significantly decreased in the skin tissue of mice that received anti-Ly6G antibody compared to mice injected with isotype antibody (Fig 5H and 5I). However, surprisingly, we observed no significant difference in IL-1Ra levels in the skin tissue of mice that received anti-Ly6G antibody and isotype antibody (Fig 5J). On the other hand, fungal load was significantly increased in the skin tissue of mice that received anti-Ly6G antibody compared with mice that received isotype antibody (Fig 5K). These findings suggest neutrophils play a critical role in host defense against C. auris skin infection in vivo. Furthermore, depleting neutrophils alone did not considerably affect IL-1Ra levels in the skin tissue of mice infected with C. auris. Next, we depleted macrophages using clodronate liposomes to identify if macrophages are the source of IL-1Ra during C. auris skin infection. The percentage and absolute number of CD11b+ MHCII+ F4/80+ macrophages were significantly decreased in the skin tissue of mice that received clodronate liposomes compared to control groups (Fig 5L) (S6D Fig). Interestingly, we identified that IL-1Ra was significantly decreased in the skin tissue of mice after they received clodronate liposome compared with control groups (Fig 5M). Furthermore, the fungal load was significantly decreased in the skin tissue of mice that received clodronate liposome compared with control groups (Fig 5N). A difference of 1.6-fold in CFU/g of tissue was observed between the control and C.L. group. A similar trend was observed at a late time point, which was 12 DPI (Fig 5O and 5P). These findings suggest that macrophages are the major source of IL-1Ra during the early and late time points of C. auris skin infection. Next, we cultured the macrophages in vitro, stimulated with C. auris, and measured the IL-1Ra level in the culture supernatants after 16 hours. The IL-1Ra level in the culture supernatant of macrophages infected with C. auris was significantly higher than the uninfected macrophages. These results suggest that C. auris infection induces IL-1Ra production in the macrophage ex vivo (S6E Fig). To examine if the IL-1Ra produced by the macrophages can affect the neutrophil activity ex vivo, we collected the culture supernatant from the macrophages stimulated or unstimulated with C. auris, and this macrophage conditioned media (MΦ CM) was used to access the neutrophil killing activity of C. auris. The survival of C. auris in the neutrophil incubated with MΦ CM from infected macrophages was significantly increased compared to MΦ CM from uninfected macrophages (S6F Fig). This finding suggests that the macrophage-produced IL-1Ra limits the neutrophil-killing activity of C. auris. Collectively, our results indicate that the IL-1Ra produced by macrophages during C. auris skin infection decreases the neutrophil function, which is important for antifungal host defense against this emerging skin tropic fungal pathogen (S7 Fig).
C. auris uses a unique cell wall mannan layer to evade IL-1R signaling mediated host defense
IL-1R signaling is critical for neutrophil recruitment and function and plays an important role in the host defense against C. albicans in oral mucosa and systemic infection [31, 32]. However, the role of IL-1R signaling in host defense against C. auris skin infection is unknown. Therefore, we used mice lacking the IL-1 receptor (IL-1R) to investigate the IL-1R signaling in skin defense against C. auris. Surprisingly, we found that the percentage and absolute numbers of neutrophils in the Il1r1-/- mice infected with C. auris were not significantly affected and showed similar levels to wild-type (WT) infected groups after 3 DPI and 12 DPI (Fig 6A–6C). We also observed no significant difference in the percentage and absolute numbers of macrophages in the Il1r1-/- mice after 3 DPI and 12 DPI (Fig 6D and 6E). Unlike C. albicans [31,32], these findings indicate that neutrophil recruitment was not affected in Il1r1-/- mice infected with wild-type C. auris strain 0387. Since fungal PAMPs modulate the host response [12], and C. auris possesses a unique outer mannan layer compared to C. albicans [13], we hypothesized that the C. auris outer cell wall mannan layer controls IL-1R signaling. We used the CRISPR-Cas9 system to generate a C. auris mutant strain (pmr1Δ) that lacks an outer mannan layer as described previously [33]. The growth kinetics was unaltered in C. auris pmr1Δ [34]. WT and Il1r1-/- mice were infected with pmr1Δ strain to examine the neutrophil and macrophage recruitment. Interestingly, we found that neutrophil recruitment was significantly decreased in the Il1r1-/- mice infected with the pmr1Δ strain compared to wild-type (WT) mice after 3 DPI and 12 DPI (Fig 6F to 6H). No significant differences were observed in the recruitment of macrophages in the Il1r1-/- mice infected with the pmr1Δ strain (Fig 6I and 6J). These findings indicate that the unique mannan outer layer of C. auris controls IL-1R signaling-dependent neutrophil recruitment.
Next, we investigated if C. auris mannan outer layer controls IL-1R signaling-dependent neutrophil function. Neutrophils isolated from WT and Il1r1-/- mice were infected with C. auris 0387 and 0387 pmr1Δ to determine fungal survival (Fig 6K). We identified no difference in fungal survival in neutrophils from WT and Il1r1-/- mice infected with the C. auris 0387 strain. However, we observed significantly higher fungal survival of the 0387 pmr1Δ strain in the Il1r1-/- neutrophils compared to the WT neutrophils (Fig 6K). These findings demonstrate that C. auris, through the outer mannan layer, evades IL-1R signaling mediated neutrophil killing. Since macrophages but not neutrophils are known to produce IL-1Ra during C. albicans systemic infection [35], we investigated whether the outer mannan layer controls macrophage IL-Ra production. BMDMs (bone marrow-derived macrophages) were infected with wild-type C. auris 0387 or 0387 pmr1Δ strain to examine IL-1Ra levels. We found a significant increase in the IL-Ra level in supernatants of macrophages infected with live wild-type C. auris 0387 strain compared to 0387 pmr1Δ strain, which lacks an outer mannan layer (Fig 6L). These findings suggest that outer mannan regulates IL-1Ra production. Taken together, our results revealed that through the unique mannan outer layer, C. auris evades IL-1R-signaling mediated neutrophil recruitment and killing to persist in the skin (S7 Fig).
