Abstract
Since the brain is separated from the blood immune system by a tight barrier, the brain-resident complement system may represent a central player in the immune defense of this compartment against human immunodeficiency virus (HIV). Chronic complement activation, however, may participate in HIV-associated neurodegeneration. Since the level of complement factors in the cerebrospinal fluid is known to be elevated in AIDS-associated neurological disorders, we evaluated the effect of HIV type 1 (HIV-1) on the complement synthesis of brain astrocytes. Incubation of different astrocytic cell lines and primary astrocytes with HIV-1 induced a marked upregulation of the expression of the complement factors C2 and C3. The synthesis of other secreted or membrane-bound complement proteins was not found to be altered. The enhancement of C3 production was measured both on the mRNA level and as secreted protein in the culture supernatants. HIV-1 laboratory strains as well as primary isolates were capable of inducing C3 production with varied effectiveness. The usage of viral coreceptors by HIV-1 was proved to be a prerequisite for the upregulation of C3 synthesis, which was modulated by the simultaneous addition of cytokines. The C3 protein which is secreted after incubation of the cells with HIV was shown to be biologically active as it can participate in the complement cascade.
The human immunodeficiency virus type 1 (HIV-1) is detected in more than 80% of the brains of patients with AIDS (12, 17, 29) and induces neurological manifestations in 20 to 30% of HIV-1-infected individuals. The complex of cognitive, motor, and behavioral dysfunction is summarized as AIDS dementia complex. Since HIV interacts with the complement system in many ways, we were interested in studying this interaction in the brain. The brain is an immunoprivileged compartment which is separated from the immune system of the blood. For this reason the brain-resident complement system may be considered an important mediator of the immune defense against invading pathogens. The complement system is an antimicrobial enzyme system that can recognize a large variety of pathogens and target them for destruction either directly by action of the membrane attack complex or by opsonization with C3 fragments and recruiting of phagocytic cells. The complement factor C3 is a central protein of the cascade, and its degradation products, like C3b, iC3b, C3d, and C3a, harbor a variety of biological functions, including cell activation, initiation of phagocytosis, and transport of immune complexes on erythrocytes (31). Similar to the situation in the blood, the complement system of the brain harbors a broad spectrum of functions. Besides the lysis of pathogens, the complement activation products and anaphylatoxins C3a and C5a can exert important functional effects on brain cells like the modulation of cytokine expression (27), the induction of nerve growth factor synthesis (15), and the activation of signal transduction pathways (19, 22). On the other hand, aberrant activation of complement was found in brain-associated pathological conditions such as multiple sclerosis or Alzheimer's disease. Activation of the complement by fibrillar amyloid β-protein is discussed as a mechanism for neuronal loss and neuritic dystrophy in Alzheimer's disease (35). In multiple sclerosis, complement activation by the myelin oligodendrocyte glycoprotein mediates degradation of the neuronal myelin sheath (34).
The primary site of complement synthesis is the liver, but no plasma complement protein will reach the central nervous system (CNS) tissue in the presence of an intact blood-brain barrier. Instead, there is increasing evidence that complement biosynthesis also occurs in the CNS and that all components of the cascade can be synthesized locally in the brain by the major cell types including astrocytes, microglia, neurons, and oligodendrocytes (21). Astrocytes have a pivotal position in local complement synthesis since they can express and secrete all the components of the classical, alternative, and terminal pathways (21). For most components, the level of constitutive expression by the cells is very low but synthesis is enhanced by various triggers, mainly inflammatory cytokines. Complement components like C3 can also be detected in the cerebrospinal fluid (CSF), where the concentration is 300 times lower than in the blood (18). Increased levels of C3 and C4 were found in the CSF of HIV-infected patients with neurological symptoms and signs of CNS dysfunction. The CSF index was shown to be a valid tool to detect intrathecal C3 and C4 production (18). Nothing was known about the mechanism(s) of this enhancement.
In the present study we investigated the effect of HIV infection on the complement synthesis of astrocytes. Incubation of the cells with HIV specifically upregulated the synthesis of complement factor C3 on the protein and mRNA levels. The time- and dose-dependent modulation of C3 expression was not restricted to one special astrocyte lineage or a certain viral strain. Inhibition of the chemokine receptors by neutralizing antibodies inhibited the HIV activity in complement production. The HIV-induced secreted C3 was proven to be biologically active, indicating a variety of possible interactions between the complement system and HIV in the brain.
MATERIALS AND METHODS
Cell lines and primary astrocyte isolation.
The human glioblastoma/astrocytoma cell line U373MG, the glioblastoma cell line U138MG, and the astroglioma cell line U87MG were purchased from the MRC (Centralised Facility for AIDS Reagents, Hertfordshire, United Kingdom) and cultured in either Ham's F12 medium or Dulbecco modified Eagle medium (Life Technologies, Vienna, Austria) supplemented with 10% fetal calf serum (FCS; BioWhittaker, Verviers, Belgium), penicillin (100 U/ml), streptomycin (100 μg/ml), 0.1 mM nonessential amino acids, and 2 mM l-glutamine (Life Technologies). The glioblastoma/astrocytoma cell line U118 and the glioblastoma multiforme cell line T98 were from the American Type Culture Collection (ATCC; HTB-15 and CRL1690) and were grown in the same medium. The human glioblastoma cell line HTR17 was kindly provided by G. Stockhammer (Innsbruck, Austria).
The T-lymphoblastoid cell line M8166 used for virus propagation was cultivated in RPMI 1640 medium (Life Technologies) supplemented with 10% FCS, penicillin, streptomycin, and 2 mM l-glutamine.
Primary adult astrocytes (preparation AP28) were isolated from human adult brain derived from surgery material. The tissue was dissected using a syringe and trypsin-EDTA enzymatic digestion and was cultivated on polylysine-coated flasks with minimal essential D-Val medium (Life Technologies) containing the same supplements as described above except 20% instead of 10% FCS. After approximately 4 weeks of culture, homogeneity was tested by immunofluorescence using an antibody specific for glial fibrillary acidic protein, an astrocyte-specific marker (Sigma, St. Louis, Mo.).
Antibodies and cytokines.
Monoclonal antibodies 37G12 and Mol for quantification of HIV p24 were from H. Katinger et al. at the Institute for Applied Microbiology, Vienna, Austria. The C3 sandwich enzyme-linked immunosorbent assay (ELISA) was established using the monoclonal C3d antibody BB5 (W. Prodinger, Innsbruck, Austria) as catching antibody and monoclonal C3c antibody (Dako, Glostrup, Denmark) as detection antibody.
For some experiments the cells were incubated with the cytokines interleukin-1β (IL-1β) (specific activity, 1 × 107 U/mg), tumor necrosis factor alpha (TNF-α) (2 × 107 U/mg), transforming growth factor β (TGF-β) (2.5 × 107 U/mg), gamma interferon (IFN-γ) (3 × 107 U/mg), IL-2 (>107 U/mg), and IL-10 (1 × 107 U/mg). All cytokines were purchased from Peprotech (London, United Kingdom). For other experimental settings, the cells were preincubated for 45 min at 4°C with neutralizing monoclonal antibodies against CCR5 and CXCR4 or the unspecific isotype control immunoglobulin G2a (IgG2a) (Pharmingen, San Diego, Calif.) before addition of the virus. The antibody against CXCR4 is the clone 12G5, which has been reported to block HIV infection, according to the manufacturer's description. Similarly the anti-CCR5 antibody (clone 2D7) blocks ligand and gp120 binding to cells.
