Abstract
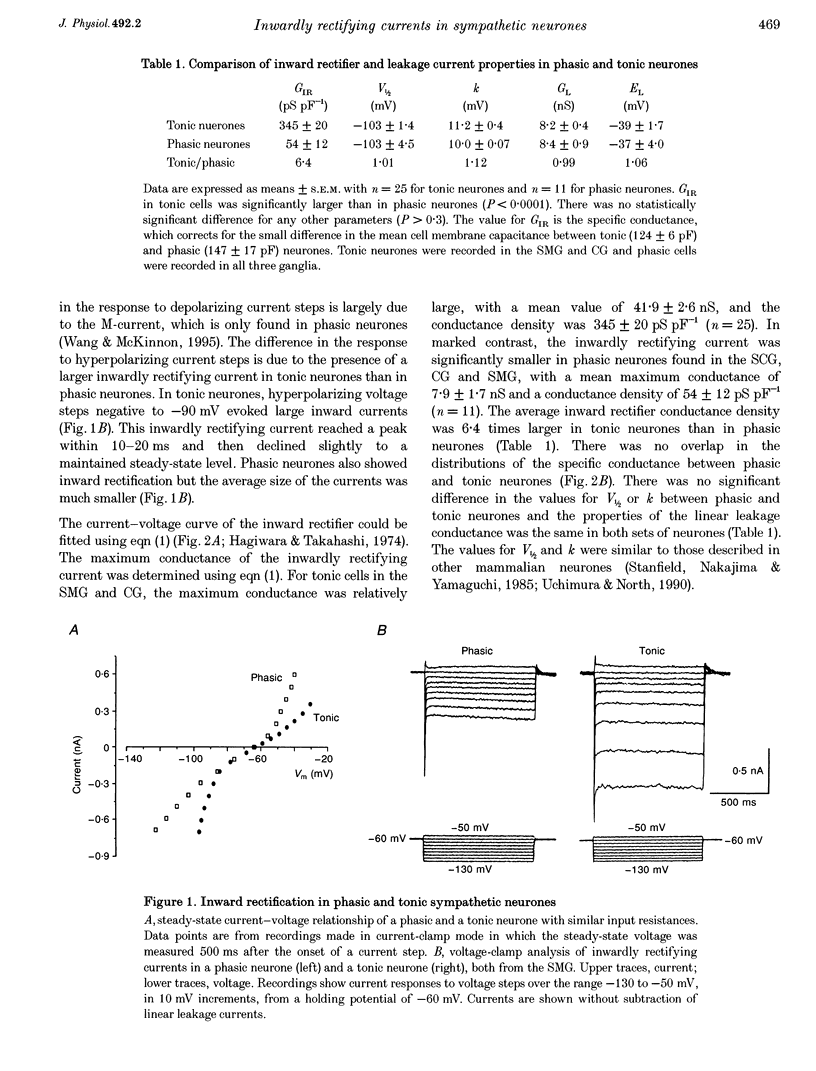
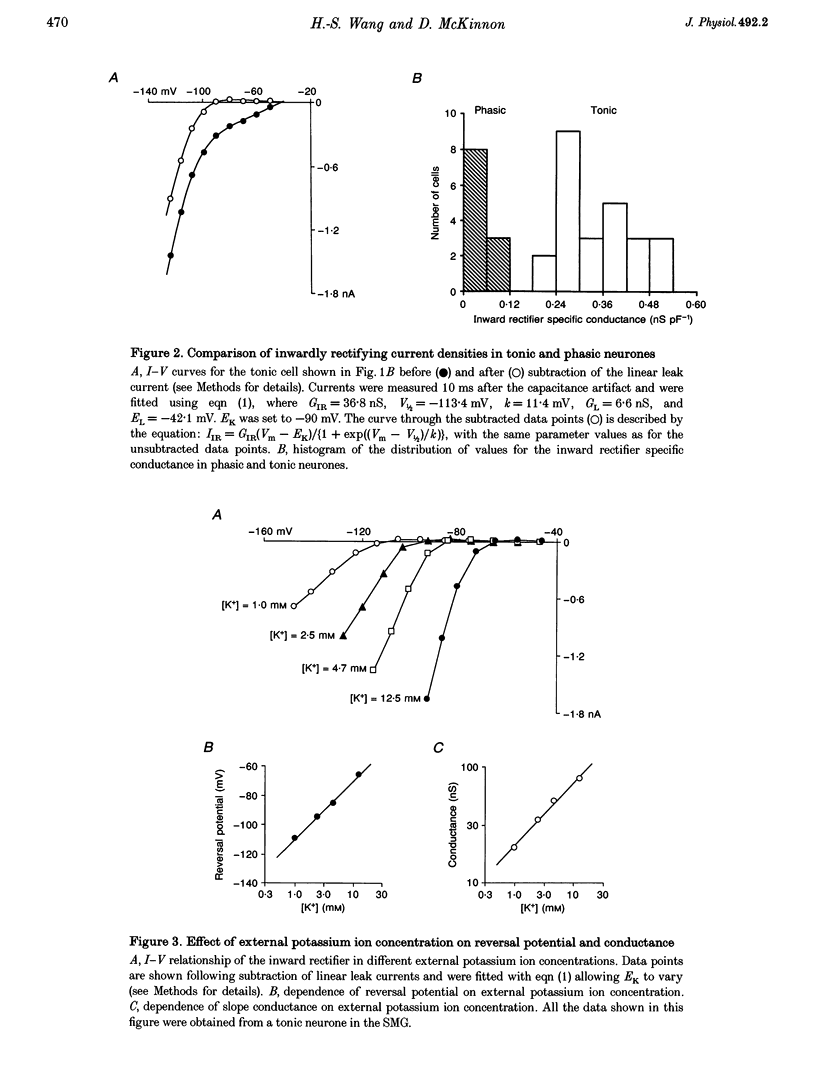
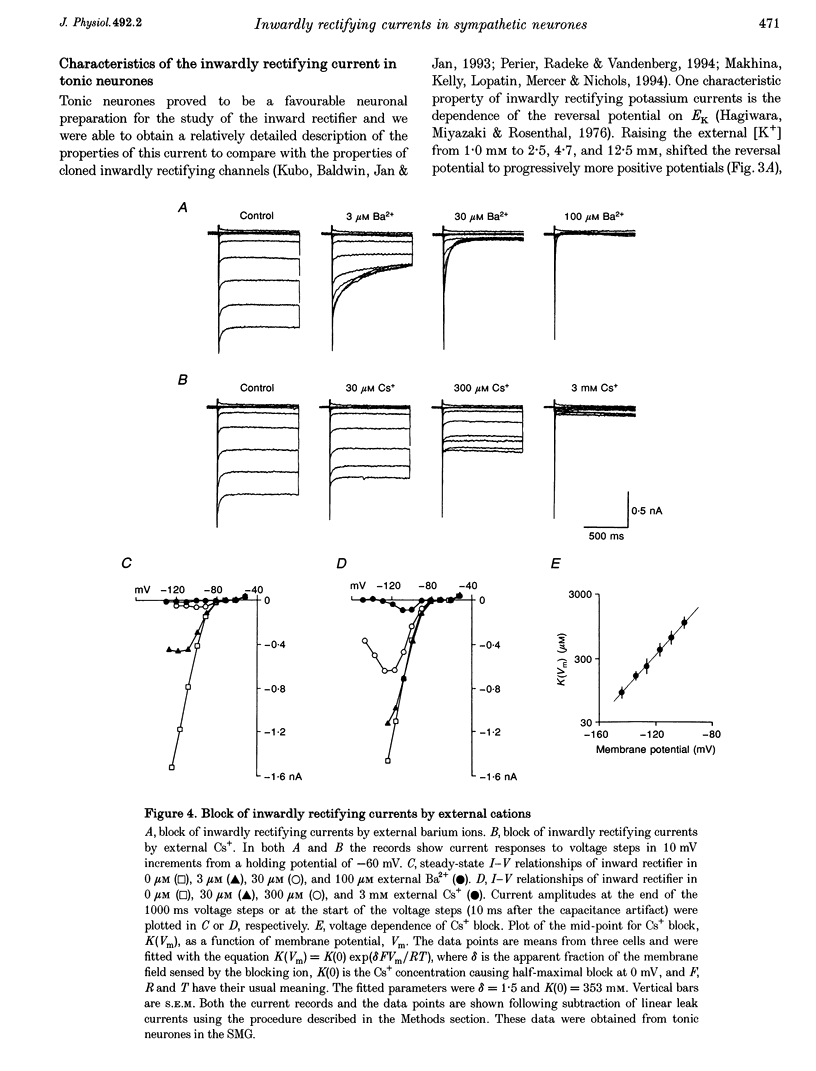
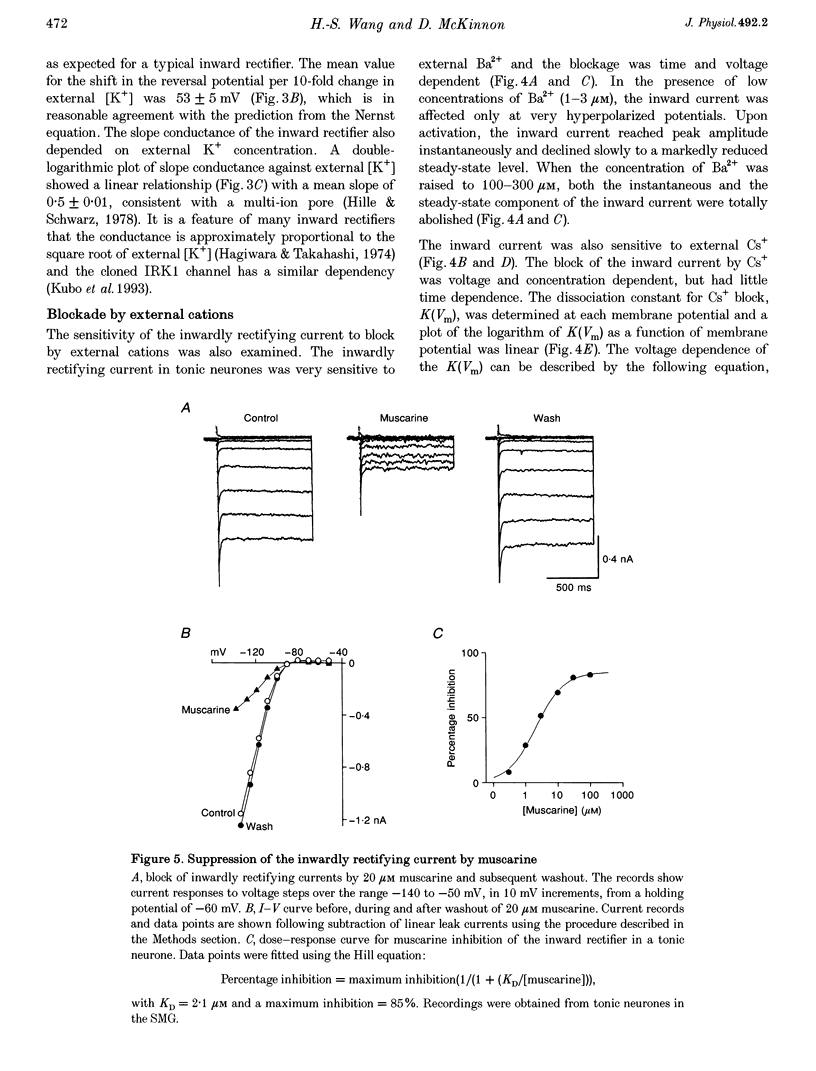
1. Intracellular recordings were made from rat sympathetic neurones in isolated superior cervical ganglia (SCG), coeliac ganglia (CG), and superior mesenteric ganglia (SMG). 2. Following classification of the firing properties of these neurones as either 'phasic' or 'tonic', single-electrode voltage-clamp recordings of the inwardly rectifying current were performed. The inward rectifier conductance was 6.4 times larger in tonic neurones than in phasic neurones. 3. The basic features of the inward rectifier in sympathetic neurones were similar to those of the classic inward rectifier described in several neuronal and non-neuronal preparations. The properties of the native channel were also similar to a subset of recently cloned inwardly rectifying channels. The reversal potential and the slope conductance were both dependent on external potassium ion concentration. The conductance was blocked by low concentrations of external Ba(2+) and Cs(+) ions. 4. A striking feature of the