Discussion
The single-cell transcriptomics platform is invaluable for capturing specific biological mechanisms and cellular heterogeneity among different cell populations in a tissue microenvironment. Given that C. auris possesses a unique cell wall, adhesin proteins, and colonizing factors to thrive in the skin [13, 17, 36], understanding the cell-type specific skin immune response to C. auris is critical to understanding the pathogenesis. In this study, comprehensive profiling of C. auris murine skin infection generated a single-cell transcriptome atlas encompassing all major immune cells and non-immune cells in the skin. Our scRNA dataset comprises ~70,300 cells regulated during fungal infection of the skin tissue, revealing cell type-specific immune response during C. auris infection in the skin. C. auris can persist in the dermis for several months [4]. Therefore, we used the intradermal mouse model of infection that could potentially identify the host immune factors that regulate C. auris burden in skin tissue [20]. Our scRNA seq identified the accumulation of various innate and adaptive immune cells at the site of infection. In addition to phagocytic cells, DCs, and T cells, we identified that Th1 cells, NK cells, and non-immune cells such as fibroblast 3 cell type were highly accumulated in the skin tissue of C. auris infected mice. Recent studies suggest that IL-17-producing T cells are critical for skin defense against C. auris [4]. In this study, we observed that the depletion of neutrophils but not macrophages significantly increased the C. auris burden in skin tissue. These findings suggest neutrophils play a critical role in skin defense against C. auris in vivo. Future studies to understand the role of DCs, Th1 cells, NK cells, and fibroblasts in C. auris pathogenesis are important to understand the contribution of specific immune cells in skin defense against this emerging fungal pathogen.
Next, we examined the transcriptomic signature in individual cell types altered during C. auris skin infection in vivo. In myeloid cells, the cytokines and chemokines mediating the recruitment of phagocytic cells at the infection site and activation of antigen-presenting cells were highly upregulated during C. auris skin infection. The PRRs in the myeloid cells facilitate fungal recognition in the host [24, 37]. We observed the key PRRs, including TLRs, C-type lectin receptors, galectin receptors, and NLRs, were upregulated in myeloid cells, revealing the participation of selective immune receptors in host defense against C. auris during skin infection. Host AMPs such as Lcn2, S100a8, and S100a9, known to have direct antifungal activity, were highly upregulated in neutrophils. In addition, we identified the genes involved in nitric oxide metabolism and acute phase proteins were highly upregulated in different myeloid cells during C. auris skin infection. Our analysis of lymphoid cells revealed the expression of cytokines, chemokines, and TFs in CD4+ T cells, CD8+ T cells, and γδT cells during C. auris infection. In addition to the IL-17 pathway, which plays a critical role in antifungal host defense, including C. auris [4,38], our findings suggest that C. auris infection highly upregulates the expression of Ifng in Th1 cells and NK cells. Previous findings indicate that IL-17 (Th17) but not IL-12 (Th1) is critical for host defense against Candida albicans in the skin tissue [39]. In addition to IL-17, our scRNA seq data highlights the potential involvement of IFNγ in C. auris infection. This could be partly due to the route of infection, as epicutaneous and intradermal infection elicits different immune responses [40,41], and future studies are required to understand the functional importance of IFNγ (Th1) in C. auris skin infection. Non-immune cells, mainly fibroblast 1 and 3, showed increased expression of cytokines and chemokines. During Staphylococcus aureus skin infection, the dermal fibroblasts differentiate into adipocytes and produce AMP, mainly CAMP, for host defense [42]. Although CAMP was not highly expressed in fibroblasts during C. auris skin infection, our analysis identified the increased expression of other AMPs such as Lcn2, Adm, and Ang in fibroblast 1 and 3 cell types. The chemoattractants such as Cxcl1, Cxcl2, Cxcl5, Cxcl12, Lcn2, and Il33 involved in recruiting immune cells such as neutrophils were highly expressed in various non-immune cells. These findings suggest that non-immune cells exhibit an active role in skin defense against C. auris through AMPs and by secreting soluble factors that assist in recruiting neutrophils to the site of infection. Collectively, our scRNA seq revealed the transcriptome changes in different immune and non-immune cell types during C. auris infection in mice skin tissue in vivo and identified the previously unknown immune and non-immune cell type-specific host responses against C. auris skin infection.
In the recent studies on the host transcriptome of C. auris in human peripheral blood mononuclear cells (PBMCs) and mouse BMDMs, the NOD-like receptor signaling pathway was only enriched in the C. auris infection but not in C. albicans infection [13,14]. Similarly, in our scRNA seq dataset, the NOD-like receptor signaling pathway was significantly enriched in the myeloid subsets. Furthermore, the transcriptome of human PBMCs exposed in vitro with a purified manna layer of C. auris induces higher Il1rn expression compared to the C. albicans [13]. The Il1rn gene encoding IL-1Ra, which is significantly upregulated in different cell types identified in our scRNA seq data, was investigated for its role in host defense against C. auris skin infection. We identified that the depletion of macrophages, but not neutrophils, significantly decreased IL-1Ra concentrations in the skin tissue of mice infected with C. auris, suggesting the macrophage as the major source of IL-1Ra during C. auris skin infection. Furthermore, the neutrophil-killing activity of C. auris was significantly decreased in the presence of recombinant IL-1Ra, and mice treated with neutralizing antibodies significantly decreased the fungal load in vivo. A recent study on C. albicans using a systemic mouse model of infection revealed the role of secreted IL-1Ra by splenic macrophages limits the neutrophil activity in kidneys by affecting its reactive oxygen species (ROS) and myeloperoxidase (MPO) activity, contributing to the immune evasion of fungi [35]. We observed that the culture supernatant collected after C. auris stimulation of macrophage in vitro induced robust IL-1Ra production and limited the neutrophil-killing activity of C. auris. Our results, along with others [35], suggest that C. albicans and C. auris infection induces IL-1Ra in macrophages and decreases the killing activity of neutrophils to different host niches, including the kidney and skin, respectively. A recent study demonstrated that C. auris was less potent in activating IL-1β than C. albicans, evading macrophages through metabolic adaptation [43]. Furthermore, compared to C. albicans, we identified that C. auris infection induces significantly increased levels of IL-1Ra in the skin tissue. Although the intradermal infection of mice leads to the dissemination of fungi to kidneys and spleen [9], the systemic IL-1Ra level in serum in the mice infected with C. auris was unaltered. Previous findings suggest that IL-1Ra also favors the polarization of macrophages towards immunosuppressive M2 phenotype [44]. Macrophages play a protective role when a host encounters Candida pathogens. Our findings indicate the IL-1Ra-mediated immune evasion mechanisms employed by C. auris to modulate macrophages and persist in the skin. Furthermore, IL-1R signaling is critical for neutrophil recruitment and function and is important in the host defense against C. albicans [31,32]. Surprisingly, our findings revealed that C. auris, through the unique mannan outer layer, evades IL-1R-mediated neutrophil recruitment and function in the skin. Collectively, our results demonstrate the IL-1Ra and IL-1R-mediated immune evasion mechanisms employed by C. auris to persist in the skin. However, IL-1Ra induction may differ between the murine and human in the skin, and intraspecies variation in the IL-1 regulation of innate immune response may exist [45]. Future studies using an ex vivo human skin infection model of C. auris might help to understand the IL-1Ra-mediated immune evasion. They may potentially correlate the findings from our mice studies to humans.
