ABSTRACT
Pomacea canaliculata is an invasive species which has significantly impacted native ecosystems globally. The benthic worm Limnodrilus hoffmeisteri is essential for the stability of the native aquatic ecosystem, facilitating the nutrient cycle dynamics through bioturbation. Nevertheless, limited information exists regarding the impact of P. canaliculata on those key native benthic species. Present study evaluated the impacts of P. canaliculata on L. hoffmeisteri by exposing L. hoffmeisteri to P. canaliculata (PC group) and the native snail Bellamya aeruginosa (BA group), with a control group consisting of no snails (NS group). The survival rate of L. hoffmeisteri in the PC group persisted diminished over 14 days, with notable declines in the rates of successful food acquisition and aggregation, an increase in migration, and a decrease in swing frequency. Elevated oxidative stress levels were linked to these alterations in L. hoffmeisteri behavior. Additionally, the presence of P. canaliculata increased the abundance of intestinal pathogenic bacteria in L. hoffmeisteri , with Aeromonas being one of the most lethal. Experimental models of Aeromonas‐free P. canaliculata (AFPC), re‐infected AFPC (IPC), and Aeromonas (As) were established to illustrate the role of Aeromonas in the decline of L. hoffmeisteri . Similar patterns in L. hoffmeisteri survival, behavior, and oxidative stress were observed in As, IPC, and PC group; however, these effects were mitigated by the elimination of Aeromonas in the AFPC group. Furthermore, L. hoffmeisteri was fatally affected by the four Aeromonas strains that were obtained from P. canaliculata intestine. These findings indicate that P. canaliculata exerts a deleterious impact on L. hoffmeisteri , and Aeromonas colonizing in intestine plays an important role. This study reveals a novel invasion mechanism of P. canaliculata .
Keywords: Aeromonas, benthic worm, intestinal microbiota, invasion mechanism, Pomacea canaliculata
Behavior pattern changes of L. hoffmeisteri exposed to non‐free Pomacea canaliculata (PC) were found PC caused oxidative stress in L. hoffmeisteri PC changed the intestinal microbiota community structure of L. hoffmeisteri Aeromonas from the intestinal tract of P. canaliculata (As) had similar effects. As is the main stress source.
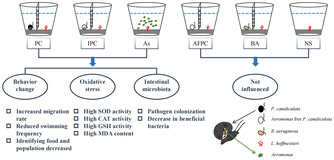
1. Introduction
Biological invasions have become a prominent environmental concern in the 21st century, leading to profound economic losses and ecosystem disruptions (Simberloff et al. 2013). In China, invasive species have been widely documented across diverse ecosystems, exerting widespread, and persistent impacts (Oya, Hirai, and Miyahara 1987; Susin 2004). The process of biological invasion is a dynamic and complicated phenomenon, the success of invasion depends not only on the biological characteristics of the invasive species, but also on the ecological environment of the habitat (Alpert, Bone, and Holzapfel 2000). Mounting evidence points that complex non‐additive interaction effects exist among the drivers contributing to the decline of native species. Clearly, invasive species are one of the direct causes of biodiversity loss; however, the relationship between invasive and native species is not simply one of trade‐offs. In fact, invasive species may lead to environmental changes in habitats, resulting in significant indirect impacts on native species (Didham et al. 2005, 2007), they can affect native species directly by predation or competition resource within the ecosystem, or indirectly affect native species by secreting allelochemicals or microorganisms. For instance, native tadpoles have to adapt to the substances secreted by the invasive crayfish ( Procambarus clarkii ), potentially leading to phenotypic changes in the population over time due to heightened stress (Melotto, Manenti, and Ficetola 2020). Furthermore, the biological composition such as microbial communities and phytoplankton communities changes in the native ecosystem were observed in response to the presence of the invasive phytophagous fish Siganus rivulatus , as evidenced by alterations in the nutrient content of its feces and other released materials (Escalas et al. 2022). It has been reported that native species may also be influenced by visual cues from invasive predators, causing them to remain in a prolonged state of alertness, which reduces their feeding activities and results in population decline (Nunes et al. 2013).