inward rectifier in sympathetic neurones was its modulation by muscarine. Application of 20 microM muscarine produced a mean 78 +/- 1.4% inhibition of the current. From dose-response curves for muscarine a mean dissociation constant of K(D) = 1.95 +/- 0.2 microM was determined. Schild plot analysis using the competitive antagonists pirenzepine and himbacine indicated that the effect of muscarine was mediated by the M(1) class of muscarinic receptors. 5. The inward rectifier was also inhibited by repetitive nerve stimulation which produced a block of the conductance similar to that seen in response to bath-applied muscarine. The onset of inhibition was relatively slow, 20-30 s, suggesting that it is mediated by a soluble second messenger pathway.
Full text
PDF

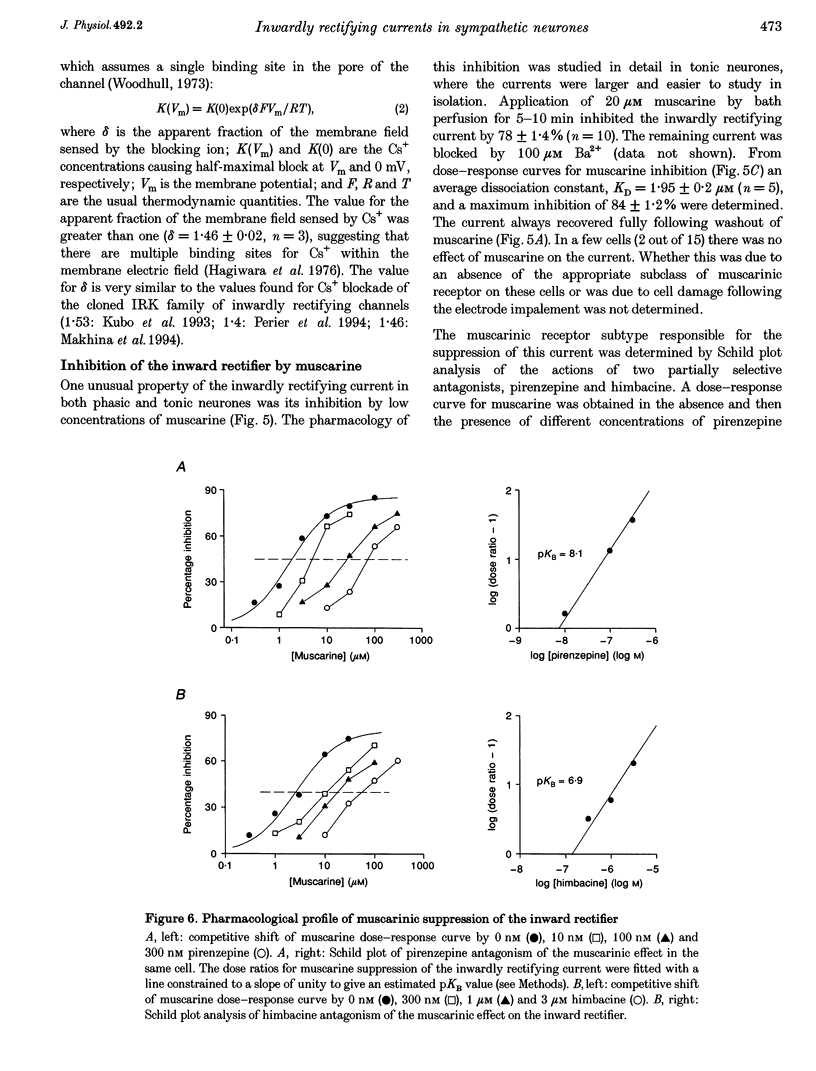
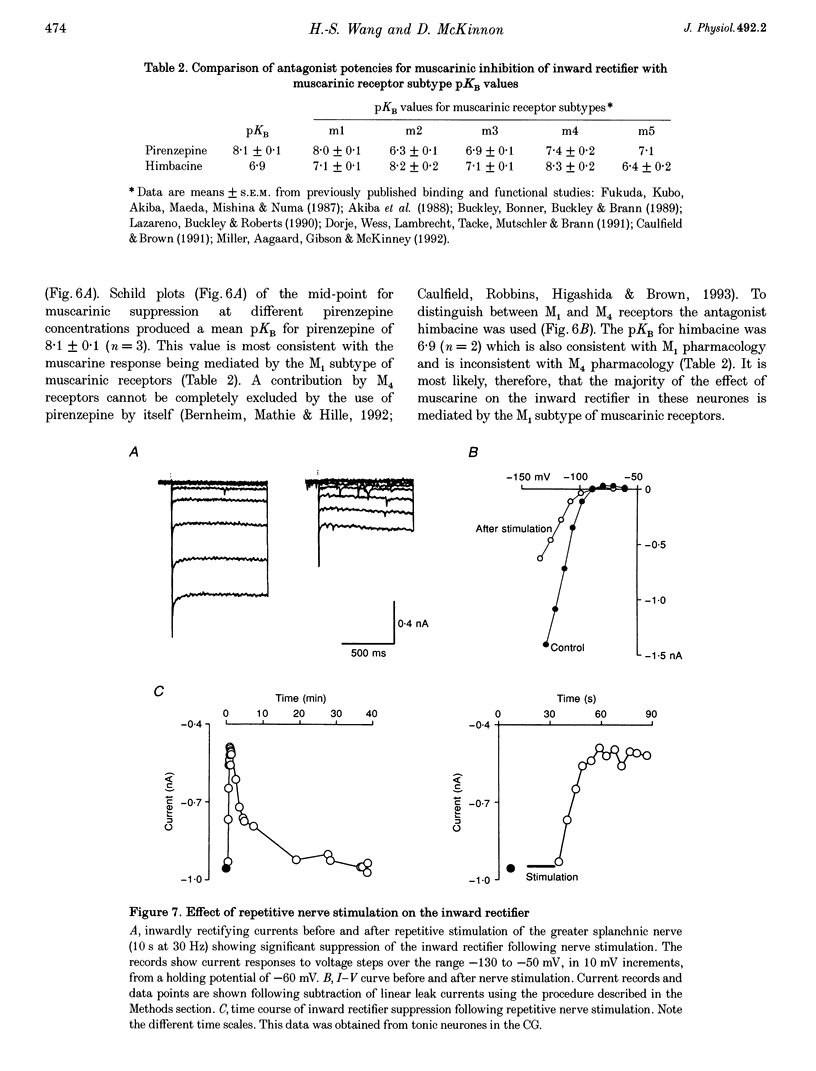
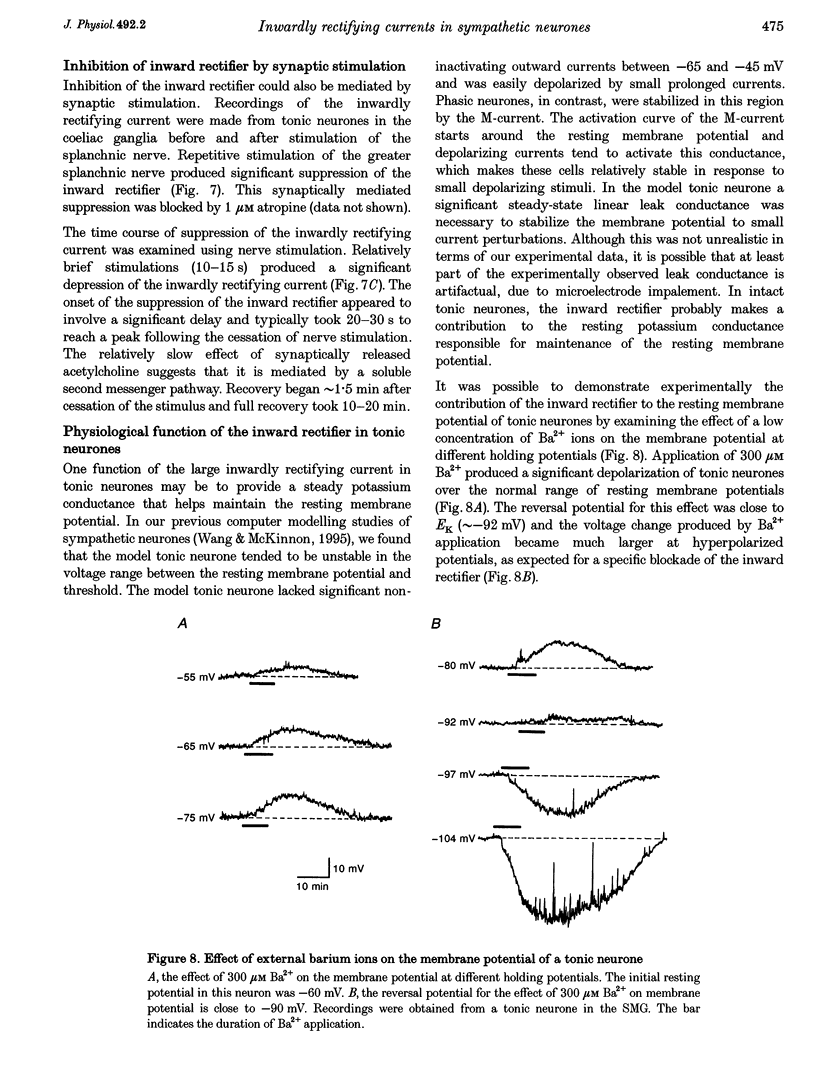



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba I., Kubo T., Maeda A., Bujo H., Nakai J., Mishina M., Numa S. Primary structure of porcine muscarinic acetylcholine receptor III and antagonist binding studies. FEBS Lett. 1988 Aug 1;235(1-2):257–261. doi: 10.1016/0014-5793(88)81274-2. [DOI] [PubMed] [Google Scholar]