The overall conclusion is that in addition to known Th17-mediated antifungal defense mechanisms, our single-cell transcriptomics analysis identified Th1 cells, NK cells, and non-immune cells, such as fibroblast role in skin defense against C. auris. Our findings demonstrate that C. auris induces IL-1Ra in macrophages to decrease neutrophil function and evades IL-1R-mediated immune defense against C. auris. Taken together, our data from the comprehensive single-cell profiling of skin tissue revealed the previously unknown mechanisms by which C. auris modulates myeloid cells to persist in the skin. Furthermore, our unbiased transcriptomics identified the potential contribution of the IFNγ pathway in lymphoid cells and the involvement of non-immune cells in skin defense against C. auris. Findings from this study will form a strong platform for developing novel host and pathogen-directed antifungal therapeutics that potentially target IL-1Ra and fungal mannan biosynthesis, respectively. Furthermore, the host receptors and immune pathways identified in this study in individual skin cell types during C. auris infection in vivo will open the door for future studies to understand the pathogenesis of C. auris in the skin and develop novel therapeutics to prevent and treat this emerging skin tropic fungal pathogen.
Experimental model and subject details
Mice
The C57BL/6J and Il1r1-/- C57BL/6J strains of mice were bred and housed in pathogen-free conditions at the centrally managed animal facilities at Purdue University. C57BL/6 and Il1r1-/- C57BL/6J strains were obtained from the Jackson Laboratory. Both old male and female mice of 6–8 weeks of age were used in the experiments.
Fungal culture
Candida auris AR0387, AR0381, AR0383, and AR0385 strains used in the study were procured from CDC AR Isolate Bank, USA, and C. albicans SC5314 strain was gifted from Dr. Andrew Koh, University of Texas Southwestern Medical Center, USA. Stock cultures of C. auris, C. albicans or C. auris 0387 pmr1Δ was stored at -80°C and subcultured on yeast peptone dextrose (YPD) agar plates at 37°C for 24 hours. Colonies from the YPD plates were cultured in YPD medium overnight at 37°C, shaking at 250 rpm. The fungal cells were harvested and washed twice with sterile 1x PBS before murine infection. For ex vitro killing assays, C. auris was cultured in YPD at 30°C for 24 hours. The fungal cells were washed with sterile 1x PBS and enumerated in a hemocytometer. The C. auris were preincubated with 10% mouse serum (Invitrogen, USA) at 37°C for 30 min. All the antibodies, ELISA kit, recombinant proteins, oligonucleotides, plasmids, and other reagents used in this study were provided in the (S6–S9 Tables).
Method details
Ethics statement
All experiments conducted on animals were in accordance with the approved protocols. Animal studies and experimental protocols were approved by the Purdue University Institutional Animal Care and Use Committee (IACUC Protocol no. 2110002211).
C. auris murine skin infection
For scRNA seq, C. auris AR0387 was used for murine infection. Yeast cells were washed and resuspended in sterile 1x PBS before murine infection. The fungal cells were enumerated in a hemocytometer and diluted to 1–2 x 107 yeast cells/ml. A six-to-eight-week-old C57BL/6J strain of mice was anesthetized, and dorsal skin hair was shaved as previously described [20]. Mice were injected intradermally with either 1–2 x 106 yeast cells (C. auris infected group) or sterile 1× PBS (Uninfected group) using a 27 G 1’ hypodermic needle on the shaved area.
Preparation of single-cell suspension from murine skin tissue for scRNA-seq
After 12 days post-infection (DPI), the mice were euthanized, and an approximately 80–120 mg weight of the dorsal skin tissue within 2 cm around the area of intradermal injection was collected in a 1.5 ml tube with digestion media (RPMI-1640 with 0.25-mg/mL Liberase TL and 1-μg/mL DNase). The tissue was transferred to a 6-well tissue culture plate with 5ml of digestion media and minced using ophthalmic scissors. The suspension is incubated for 1 hour 50 min at 37°C with 5% CO2 to ensure digestion of skin tissue. Then 1 mL of 0.25% trypsin-1-mM EDTA was added and incubated for another 10 min to separate the dermis from the epidermal surface as described previously [46]. After 2 hours of digestion, 4 ml of 1x PBS with 5% FBS was added to the suspension and pumped 8 times with a 10-mL syringe to mechanically dissociate the release of single cells from the tissues. Then the tissue clumps were removed by filtering with a 70 μm cell strainer, centrifuged at 300 x g for 7 min, and resuspended in RPMI-1640 with 10% FBS. Further, the fine tissue particles were removed by passing the single-cell suspension in a 40 μm cell strainer and a 30 μm cell strainer. The viability of the cells, presence of cell debris, and aggregating cells were assessed using trypan blue staining under a microscope. Two technical replicates for each sample were processed. Samples with more than 85% cell viability and less debris and aggregates were enumerated using a hemocytometer and adjusted to ~10,000,000/mL for further Single-cell partition and RNA library preparation.
Single-cell capturing, library construction, and 3’ RNA-sequencing
The Single-cell suspensions from the samples were diluted to 17,000 cells and loaded on a microfluidics chip Chromium Single Cell Instrument (10x Genomics) to target 10,000 cells. Single-cell GEMs (Gel Bead-In Emulsions) containing barcoded oligonucleotides and reverse transcriptase reagents were generated with the Next Gem single-cell reagent kit (10X Genomics). Following cell capture and cell lysis, cDNA was synthesized and amplified. At each step, the quality of cDNA and library was examined by a Bioanalyzer [47, 48]. The final libraries were sequenced on an Illumina NovaSeq 6000. 100-bp reads, including cell barcode and UMI sequences, and 100-bp RNA reads were generated.
