Abstract
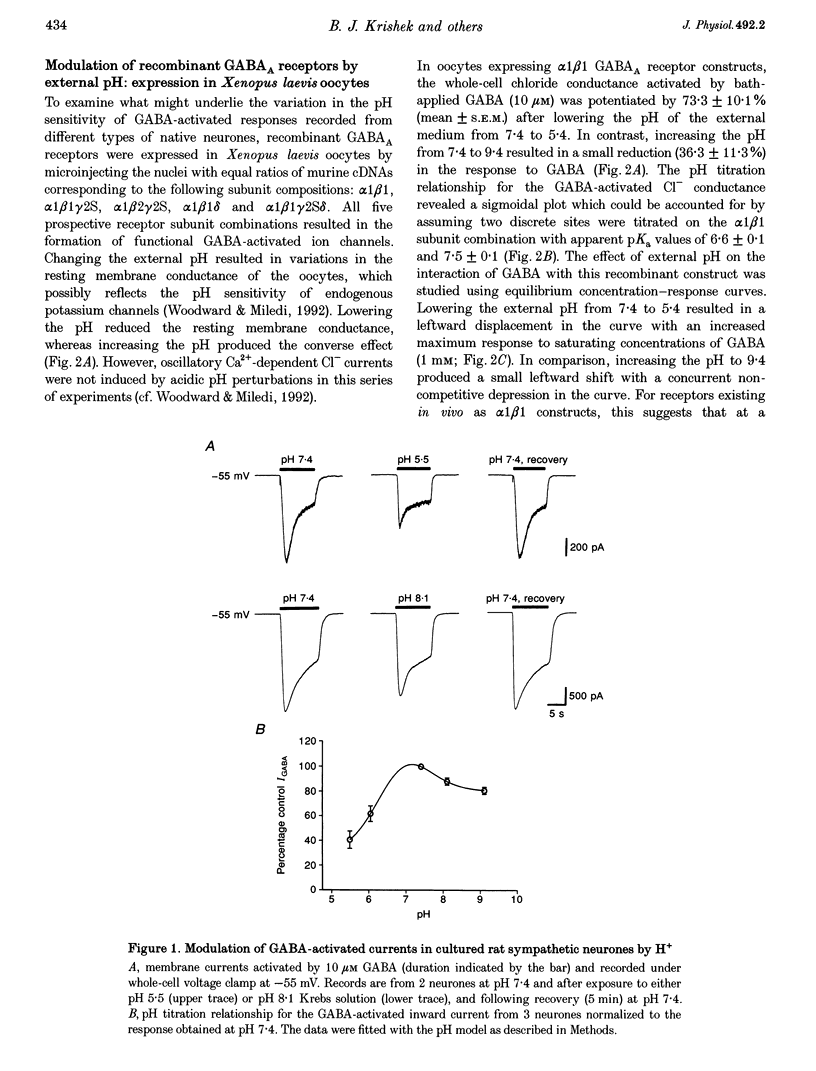
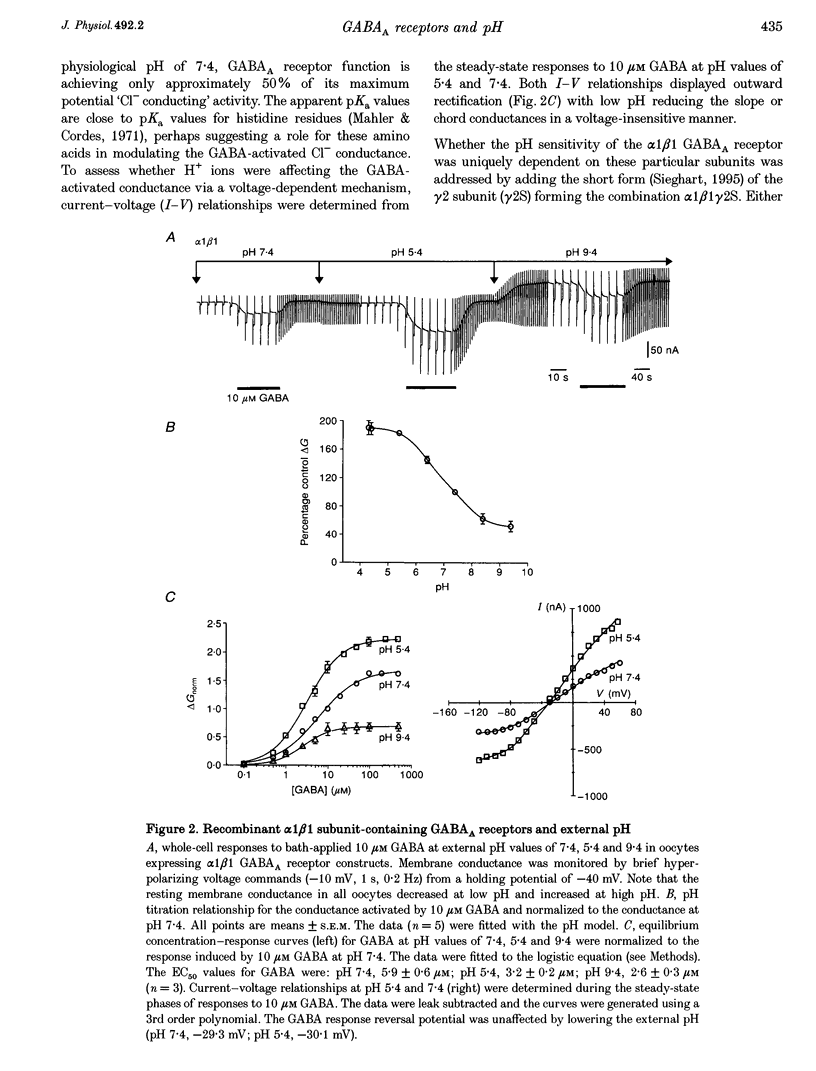
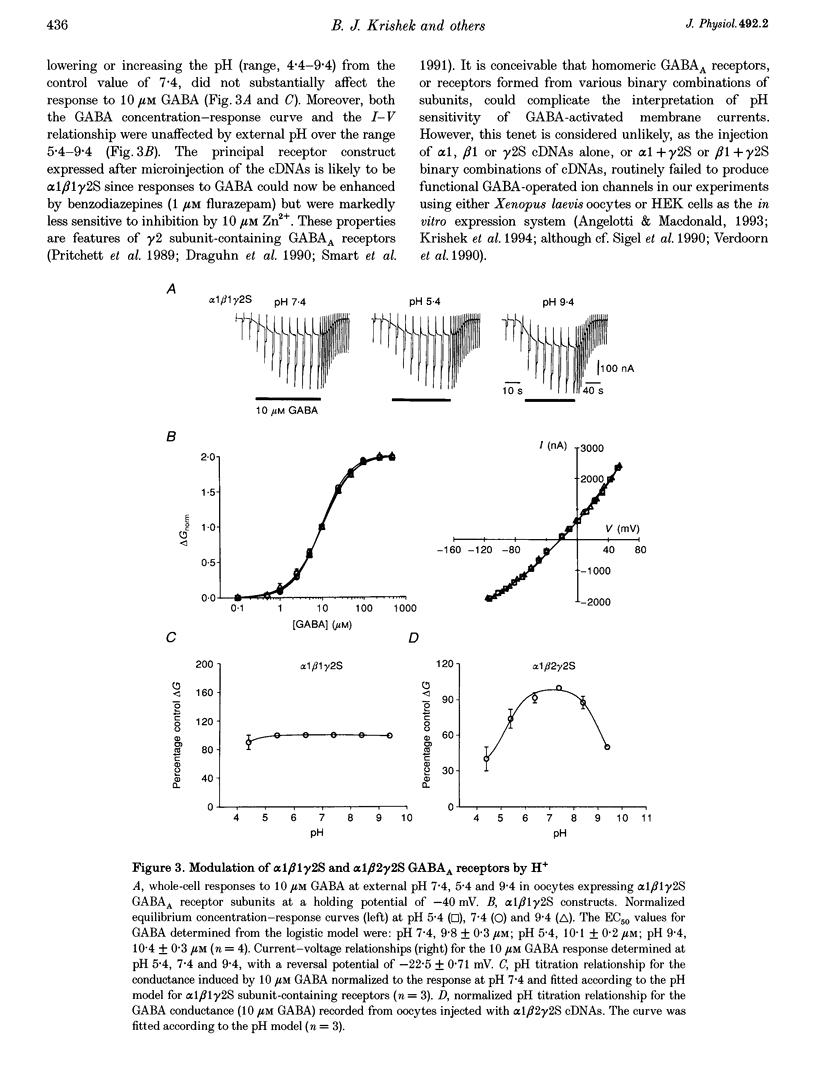
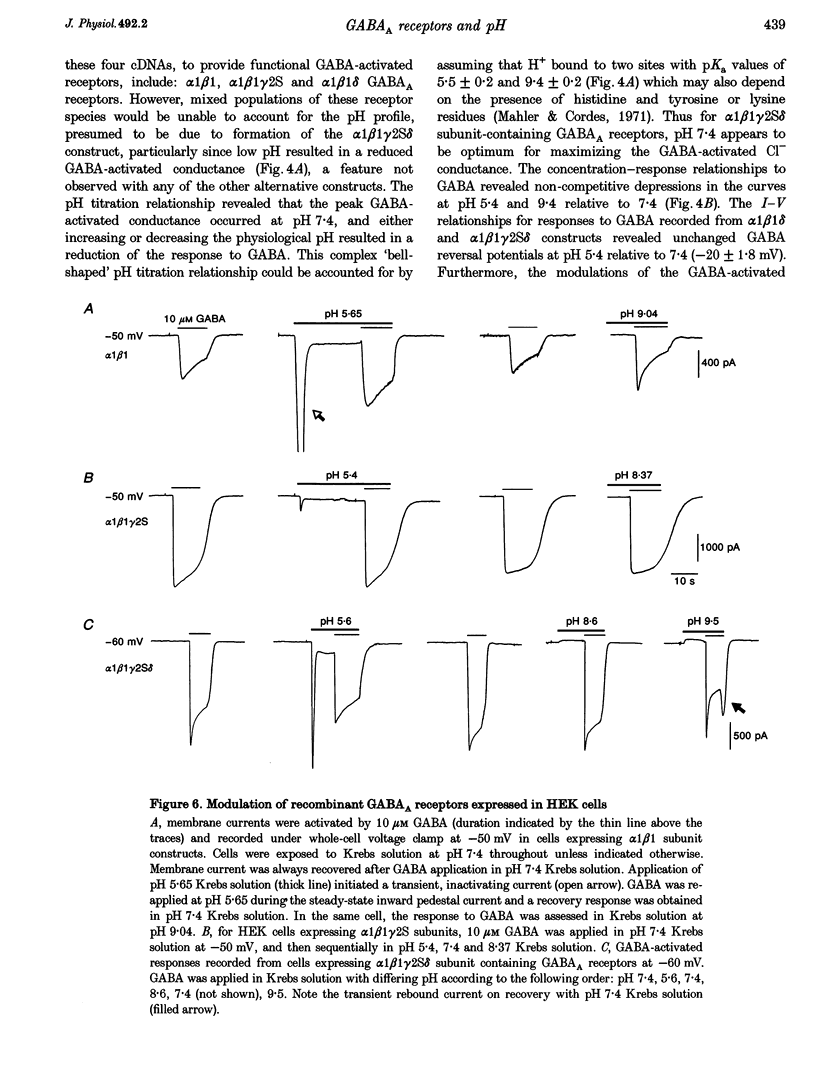
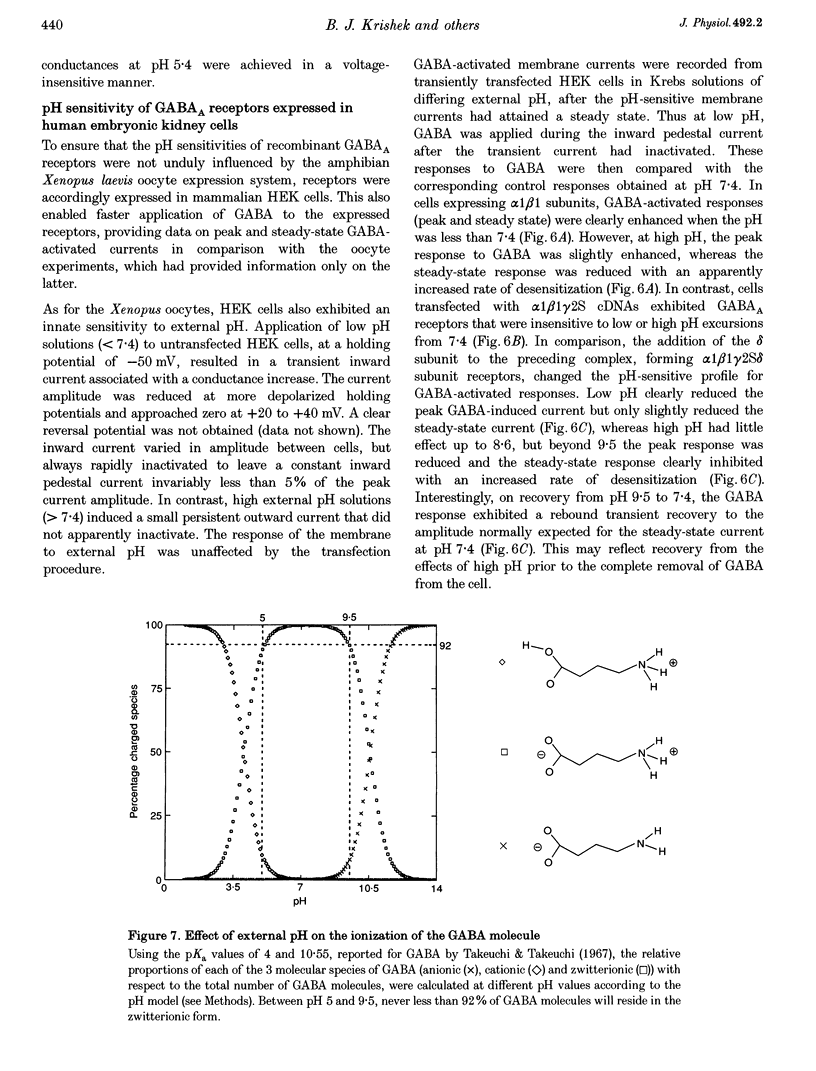
1. Modulation of GABA(A) receptors by external H(+) was examined in cultured rat sympathetic neurones, and in Xenopus laevis oocytes and human embryonic kidney (HEK) cells expressing recombinant GABA(A) receptors composed of combinations of alpha 1, beta 1, beta 2, gamma 2S and delta subunits. 2. Changing the external pH from 7.4 reduced GABA-activated currents in sympathetic neurones. pH titration of the GABA-induced current was fitted with a pH model which predicted that H(+) interact with two sites (PK(a) values of 6.4 and 7.2). 