Viral propagation and purification.
Different viral strains were used for these studies. HIV-1 IIIB and RF were from the MRC (2, 24). Isolate SF162 was a gift of J. Levy (San Francisco, Calif.). HIV-1 PI21 was a primary isolate derived from a patient from Innsbruck (10). Primary isolates 92RW021 and WSC were a kind gift of M. Purtscher (Vienna, Austria). All viral strains were grown in the cell line M8166 (obtained from the MRC). M8166 cells were infected with the different viral stocks. Virus-containing culture supernatants were harvested after 3 days and frozen at −70°C. For mock treatment of cells, culture supernatant of noninfected M8166 was collected at the same time points. For some experiments, virus was purified by ultracentrifugation at 20,000 × g for 60 min. The pellet was resuspended in phosphate-buffered saline (PBS) and stored at −70°C.
Viral yield was determined by p24 antigen ELISA developed at the Institute of Applied Microbiology. Briefly, microplates were coated overnight with the first monoclonal antibody and washed three times with PBS-0.1% Tween 20 (Serva, Heidelberg, Germany). A series of dilutions of M8166 culture supernatants or purified viral stocks were applied on the plate, together with the second antibody conjugated with biotin, for 1 h. The amount of bound p24 was quantified by adding resorufin-β-d-galactopyranoside substrate solution and measuring the optical density at 550 nm using an ELISA reader. The p24 assay was calibrated using baculovirus-derived recombinant p24 as a standard.
RNA preparation, reverse transcription, and PCR.
At different time points of incubation cells were lysed and RNA was isolated using the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. For quantification, 2 μg of total RNA was reverse transcribed into cDNA using oligo(dT) as a primer and Moloney murine leukemia virus reverse transcriptase (Life Technologies) in a final volume of 25 μl. PCR was performed in a total volume of 50 μl containing 2 μl of cDNA, 15 pmol of each primer, 200 uM each deoxynucleotide, 2.5 U of Taq polymerase (Promega, Mannheim, Germany), and buffer supplied with the polymerase. PCR conditions were optimized for quantification of the different complement factors. The following primers and conditions were chosen: 5′-CCCAGAAAATCGCCTTCTCTGC-3′ and 5′-CGGAAAAGATGCTGTTGGCACC-3′ for C1q; 5′-CTCACCATCCTTCTTGGACCC-3′ and 5′-GAGATCAATGTGTGAGCGTTGCC-3′ for C2; 5′-AGAACCCCATGAGGTTCTCGT-3′ and 5′-GTAGTTCCACCCTCACCTTGA-3′ for C3; 5′-CGGGTCTTTGCACTGGATCA-3′ and 5′-CTTCACCTCGAAGTTGGGAA-3′ for C4; 5′-TGAAGCCAGCCAAAAGAGAA-3′ and 5′-TCCAGCACCGTCACTCCAGA-3′ for C5; 5′-CGCCTCTGGTAGCCTTTCAA-3′ and 5′-AGCCGACATTTTCCAGATTG-3′ for C6; 5′-TAGCATTTGTGTGGAACTGAACGGC-3′ and 5′-CCAAGGCCCAGAGAAGGTAACACC-3′ for C7; 5′-ACCTGGCTCCTTGTGGCTGT-3′ and 5′-GGTTTCACGGAGGACAGGCA-3′ for C8γ; 5′-GTGACTGACTTTGTCAACTGGGC-3′ and 5′-GCATGTTGCTATTTACTTGGC-3′ for C9; 5′-GTGAAGGTCGGAGTCAACG-3′ and 5′-GGTGAAGACGCCAGTGGACTC-3′ for GAPDH (glyceraldehyde-3-phosphate dehydrogenase); and 5′-ACCTCAGGTACCTTTAAGACCAATG-3′ and 5′-TGTGTAGTTCTGCCAATCAGGGAA-3′ for HIV-1 nef. Hybridization probes were generated with the same primers. PCR conditions were as follows: 1 min at 94°C, 1 min at 59°C, and 1 min at 72°C for C1q (30 cycles); 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C for C2 and C9; 2 min at 94°C (1 cycle) followed by 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C (35 cycles) for C3; 2 min at 94°C (1 cycle) followed by 1 min at 94°C, 1 min at 61°C, and 1 min at 72°C (35 cycles) for C4 and C7; 5 min at 94°C (1 cycle) followed by 1 min at 94°C, 1 min at 60°C, and 2 min at 72°C (5 cycles) followed by 30s at 94°C, 45s at 60°C, and 1 min at 72°C (25 cycles) followed by 15 min at 72°C and 1 min at 30°C for C5, C6, and C8.
Half of the PCR mixture was applied on an agarose gel and transferred to a nylon membrane (Bio-Rad, Munich, Germany) by alkaline capillary blotting. Filters were cross-linked by UV and hybridized with 32P-labeled probes under stringent conditions according to the method of Church and Gilbert (3). The radioactive signal obtained after hybridization was quantified using a PhosphorImager (Fuji) and expressed as photosensitive luminescence.
Experimental settings and measurement of C3 by ELISA.
Astrocytes were seeded in 96-well plates at a concentration of 1.5 × 104 cells/ml and incubated with HIV either directly as virus-containing M8166 culture supernatants or purified by ultracentrifugation. Control cells were incubated with culture supernatant from uninfected M8166 or PBS. Culture supernatants were harvested at indicated time points. To quantify C3 in cell culture supernatants, ELISA microplates were coated overnight at 4°C with the monoclonal C3d antibody in 0.1 M NaHCO3 (pH 9.6). Unspecific binding was inhibited by saturation with 1% bovine serum albumin (BSA) (Sigma) in PBS. Cell culture supernatants (50 μl) were added to the coated wells and incubated for 1 h at room temperature. After the washing procedure, bound C3 was detected with monoclonal C3c antibody and a second peroxidase-labeled anti-mouse IgG antibody (Dako).
Western blot analysis.
Cell culture supernatants were harvested at different time points and subjected to 9.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (9.5% SDS-PAGE) under reducing conditions and then electroblotted onto nitrocellulose. Before probing, blots were blocked in PBS supplemented with 3% BSA for at least 1 h. For staining of the Western blot, a polyclonal anti-C3 antiserum (Sigma) was used with normal human serum as a positive control. Subsequent detection of the bands was performed with a second alkaline phosphatase-coupled antibody (Sigma).
Fluorescence-activated cell sorter (FACS) analysis of membrane-bound regulator protein expression.
To analyze the surface expression of various molecules, cells were detached by trypsin-EDTA, washed twice with PBS–1% BSA– 0.1% sodium azide and incubated with either unspecific IgG as the isotype control or with anti-CD55, anti-CD46, anti-CD59, or anti-CD35 antibodies (Pharmingen) for 30 min at 4°C. After subsequent washing in PBS-BSA, the cell-bound antibodies were stained with a second fluorescein isothiocyanate-labeled antibody (DAKO, Vienna, Austria). The analysis was performed using a FACScan flow cytometer (Becton Dickinson, San Diego, Calif.). The specific mean fluorescence intensity (MFI) of the samples was determined by subtracting the MFI of the isotype control.