- Bernheim L., Mathie A., Hille B. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980 Dec;70(4):593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A. M currents. Ion Channels. 1988;1:55–94. doi: 10.1007/978-1-4615-7302-9_2. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. Membrane currents underlying the cholinergic slow excitatory post-synaptic potential in the rat sympathetic ganglion. J Physiol. 1985 Aug;365:365–387. doi: 10.1113/jphysiol.1985.sp015777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Cassell J. F., Clark A. L., McLachlan E. M. Characteristics of phasic and tonic sympathetic ganglion cells of the guinea-pig. J Physiol. 1986 Mar;372:457–483. doi: 10.1113/jphysiol.1986.sp016020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. P., Brown D. A. Pharmacology of the putative M4 muscarinic receptor mediating Ca-current inhibition in neuroblastoma x glioma hybrid (NG 108-15) cells. Br J Pharmacol. 1991 Sep;104(1):39–44. doi: 10.1111/j.1476-5381.1991.tb12381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. P., Robbins J., Higashida H., Brown D. A. Postsynaptic actions of acetylcholine: the coupling of muscarinic receptor subtypes to neuronal ion channels. Prog Brain Res. 1993;98:293–301. doi: 10.1016/s0079-6123(08)62411-5. [DOI] [PubMed] [Google Scholar]
- Crowcroft P. J., Holman M. E., Szurszewski J. H. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol. 1971 Dec;219(2):443–461. doi: 10.1113/jphysiol.1971.sp009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N. B., Gintant G. A., Cohen I. S. Versatile temperature controlled tissue bath for studies of isolated cells using an inverted microscope. Pflugers Arch. 1985 Mar;403(3):318–323. doi: 10.1007/BF00583607. [DOI] [PubMed] [Google Scholar]
- Dörje F., Wess J., Lambrecht G., Tacke R., Mutschler E., Brann M. R. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 1991 Feb;256(2):727–733. [PubMed] [Google Scholar]
- Finkel A. S., Redman S. Theory and operation of a single microelectrode voltage clamp. J Neurosci Methods. 1984 Jun;11(2):101–127. doi: 10.1016/0165-0270(84)90029-3. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kubo T., Akiba I., Maeda A., Mishina M., Numa S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987 Jun 18;327(6123):623–625. doi: 