3. For alpha 1 beta 1 GABA(A) receptors, low external pH (< 7.4) enhanced responses to GABA. pH titration predicted the existence of two sites with PK(a) values of 6.6 and 7.5. The GABA concentration-response curve was shifted to the left by low pH and non-competitively inhibited at high pH (> 7.4). 4. alpha 1 beta 1 gamma 2S receptor constructs were not affected by external pH, whereas exchanging the beta 1 subunit for beta 2 conferred a sensitivity to pH, with predicted PK(a) values of 5.16 and 9.44. 5. Low pH enhanced the responses to GABA on alpha 1 beta 1 delta subunits, whilst high pH caused an inhibition (PK(a) values of 6.6 and 9.9). The GABA concentration-response curves were enhanced (pH 5.4) or reduced (pH 9.4) with no changes in the GABA EC(50). 6. Immunoprecipitation with subunit and epitope-specific antisera to alpha 1, beta 1 and delta subunits demonstrated that these subunits could co-assemble in cell membranes. 7. Expression of alpha 1 beta 1 gamma 2S delta constructs resulted in a 'bell-shaped' pH titration relationship. Increasing or decreasing external pH inhibited the responses to GABA. 8. The pH sensitivity of recombinant GABA(A) receptors expressed in HEK cells was generally in accordance with data accrued from Xenopus oocytes. However, rapid application of GABA to alpha 1 beta 1 constructs at high pH (> 7.4) caused an increased peak and reduced steady-state current, with a correspondingly increased rate of desensitization. 9. Modulation of GABA(A) receptor function was apparently unaffected by the internal pH. Moreover, pH values between 5 and 9.5 did not significantly affect the charge distribution on the zwitterionic GABA molecules. 10. In conclusion, this study demonstrates that external pH can either enhance, have little effect, or reduce GABA-activated responses, and this is apparently dependent on the receptor subunit composition. The potential importance of H(+) sensitivity of GABA(A) receptors is discussed.