Bioinformatics analysis
The FASTQ files generated by Illumina sequencing were processed using the CellRanger (v7.1.0) pipeline from 10X Genomics, and the reads were mapped to mouse reference genome mm10-2020-A. A digital gene expression matrix was generated containing the raw UMI counts for each cell for each sample. Downstream analysis was performed using various functions in the Seurat package (v 4.0) [49–52]. Cells with fewer than 300 or more than 7500 unique feature counts or more than 20% mitochondrial reads were excluded. To address the ambient RNA contamination SoupX version 1.5.2 [53] was utilized to identify and remove ambient RNA background noise from the data. After excluding low-quality cells, the count data was normalized using the SC Transform normalization workflow. Cell clusters were identified using ‘FindNeighbors’ and ‘FindClusters’ functions with the first 30 principal components at 0.6 resolution. We visualized our results in a two-dimensional space using UMAP. SingleR (v 2.0.0) package [54] was used for cell type annotation of the clusters using reference dataset GSE181720 [23]. Differential gene expression between infected and uninfected cells was performed using edgeR (v 3.38.4) [55]. Subclusters of lymphoid cell clusters identified above were identified by using the ‘resolution’ parameter from 0.1 to 1.0 in ‘FindClusters’ function to generate the detailed substructures of the clusters. We used pseudo-bulk analysis to combine single-cell RNA sequencing data by cell type and condition status, uninfected and infected. For each identified cell type within the seurat object, we extracted count matrices from both infected and uninfected samples. This extraction involved summing the UMIs (unique molecular identifiers) for each gene across all cells within a given cell type and condition, thereby creating pseudo-bulk samples representing each cell population’s collective gene expression profile under different experimental conditions. The differential expression analysis was performed using the edgeR package. We first normalized the data to account for differences in library sizes. Then we applied statistical models to identify genes that were significantly differentially expressed based on FDR ≤ 0.05 and Log2FC ≥ 2 or ≤ -2 between infected and uninfected conditions for each cell type. To visualize the results of our differential expression analysis, we generated volcano plots using the ggplot2 package in R. An ‘R’ package clusterProfiler (clusterProfiler_4.6.2) [56] was used to identify up-regulated KEGG pathways using differentially expressed genes between infected and uninfected cells when comparing different clusters and/or cell types.
Depletion of neutrophils and macrophages in C57BL/6J mice
To deplete neutrophils in C57BL/6J mice, 200 μg of anti-mouse Ly-6G antibody (Biolegend, USA) were injected intraperitoneally on day -1 and +1 following C. auris skin infection. The control groups received isotype-matched control Rat IgG2a (Biolegend, USA) on the following days [57]. To deplete macrophages, C57BL/6J mice were injected with 1 mg of Clodrosome (Encapsula Nano Sciences) intraperitoneally on day -2 and +1 for 2 DPI, and alternative 3–4 days for 12 DPI following C. auris skin infection [58,59]. PBS was injected into the control groups. Depletion of neutrophils and macrophages was assessed by flow cytometry. The skin fungal burden of the neutrophil or macrophage-depleted mice was determined from the infected skin tissue samples by homogenizing it in 1X PBS and plating in YPD supplemented with antibiotics, as described previously [20].
Immune cell quantification by Flow Cytometry
Murine skin tissue from scRNA Seq validation and depletion experiments were subjected to immune cell quantification using flow cytometry. Cells were isolated from murine skin as described previously [20]. The single-cell suspension from the murine skin tissue was stained with LIVE/DEAD Fixable Yellow (Invitrogen, Waltham, MA, USA), followed by surface and intracellular markers for innate and adaptive panels as described previously [20]. For the depletion experiments, the macrophage population was determined by F4/80 antibody. Then the stained samples were acquired through Attune NxT Flow Cytometer (Invitrogen, Carlsbad, CA, USA) and analyzed using FlowJo software (Eugene, OR, USA).
In vivo neutralization of IL-1Ra in the murine skin
To neutralize the IL-1Ra, 200 μg of neutralizing anti-mouse IL-1Ra monoclonal antibody were intradermally injected in the mice on day 0 (6 h before infection), +1 and +2 following C. auris skin infection [35,60]. 1X PBS was given to the control groups on the following days. On 3 DPI the mice were euthanized, and skin fungal burden was determined by homogenizing the skin tissue and plating it in YPD plates supplemented with antibiotics as described previously [20].
Blood sampling and serum separation
Peripheral blood was collected from uninfected and C. albicans and C. auris-infected mice on day 2 through the retro-orbital venous plexus route. Mice were anesthetized under isoflurane, and blood was collected using a heparinized microhematocrit capillary tube (Fisher Scientific, USA) in a microcentrifuge tube. Then, the blood was centrifuged at 500 × g for 10 min, and the separated serum was subjected to IL-1Ra ELISA.
IL-1Ra quantification using enzyme-linked immunosorbent assays (ELISA)
IL-1Ra production in skin homogenate, culture supernatant, and serum was quantified using a mouse IL-1Ra/IL-1F3 DuoSet kit (RnD Systems) by following the manufacturer’s standard operating protocol. Skin tissue collected from mice was homogenized in PBS. The homogenates were centrifuged at 2500 × g for 10 min at 4°C, and the Halt protease inhibitor cocktail (ThermoFisher Scientific) was added to the supernatant and stored at -80°C. 20 μl of supernatant from skin homogenate, 50 μl of serum, 100 μl of BMDM culture supernatant were used for IL-1Ra ELISA.
Isolation and culturing of murine primary neutrophils
Six to eight weeks old C57BL/6J or Il1r1-/- mice were used for neutrophil isolation. As described previously, immune cells were isolated from mice’s peritoneal cavity [61]. Briefly, 7.5% of casein solution was injected intraperitoneally to stimulate immune cell infiltration. After 1–4 days, mice were euthanized, and the peritoneal cavity was washed with 1xPBS with 0.5% BSA, and the leukocytes were harvested from peritoneal lavage. Then the neutrophil was isolated from the buffy coat after centrifugation with Percoll at 1000 × g for 20 min as described previously [61]. For bone marrow-derived neutrophil isolation, the bone marrow cells were isolated from the femur by flushing the bone cavity with RPMI supplemented with 10% FBS and 2 mM EDTA. Then the RBC was lysed in 1.6% NaCl, and subjected to histopaque density gradient centrifugation for neutrophil separation as described previously [61].
Construction of pmr1Δ deletion strain
The PMR1 deletion in C. auris AR0387 was performed by CRISPR-mediated gene editing in C. auris, as described previously [33]. Briefly, the upstream and downstream regions of the PMR1 gene were amplified and stretched with a 23 bp barcode, replacing the Open reading frame (ORF) to construct the repair template. Then, the gRNA cassette, Cas9 cassette, and the repair template were transformed into C. auris using lithium acetate, single-stranded carrier DNA, and polyethylene glycol method of competence induction. Then the transformed colonies were selected in the presence of NAT and screened for the PMR1 deletion by colony PCR. The deletion of PMR1 was confirmed using Sanger sequencing (S7 Table).