Pomacea canaliculata Lamarck, 1819 (Neogastropoda, Ampullariidae), commonly known as the golden apple snail, is a highly invasive aquatic species that has established a strong presence not only in China but also in numerous other regions around the world. It is remarkable adaptability and prolific reproductive capacity enabled it to invade a wide range of freshwater ecosystems in China (Oya, Hirai, and Miyahara 1987; Susin 2004). P. canaliculata is an omnivorous snail with a preference for plants consumption, which poses a substantial menace to rice crops. Additionally, it preys on native snails and other aquatic animals such as Paramecium caudatum , disrupting the ecological balance and, consequently, endangering the biodiversity of aquatic ecosystems (Carlsson, Brönmark, and Hansson 2004; Escalas et al. 2022; King‐Lun, Chan, and Qiu 2009; Matsukura et al. 2016). Moreover, P. canaliculata indirectly influences the density of primary producers and the primary productivity of the entire ecosystem by consuming algae and phytoplankton (Ling et al. 2010). Furthermore, the metabolites and excreta of P. canaliculata contribute to increase in nitrogen and phosphorus content, stimulating the proliferation of microorganisms and leading to the water quality deterioration or eutrophication (Carlsson, Brönmark, and Hansson 2004; O'Neil et al. 2023). It has also been reported that the secretions of P. canaliculata directly impact the structure of bacterial communities and the survival of adjacent species (Maldonado and Martín 2019; Wang et al. 2020).
Bioturbation is a crucial process in the modification of material cycling and energy flow within the sediment/water interface and is conducted benthic organisms through various activities such as feeding, excavation, and exploration (Lohrer, Thrush, and Gibbs 2004). These activities profoundly influence the physical and chemical properties of both the sediment and water (Pratihary et al. 2009). The bioturbation rate varies among different functional groups of macroinvertebrates, with Tubificidae exhibiting a notably stronger bioturbation ability compared to other benthic animals (Michaud et al. 2005, 2006). Limnodrilus hoffmeisteri Claparède, 1862 (Tubificida, Tubificidae) is the most abundant freshwater worms, it dominates in many freshwater environments throughout China (Zhang et al. 2010). The swing movement of L. hoffmeisteri facilitates oxygenation, nesting and feeding, resulting in the transportation and mixing of sediment particles, as well as the changing the pH, oxidation–reduction potential and dissolved oxygen content of sediment–water interface (Zhang et al. 2010). Therefore, L. hoffmeisteri plays a pivotal role in maintaining the stability of the aquatic environment. Nevertheless, the widespread distribution of L. hoffmeisteri has led to its dominance in water body invaded by the P. canaliculata . However, the effect of the invasion of P. canaliculata on the population of L. hoffmeisteri remains unclear.
Throughout the extensive history of co‐evolution between hosts and microorganisms, the intestinal microbiota has progressively established a mutually cooperative and symbiotic relationship with the host organism. In usual conditions, the intestinal microbiota maintains a dynamic balance state in response to complex and changeable environments. This balance is crucial for the host organism's assimilation and metabolism of nutrients, as well as the regulation of its immune response (Arthur et al. 2013; Fassarella et al. 2021; Yang, Yang, and Li 2022). However, the intestinal microbiota of aquatic invertebrates is highly susceptible to environmental changes, providing a potential gateway for pathogen such as Aeromonas invasion and colonization within the intestine (Chen et al. 2021; Chen et al. 2008; Wang et al. 2019). The primitive immune system of L. hoffmeisteri heavily relies on the assistance of its intestinal microbiota, making it vulnerable to pathogenic bacteria, including Aeromonas, which colonize the intestine of P. canaliculata (Chen et al. 2021; Li et al. 2022). The release of these pathogenic bacteria to the aquatic environment may potentially result in infections, illness, and even death among other aquatic organisms.