Activity testing of secreted C3.
U373 astrocytes were either mock treated or incubated with 10 ng of HIV IIIB/ml, and culture supernatants were collected after 4 days. Purified viral particles were preincubated with an anti-gp120 antibody (MRC; ARP389) for 30 min at 37°C. CSF derived from lumbal puncture of healthy persons was added with or without the addition of U373 culture supernatants. Complement activation was measured as the generation of C3a. Quantification of C3a was performed by an enzyme immunoassay (Quidel, San Diego, Calif.) according to the manufacturer's instructions. The antibody used in this kit to catch C3a from culture supernatants is specific for C3a and does not recognize other forms of C3. Bound C3a was detected with a peroxidase-conjugated rabbit anti-C3a antiserum.
Statistical analysis.
Statistical analysis (Student's t test) was performed using the Origin 4.1 software (serial no. 6014906).
RESULTS
HIV-1 selectively increases mRNA levels of C2 and C3 in the astrocytic cell line U373.
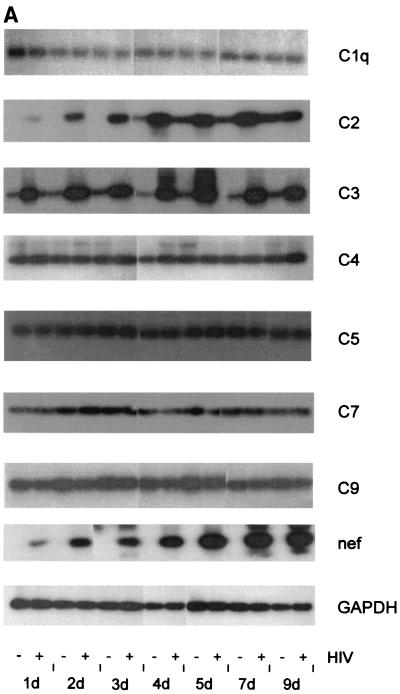
U373 astrocytes were incubated with HIV-1 strain IIIB, and the production of several complement factors of classical, alternative, and terminal pathways was measured on the mRNA level at different time points. HIV-1 specifically upregulated the synthesis of the complement factors C2 and C3, leaving the expressing of the other factors unaltered (Fig. 1). An enhancement of C2 mRNA expression by HIV-1 was seen already after 1 day of incubation; the maximal difference was a 6-to-10-times higher level of infected astrocytes than of uninfected control cells. For C3 the increase in mRNA synthesis was even higher, with HIV-infected cells synthesizing more than 14 times more C3 mRNA than mock-treated control cells. Again, the upregulation was visible already at day 1 after the addition of the virus. The mRNA levels of C1q, C4, C5, C7, and C9 did not change over the incubation period. The expression signals of C6 and C8 were very faint and did not show any significant difference within 9 days of incubation (data not shown). No difference was found between the addition of cell culture supernatants of infected M8166 cells and the addition of purified viral particles. The infection of the astrocytes during the experiment was ensured by measuring the expression of the viral HIV-1 nef gene (Fig. 1A and B).
FIG. 1.
mRNA expression of different complement factors by HIV-infected and mock-treated U373 astrocytes. Cells were incubated with 50 ng of HIV IIIB/ml (+) or were mock treated with medium (−). RNA was isolated at different time points, and expression levels were quantified by reverse transcription-PCR. (A) PCR amplification products were resolved on a 1.5% agarose gel and detected by hybridization with specific 32P-labeled probes. (B) Densitometrical quantification of the PCR signals with a phosphorimager after hybridization with a 32P-labeled probe. The signals are calculated as photosensitive luminescence units. OD, optical density.
Since a significant enhancement of C2 and C3 mRNA level was seen already 24 h after the addition of the virus, we evaluated the minimal incubation time for differences to appear between infected and uninfected cells. An induction of C2 and C3 expression on the mRNA level was visible for both complement components as early as 5 h after the addition of HIV (data not shown), indicating that this very prompt increase is a rather direct response of the cells.
Complement factor C3 protein is synthesized and secreted after incubation with HIV.
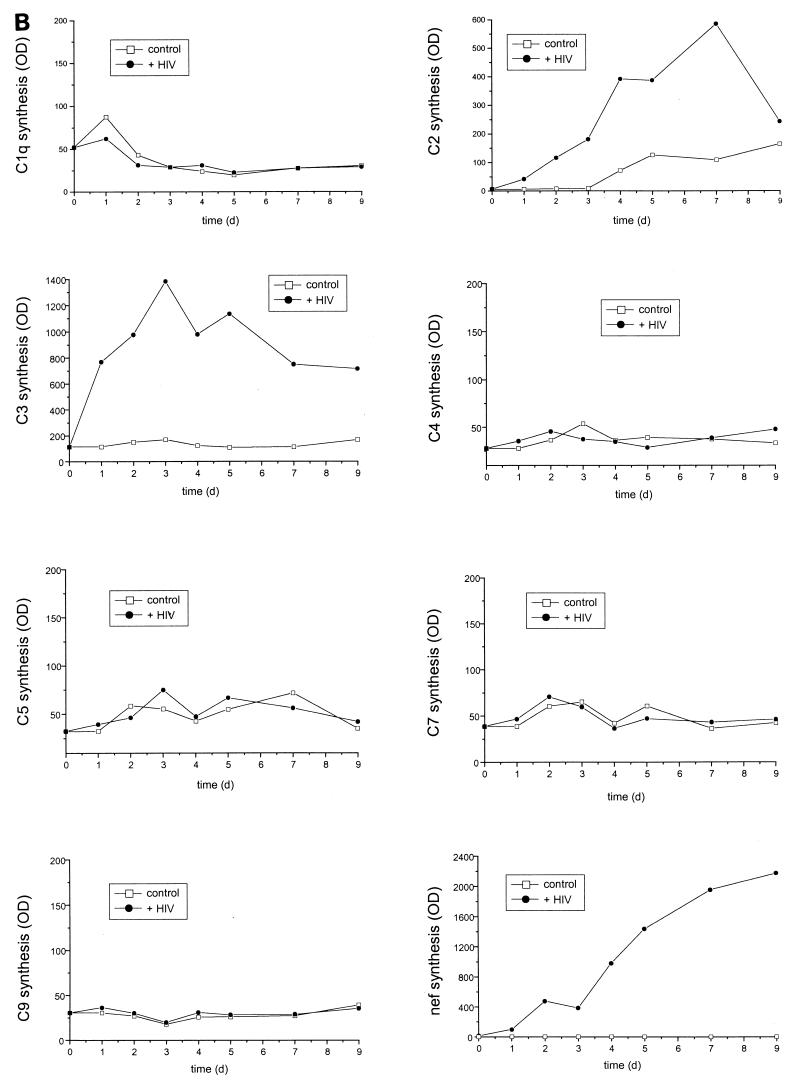
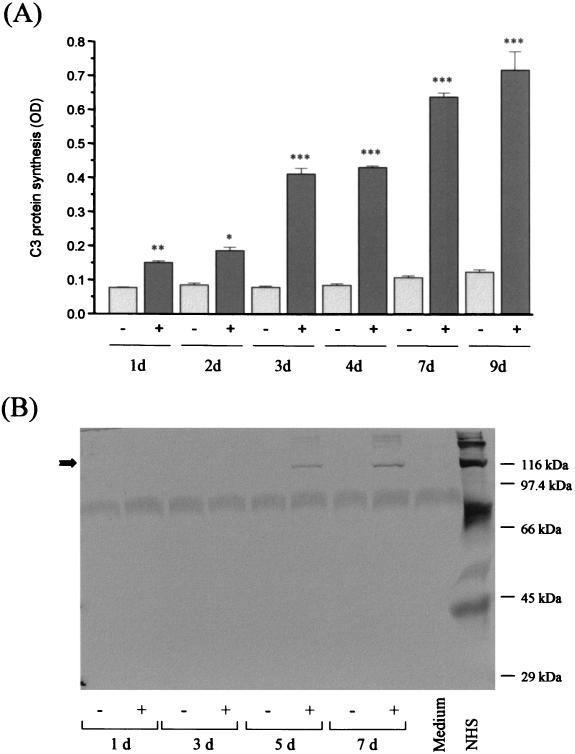
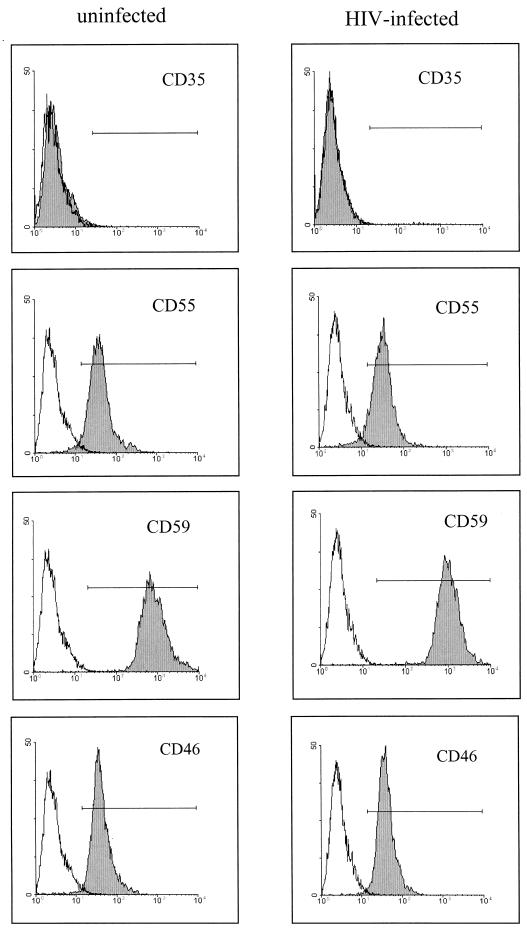