10.1038/327623a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994 Dec;17(12):531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H. How we describe competitive antagonists: three questions of usage. Trends Pharmacol Sci. 1991 Feb;12(2):53–54. doi: 10.1016/0165-6147(91)90497-g. [DOI] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. M. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 1992 Dec;15(12):475–481. doi: 10.1016/0166-2236(92)90092-m. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Lazareno S., Buckley N. J., Roberts F. F. Characterization of muscarinic M4 binding sites in rabbit lung, chicken heart, and NG108-15 cells. Mol Pharmacol. 1990 Dec;38(6):805–815. [PubMed] [Google Scholar]
- Makhina E. N., Kelly A. J., Lopatin A. N., Mercer R. W., Nichols C. G. Cloning and expression of a novel human brain inward rectifier potassium channel. J Biol Chem. 1994 Aug 12;269(32):20468–20474. [PubMed] [Google Scholar]
- Marrion N. V., Smart T. G., Marsh S. J., Brown D. A. Muscarinic suppression of the M-current in the rat sympathetic ganglion is mediated by receptors of the M1-subtype. Br J Pharmacol. 1989 Oct;98(2):557–573. doi: 10.1111/j.1476-5381.1989.tb12630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Meckler R. L. Characteristics of synaptic input to three classes of sympathetic neurone in the coeliac ganglion of the guinea-pig. J Physiol. 1989 Aug;415:109–129. doi: 10.1113/jphysiol.1989.sp017714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Aagaard P. J., Gibson V. A., McKinney M. Binding and functional selectivity of himbacine for cloned and neuronal muscarinic receptors. J Pharmacol Exp Ther. 1992 Nov;263(2):663–667. [PubMed] [Google Scholar]
- North R. A., Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. J Physiol. 1989 Oct;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périer F., Radeke C. M., Vandenberg C. A. Primary structure and characterization of a small-conductance inwardly rectifying potassium channel from human hippocampus. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6240–6244. doi: 10.1073/pnas.91.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. E., Modert C. W., Yip H. K., Johnson E. M., Jr Retrograde axonal transport of intravenously administered 125I-nerve growth factor in rats with streptozotocin-induced diabetes. Diabetes. 1983 Jul;32(7):654–663. doi: 10.2337/diab.32.7.654. [DOI] [PubMed] [Google Scholar]
- Skok V. I., Ivanov A. Y. What is the ongoing activity of sympathetic neurons? J Auton Nerv Syst. 1983 Mar-Apr;7(3-4):263–270. doi: 10.1016/0165-1838(83)90079-6. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R., Nakajima Y., Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985 Jun 6;315(6019):498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Uchimura N., North R. A. Muscarine reduces inwardly rectifying potassium conductance in rat nucleus accumbens neurones. J Physiol. 1990 Mar;422:369–380. doi: 10.1113/jphysiol.1990.sp017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. S., McKinnon D. Potassium currents in rat prevertebral and paravertebral sympathetic neurones: control of firing properties. J Physiol. 1995 Jun 1;485(Pt 2):319–335. doi: 10.1113/jphysiol.1995.sp020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems W. A., Szurszewski J. H. An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J Neurophysiol. 1978 Mar;41(2):305–321. doi: 10.1152/jn.1978.41.2.305. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


