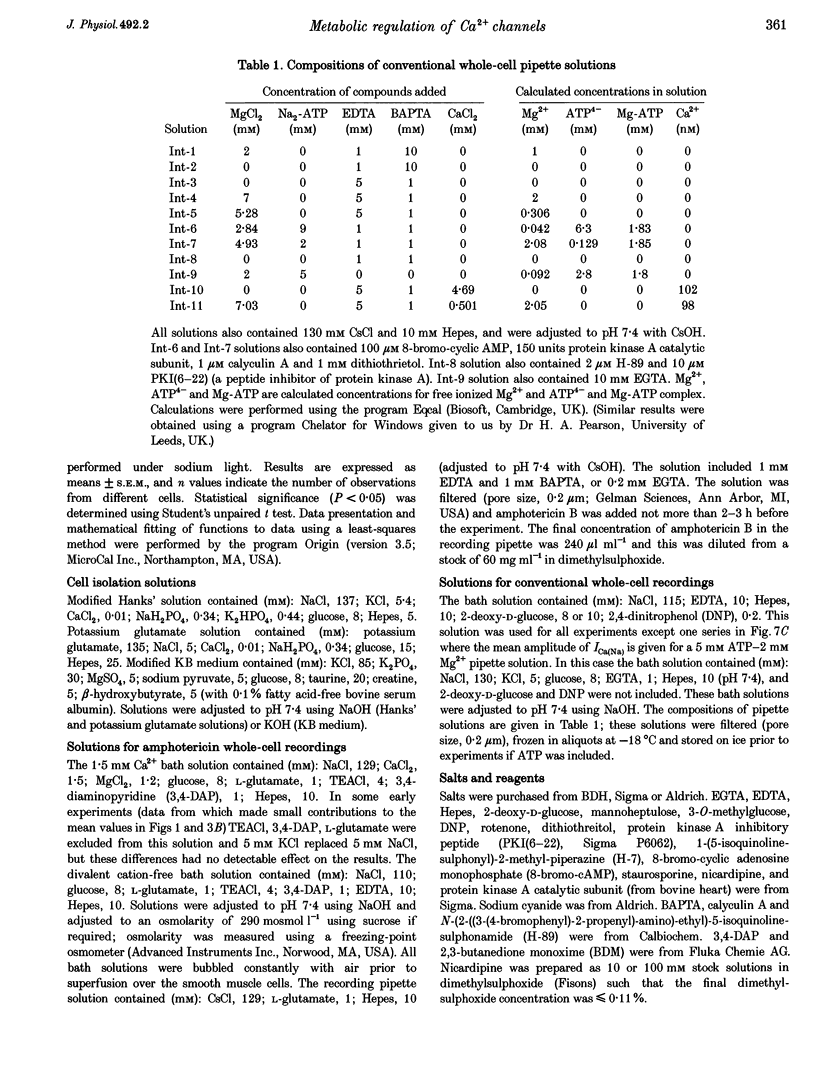
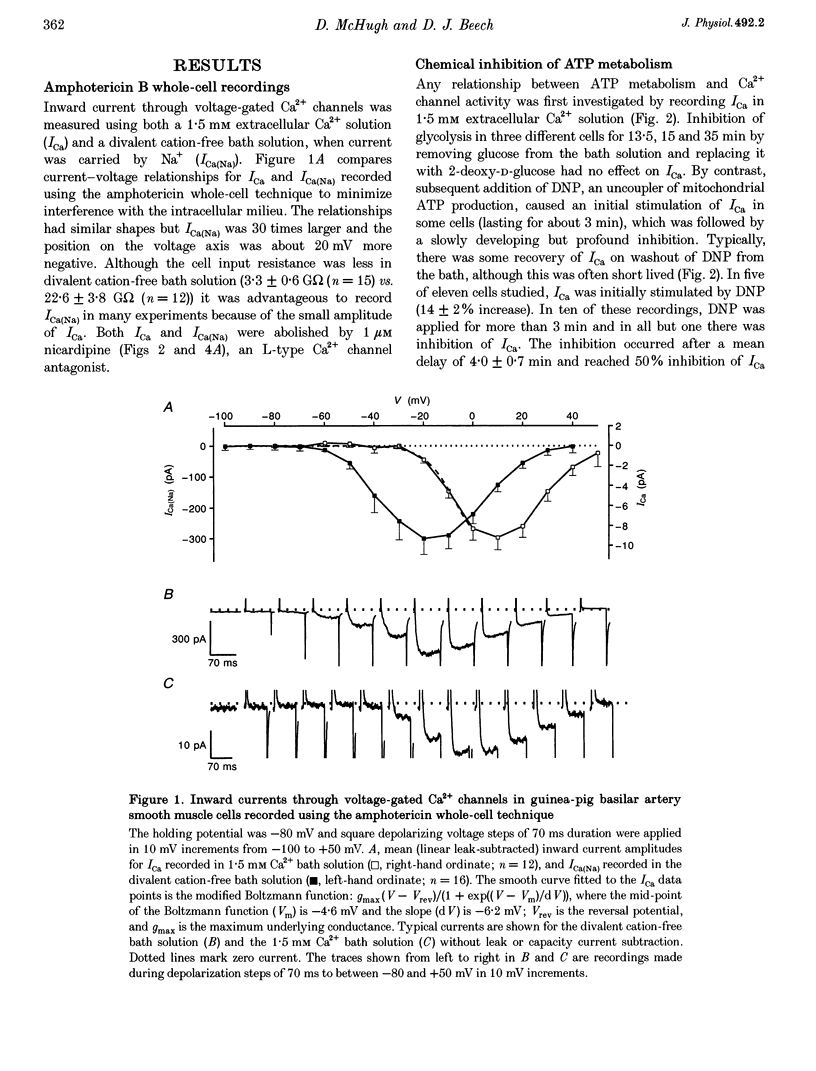
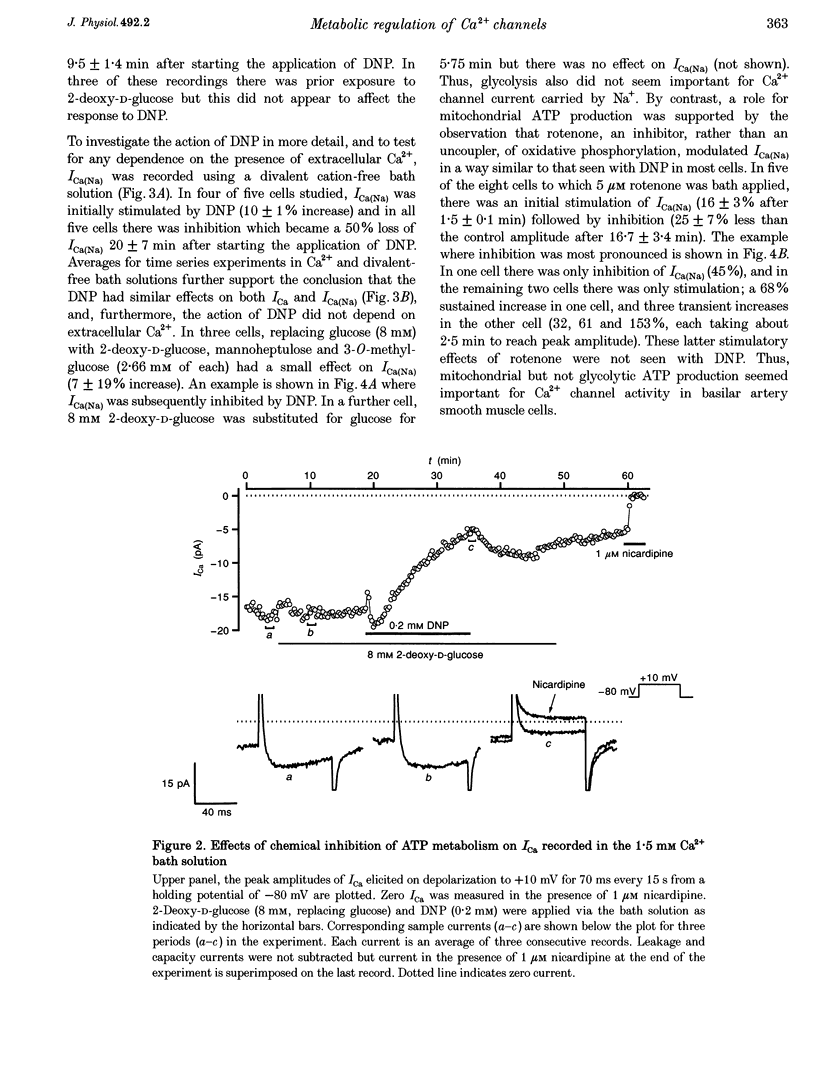
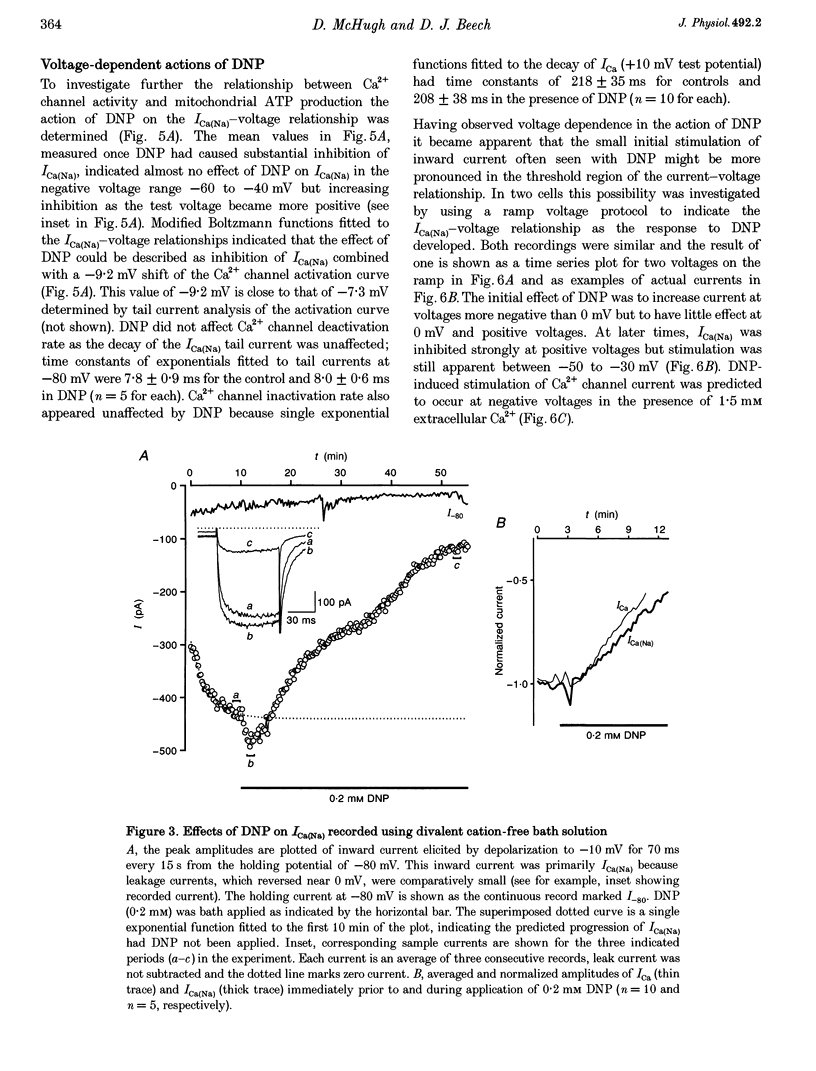
Abstract
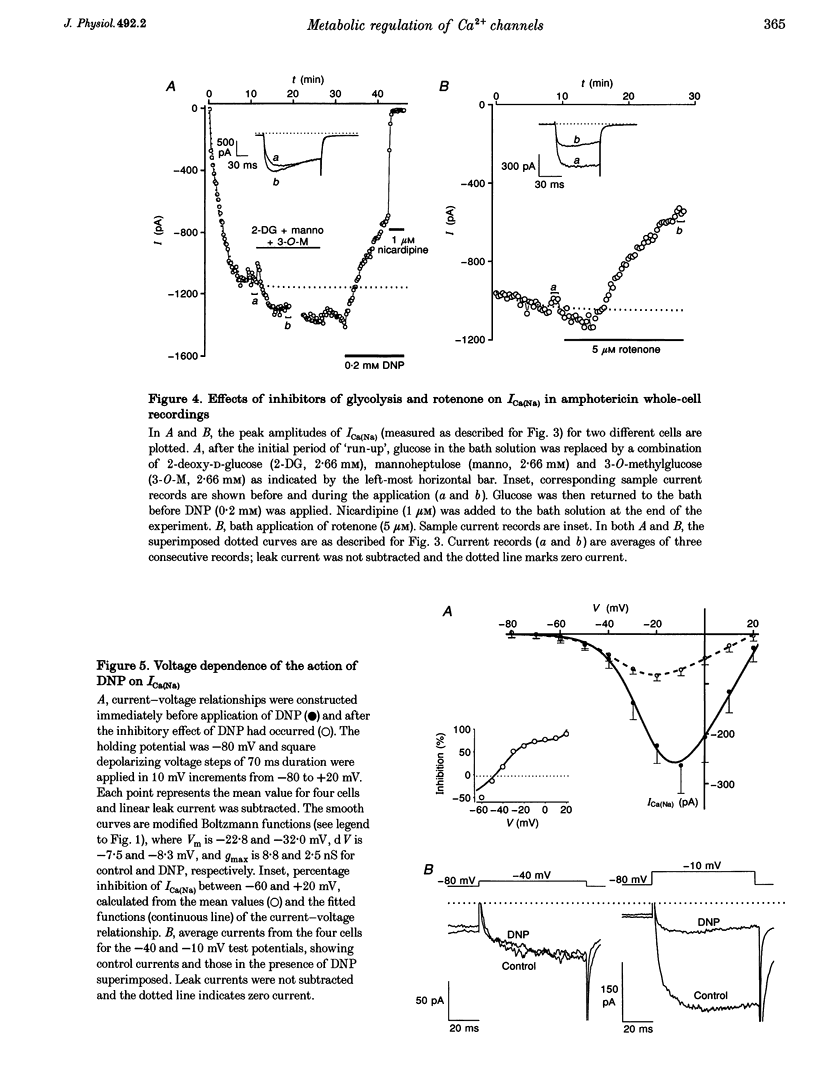
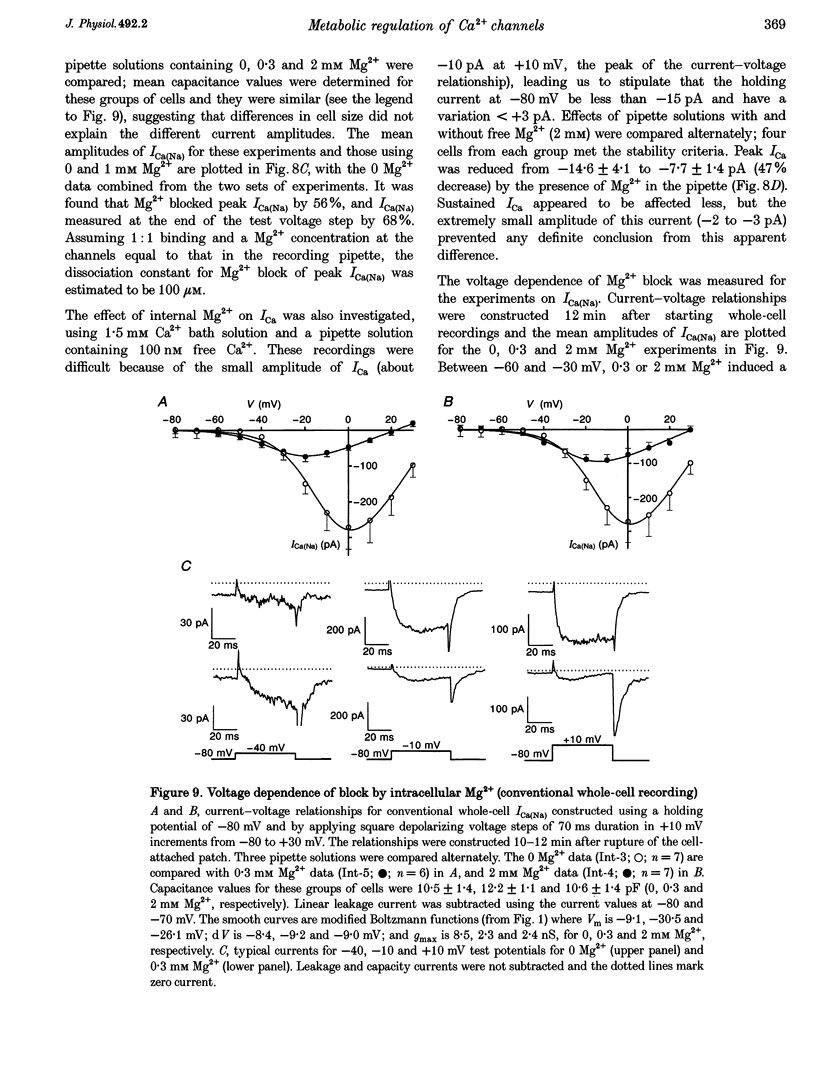
1. Single smooth muscle cells were isolated from the basilar artery of the guinea-pig and, within 10 h, inward currents through voltage-gated Ca2+ channels were recorded using the amphotericin or conventional whole-cell voltage-clamp techniques. 2. In amphotericin whole-cell recordings, bath application of 2,4-dinitrophenol (DNP, an uncoupler of mitochondrial ATP production) induced an initial stimulation (14% increase in 5 of 11 cells) and then