Since C3 harbors many different biological activities and since C3 fragments are bound by various cell surface receptors, our further experiments concentrated on the induction of C3 production by HIV-infected astrocytes. A C3-specific ELISA with cell culture supernatants of infected and uninfected U373 cells revealed that incubation with HIV alters C3 expression not only at the mRNA level but also at the protein level. In a time-dependent manner C3 protein expression was up to seven times higher in U373 cells incubated with HIV than in mock-treated control cells (Fig. 2A). A similar increase was visible in a Western blot using a C3c-specific polyclonal antiserum (Fig. 2B). A faint band corresponding to the size of the α-chain of C3 appeared at day 3 after the addition of the virus, and a marked signal was present in the supernatants of infected cells at days 5 and 7. We also examined the synthesis of the membrane-bound inhibitory regulators of the complement cascade by FACS analysis. U373 cells were negative for CD35 (CR1) expression whereas significant amounts of CD55, CD59, and CD46 were detected on their surface (Fig. 3). No alteration of expression was observed after the incubation of the cells with HIV-1 for 7 days; the MFI was comparable for infected and uninfected astrocytes.
FIG. 2.
C3 protein synthesis after incubation of U373 astrocytes with HIV IIIB. U373 astrocytes were either mock treated (−) or incubated with 50 ng of HIV IIIB/ml (+). Cell culture supernatants were harvested at different time points. (A) The amount of C3 protein was quantified by a specific ELISA. Results are the mean ± the standard deviation of triplicate determinations; the statistical significance of HIV-induced C3 production versus that of mock-treated cells was evaluated by Student's t test. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005; OD, optical density. (B) Supernatants were applied on a 9.5% acrylamide gel under reducing conditions, and Western blots were performed using a polyclonal C3-specific antibody. The arrow indicates the size of the C3 α-chain (113 kDa). Normal human serum (NHS) was applied on the gel as a positive control.
FIG. 3.
Expression of membrane-bound complement regulator proteins on U373 astrocytes. Cells were either mock treated or incubated with 50 ng of HIV IIIB/ml for 7 days. Membrane protein expression of CD35, CD55, CD59, and CD46 was monitored by FACS analysis using specific antibodies (shaded area) as described in Materials and Methods. As control, unspecific binding of immunoglobulin of the same isotype was measured (white area).
Dose dependence of the HIV-induced C3 synthesis in U373 astrocytes.
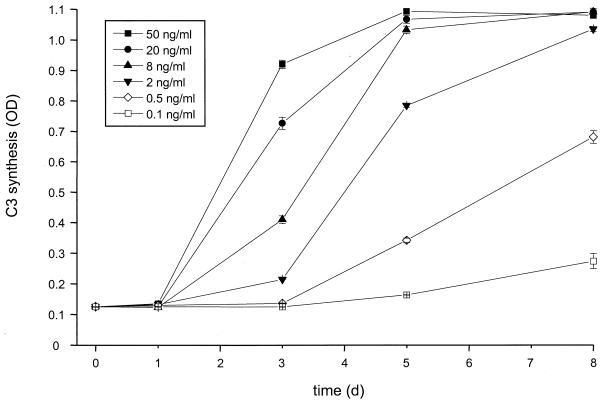
To evaluate the minimal virus amounts necessary to stimulate C3 production, we incubated U373 astrocytes with virus at various concentrations. The C3 level in culture supernatant was quantified by the specific ELISA. Even virus concentrations as low as 100 pg of p24/ml were sufficient to induce a small but significant upregulation of C3 synthesis after 8 days of incubation (P < 0.05, virus- versus mock-treated cells) (Fig. 4). The increase of C3 synthesis started earlier with higher amounts of viral particles, in some cases after only 2 days (data not shown). With the highest virus concentration tested of 50 ng of p24/ml the cells reached the plateau phase of maximum C3 production and secretion after only 3 days of incubation.
FIG. 4.
Dose-dependent induction of C3 synthesis by HIV IIIB. U373 astrocytes were incubated with increasing amounts of virus as quantified by p24 ELISA. Culture supernatants were harvested at the indicated time points, and the C3 level was determined by ELISA. Results are the mean ± the standard deviation of triplicate determinations. OD, optical density.