This study focused on the non‐predatory interactions between P. canaliculata and the key native bioturbator‐ L. hoffmeisteri . To investigate the effects and underlying mechanisms of P. canaliculata invasion on native bioturbator, the behavioral reactions, oxidative stress levels, and alterations in the intestinal microbiota of L. hoffmeisteri in response to P. canaliculata and native snail Bellaya aeruginosa exposure were analyzed. Aeromonas represents one of the most abundant bacterial communities in the gut of P. canaliculata , may enter aquatic ecosystems through the secretion and excretion processes of P. canaliculata , potentially leading to infections and mortality among L. hoffmeisteri . We constructed an Aeromonas‐free P. canaliculata model (AFPC) and an Aeromonas‐infected AFPC model (IPC) to further clarify the significance of Aeromonas from the gut of P. canaliculata by exposing L. hoffmeisteri to the two animal models. At last, we isolated three Aeromonas strains from the gut of P. canaliculata and exposed L. hoffmeisteri to these strains to demonstrate the direct pathogenicity of the Aeromonas isolates present in the invasive snail's gut. Our findings establish a theoretical basis for evaluating the ecological dangers posed by the invasion of P. canaliculata on bioturbators and clarify the probable pathways by which the gut microbiota of P. canaliculata affects these interactions. This method enriches the theoretical evidence that an interaction modification effect between invasive species and native species.
2. Materials and Methods
2.1. Test Organism
The invasive snail Pomacea canaliculata was collected from a lake in Zhaoqing (Guangdong Province, China). The native snail Bellaya aeruginosa Reeve, 1863 (Mesogastropoda, Viviparidae), which is the near niche species of P. canaliculata in China, is a wild population in Weishan Lake obtained from local fishermen, which are set as control group (Shandong Province, China). Limnodrilus hoffmeisteri were collected from the sediment of Dalian Qianguan Wetland Park (Liaoning province, China). No natural contact occurred between the populations of these three animals. All animals were immediately transported to the laboratory under cool conditions.
The snails were kept in a 76 L water tank containing 50 L aerated tap water, accommodating 100 animals per tank. And L. hoffmeisteri were kept in 3.5 L water tanks with 3 L aerated tap water, containing approximately 1000 ind per tank. All species were acclimated in deionized water separately, and maintained under a 12:12 light/dark (L:D) cycle at a temperature of 20°C ± 2°C for a period over 14 days. All animals were fed with homogeneous commercial fish food (Tetramin, Germany) at a rate of 0.1 mg/ind/d.
2.2. Establishment of Animal Model
Our previous study has demonstrated that Aeromonas from the intestine of P. canaliculata plays a crucial role in the infection of native snails (Data not published). Four major Aeromonas strains were isolated from the intestinal tract of P. canaliculata : Aeromonas sp., Aeromonas hydrophila , Aeromonas media , and Aeromonas veronii . These strains were sequenced and preserved in the laboratory. For the present study, a mixed Aeromonas culture was created by blending the four strains in equal proportions (1:1:1:1) in the L. hoffmeisteri infection group (As).
To establish the Aeromonas‐free P. canaliculata model (AFPC), P. canaliculata individuals were cultured at a density of 4 ind/L and treated with florfenicol at a concentration of 2 g/L for 4 days, with the treatment solution renewed daily. The successful construction of the AFPC model was confirmed by the absence of Aeromonas colonies in the microbial culture of the AFPC intestinal tract. All the snails survived after treatment, and the behavior patterns did not change.
To establish the Aeromonas‐infected AFPC model (IPC), the established AFPC were exposed to a culture of mixed Aeromonas at a concentration of 1 × 108 CFU/mL for 24 h. The establishment of the IPC model was confirmed by the detection of Aeromonas colonies in the microbial culture of the IPC intestinal tract.
2.3. Experimental Design
For the non‐contact exposure experiment, round polyethylene box (1250 mL) with small polyethylene boxes with holes inside, allowing water circulation and keeping the animals isolated was used as the experimental system. Sixty experimental systems were established and randomly divided into three groups: the invasion group with one P. canaliculata (PC) (7.00 ± 0.30 g), the native group with one B. aeruginosa (BA) (2.50 ± 0.20 g), and the control group without snails (NS). Initially, the bottom of the round polyethylene box was layered with sterilized and cooled fine river sand (diameter 1–2 mm) at a thickness of 1.5 cm. Subsequently, 800 mL of aerated tap water and 1000 ind L. hoffmeisteri individuals were introduced into the system after acclimation for 24 h. To further assess the impact of gut microbiota from P. canaliculata on L. hoffmeisteri. One of the representative model animals from AFPC group, IPC group and As group were introduced into the small polyethylene boxes. And the other experimental settings kept the same.