Isolation and culturing of murine bone marrow-derived macrophage (BMDM)
BMDM were isolated from 6–8 weeks old C57BL/6 mice as described previously[62]. Briefly, the bone marrow was harvested by flushing the bone cavity of the femur. Cells were cultured in DMEM supplemented with 10% FBS, 1× GlutaMAX, 1× Penicillin/Streptomycin, and 50 ng/mL M-CSF to induce macrophage differentiation as described [63]. After 5–8 days, the adherent cells were separated, washed, and confirmed for CD11b+ MHCII+ by flow cytometry. 5 × 104 cells/well of BMDM were cultured in a 96-well treated plate for 24 hours for efficient attachment. Then the macrophages were either stimulated at 1:1 MOI with live C. auris WT or pmr1Δ for 16 hours for the IL-1Ra ELISA. For preparation of macrophage conditioned media (MΦ CM), 2 × 105 cells/well of BMDM were cultured in 48-well plate for 16 hours. The C. auris 0387 were stimulated with 1:1 MOI the C. auris MΦ CM, and the control conditioned media (ctrl MΦ CM) were unstimulated. Then 100 μl of supernatant was used for IL-1Ra level determination using ELISA.
Neutrophil killing assay
To determine the ex vivo neutrophil killing of C. auris in the presence of IL-1Ra, 5 × 104 neutrophils were seeded in a 96-well plate with RPMI 1640 media (ThermoFisher, USA) and pretreated with 100 ng/ml recombinant IL-1Ra for 30 min. Further, C. auris AR0387 was incubated with 10% mouse serum for 30 min and transferred to the 96-well plate at MOI of 1: 0.25. The plates were incubated at 37°C for 3 hours. Then, the cells were lysed by resuspending in 0.02% triton X-100 for 5 min, and the lysate was serially diluted and plated in YPD agar with antibiotics. The colonies were enumerated after incubating plates at 37°C for 24 hours to calculate the neutrophil-killing activity. For fungal survival assay, C. auris 0387 or 0387 pmr1Δ were primed with 5 × 104 bone marrow neutrophils isolated from WT or Il1r1-/- mice at 1: 2 MOI and the percentage of fungal survival were determined by plated in YPD agar with antibiotics [64,65]. For fungal survival in macrophage conditioned media (MΦ CM), the neutrophil and C. auris were primed at 1:0.25 MOI in presence of MΦ CM at various concentrations and for 50% MΦ CM, the MΦ CM were diluted in DMEM supplemented with 10% FBS. The percentage of fungal survival were determined by plated in YPD agar with antibiotics.
Supporting information
The representative flow plot and the bar graph represent the percentage and absolute number of (A) neutrophils, (B) Monocytes, (C) Macrophages, and (D) dendritic cells in the C. auris infected group compared to the uninfected group. The bar graph represents the percentage and absolute number of (E) γδ+ cells, γδ+ IFNγ+ cells, γδ+ IL17A+ cells, and γδ+ IL17F+ cells, (F) CD4+ cells, CD4+ IFNγ+ cells, CD4+ IL17A+ cells, CD4+ and IL17F+ cells, (G) CD8+ cells, CD8+ IFNγ+ cells, CD8+ IL17A+ cells, CD8+ and IL17F+ cells. Twelve mice were used from each group, and the error bar represents the mean ± SEM. * p < 0.05, ** p <0.01, *** p <0.001, **** p <0.0001.
(TIF)
Venn diagram showing the number of upregulated genes shared within the (A) phagocytic cells and (B) antigen presenting cells upon C. auris infection.
(TIF)
(A) Venn diagram showing the number of upregulated genes shared within lymphoid subsets. The heatmap represents the expression of the significantly upregulated genes in (B) CD8+ and (C) NK cells in uninfected and infected groups. Upregulated genes with Log 2-fold change + 2 and FDR > 5% were represented. The normalized gene counts were plotted in the heatmap, and the scale indicates red for high, blue for low, and white for moderate expression in the samples. Each column represents a different sample.
(TIF)
The heatmap represents the expression of the significantly upregulated genes in (A) BEC (B) outer bulge cells and (C) pericytes cells in uninfected and infected groups. Upregulated genes with Log 2-fold change ≥ 2 and FDR > 5% were represented. The normalized gene counts were plotted in the heatmap, and the scale indicates red for high, blue for low, and white for moderate expression in the samples. Each column represents a different sample.
(TIF)
(A) Venn diagram showing the number of significantly upregulated genes shared within the identified fibroblast subsets. The bubble plot represents the KEGG pathways of the enriched upregulated genes of the (B) BEC, (C) pericytes and (D) outer bulge cells. The X-axis denotes the percentage enrichment of the KEGG pathways.
(TIF)
(A) The normalized read counts of Il1rn gene in the hematopoietic and non-hematopoietic cell types from the scRNA-seq dataset. Both uninfected and infected samples were plotted and the mean were represented with the error bar. The IL-1Ra level in skin tissues of mice groups received PBS, Candida auris South Asian clade AR0387, East Asian clade AR0381, African clade AR0383, or South American clade AR0385 after day. (B) 3 p.i. and (C) 14 p.i. (n = 5–7 mice/group). (D) Percentage and absolute number of F4/80+ MHCII+ macrophage in the infected skin tissue of mice injected with clodrosome (C.L) or 1X PBS (Ctrl) injected mice groups. (E) Measurement IL-1Ra levels from the culture supernatant of BMDM alone or BMDM stimulated with C. auris 0387 for 16 h. (n = 12). Error bars represent mean ± SEM. ** p <0.01. (F) The bar graph represents the fungal survival of C. auris 0387 primed with neutrophils in the presence of MΦ CM collected from BMDM alone (Ctrl MΦ CM) or BMDM stimulated with C. auris 0387 (C. auris MΦ CM) for 16 h (n = 16 to 19). 50% CM was diluted with complete DMEM. Error bars represent mean ± SEM. * p <0.05, **** p <0.0001. Abbreviations—BMDM, bone marrow-derived macrophages; MΦ CM, macrophage conditioned medium. Statistical significances were calculated using Mann–Whitney U.
(TIF)
(TIF)
The mean cell numbers of the cell type in the uninfected and infected samples were represented. The fold changes in the cell types recruited in the infected samples compared to the uninfected control were represented.
(DOCX)
The mean UMIs in uninfected and infected sample are represented in the table.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability
After our request authors have provided the following update: "The Seurat object file with annotated single-cell RNA-seq data after cell type assignments is made available through figshare: https://figshare.com/articles/dataset/Seurat_sct_slot_with_annotation_RDS/27320781?file=50047740. DOI: 10.6084/m9.figshare.27320781. The differentially expressed genes from pseudo-bulk analysis are provided as S10 Table.xlsx. All other data is provided in the manuscript and Supporting Information files.