pronounced inhibition (50% decrease in 9 of 11 cells within 9.5 min) of voltage-dependent Ca2+ current (I(Ca)) elicited by depolarizing to +10 mV in 1.5 mM extracellular Ca2+ solution. By contrast, inhibition of glycolysis by replacing glucose in the bath with 2-deoxy-D-glucose had no effect. 3. Na+ current through Ca2+ channels (I[(Ca)(Na)]) recorded in the absence of extracellular divalent cations also responded to DNP, again with stimulation followed by inhibition of current. The stimulation of I[(Ca)(Na)] was associated with a leftward shift of the Ca2+ channel activation curve which averaged -9 mV. A combination of 2-deoxy-D-glucose, mannoheptulose and 3-0-methyl-glucose had only minor effects on I[(Ca)(Na)], whereas rotenone had an effect similar to that of DNP in six of eight cells. 4. The amplitude of I[(Ca)(Na)] in conventional whole-cell recordings was not different from that in amphotericin whole-cell recordings, even without ATP in the recording pipette and with metabolic poisons in the bath solution. Furthermore, attempts to dephosphorylate the Ca2+ channels in ATP-free conditions did not prevent I[(Ca)(Na)], and a high concentration of Mg-ATP with or without a phosphorylation-supporting medium in the recording pipette did not increase its amplitude. 5. In the absence of ATP, Mg2+ inhibited whole-cell I[Ca)(Na)] with a K(d) of about 100 mu M at -10 mV and induced a leftward shift of the Ca2+ channel activation curve. When ATP and a phosphorylation-supporting medium were in the recording pipette the blocking effect of free Mg2+ was reduced but the shift in the Ca2+ channel activation curve was unaffected. 6. From these data it is suggested that inhibition of mitochondrial, but not glycolytic, ATP production has stimulatory and inhibitory effects on voltage-gated Ca2+ channels of basilar artery smooth muscle cells. Effects of intracellular Mg2+ on the Ca2+ channels were modulated by ATP and mimicked the effects of metabolic poisoning by DNP. A hypothesis is discussed in which the intracellular free Mg2+ concentration may be a key factor coupling ATP production to Ca2+ channels.