The two experiments lasted for 14 days. At D7 and D14, 10 repetitions systems from each experimental group were collected for subsequent analysis. L. hoffmeisteri were isolated from the sand and their survival rates were counted, then the behavioral indicators and antioxidant enzymes activity were assessed. L. hoffmeisteri individuals form the PC, BP, and NS group were collected for 16S rRNA microbiota sequencing.
2.4. Survival Rate of L. hoffmeisteri
At D7 and D14, 10 experimental systems of PC, BP, NS and AFPC, IPC, AS group were sampled, following which the bottom sediments were thoroughly transferred onto a white porcelain plate, all the L. hoffmeisteri were isolated from the sand. Subsequently, all surviving L. hoffmeisteri were identified and quantified. The survival rates for each group at D7 and D14 were calculated.
2.5. Behavior Responses of L. hoffmeisteri
L. hoffmeisteri typically form a large nucleus population, and individual worms exhibit migration behaviors to avoid potentially hazardous environmental conditions. Thus, the migration patterns of L. hoffmeisteri from the nucleus population can serve as an indicator for assessing environmental conditions, and their swing behavior can be used to evaluate their vitality.
A total of 500 L. hoffmeisteri individuals were collected from all experimental groups at D7 and D14. These worms were placed in a 6‐well cell culture plate filled with 10 mL of deionized water per well. Worms observed swinging alone far away from the nucleus population, or those formed a small population, were categorized as migratory individuals. After holding for 24 h, the proportion of migratory L. hoffmeisteri in total L. hoffmeisteri was observed in 10 wells. The swing frequency of L. hoffmeisteri from migratory individuals or nucleus population individuals was then recorded within 1 min. The behavior of 20 randomly selected L. hoffmeisteri was observed from at least five wells, and the average number was recorded.
The acquisition of food is an innate reflexive behavior of L. hoffmeisteri , with the speed of exploring and capturing food reflecting their vitality and physiological state. To investigate the effects of the treated groups on this behavior, 500 L. hoffmeisteri individuals were collected and placed in the center of a well. A piece of fish food was placed on the opposite edge of the well. The time it took for the individuals to extend out of their bodies and retrieve the food to the central location was recorded as a successful food capture event. Food acquisition rates were calculated at different time points, and the NS, BA and PC group was repeated for six times at D7 and 10 times at D10. The AFPC, As, and IPC group was repeated for 10 times at both D7 and D10.
L. hoffmeisteri individuals tend to aggregate into large nucleus populations. However, their ability to recognize population boundaries might be influenced by environment deterioration or exposure to stressors. To assess this, L. hoffmeisteri collected from the different experimental groups on D7 and D14 were placed on the leftmost and rightmost edges of a culture plate, with 250 individuals on each side. The time taken for the two populations to aggregate into one nucleus population was recorded, and the NS, BA, and PC groups were repeated six times at D7 and 10 times at D10. The AFPC, As, and IPC groups were repeated 10 times on both D7 and D10.
2.6. Antioxidant Enzyme Activity Detection
To assess the oxidative stress in L. hoffmeisteri at D7 and D14, 200 L. hoffmeisteri individuals from each experimental group were collected in experimental systems triplicate. The collected samples were homogenized with physiological saline, followed by centrifugation at 2500 rpm/min at 4°C for 10 min. The supernatant was collected to detect malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) following the instruction of the commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Additionally, protein concentrations were determined using the bicinchoninic acid (BCA) protein kit as standard.