Funding Statement
This study was supported by NIAID (1R01AI177604 to ST) and the Division of Intramural Research of the NIAID, NIH (ZIA AI001175 to MSL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ahmad S, Alfouzan W. Candida auris: Epidemiology, Diagnosis, Pathogenesis, Antifungal Susceptibility, and Infection Control Measures to Combat the Spread of Infections in Healthcare Facilities. Microorganisms. 2021;9(4). Epub 2021/05/01. doi: 10.3390/microorganisms9040807 ; PubMed Central PMCID: PMC8069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO fungal priority pathogens list to guide research, development and public health action, Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 30 IGO, https://www.who.int/publications/i/item/9789240060241. WHO, 2022. [Google Scholar]
- 3.Kadri SS. Key Takeaways From the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit Care Med. 2020;48(7):939–45. doi: 10.1097/CCM.0000000000004371 ; PubMed Central PMCID: PMC7176261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang X, Hurabielle C, Drummond RA, Bouladoux N, Desai JV, Sim CK, et al. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe. 2021;29(2):210–21 e6. Epub 2021/01/02. doi: 10.1016/j.chom.2020.12.002 ; PubMed Central PMCID: PMC7878403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shastri PS, Shankarnarayan SA, Oberoi J, Rudramurthy SM, Wattal C, Chakrabarti A. Candida auris candidaemia in an intensive care unit—Prospective observational study to evaluate epidemiology, risk factors, and outcome. J Crit Care. 2020;57:42–8. Epub 20200109. doi: 10.1016/j.jcrc.2020.01.004 . [DOI] [PubMed] [Google Scholar]
- 6.Proctor DM, Drummond RA, Lionakis MS, Segre JA. One population, multiple lifestyles: Commensalism and pathogenesis in the human mycobiome. Cell Host Microbe. 2023;31(4):539–53. Epub 2023/04/14. doi: 10.1016/j.chom.2023.02.010 ; PubMed Central PMCID: PMC10155287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparber F, De Gregorio C, Steckholzer S, Ferreira FM, Dolowschiak T, Ruchti F, et al. The Skin Commensal Yeast Malassezia Triggers a Type 17 Response that Coordinates Anti-fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe. 2019;25(3):389–403 e6. doi: 10.1016/j.chom.2019.02.002 . [DOI] [PubMed] [Google Scholar]
- 8.Proctor DM, Dangana T, Sexton DJ, Fukuda C, Yelin RD, Stanley M, et al. Integrated genomic, epidemiologic investigation of Candida auris skin colonization in a skilled nursing facility. Nature Medicine. 2021;27(8):1401–9. doi: 10.1038/s41591-021-01383-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towns KA, Datta A, Thangamani S. Intradermal infection and dissemination of Candida auris in immunocompetent and immunocompromised mouse models. Microbiol Spectr. 2024:e0012724. Epub 20240624. doi: 10.1128/spectrum.00127-24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pekmezovic M, Hovhannisyan H, Gresnigt MS, Iracane E, Oliveira-Pacheco J, Siscar-Lewin S, et al. Candida pathogens induce protective mitochondria-associated type I interferon signalling and a damage-driven response in vaginal epithelial cells. Nat Microbiol. 2021;6(5):643–57. Epub 20210322. doi: 10.1038/s41564-021-00875-2 . [DOI] [PubMed] [Google Scholar]
- 11.Lionakis MS, Drummond RA, Hohl TM. Immune responses to human fungal pathogens and therapeutic prospects. Nat Rev Immunol. 2023;23(7):433–52. Epub 20230104. doi: 10.1038/s41577-022-00826-w ; PubMed Central PMCID: PMC9812358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briard B, Fontaine T, Kanneganti TD, Gow NAR, Papon N. Fungal cell wall components modulate our immune system. Cell Surf. 2021;7:100067. Epub 20211106. doi: 10.1016/j.tcsw.2021.100067 ; PubMed Central PMCID: PMC8603304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruno M, Kersten S, Bain JM, Jaeger M, Rosati D, Kruppa MD, et al. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen Candida auris. Nat Microbiol. 2020;5(12):1516–31. Epub 20200824. doi: 10.1038/s41564-020-0780-3 ; PubMed Central PMCID: PMC9204844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Zou Y, Chen X, Li H, Yin Z, Zhang B, et al. Innate immune responses against the fungal pathogen Candida auris. Nat Commun. 2022;13(1):3553. Epub 20220621. doi: 10.1038/s41467-022-31201-x ; PubMed Central PMCID: PMC9213489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan L, Xia K, Yu Y, Miliakos A, Chaturvedi S, Zhang F, et al. Unique Cell Surface Mannan of Yeast Pathogen Candida auris with Selective Binding to IgG. ACS Infect Dis. 2020;6(5):1018–31. Epub 20200410. doi: 10.1021/acsinfecdis.9b00450 . [DOI] [PubMed] [Google Scholar]
- 16.Seiser S, Arzani H, Ayub T, Phan-Canh T, Staud C, Worda C, et al. Native human and mouse skin infection models to study Candida auris-host interactions. Microbes Infect. 2024;26(1–2):105234. Epub 20231007. doi: 10.1016/j.micinf.2023.105234 . [DOI] [PubMed] [Google Scholar]
- 17.Santana DJ, Anku JAE, Zhao G, Zarnowski R, Johnson CJ, Hautau H, et al. A Candida auris-specific adhesin, Scf1, governs surface association, colonization, and virulence. Science. 2023;381(6665):1461–7. Epub 20230928. doi: 10.1126/science.adf8972 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrada J, Gamal A, Long L, Ghannoum MA. In Vivo Skin Colonization and Decolonization Models for Candida auris. Methods Mol Biol. 2022;2517:269–85. doi: 10.1007/978-1-0716-2417-3_22 . [DOI] [PubMed] [Google Scholar]
- 19.Bryak G, Cox A, Lionakis MS, Thangamani S. Yeast and filamentous Candida auris stimulate distinct immune responses in the skin. mSphere. 2024:e0005524. Epub 20240621. doi: 10.1128/msphere.00055-24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datta A, Das D, Nett JE, Vyas JM, Lionakis MS, Thangamani S. Differential skin immune responses in mice intradermally infected with Candida auris and Candida albicans. Microbiol Spectr. 2023:e0221523. Epub 2023/10/09. doi: 10.1128/spectrum.02215-23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solá P, Mereu E, Bonjoch J, Casado-Peláez M, Prats N, Aguilera M, et al. Targeting lymphoid-derived IL-17 signaling to delay skin aging. Nature Aging. 2023;3(6):688–704. doi: 10.1038/s43587-023-00431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui A, Huang T, Li S, Ma A, Pérez JL, Sander C, et al. Dictionary of immune responses to cytokines at single-cell resolution. Nature. 2024;625(7994):377–84. doi: 10.1038/s41586-023-06816-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venugopal G, Bird JT, Washam CL, Roys H, Bowlin A, Byrum SD, et al. In vivo transcriptional analysis of mice infected with Leishmania major unveils cellular heterogeneity and altered transcriptomic profiling at single-cell resolution. PLOS Neglected Tropical Diseases. 2022;16(7):e0010518. doi: 10.1371/journal.pntd.0010518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netea MG, Joosten LAB, van der Meer JWM, Kullberg B-J, van de Veerdonk FL. Immune defence against Candida fungal infections. Nature Reviews Immunology. 2015;15(10):630–42. doi: 10.1038/nri3897 [DOI] [PubMed] [Google Scholar]
