Abstract
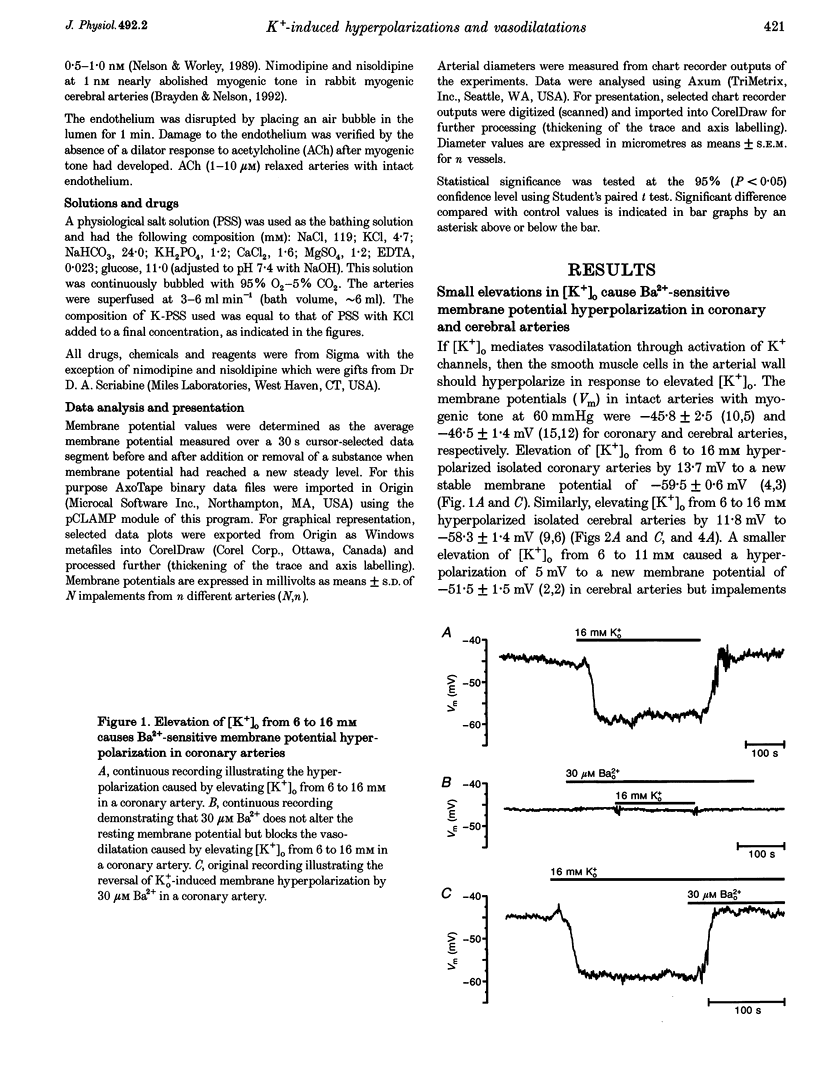
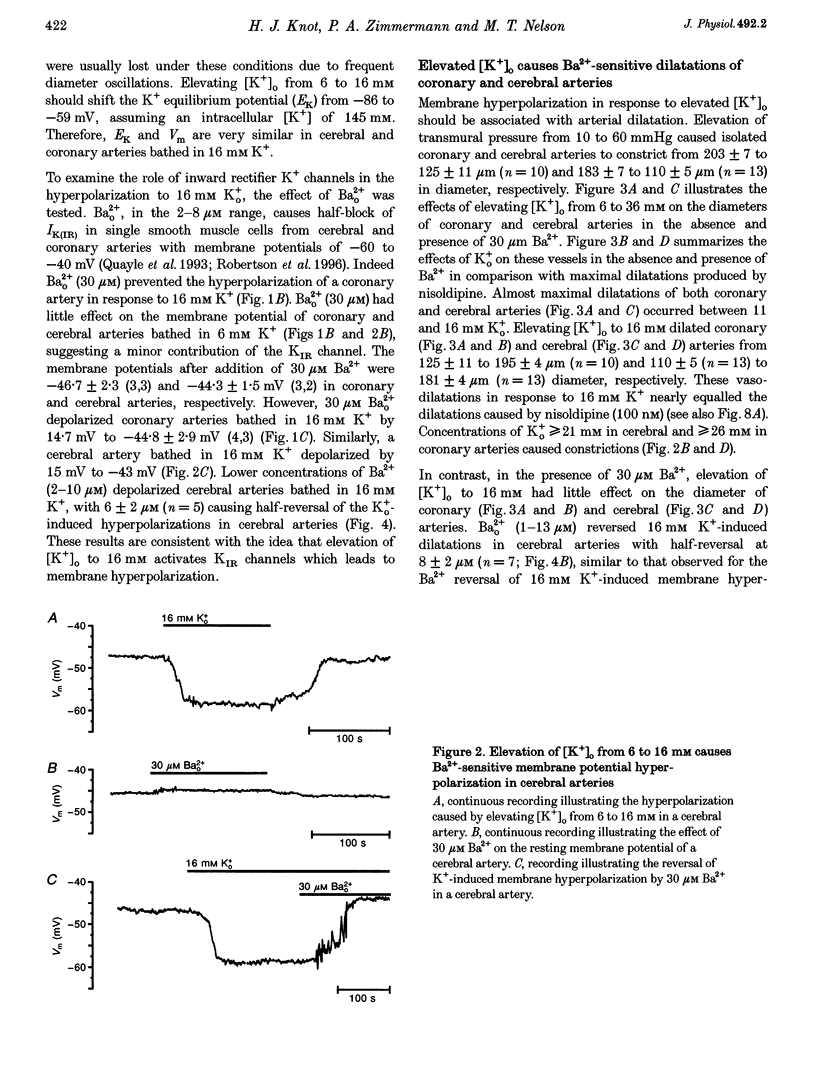
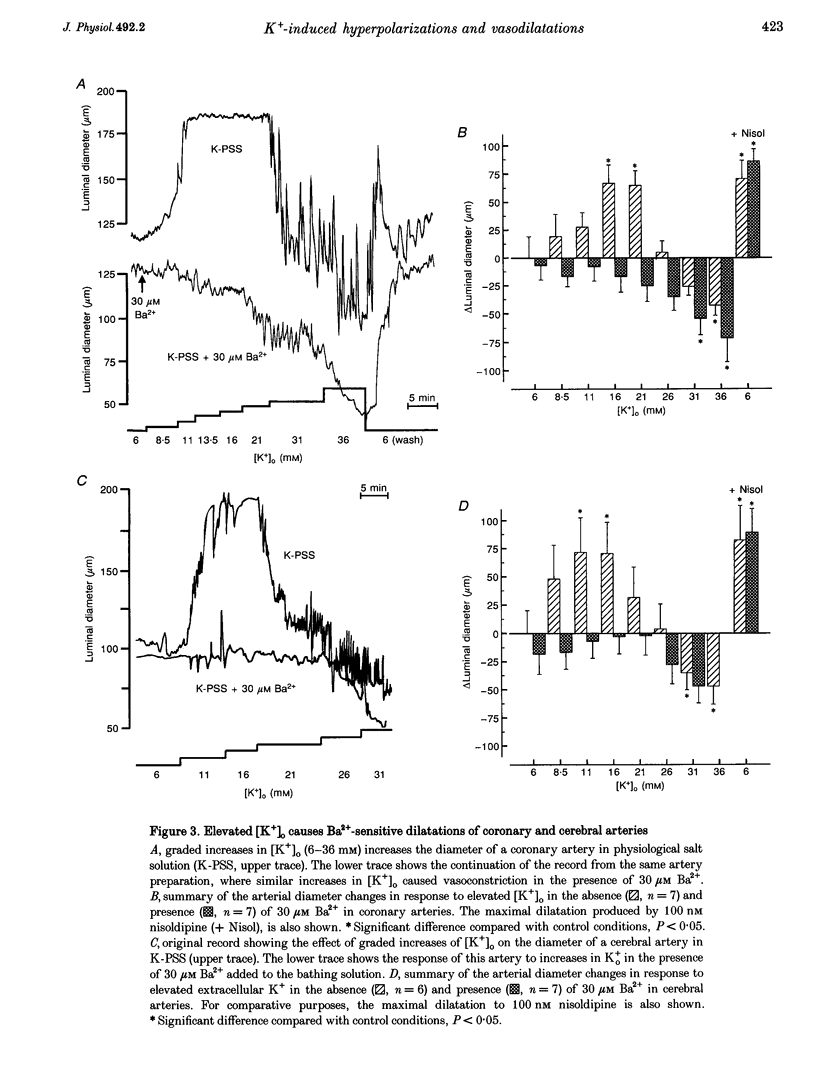
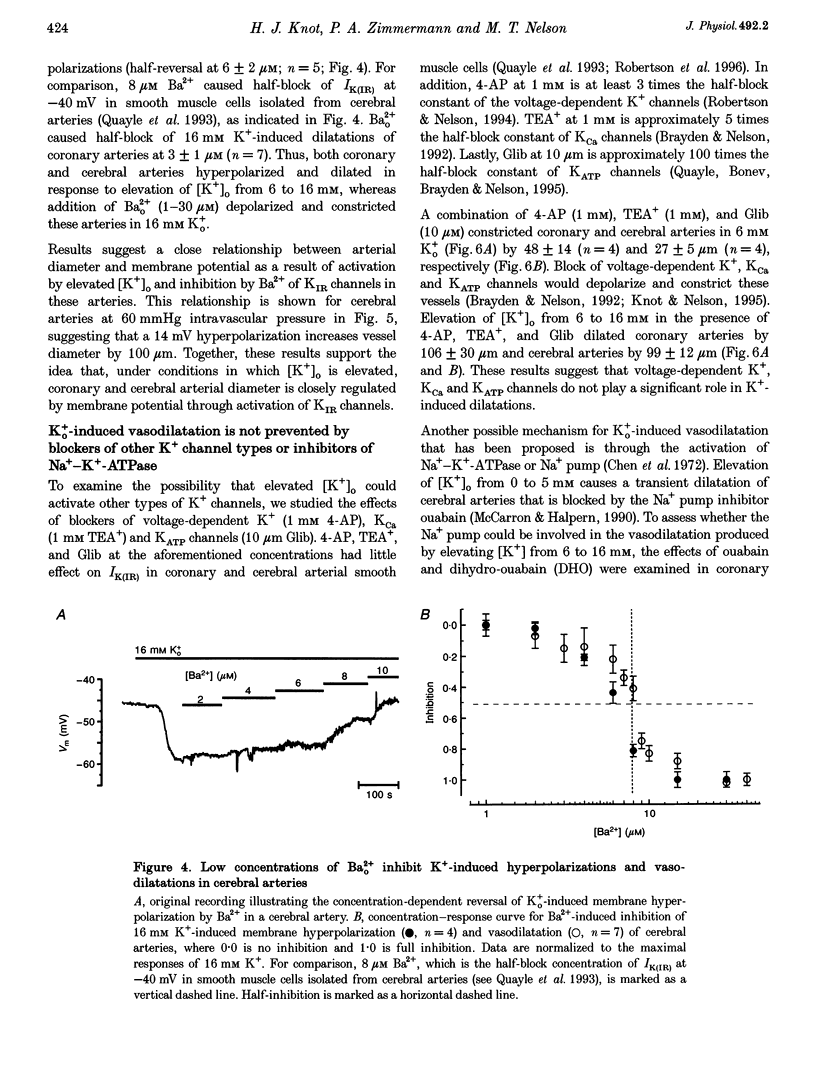
1. The hypothesis that inward rectifier K(+) channels are involved in the vasodilatation of small coronary and cerebral arteries (100-200 microm diameter) in response to elevated [K+]o was tested. The diameters and membrane potentials of pressurized arteries from rat were measured using a video-imaging system and conventional microelectrodes, respectively. 2. Elevation of [K+]o from 6 to 16 mM caused the membrane potential of pressurized (60 mmHg) arteries to hyperpolarize by 12-14 mV. Extracellular Ba(2+) (Ba2+(o)) blocked K(+)-induced membrane potential hyperpolarizations at concentrations (IC(50), 6 microM) that block inward rectifier K(+) currents in smooth muscle cells isolated from these arteries. 3. Elevation of [K+]o from 6 to 16 mM caused sustained dilatations of pressurized coronary and cerebral arteries with diameters increasing from 125 to 192 microm and 110 to 180 microm in coronary and cerebral arteries, respectively. Ba2+(o) blocked K(+)-induced dilatations of pressurized coronary and cerebral arteries (IC50, 3-8 microM). 