2.7. Intestinal Microbiota Analysis
L. hoffmeisteri were fasted for 24 h and cleaned before sampling. The intestinal contents were extracted, immediately frozen in liquid nitrogen, and stored at −80°C until use. All samplings in the experiment were performed under sterile conditions. For the NS, BA, and PC groups at D7 and D14, L. hoffmeisteri were washed three times with sterile physiological saline, and 150 worms were pooled into a single sample. The total genome DNA were extracted and the DNA concentration and purity were measured. Then the bacterial 16S rRNA gene V4 region was amplified with the primers 515F‐806R (F: CCTAYGGGRBGCASCAG, R: GGACTACNNGGGTATCTAAT). The amplified DNA was subsequently sequenced on an Illumina MiSeq platform of Novogene (Beijing, China), generating 250 bp paired‐end reads for intestinal microbiota analysis. The raw sequencing data is assembled and filtered to obtain Clean Data; then denoising is performed using DADA2 based on the Clean Data, filtering out sequences with abundances lower than five to obtain the final ASVs.
2.8. Acute Toxicological Experiment of Aeromonas on L. hoffmeisteri
The cumulative mortality rate of L. hoffmeisteri with the exposure to Aeromonas from the intestinal tract of P. canaliculata was investigated. Aeromonas sp., Aeromonas hydrophila , Aeromonas media , and Aeromonas veronii , and the mixed Aeromonas in a 1:1:1:1 ratio of four Aeromonas with a concentration of 108 CFU/mL were used as experimental bacteria. A six‐well cell culture plate was used as the experimental system. Ten individual L. hoffmeisteri without any prior experimental treatment were placed into the wells. The control group (CK) received sterile water only. Repeat six times in each group. All the surviving L. hoffmeisteri individuals were observed, and the cumulative mortality rate of L. hoffmeisteri was calculated.
2.9. Statistical Analysis
Data were presented as mean ± standard deviation (SD) and tested for statistical significance using Paired‐sample t‐tests. The statistical values were considered significantly different when the calculated probability (p) level was below 0.05. The results were plotted using GraphPad Prism 9.
3. Results
3.1. Effects of the P. canaliculata on the Survival Rate and the Behavior of L. hoffmeisteri
The survival rate of L. hoffmeisteri in all experimental groups exceeded 80% at D7 (Figure 1a). Specifically, the NS group, BA group, and PC group exhibited survival rates of 96.4%, 89.7%, and 82.28%, respectively. With the extension of treatment duration, the survival rates of L. hoffmeisteri decreased to 90.68%, 74.8%, and 58.71% at D14, respectively. It is worth noting that the PC group exhibited significantly lower survival rate compared to the BA group and NS group at both D7 and D14 (p < 0.05).
FIGURE 1.
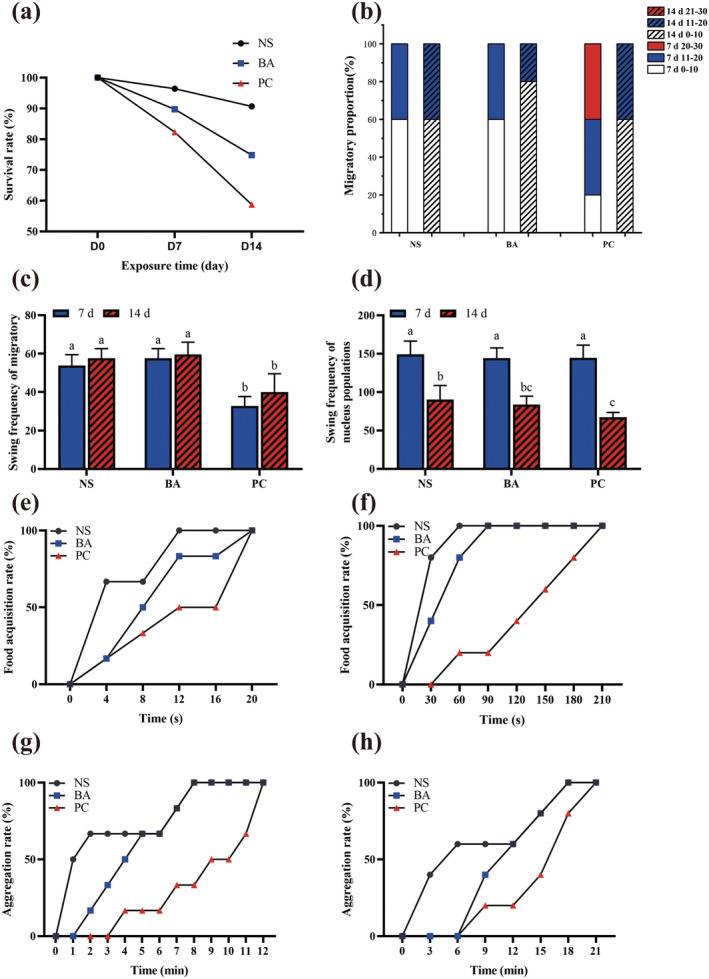
The survival rate and behavior of Limnodrilus hoffmeisteri after different treatments. (a) The survival rate of L. hoffmeisteri ; (b) Proportion of the migratory numbers of L. hoffmeisteri ; (c) Swing frequency of migratory L. hoffmeisteri ; (d) Swing frequency of nucleus populations L. hoffmeisteri ; (e) Successful food acquisition rate of L. hoffmeisteri at D7; (f) Successful food acquisition rate of L. hoffmeisteri at D14; (g) Aggregation rate of L. hoffmeisteri at D7; (h) Aggregation rate of L. hoffmeisteri at D14. BA: B. aeruginosa; NS: None snails; PC: P. canaliculata . Different superscript letters indicate significant difference between groups (p < 0.05).