Full text
PDF


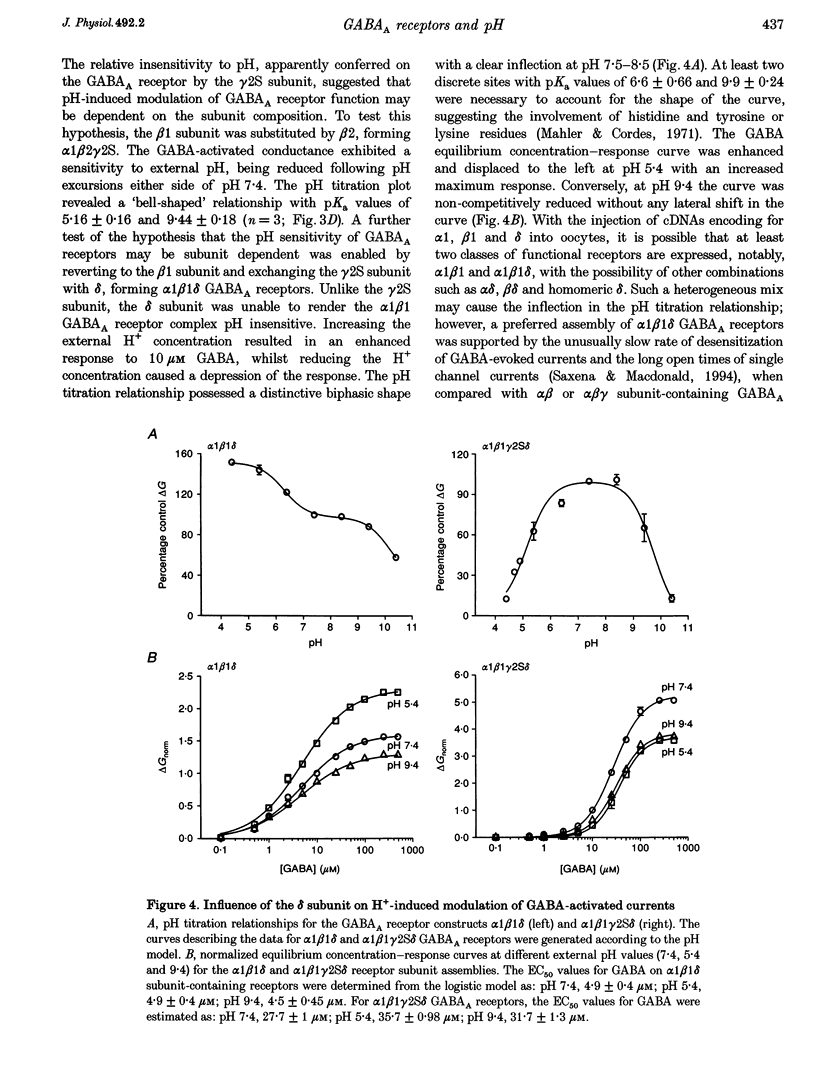




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelotti T. P., Macdonald R. L. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993 Apr;13(4):1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antosiewicz J., McCammon J. A., Gilson M. K. Prediction of pH-dependent properties of proteins. J Mol Biol. 1994 May 6;238(3):415–436. doi: 10.1006/jmbi.1994.1301. [DOI] [PubMed] [Google Scholar]
- Chen J. C., Chesler M. Modulation of extracellular pH by glutamate and GABA in rat hippocampal slices. J Neurophysiol. 1992 Jan;67(1):29–36. doi: 10.1152/jn.1992.67.1.29. [DOI] [PubMed] [Google Scholar]
- Chesler M., Chan C. Y. Stimulus-induced extracellular pH transients in the in vitro turtle cerebellum. Neuroscience. 1988 Dec;27(3):941–948. doi: 10.1016/0306-4522(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Chesler M., Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992 Oct;15(10):396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34(5):401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Draguhn A., Verdorn T. A., Ewert M., Seeburg P. H., Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990 Dec;5(6):781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Kenning N. A., O'Neill S. C., Pocock G., Richards C. D., Valdeolmillos M. A novel method for absolute calibration of intracellular pH indicators. Pflugers Arch. 1989 Mar;413(5):553–558. doi: 10.1007/BF00594188. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol D. L., Barker J. L., Huang L. Y., MacDonald J. F., Smith T. G., Jr Hydrogen ions have multiple effects on the excitability of cultured mammalian neurons. Brain Res. 1980 Feb 3;183(1):247–252. doi: 10.1016/0006-8993(80)90138-9. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Gibbons S. J. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994 Aug;33(8):935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994 Mar;42(4):489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kraig R. P., Ferreira-Filho C. R., Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983 Mar;49(3):831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Krishek B. J., Xie X., Blackstone C., Huganir R. L., Moss S. J., Smart T. G. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994 May;12(5):1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J Physiol. 1989 Aug;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellergård P. E., Ouyang Y. B., Siesjö B. K. The regulation of intracellular pH in cultured astrocytes and neuroblastoma cells, and its dependence on extracellular pH in a HCO3-free solution. Can J Physiol Pharmacol. 1992;70 (Suppl):S293–S300. doi: 10.1139/y92-275. [DOI] [PubMed] [Google Scholar]