4. Elevated [K+]o-induced vasodilatation was not prevented by blockers of other types of K(+) channels (1 mM 4-aminopyridine, 1 mM TEA+, and 10 mu M glibenclamide), and blockers of Na(+)-K(+)-ATPase. Elevated [K+]o-induced vasodilatation was unaffected by removal of the endothelium. 5. These findings suggest that K+(o) dilates small rat coronary and cerebral arteries through activation of inward rectifier K(+) channels. Furthermore, these results support the hypothesis that inward rectifier K(+) channels may be involved in metabolic regulation of coronary and cerebral blood flow in response to changes in [K+]o.
Full text
PDF


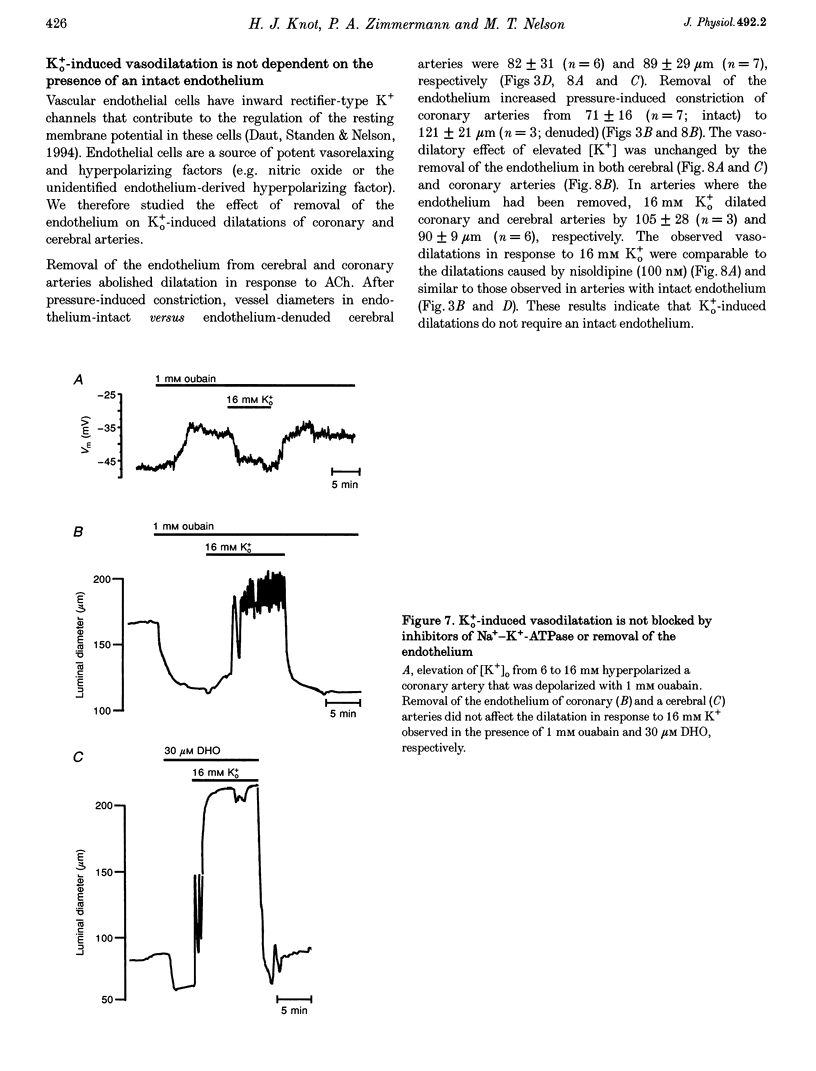
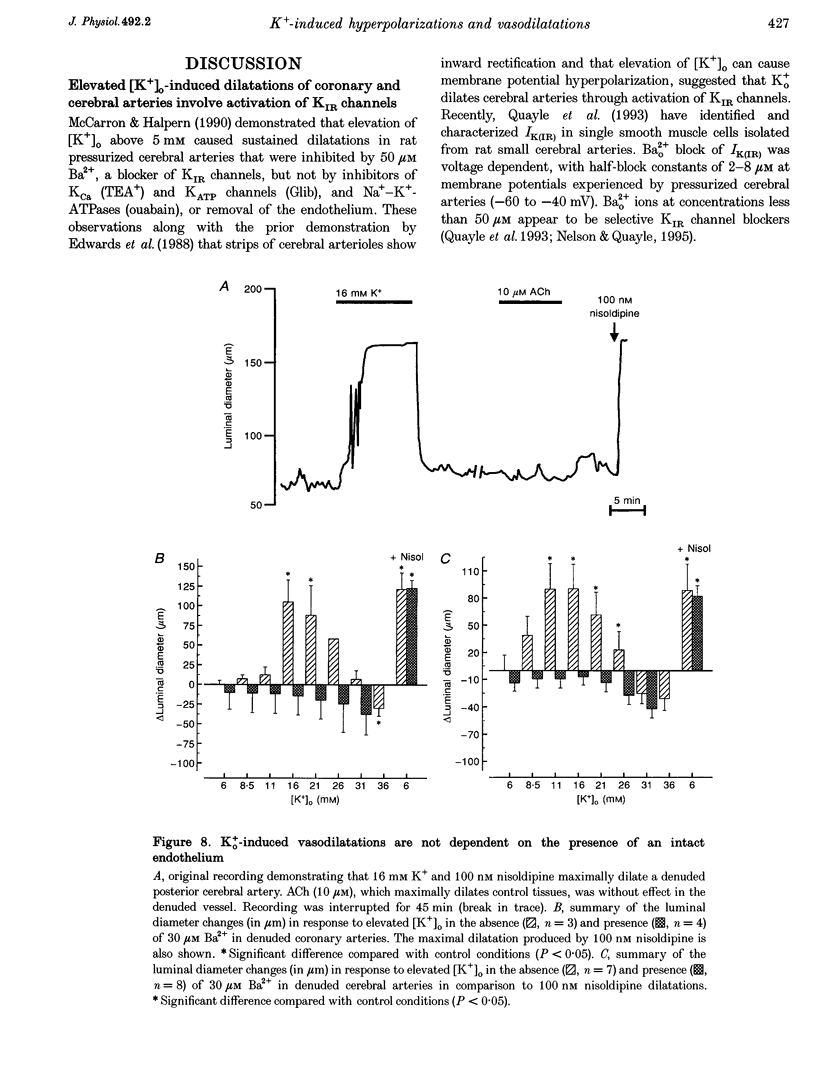



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Bünger R., Haddy R. J., Querengässer A., Gerlach E. Studies on potassium induced coronary dilation in the isolated guinea pig heart. Pflugers Arch. 1976 May 6;363(1):27–31. doi: 10.1007/BF00587398. [DOI] [PubMed] [Google Scholar]