Full text
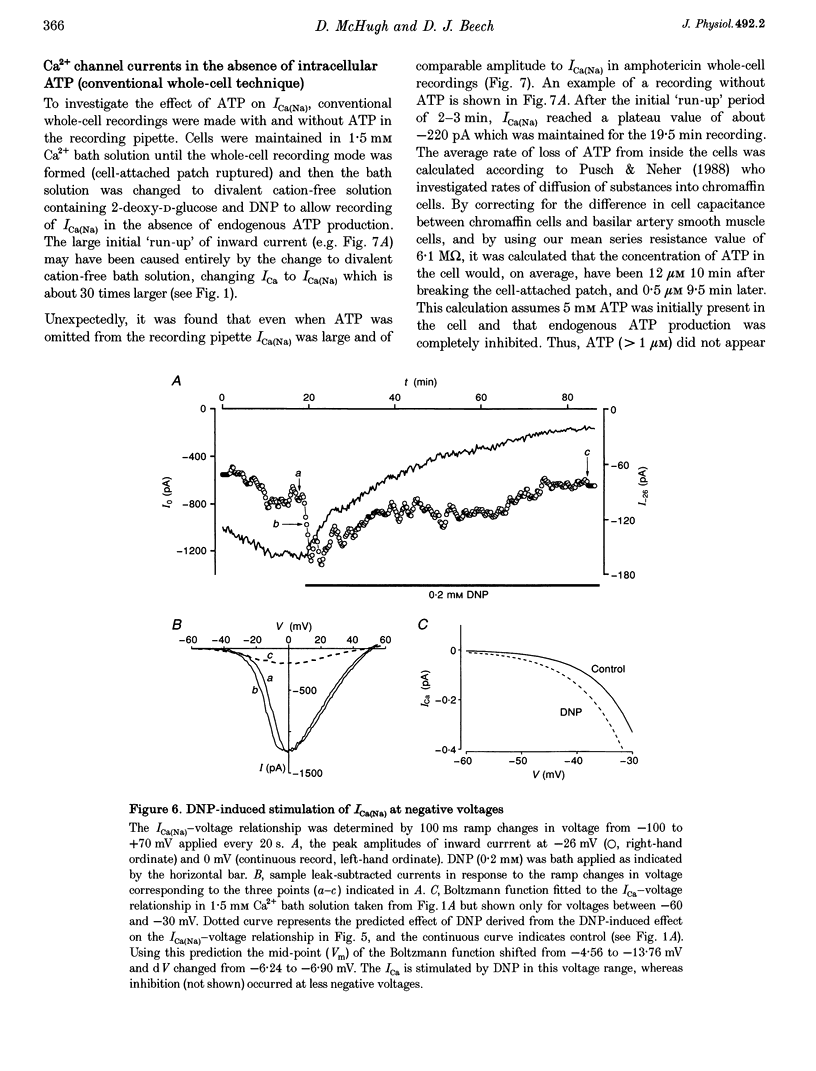
PDF

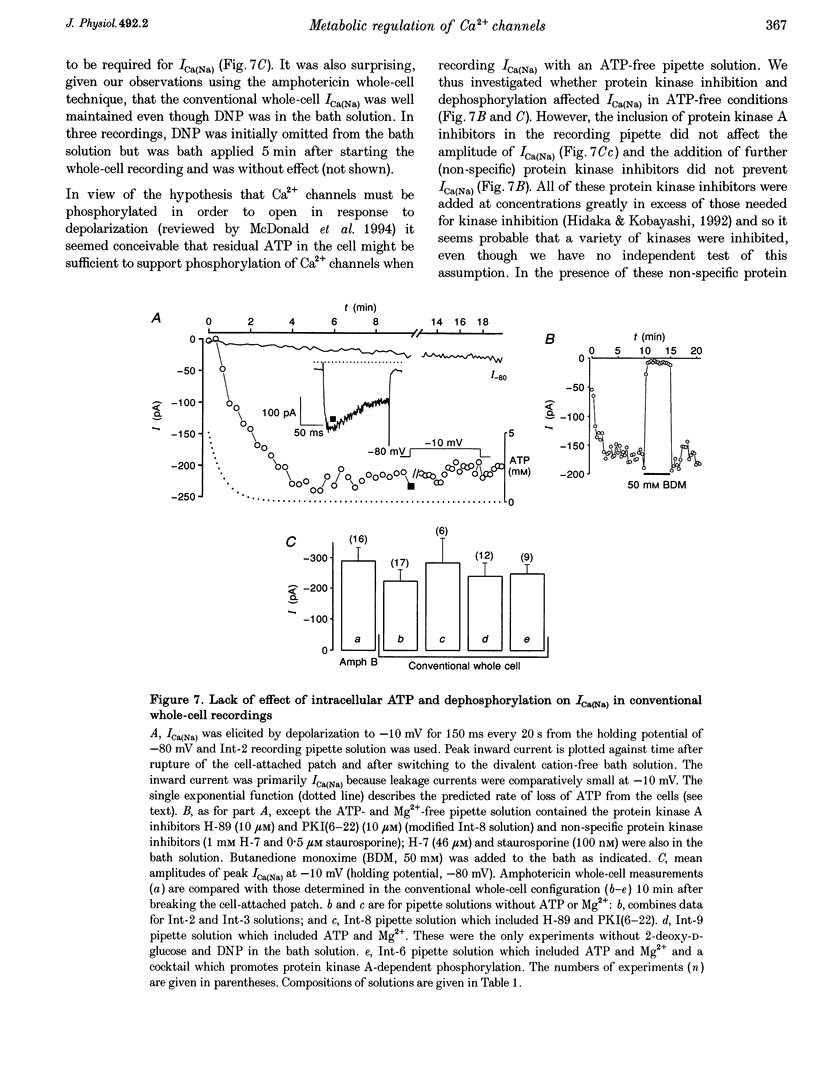
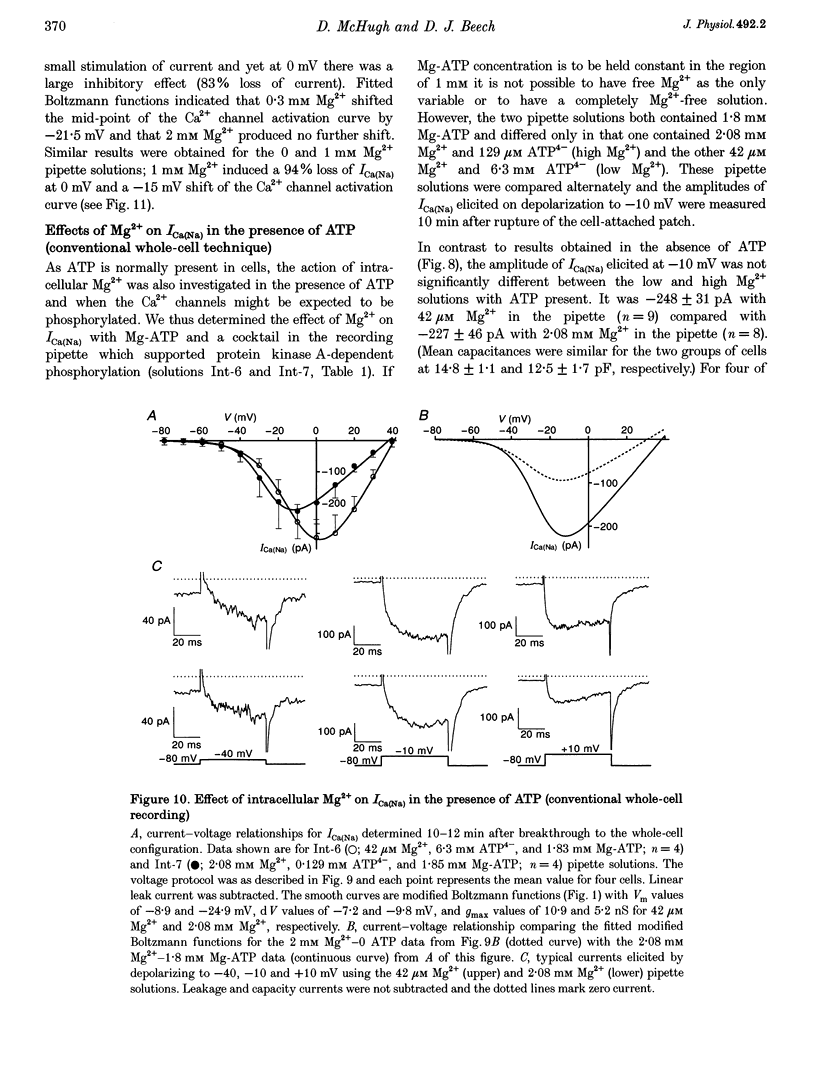