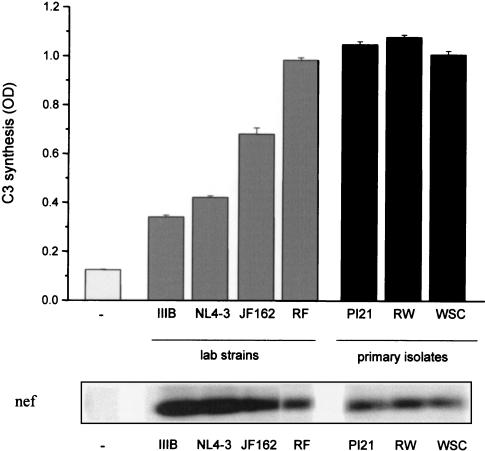
Different viral strains and isolates can induce C3 production in astrocytes.
To evaluate whether the capacity to induce C3 synthesis is inherent only for HIV IIIB, we incubated U373 astrocytes in parallel with different viral strains and primary isolates. Both the established laboratory strains IIIB, NL4-3, RF, and SF162 and the primary isolates PI21, 92RW021, and WSC increased C3 production and secretion by U373 astrocytes significantly (Fig. 5). Nevertheless, the level of induction varied among the isolates. Whereas low amounts (0.5 ng of p24/ml) of established viral strains IIIB and NL4-3 induced three- to fourfold increases in C3 amounts in the culture supernatants after an incubation period of 5 days, the effect was more pronounced for the other isolates. Astrocytes incubated with strain RF and with the primary isolates PI21, 92RW021, and WSC had already reached the maximum induction of C3 synthesis 3 days after virus addition. Viral infection was monitored by expression of viral nef mRNA (Fig. 5).
FIG. 5.
Induction of C3 synthesis by different virus strains. U373 astrocytes were incubated with 0.5 ng of p24/ml of different HIV-1 laboratory strains (HIV IIIB, NL4-3, RF, and SF162) or primary isolates (PI21, 92RW021 [RW], and WSC). Cell culture supernatants were harvested after 5 days, and the C3 levels were quantified by ELISA. Results are the mean ± the standard deviation of triplicate determinations. As infection control, expression of viral nef was measured by RNA isolation, quantitative PCR amplification with specific primers, and hybridization with a specific 32P-labeled probe as described in Fig. 1. OD, optical density.
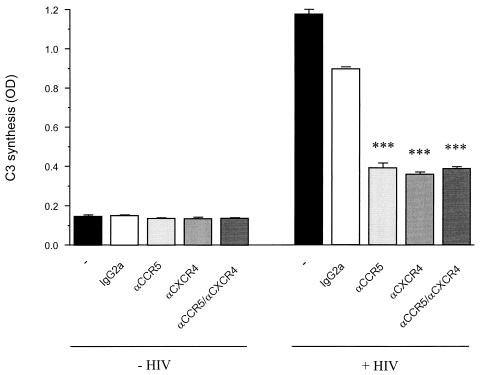
Blocking of HIV-induced C3 synthesis by chemokine receptor antibodies.
Further experiments aimed to confirm the role of HIV binding to the cells causing C3 induction. U373 astrocytes were preincubated with antibodies against the HIV coreceptors CCR5, CXCR4, or both prior to the addition of the virus. Both antibodies had the ability to block ligand binding and gp120 binding to the receptors. As a control the cells were pre-incubated with an unspecific antibody of the same isotype IgG2a. C3 levels in the supernatants were quantified after 6 days. Whereas all these antibodies alone did not affect C3 synthesis of the astrocytes, the antibodies against CCR5 and CXCR4 both suppressed the HIV-induced C3 synthesis (Fig. 6). The unspecific IgG2a control antibody had only a slight effect on C3 production. These results imply that the binding of HIV to coreceptors on the astrocytes is a prerequisite for the induction of C3 synthesis.
FIG. 6.
HIV-induced C3 expression after the blocking of chemokine receptors in astrocytes. U373 astrocytes were incubated with either medium or HIV IIIB (20 ng of p24/ml). A part of the cells had been preincubated for 45 min at 4°C with neutralizing antibodies (10 μg/ml) against CCR5, CXCR4, or both, prior to addition of the virus. Control cells were either mock treated or incubated with the same amount of an unspecific IgG2a antibody. Secreted C3 was quantified after 6 days by ELISA. Results are the mean ± the standard deviation of duplicate determinations of a representative experiment. ∗∗∗, P < 0.005 versus the IgG2a control. OD, optical density.
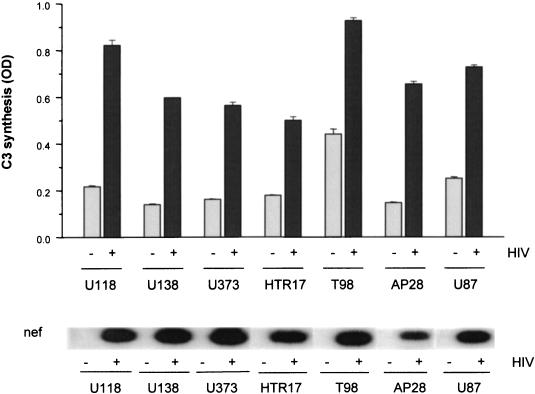
Various astrocytic lines and primary astrocytes are responsive toward HIV.
To examine whether the modulation of C3 production in astrocytes is a general mechanism or restricted to the cell line U373, various astrocytic cell lines and a preparation of adult primary astrocytes (AP28) were incubated with 20 ng of HIV IIIB/ml. Control cells were mock treated with the same amount of medium. C3 production was quantified 3 days after virus addition (Fig. 7). Three of the tested astrocytic cell lines showed a significant spontaneous C3 production (U118, T98, and U87) (Fig. 7) where a plateau phase was reached after 5 to 10 days in culture without external stimulus (data not shown). Nevertheless, HIV increased the C3 protein expression significantly (P < 0.001) in these cell lines with a visible difference between infected and mock-treated cells 3 days (Fig. 7) and 5 days (data not shown) after infection. The other cell lines, U138, U373, and HTR17, did not show spontaneous C3 production above the medium background signal during the cultivation period of 10 days. When HIV was added to the cells, a pronounced upregulation of the C3 levels in the medium was seen for all cell lines. Furthermore, a preparation of adult primary astrocytes (AP28) also reacted with significantly enhanced C3 synthesis upon the addition of virus to the cells. In all cell lines viral infection was assessed by mRNA isolation and nef quantification by PCR with subsequent gel and hybridization (Fig. 7).
FIG. 7.
Induction of C3 synthesis in different astrocytic cell lines or primary astrocytes by HIV. Various astrocytoma cell lines were incubated with HIV IIIB (20 ng of p24/ml); culture supernatants were harvested after 3 days, and the amounts of C3 were measured by ELISA. Results are the mean ± the standard deviation of triplicate determinations. As infection control, expression of viral nef was measured by RNA isolation, quantitative PCR amplification, and hybridization with a specific 32P-labeled probe as described in Fig. 1. OD, optical density.
Modulation of HIV-induced complement production by cytokines.