- Cameron I. R., Caronna J. The effect of local changes in potassium and bicarbonate concentration on hypothalamic blood flow in the rabbit. J Physiol. 1976 Nov;262(2):415–430. doi: 10.1113/jphysiol.1976.sp011602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Brace R. A., Scott J. B., Anderson D. K., Haddy F. J. The mechanism of the vasodilator action of potassium. Proc Soc Exp Biol Med. 1972 Jul;140(3):820–824. doi: 10.3181/00379727-140-36560. [DOI] [PubMed] [Google Scholar]
- Daut J., Standen N. B., Nelson M. T. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J Cardiovasc Electrophysiol. 1994 Feb;5(2):154–181. doi: 10.1111/j.1540-8167.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D. Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol. 1988 Oct;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D., Silverberg G. D. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol. 1988 Oct;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994 Nov 11;266(5187):1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Haddy F. J., Scott J. B. Metabolically linked vasoactive chemicals in local regulation of blood flow. Physiol Rev. 1968 Oct;48(4):688–707. doi: 10.1152/physrev.1968.48.4.688. [DOI] [PubMed] [Google Scholar]
- Harik S. I., Doull G. H., Dick A. P. Specific ouabain binding to brain microvessels and choroid plexus. J Cereb Blood Flow Metab. 1985 Mar;5(1):156–160. doi: 10.1038/jcbfm.1985.20. [DOI] [PubMed] [Google Scholar]
- Hexum T. D. Characterization of NaK-ATPase from vascular smooth muscle. Gen Pharmacol. 1981;12(5):393–396. doi: 10.1016/0306-3623(81)90098-7. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., van Helden D. F. Ionic basis of the resting potential of submucosal arterioles in the ileum of the guinea-pig. J Physiol. 1982 Dec;333:53–67. doi: 10.1113/jphysiol.1982.sp014438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Knot H. J., Nelson M. T. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol. 1995 Jul;269(1 Pt 2):H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W., Wahl M., Bosse O., Thurau K. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ Res. 1972 Aug;31(2):240–247. doi: 10.1161/01.res.31.2.240. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994 Nov 24;372(6504):366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- McCarron J. G., Halpern W. Potassium dilates rat cerebral arteries by two independent mechanisms. Am J Physiol. 