Selected References
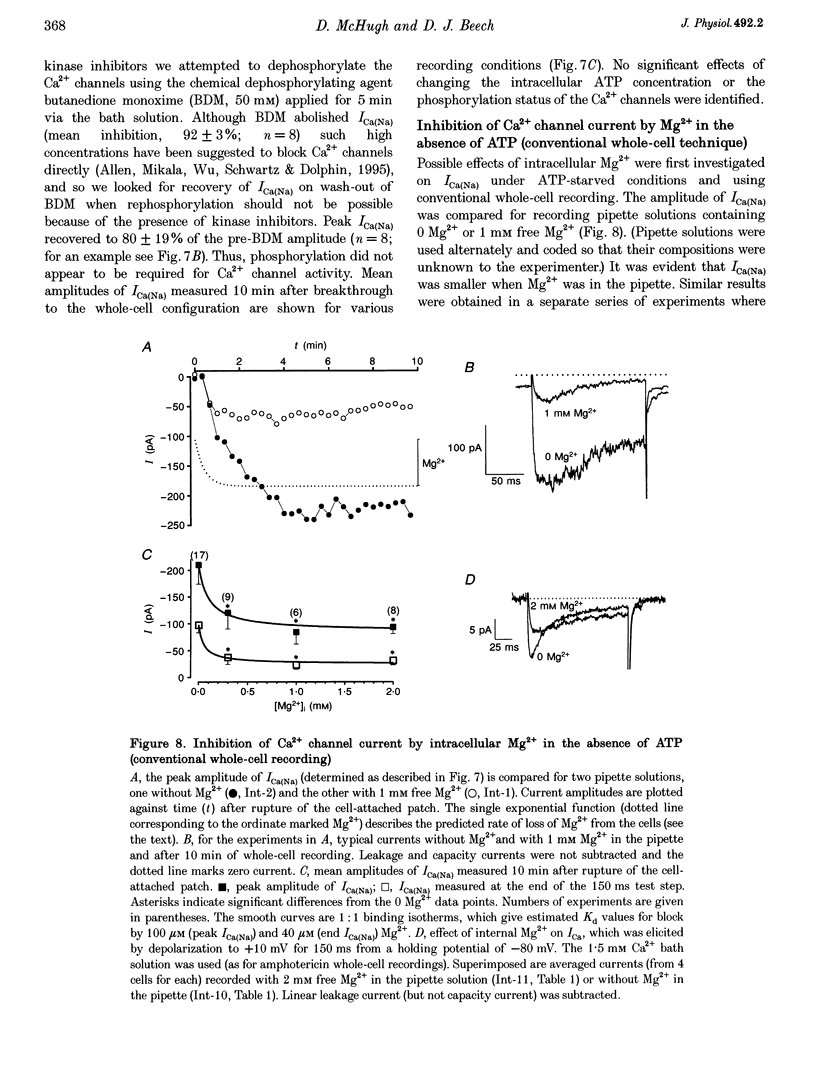
These references are in PubMed. This may not be the complete list of references from this article.