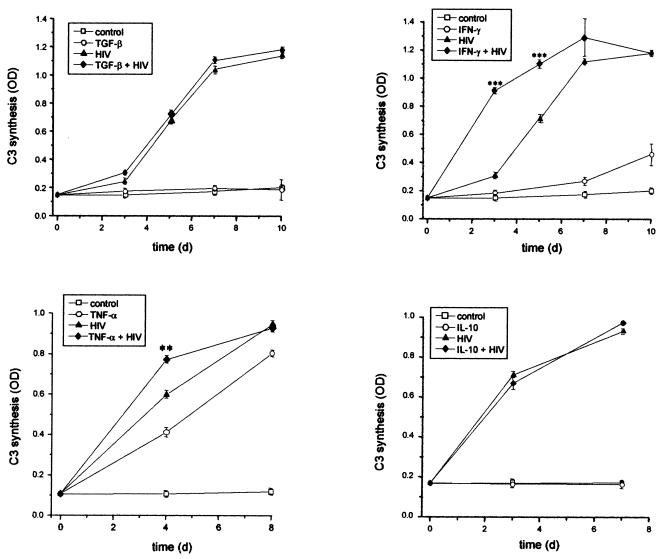
Since it is known that cytokines can modulate C3 expression, we investigated the influence of different cytokines on C3 induction by HIV, including IFN-γ, TNF-α, TGF-β, IL-1β, IL-2, and IL-10. The results for some of these cytokines are given in Fig. 8. TGF-β, IL-10 (Fig. 8), or IL-2 (data not shown) alone did not induce the synthesis of C3 in U373 astrocytes; the C3 levels in the supernatants corresponded to that of the mock-treated cells. Furthermore, these cytokines did not modulate the HIV-induced upregulation of C3 production. In contrast, both TNF-α (Fig. 8) and IL-1β (data not shown) enhanced the C3 synthesis on their own. Coincubation of TNF-α or IL-1β with HIV enhanced C3 production above the level of cells treated with cytokines or HIV alone. This effect was an additive one. The addition of IFN-γ to the cells (Fig. 8) upregulated C3 production in U373 astrocytes late in the experiment. Simultaneous incubation with HIV and IFN-γ increased the C3 amounts in the culture supernatants in a more than additive manner, indicating that HIV and IFN-γ have a synergistic effect on C3 production in astrocytes.
FIG. 8.
Effect of various cytokines on HIV-induced C3 production. U373 astrocytes were either mock treated or incubated with IL-10 (10 ng/ml), TGF-β (10 ng/ml), TNF-α (10 ng/ml), IFN-γ (250 U/ml), and/or HIV IIIB (5 ng of p24/ml). C3 levels in the supernatants were quantified by ELISA. The results are the mean ± the standard deviation of triplicate determinations; the statistical significance of C3 production in cells induced by HIV and cytokines versus cells treated with HIV only was evaluated by Student's t test. ∗∗, P < 0.01; ∗∗∗, P < 0.005. OD, optical density.
Activity test of HIV-induced C3.
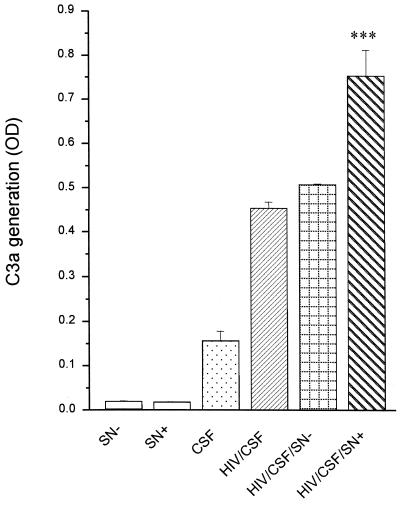
We evaluated whether the virus-induced secreted C3 is active and able to participate in the complement cascade of the CSF. HIV was used as a stimulus for the complement activation to clarify the role of secreted C3 in the immune defense against the virus. The generation of the C3 cleavage product C3a in CSF with and without the addition of C3-containing cell culture supernatants was used as a parameter for complement activation and was quantified by a specific ELISA.
Purified HIV was preincubated with a gp120-specific antibody and was added to CSF derived from lumbal puncture of healthy persons to activate the complement cascade. The addition of astrocyte supernatant of uninfected cells did not alter C3a generation in the CSF in response to HIV (Fig. 9). In contrast, C3-containing supernatants of infected astrocytes enhanced the formation of C3a significantly. This enhancement is not due to viral particles in the supernatant of infected astrocytes, since further addition of virus to the reactions with mock-treated supernatant did not upregulate C3a generation. These results indicate that the C3 which is induced in astrocytes by incubation with HIV is active and can participate in the course of the complement cascade to defend the host against the viral infection.
FIG. 9.
Activity of HIV-induced secreted C3 of astrocytes. CSF gained from lumbal puncture of healthy persons was incubated either alone or with 50 ng of HIV/ml pretreated with an anti-gp120 antibody or supplemented with supernatants from either infected (SN+) or uninfected (SN−) U373 astrocytes. Complement activation was measured as the generation of C3a after 45 min of incubation. Results are the mean ± the standard deviation of duplicate determinations. ∗∗∗, P < 0.01 of C3a generation in CSF supplemented with SN+ versus CSF supplemented with SN−. OD, optical density.
DISCUSSION
The complement system is an important part of the brain-resident immune system, which is separated from the blood immune system by the blood-brain barrier. All components of the complement cascade can be synthesized by brain cells (11, 21), but the amounts in CSF are normally much lower than in serum. The significance of complement for the brain defense against pathogens is possibly further enhanced by compensating the astrocyte-mediated deactivation of invading monocytes/macrophages and T cells (14, 36).
Our experiments show that incubation of astrocytes with HIV-1 markedly upregulates the expression of complement factors C2 and C3. This increase is, at least for C3, seen on both the mRNA and the protein levels. Several viral laboratory strains as well as primary isolates were able to induce this reaction, thus underlining the general importance of this finding. The C3 protein which is released by infected astrocytes was shown to be biologically active and to be capable of participating in the complement cascade. The enhanced generation of complement activation products was visible when CSF was supplemented with the supernatant of infected astrocytes.
These results imply that complement might play a pivotal role in the HIV infection of the brain. Characteristics of HIV-affected brains are neuronal loss, reactive astrocytosis, and myelin pallor (9). The role of complement in the development of these symptoms may be a dual one including both protective and detrimental aspects. On the positive side, enhanced complement synthesis and activation may represent a defense mechanism of the brain, thus limiting viral spread within this organ and decreasing the viral burden. Complement activation products such as the anaphylatoxins C3a and C5a have a chemotactic activity toward macrophages and microglial cells (9a, 21a) and thus may compensate the downmodulation of phagocytic cells by astrocytes. Furthermore, different complement activation products are known to interact with brain cells to inhibit apoptosis or to induce production of neuroprotective factors (15, 23, 30). On the other hand, chronic complement synthesis and activation may represent a mechanism for the induction of AIDS-associated neurodegeneration and dementia complex. There is considerable evidence that enhanced complement synthesis and activation occur in various inflammatory and degenerative diseases of the brain. A role in various pathological conditions including multiple sclerosis, Alzheimer's disease, stroke, and cerebral trauma has been discussed for the complement system (8, 16, 20, 25, 38). The mRNA levels of various complement factors including C3 were markedly upregulated in affected areas of Alzheimer brains (38). In addition, there are indications that this process of complement-induced neurodegeneration in the course of an HIV infection may also occur in vivo since increased C3 levels are described in patients with HIV infection and signs of CNS dysfunction (18).