- Mertens S., Benke D., Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by alpha 5- and delta-subunit-specific immunopurification. J Biol Chem. 1993 Mar 15;268(8):5965–5973. [PubMed] [Google Scholar]
- Nakagawa T., Wakamori M., Shirasaki T., Nakaye T., Akaike N. gamma-Aminobutyric acid-induced response in acutely isolated nucleus solitarii neurons of the rat. Am J Physiol. 1991 Apr;260(4 Pt 1):C745–C749. doi: 10.1152/ajpcell.1991.260.4.C745. [DOI] [PubMed] [Google Scholar]
- Nayeem N., Green T. P., Martin I. L., Barnard E. A. Quaternary structure of the native GABAA receptor determined by electron microscopic image analysis. J Neurochem. 1994 Feb;62(2):815–818. doi: 10.1046/j.1471-4159.1994.62020815.x. [DOI] [PubMed] [Google Scholar]
- Pasternack M., Bountra C., Voipio J., Kaila K. Influence of extracellular and intracellular pH on GABA-gated chloride conductance in crayfish muscle fibres. Neuroscience. 1992;47(4):921–929. doi: 10.1016/0306-4522(92)90040-9. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N. C., Macdonald R. L. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994 Nov;14(11 Pt 2):7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995 Jun;47(2):181–234. [PubMed] [Google Scholar]
- Sigel E., Baur R., Trube G., Möhler H., Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990 Nov;5(5):703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Smart T. G. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol. 1992 Feb;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G., Xie X., Krishek B. J. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994 Feb;42(3):393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990 May 24;345(6273):347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Urbanics R., Leniger-Follert E., Lübbers D. W. Time course of changes of extracellular H+ and K+ activities during and after direct electrical stimulation of the brain cortex. Pflugers Arch. 1978 Dec 15;378(1):47–53. doi: 10.1007/BF00581957. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Sensitivity of Xenopus oocytes to changes in extracellular pH: possible relevance to proposed expression of atypical mammalian GABAB receptors. Brain Res Mol Brain Res. 1992 Dec;16(3-4):204–210. doi: 10.1016/0169-328x(92)90226-2. [DOI] [PubMed] [Google Scholar]