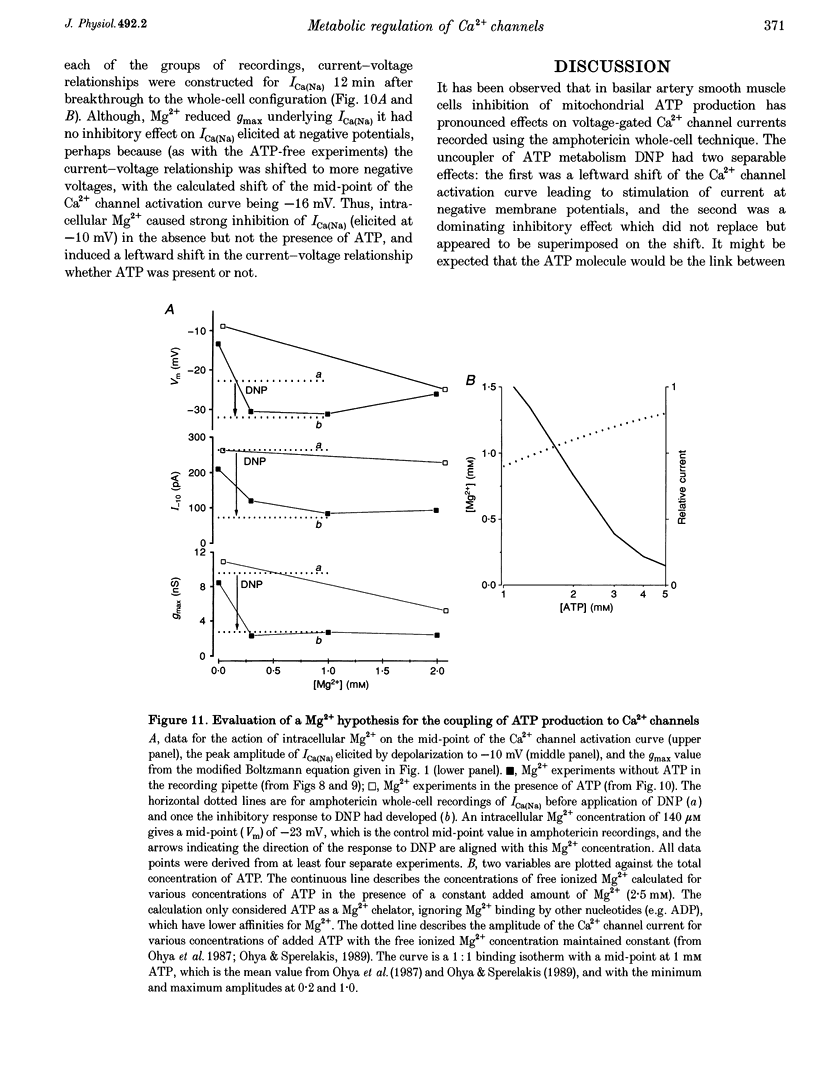
- Beech D. J., Zhang H., Nakao K., Bolton T. B. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993 Oct;110(2):573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D., Bonnet P., Greene A. S., England S. K., Rusch N. J., Lombard J. H., Harder D. R. Hypoxia increases the activity of Ca(2+)-sensitive K+ channels in cat cerebral arterial muscle cell membranes. Pflugers Arch. 1994 Oct;428(5-6):621–630. doi: 10.1007/BF00374586. [DOI] [PubMed] [Google Scholar]
- Goldhaber J. I., Parker J. M., Weiss J. N. Mechanisms of excitation-contraction coupling failure during metabolic inhibition in guinea-pig ventricular myocytes. J Physiol. 1991 Nov;443:371–386. doi: 10.1113/jphysiol.1991.sp018838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., White R. E. Effects of magnesium on inactivation of the voltage-gated calcium current in cardiac myocytes. J Gen Physiol. 1989 Oct;94(4):745–767. doi: 10.1085/jgp.94.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Effects of hypoxia on high-energy phosphagen content, energy metabolism and isometric force in guinea-pig taenia caeci. J Physiol. 1990 May;424:41–56. doi: 10.1113/jphysiol.1990.sp018054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. D., Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+. Neuron. 1993 May;10(5):797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Klaas M., Wadsworth R. Contraction followed by relaxation in response to hypoxia in the sheep isolated middle cerebral artery. Eur J Pharmacol. 1989 Sep 13;168(2):187–192. doi: 10.1016/0014-2999(89)90564-5. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Intracellular pH modulates the availability of vascular L-type Ca2+ channels. J Gen Physiol. 1994 Apr;103(4):647–663. doi: 10.1085/jgp.103.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Raper A. J., Rosenblum W. I., Navari R. M., Patterson J. L., Jr Role of tissue hypoxia in local regulation of cerebral microcirculation. Am J Physiol. 1978 May;234(5):H582–H591. doi: 10.1152/ajpheart.1978.234.5.H582. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Hess P. Block of the L-type Ca2+ channel pore by external and internal Mg2+ in rat phaeochromocytoma cells. J Physiol. 1993 Jul;466:683–706. [PMC free article] [PubMed] [Google Scholar]
- Lombard J. H., Smeda J., Madden J. A., Harder D. R. Effect of reduced oxygen availability upon myogenic depolarization and contraction of cat middle cerebral artery. Circ Res. 1986 Apr;58(4):565–569. doi: 10.1161/01.res.58.4.565. [DOI] [PubMed] [Google Scholar]
- Loutzenhiser R. D., Parker M. J. Hypoxia inhibits myogenic reactivity of renal afferent arterioles by activating ATP-sensitive K+ channels. Circ Res. 1994 May;74(5):861–869. doi: 10.1161/01.res.74.5.861. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer S., Trautwein W., Pelzer D. J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994 Apr;74(2):365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Murphy E., Steenbergen C., Levy L. A., Raju B., London R. E. Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem. 1989 Apr 5;264(10):5622–5627. [PubMed] [Google Scholar]