According to our experiments HIV-infected astrocytes may represent a dominant source of this increase in complement factor C3 levels. Different astrocytic cell lines as well as primary astrocytes responded to HIV infection with enhancement of C3 synthesis. Astrocytes, the major glial cell type in the CNS, play a pivotal role in CNS immunological processes including antigen presentation and cytokine production, suppression of T-cell proliferation, and monocyte deactivation (1a). On the other hand astrocytes are the main source of complement production in the brain (21), thus enabling the innate immunity to represent a key system in the immune defense of this immunoprivileged organ. Our results add the enhancement of complement synthesis after viral infection to this spectrum of immunological functions.
The exact mechanism of HIV-induced upregulation of complement synthesis is not known. Blocking of the two predominant HIV coreceptors, CCR5 and CXCR4, significantly suppressed the upregulation of C3 expression, indicating that viral infection or at least binding of the virus to the cell is a prerequisite. The fact that both antibodies blocked C3 expression is rather surprising since IIIB is described as a primarily T-cell tropic isolate. However, IIIB is rather heterogeneous and has also been shown to successfully infect monocytic cells (5, 37; our unpublished results). For that reason we hypothesize that our IIIB isolate is adapted for both coreceptors. Since the neutralizing antibodies did not suppress the C3 induction completely one might speculate that other chemokine receptors could also be able to mediate HIV binding to the astrocytes and, thus, modulate C3 synthesis.
Complement gene expression has been shown to be differentially regulated by cytokines like IL-1β, TNF-α, and IFN-γ (1, 28). IFN-γ is described as having little effect or even a suppressive effect on C3 synthesis in most cell types. Astrocytes, however, respond to IFN-γ with an increase of C3 expression (1, 13). We were able to show that HIV and IFN-γ influence C3 production in a synergistic manner. In contrast, IL-1β and TNF-α had only an additive effect when coincubated with HIV.
There are several possibilities to explain the stimulatory effect of HIV on the complement factor synthesis of astrocytes. Modulation of C2 and C3 production could be due to the effect of a viral protein on the activity of the complement promoter. The viral proteins Nef, Rev, and Tat are good candidates for this activation since they are known to be highly expressed in astrocytes and are able to modulate protein synthesis of the host cell. But also, structural proteins such as gp120 and gp41, although only moderately expressed by infected astrocytes, are known to harbor various biological functions including the modulation of protein expression (4, 32).
As a second possibility, the modulation of C2 and C3 production may represent an indirect consequence of HIV-induced transcription factor activation. This hypothesis concentrates on transcription factor NFκB, the central regulatory factor for viral transcription. However, IL-10, which has been described as inhibiting NF-κB activation (7), had no effect on C3 induction in astrocytes by HIV.
As a third possibility, this enhancement of complement factor expression may represent an early defense mechanism of the astrocytes in response to viral infection, regardless of the identity of the virus. In this case the induction of complement synthesis may occur via a general unspecific mechanism similar to the induction of heat stress proteins by temperature increase or similar to IFN-γ induction by viral infection in monocytes. This hypothesis of a general defense mechanism is supported by the finding that Newcastle disease virus, a neurotropic paramyxovirus, also induces C3 expression in rat astrocytes (26).
In conclusion, our experiments show that infection of astrocytes with HIV-1 strongly affects complement synthesis of these cells in a dose- and time-dependent manner. Since the secreted C3 is biologically active and can participate in the complement cascade, further experiments will focus on the role of induced complement synthesis in the antiviral defense mechanisms of the brain and in the development of neurodegenerative AIDS dementia complex.
ACKNOWLEDGMENTS
We gratefully acknowledge the generous gift of the monoclonal anti-C3 antibody BB5 from Wolfgang Prodinger (Innsbruck, Austria). We are grateful to the MRC for providing various reagents. We thank G. Stockhammer for kindly providing the cell line HTR17 and H. Katinger of the Institute of Applied Microbiology for the p24 antibodies.
This study was supported by the FWF (P12857-GEN), the Ludwig Boltzmann Society, the BMAGS, and the State of Tyrol.
REFERENCES
- 1.Barnum S R, Jones J L. Differential regulation of C3 gene expression in human astroglioma cells by interferon-γ and interleukin-1β. Neurosci Lett. 1995;197:121–124. doi: 10.1016/0304-3940(95)11923-k. [DOI] [PubMed] [Google Scholar]
- 1a.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 2.Cheng-Mayer C, Levy J A. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Ann Neurol. 1988;23:58–61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 3.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corasaniti M T, Bagetta G, Rotiroti D, Nistoco G. The HIV envelope protein gp120 in the nervous system. Biochem Pharmacol. 1998;56:153–156. doi: 10.1016/s0006-2952(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 5.DeLuca C, Kwon H, Pelletier N, Wainberg M A, Hiscott J. NF-kappaB protects HIV-1 infected myeloid cells from apoptosis. Virology. 1998;244:27–38. doi: 10.1006/viro.1998.9085. [DOI] [PubMed] [Google Scholar]
- 6.Ebenbichler C F, Thielens N M, Vornhagen R, Marschang P, Arlaud G J, Dierich M P. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J Exp Med. 1991;174:1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrlich L C, Hu S, Peterson P K, Chao C C. IL-10 down-regulates human microglial IL-8 by inhibition of NF-kappaB activation. Neuroreport. 1998;9:1723–1726. doi: 10.1097/00001756-199806010-00010. [DOI] [PubMed] [Google Scholar]
- 8.Eikelbloom P, Hack C E, Rozemuller J M, Stam F C. Complement activation in amyloid plaques in Alzheimer's dementia. Arch Cell Pathol. 1989;56:259–262. doi: 10.1007/BF02890024. [DOI] [PubMed] [Google Scholar]
- 9.Epstein E G, Gendelman H E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann Neurol. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- 9a.Fernandez H N, Henson P M, Otani A, Hugli T E. Chemotactic response to human C3a and C5a anaphylatoxins. I. evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978;120:109–115. [PubMed] [Google Scholar]