- 25.Blake JA, Baldarelli R, Kadin JA, Richardson JE, Smith Cynthia L, Bult CJ, et al. Mouse Genome Database (MGD): Knowledgebase for mouse–human comparative biology. Nucleic Acids Research. 2020;49(D1):D981–D7. doi: 10.1093/nar/gkaa1083 %J Nucleic Acids Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity. 2019;50(4):778–95. doi: 10.1016/j.immuni.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter DB, Deibel MR, Dunn CJ, Tomich CSC, Laborde AL, Slightom JL, et al. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344(6267):633–8. doi: 10.1038/344633a0 [DOI] [PubMed] [Google Scholar]
- 28.Martin P, Palmer G, Rodriguez E, Palomo J, Lemeille S, Goldstein J, et al. Intracellular IL-1 Receptor Antagonist Isoform 1 Released from Keratinocytes upon Cell Death Acts as an Inhibitor for the Alarmin IL-1α. The Journal of Immunology. 2020;204(4):967–79. doi: 10.4049/jimmunol.1901074 [DOI] [PubMed] [Google Scholar]
- 29.Kanangat S, Postlethwaite AE, Higgins GC, Hasty KA. Novel Functions of Intracellular IL-1ra in Human Dermal Fibroblasts: Implications in the Pathogenesis of Fibrosis. Journal of Investigative Dermatology. 2006;126(4):756–65. doi: 10.1038/sj.jid.5700097 [DOI] [PubMed] [Google Scholar]
- 30.Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, et al. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343(6256):336–40. doi: 10.1038/343336a0 [DOI] [PubMed] [Google Scholar]
- 31.Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa. PLoS Pathog. 2016;12(9):e1005882. Epub 20160915. doi: 10.1371/journal.ppat.1005882 ; PubMed Central PMCID: PMC5025078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172(5):3059–69. doi: 10.4049/jimmunol.172.5.3059 . [DOI] [PubMed] [Google Scholar]
- 33.Ennis CL, Hernday AD, Nobile CJ. A Markerless CRISPR-Mediated System for Genome Editing in Candida auris Reveals a Conserved Role for Cas5 in the Caspofungin Response. Microbiol Spectr. 2021;9(3):e0182021. Epub 2021/11/04. doi: 10.1128/Spectrum.01820-21 ; PubMed Central PMCID: PMC8567271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton MV, Johnson CJ, Zarnowski R, Andes BD, Schoen TJ, Kernien JF, et al. Candida auris Cell Wall Mannosylation Contributes to Neutrophil Evasion through Pathways Divergent from Candida albicans and Candida glabrata. mSphere. 2021;6(3):e0040621. Epub 2021/06/24. doi: 10.1128/mSphere.00406-21 ; PubMed Central PMCID: PMC8265655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gander-Bui HTT, Schlafli J, Baumgartner J, Walthert S, Genitsch V, van Geest G, et al. Targeted removal of macrophage-secreted interleukin-1 receptor antagonist protects against lethal Candida albicans sepsis. Immunity. 2023;56(8):1743–60 e9. Epub 2023/07/22. doi: 10.1016/j.immuni.2023.06.023 . [DOI] [PubMed] [Google Scholar]
- 36.Balakumar A, Bernstein D, Thangamani S. The adhesin SCF1 mediates Candida auris colonization. Trends in Microbiology. 2024;32(1):4–5. doi: 10.1016/j.tim.2023.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Netea MG, Brown GD, Kullberg BJ, Gow NAR. An integrated model of the recognition of Candida albicans by the innate immune system. Nature Reviews Microbiology. 2008;6(1):67–78. doi: 10.1038/nrmicro1815 [DOI] [PubMed] [Google Scholar]
- 38.Conti HR, Gaffen SL. IL-17–Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. The Journal of Immunology. 2015;195(3):780–8. doi: 10.4049/jimmunol.1500909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. Journal of immunology (Baltimore, Md: 1950). 2010;185(9):5453–62. Epub 2010/10/06. doi: 10.4049/jimmunol.1001153 ; PubMed Central PMCID: PMC3076054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linehan JL, Harrison OJ, Han S-J, Byrd AL, Vujkovic-Cvijin I, Villarino AV, et al. Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell. 2018;172(4):784–96.e18. doi: 10.1016/j.cell.2017.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashem SW, Igyártó BZ, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, et al. Candida albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity. 2015;42(2):356–66. doi: 10.1016/j.immuni.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L-j Guerrero-Juarez CF, Hata T Bapat SP, Ramos R Plikus MV, et al. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347(6217):67–71. doi: 10.1126/science.1260972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weerasinghe H, Simm C, Djajawi TM, Tedja I, Lo TL, Simpson DS, et al. Candida auris uses metabolic strategies to escape and kill macrophages while avoiding robust activation of the NLRP3 inflammasome response. Cell Rep. 2023;42(5):112522. Epub 2023/05/19. doi: 10.1016/j.celrep.2023.112522 . [DOI] [PubMed] [Google Scholar]
- 44.Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, et al. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells. 2016;34(2):483–92. Epub 20151231. doi: 10.1002/stem.2254 . [DOI] [PubMed] [Google Scholar]
- 45.Tahtinen S, Tong A-J, Himmels P, Oh J, Paler-Martinez A, Kim L, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nature Immunology. 2022;23(4):532–42. doi: 10.1038/s41590-022-01160-y [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto K, Goel S, Funakoshi A, Honda T, Nagao K. Flow cytometry analysis of the subpopulations of mouse keratinocytes and skin immune cells. STAR Protocols. 2022;3(1):101052. doi: 10.1016/j.xpro.2021.101052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macosko Evan Z, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161(5):1202–14. doi: 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, et al. Massively parallel digital transcriptional profiling of single cells. Nature Communications. 2017;8(1):14049. doi: 10.1038/ncomms14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, et al. Comprehensive Integration of Single-Cell Data. Cell. 2019;177(7):1888–902.e21. doi: 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nature Biotechnology. 2015;33(5):495–502. doi: 10.1038/nbt.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature Biotechnology. 2018;36(5):411–20. doi: 10.1038/nbt.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–87.e29. doi: 10.1016/j.cell.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young MD, Behjati S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. GigaScience. 2020;9(12):giaa151. doi: 10.1093/gigascience/giaa151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nature Immunology. 