- Nakayama S., Tomita T. Regulation of intracellular free magnesium concentration in the taenia of guinea-pig caecum. J Physiol. 1991 Apr;435:559–572. doi: 10.1113/jphysiol.1991.sp018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke B., Backx P. H., Marban E. Phosphorylation-independent modulation of L-type calcium channels by magnesium-nucleotide complexes. Science. 1992 Jul 10;257(5067):245–248. doi: 10.1126/science.1321495. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Modulation of ionic currents in smooth muscle balls of the rabbit intestine by intracellularly perfused ATP and cyclic AMP. Pflugers Arch. 1987 May;408(5):465–473. doi: 10.1007/BF00585070. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1988 Feb;62(2):375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Sperelakis N. ATP regulation of the slow calcium channels in vascular smooth muscle cells of guinea pig mesenteric artery. Circ Res. 1989 Jan;64(1):145–154. doi: 10.1161/01.res.64.1.145. [DOI] [PubMed] [Google Scholar]
- Okashiro T., Tokuno H., Fukumitsu T., Hayashi H., Tomita T. Effects of intracellular ATP on calcium current in freshly dispersed single cells of guinea-pig portal vein. Exp Physiol. 1992 Sep;77(5):719–731. doi: 10.1113/expphysiol.1992.sp003638. [DOI] [PubMed] [Google Scholar]
- Paul R. J. Smooth muscle energetics. Annu Rev Physiol. 1989;51:331–349. doi: 10.1146/annurev.ph.51.030189.001555. [DOI] [PubMed] [Google Scholar]
- Pearce W. J., Ashwal S., Long D. M., Cuevas J. Hypoxia inhibits calcium influx in rabbit basilar and carotid arteries. Am J Physiol. 1992 Jan;262(1 Pt 2):H106–H113. doi: 10.1152/ajpheart.1992.262.1.H106. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Simard J. M. Calcium channel currents in isolated smooth muscle cells from the basilar artery of the guinea pig. Pflugers Arch. 1991 Jan;417(5):528–536. doi: 10.1007/BF00370950. [DOI] [PubMed] [Google Scholar]
- Spurway N. C., Wray S. A phosphorus nuclear magnetic resonance study of metabolites and intracellular pH in rabbit vascular smooth muscle. J Physiol. 1987 Dec;393:57–71. doi: 10.1113/jphysiol.1987.sp016810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swärd K., Josefsson M., Lydrup M. L., Hellstrand P. Effects of metabolic inhibition on cytoplasmic calcium and contraction in smooth muscle of rat portal vein. Acta Physiol Scand. 1993 Jul;148(3):265–272. doi: 10.1111/j.1748-1716.1993.tb09557.x. [DOI] [PubMed] [Google Scholar]
- Taguchi H., Heistad D. D., Kitazono T., Faraci F. M. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994 May;74(5):1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- Tewari K., Simard J. M. Protein kinase A increases availability of calcium channels in smooth muscle cells from guinea pig basilar artery. Pflugers Arch. 1994 Aug;428(1):9–16. doi: 10.1007/BF00374746. [DOI] [PubMed] [Google Scholar]
- Trezise D. J., Weston A. H. Hypoxia-evoked dilatation of rabbit isolated basilar arteries is not mediated by the opening of glibenclamide-sensitive potassium channels. Jpn J Pharmacol. 1992;58 (Suppl 2):308P–308P. [PubMed] [Google Scholar]
- Wahl M., Schilling L. Regulation of cerebral blood flow--a brief review. Acta Neurochir Suppl (Wien) 1993;59:3–10. doi: 10.1007/978-3-7091-9302-0_1. [DOI] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science. 1988 Feb 12;239(4841 Pt 1):778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- Zong X., Schreieck J., Mehrke G., Welling A., Schuster A., Bosse E., Flockerzi V., Hofmann F. On the regulation of the expressed L-type calcium channel by cAMP-dependent phosphorylation. Pflugers Arch. 1995 Jul;430(3):340–347. doi: 10.1007/BF00373908. [DOI] [PubMed] [Google Scholar]