- 10.Frank I, Kacani L, Stoiber H, Stössel H, Spruth M, Steindl F, Romani N, Dierich M P. Human immunodeficiency virus type 1 derived from cocultures of immature dendritic cells with autologous T cells carries T-cell-specific molecules on its surface and is highly infectious. J Virol. 1999;73:3449–3454. doi: 10.1128/jvi.73.4.3449-3454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasque P, Dean Y D, McGreal E P, VanBeek J, Morgan B P. Complement components in the innate immune system in health and disease in the CNS. Immunopharmacology. 2000;49:171–186. doi: 10.1016/s0162-3109(00)80302-1. [DOI] [PubMed] [Google Scholar]
- 12.Gray F, Scaravilli F, Everall I, Chretien F, An S, Boche D, Adle-Biassette H, Wingertsmann L, Durigon M, Hurtrel B, Chiodi F, Bell J, Lantos P. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 13.Haga S, Aizawa T, Ishii T, Ikeda K. Complement gene expression in mouse microglial and astrocytes in culture: comparison with mouse peritoneal macrophages. Neurosci Lett. 1996;216:191–194. doi: 10.1016/0304-3940(96)13040-8. [DOI] [PubMed] [Google Scholar]
- 14.Hailer N P, Heppner F L, Haas D, Nitsch R. Astrocytic factors deactivate antigen presenting cells that invade the central nervous system. Brain Pathol. 1998;8:459–474. doi: 10.1111/j.1750-3639.1998.tb00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heese K, Hock C, Otten U. Inflammatory signals induce neurotrophin expression in human microglial cells. J Neurochem. 1998;70:699–707. doi: 10.1046/j.1471-4159.1998.70020699.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Kim L J, Mealey R, Marsh H C, Zhang Y, Tenner A J, Connolly E S, Pinsky D J. Neuronal protection in stroke by an sLEx-glycosylated complement inhibitory protein. Science. 1999;285:595–599. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 17.Johnson R T, Glass J D, McArthur J C, Chesebro B W. Quantitation of human immunodeficiency virus in brain of demented and nondemented patients with acquired immunodeficiency syndrome. Ann Neurol. 1996;39:392–395. doi: 10.1002/ana.410390319. [DOI] [PubMed] [Google Scholar]
- 18.Jongen P J H, Doesburg W H, Ibrahim-Stappers J L M, Lennens W A J G, Hommes O R, Lamers K J B. Cerebrospinal fluid C3 and C4 indexes in immunological disorders of the central nervous system. Acta Neurol Scand. 2000;101:116–121. doi: 10.1034/j.1600-0404.2000.101002116.x. [DOI] [PubMed] [Google Scholar]
- 19.Moller T, Nolte C, Burger R, Verkhratsky A, Kettenmann H. Mechanisms of C5a and C3a complement fragment-induced [Ca2+]I signalling in mouse microglia. J Neurosci. 1997;17:615–624. doi: 10.1523/JNEUROSCI.17-02-00615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan B P. Role of complement in the brain. In: Whaley K, Loos M, Weiler J M, editors. Complement in health and disease. 2nd ed. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 353–375. [Google Scholar]
- 21.Morgan B P, Gasque P. Expression of complement in the brain: role in health and disease. Immunol Today. 1996;17:461–466. doi: 10.1016/0167-5699(96)20028-f. [DOI] [PubMed] [Google Scholar]
- 21a.Nataf S, Stahel P F, Davoust N, Barnum S R. Complement anaphylatoxin receptors on neurons: new tricks for old receptors? Trends Neurosci. 1999;22:397–402. doi: 10.1016/s0166-2236(98)01390-3. [DOI] [PubMed] [Google Scholar]
- 22.Osaka H, McGinty A, Hoepken U E, Lu B, Gerard C, Pasinetti G M. Expression of C5a receptor in mouse brain: role in signal transduction and neurodegeneration. Neuroscience. 1999;88:1073–1082. doi: 10.1016/s0306-4522(98)00372-8. [DOI] [PubMed] [Google Scholar]
- 23.Osaka H, Mukherjee P, Aisen P S, Pasinetti G M. Complement-derived anaphylatoxin C5a protects against glutamate-mediated neurotoxicity. J Cell Biochem. 1999;73:303–311. [PubMed] [Google Scholar]
- 24.Popovic M, Sarngadharan M G, Read E, Gallo R C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 25.Rogers J, Cooper N R, Webster S, Schultz J, McGeer P L, Styren S, Civin W H, Brachova L, Bradt B, Ward P, Lieberburg I. Complement activation by β-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rus H G, Kim L M, Niculescu F I, Shin M L. Induction of C3 expression in astrocytes is regulated by cytokines and Newcastle disease virus. J Immunol. 1992;148:928–933. [PubMed] [Google Scholar]
- 27.Sayah S, Ischenko A M, Zhakhov A, Bonnard A S, Fontaine M. Expression of cytokines by human astrocytomas following stimulation by C3a and C5a anaphylatoxins: specific increase in interleukin-6 mRNA expression. J Neurochem. 1999;72:2426–2436. doi: 10.1046/j.1471-4159.1999.0722426.x. [DOI] [PubMed] [Google Scholar]
- 28.Sheerin N S, Zhou W, Adler S, Sacks S H. TNF-α regulation of C3 gene expression and protein biosynthesis in rat glomerular endothelial cells. Kidney Int. 1996;51:703–710. doi: 10.1038/ki.1997.101. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair E, Gray F, Ciardi A, Scaravilli F. Immunohistochemical changes and PCR detection of HIV provirus DNA in brains of asymptomatic HIV-positive patients. J Neuropathol Exp Neurol. 1994;53:43–50. doi: 10.1097/00005072-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Soane L, Rus H, Niculescu F, Shin M L. Inhibition of oligodendrocyte apoptosis by sublytic C5b-9 is associated with enhanced synthesis of bcl-2 and mediated by inhibition of caspase-3 activation. J Immunol. 1999;163:6132–6138. [PubMed] [Google Scholar]
- 31.Speth C, Würzner R, Stoiber H, Dierich M P. The complement system: pathophysiology and clinical relevance. Wien Klin Wochenschr. 1999;111/10:378–391. [PubMed] [Google Scholar]
- 32.Speth C, Joebstl B, Barcova M, Dierich M P. HIV-1 envelope protein gp41 modulates expression of interleukin-10 and chemokine receptors on monocytes, astrocytes and neurons. AIDS. 2000;14:629–636. doi: 10.1097/00002030-200004140-00001. [DOI] [PubMed] [Google Scholar]
- 33.Stoiber H, Clivio A, Dierich M P. Role of complement in HIV infection. Annu Rev Immunol. 1997;15:649–674. doi: 10.1146/annurev.immunol.15.1.649. [DOI] [PubMed] [Google Scholar]
- 34.Van der Laan L J W, Ruuls S R, Weber K S, Lodder I J, Döpp E A, Dijkstra C D. Macrophage phagocytosis of myelin in vitro determined by flow cytometry: phagocytosis is mediated by CR3 and induces production of tumor necrosis factor-α and nitric oxide. J Neuroimmunol. 1996;70:145–152. doi: 10.1016/s0165-5728(96)00110-5. [DOI] [PubMed] [Google Scholar]
- 35.Veerhuis R, Janssen I, Hack C E, Eikelenboom P. Early complement components in Alzheimer's disease brains. Acta Neuropathol. 1996;91:53–60. doi: 10.1007/s004019570001. [DOI] [PubMed] [Google Scholar]
- 36.Xiao B G, Diab A, Zhu J, van der Meide P, Link H. Astrocytes induce hyporesponses of myelin basic protein-reactive T and B cell function. J Neuroimmunol. 1998;89:113–121. doi: 10.1016/s0165-5728(98)00123-4. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi K, Groopman J E, Byrn R A. The regulation of HIV by retinoic acid correlates with cellular expression of the retinoic acid receptors. AIDS. 1994;8:1675–1682. doi: 10.1097/00002030-199412000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Yasojima K, Schwab C, McGeer E G, McGeer P L. Up-regulated production and activation of the complement system in Alzheimer's disease brain. Am J Pathol. 1999;154:927–936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]