1990 Sep;259(3 Pt 2):H902–H908. doi: 10.1152/ajpheart.1990.259.3.H902. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Blaustein M. P. Properties of sodium pumps in internally perfused barnacle muscle fibers. J Gen Physiol. 1980 Feb;75(2):183–206. doi: 10.1085/jgp.75.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Worley J. F. Dihydropyridine inhibition of single calcium channels and contraction in rabbit mesenteric artery depends on voltage. J Physiol. 1989 May;412:65–91. doi: 10.1113/jphysiol.1989.sp017604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson O. B., Newman E. A. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987 Aug 21;237(4817):896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. M., Bonev A. D., Brayden J. E., Nelson M. T. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. Am J Physiol. 1995 Nov;269(5 Pt 1):C1112–C1118. doi: 10.1152/ajpcell.1995.269.5.C1112. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., McCarron J. G., Brayden J. E., Nelson M. T. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993 Nov;265(5 Pt 1):C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- Robertson B. E., Nelson M. T. Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. Am J Physiol. 1994 Dec;267(6 Pt 1):C1589–C1597. doi: 10.1152/ajpcell.1994.267.6.C1589. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber F. E., Wilson D. A., Hanley D. F., Traystman R. J. Extracellular potassium activity and cerebral blood flow during moderate hypoglycemia in anesthetized dogs. Am J Physiol. 1993 Jun;264(6 Pt 2):H1774–H1780. doi: 10.1152/ajpheart.1993.264.6.H1774. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- Warner M. R., Kroeker T. S., Zipes D. P. Sympathetic stimulation and norepinephrine infusion modulate extracellular potassium concentration during acute myocardial ischemia. Circ Res. 1992 Nov;71(5):1078–1087. doi: 10.1161/01.res.71.5.1078. [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V., Mackic J. B., Wang L., McComb J. G., McDonough A. Differential expression of Na,K-ATPase alpha and beta subunit isoforms at the blood-brain barrier and the choroid plexus. J Biol Chem. 1993 Apr 15;268(11):8019–8025. [PubMed] [Google Scholar]