2019;20(2):163–72. doi: 10.1038/s41590-018-0276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. OMICS: A Journal of Integrative Biology. 2012;16(5):284–7. doi: 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carr KD, Sieve AN, Indramohan M, Break TJ, Lee S, Berg RE. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. European Journal of Immunology. 2011;41(9):2666–76. doi: 10.1002/eji.201041363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Kubes P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell. 2016;165(3):668–78. doi: 10.1016/j.cell.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 59.Hiengrach P, Panpetch W, Chindamporn A, Leelahavanichkul A. Macrophage depletion alters bacterial gut microbiota partly through fungal overgrowth in feces that worsens cecal ligation and puncture sepsis mice. Scientific Reports. 2022;12(1):9345. doi: 10.1038/s41598-022-13098-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka R, Hiramitsu M, Shimizu S, Kawashima S, Sato A, Iwase Y. Efficient drug delivery to lymph nodes by intradermal administration and enhancement of anti-tumor effects of immune checkpoint inhibitors. Cancer Treatment and Research Communications. 2023;36:100740. doi: 10.1016/j.ctarc.2023.100740 [DOI] [PubMed] [Google Scholar]
- 61.Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of Mouse Neutrophils. Curr Protoc Immunol. 2015;110:3.20.1–3..15. Epub 2015/08/04. doi: 10.1002/0471142735.im0320s110 ; PubMed Central PMCID: PMC4574512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14:14 1 1–1 Epub 2008/11/20. doi: 10.1002/0471142735.im1401s83 ; PubMed Central PMCID: PMC2834554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haag SM, Murthy A. Murine Monocyte and Macrophage Culture. Bio Protoc. 2021;11(6):e3928. Epub 2021/04/16. doi: 10.21769/BioProtoc.3928 ; PubMed Central PMCID: PMC8032491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson Chad J, Davis JM, Huttenlocher A, Kernien John F, Nett Jeniel E. Emerging Fungal Pathogen Candida auris Evades Neutrophil Attack. mBio. 2018;9(4):10.1128/mbio.01403-18. doi: 10.1128/mBio.01403-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu S-Y, Weng C-L, Jheng M-J, Kan H-W, Hsieh S-T, Liu F-T, et al. Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLOS Pathogens. 2019;15(11):e1008096. doi: 10.1371/journal.ppat.1008096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The representative flow plot and the bar graph represent the percentage and absolute number of (A) neutrophils, (B) Monocytes, (C) Macrophages, and (D) dendritic cells in the C. auris infected group compared to the uninfected group. The bar graph represents the percentage and absolute number of (E) γδ+ cells, γδ+ IFNγ+ cells, γδ+ IL17A+ cells, and γδ+ IL17F+ cells, (F) CD4+ cells, CD4+ IFNγ+ cells, CD4+ IL17A+ cells, CD4+ and IL17F+ cells, (G) CD8+ cells, CD8+ IFNγ+ cells, CD8+ IL17A+ cells, CD8+ and IL17F+ cells. Twelve mice were used from each group, and the error bar represents the mean ± SEM. * p < 0.05, ** p <0.01, *** p <0.001, **** p <0.0001.
(TIF)
Venn diagram showing the number of upregulated genes shared within the (A) phagocytic cells and (B) antigen presenting cells upon C. auris infection.
(TIF)
(A) Venn diagram showing the number of upregulated genes shared within lymphoid subsets. The heatmap represents the expression of the significantly upregulated genes in (B) CD8+ and (C) NK cells in uninfected and infected groups. Upregulated genes with Log 2-fold change + 2 and FDR > 5% were represented. The normalized gene counts were plotted in the heatmap, and the scale indicates red for high, blue for low, and white for moderate expression in the samples. Each column represents a different sample.
(TIF)
The heatmap represents the expression of the significantly upregulated genes in (A) BEC (B) outer bulge cells and (C) pericytes cells in uninfected and infected groups. Upregulated genes with Log 2-fold change ≥ 2 and FDR > 5% were represented. The normalized gene counts were plotted in the heatmap, and the scale indicates red for high, blue for low, and white for moderate expression in the samples. Each column represents a different sample.
(TIF)
(A) Venn diagram showing the number of significantly upregulated genes shared within the identified fibroblast subsets. The bubble plot represents the KEGG pathways of the enriched upregulated genes of the (B) BEC, (C) pericytes and (D) outer bulge cells. The X-axis denotes the percentage enrichment of the KEGG pathways.
(TIF)
(A) The normalized read counts of Il1rn gene in the hematopoietic and non-hematopoietic cell types from the scRNA-seq dataset. Both uninfected and infected samples were plotted and the mean were represented with the error bar. The IL-1Ra level in skin tissues of mice groups received PBS, Candida auris South Asian clade AR0387, East Asian clade AR0381, African clade AR0383, or South American clade AR0385 after day. (B) 3 p.i. and (C) 14 p.i. (n = 5–7 mice/group). (D) Percentage and absolute number of F4/80+ MHCII+ macrophage in the infected skin tissue of mice injected with clodrosome (C.L) or 1X PBS (Ctrl) injected mice groups. (E) Measurement IL-1Ra levels from the culture supernatant of BMDM alone or BMDM stimulated with C. auris 0387 for 16 h. (n = 12). Error bars represent mean ± SEM. ** p <0.01. (F) The bar graph represents the fungal survival of C. auris 0387 primed with neutrophils in the presence of MΦ CM collected from BMDM alone (Ctrl MΦ CM) or BMDM stimulated with C. auris 0387 (C. auris MΦ CM) for 16 h (n = 16 to 19). 50% CM was diluted with complete DMEM. Error bars represent mean ± SEM. * p <0.05, **** p <0.0001. Abbreviations—BMDM, bone marrow-derived macrophages; MΦ CM, macrophage conditioned medium. Statistical significances were calculated using Mann–Whitney U.
(TIF)
(TIF)
The mean cell numbers of the cell type in the uninfected and infected samples were represented. The fold changes in the cell types recruited in the infected samples compared to the uninfected control were represented.
(DOCX)
The mean UMIs in uninfected and infected sample are represented in the table.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
After our request authors have provided the following update: "The Seurat object file with annotated single-cell RNA-seq data after cell type assignments is made available through figshare: https://figshare.com/articles/dataset/Seurat_sct_slot_with_annotation_RDS/27320781?file=50047740. DOI: 10.6084/m9.figshare.27320781. The differentially expressed genes from pseudo-bulk analysis are provided as S10 Table.xlsx. All other data is provided in the manuscript and Supporting Information files.