It was observed that exposure to P. canaliculata had an irritating effect on the behavior pattern of L. hoffmeisteri . Most of the wells in NS and BA groups had only 0–10 migratory individuals at D7 (Figure 1b). Conversely, the PC group displayed more pronounced migration, with 40% of the experimental units exhibiting 21–30 migratory individuals, and 40% having 11–20 migratory individuals. The findings suggest that P. canaliculata elicits alarm response in L. hoffmeisteri . Furthermore, the proportion of highly migratory individuals of L. hoffmeisteri in the PC group exhibited a significant decrease at D14. Only 20% of the wells displayed 11–20 migratory individuals, indicating that prolonged exposure to P. canaliculata has fostered adaptability in L. hoffmeisteri , resulting in reduced vigilance behavior.
The migratory L. hoffmeisteri individuals in the PC group exhibited a significantly lower swing frequency compared to those in the NS and BA group at both D7 and D14 (p < 0.05) (Figure 1c). Similarly, the swing frequency of nuclear L. hoffmeisteri in the PC group was significantly lower than that in the NS group at D14 (p < 0.05) (Figure 1d), while no significant difference was observed in the swing frequency of nuclear L. hoffmeisteri among all groups within the nucleus population at D7 (p > 0.05). Therefore, the invasion of P. canaliculata will persistently exert a negative impact on the bioturbation performance of L. hoffmeisteri .
In terms of food acquisition, the rate of food acquisition of L. hoffmeisteri in the PC group was the slowest (Figure 1e). Only 50% of the experimental wells in the PC group successfully obtained food within 12 s at D7. In contrast, all the experimental units in the NS group successful obtained food within 12 s, and 50% of experimental units obtained food within 3 s. In the BA group, it took 9 s for 50% of the experimental units to successfully obtain food. As the treatment duration prolonged, the time required for each group to successfully acquire food also increased. In the PC group, the food acquisition rate reached 100% at 210 s at D14 (Figure 1f). These findings indicate that P. canaliculata may affect the food acquisition and exploration ability of L. hoffmeisteri .
Furthermore, L. hoffmeisteri individuals in the PC group encountered challenges in recognizing and aggregating with each other, only 50% of the experimental units successfully aggregated within 9 min at D7, while it took 11 min for all experimental units to achieve successful aggregation. In contrast, L. hoffmeisteri in the BA and NS group identified and aggregated with each other within 8 min (Figure 1g). With the extension of experiment duration, the aggregation rate between populations of L. hoffmeisteri under different treatments decreased. In the PC group, all the experimental units successfully aggregated within 18 min (Figure 1h), highlighting a diminished population recognition ability of L. hoffmeisteri in response to P. canaliculata invasion.
3.2. Effects of the P. canaliculata on the Antioxidant Responses of L. hoffmeisteri
The levels of SOD and CAT activity in L. hoffmeisteri showed a consistent trend, with significant increases in the PC group compared to the other groups at both D7 and D14 (p < 0.05). The SOD activity in the PC group at D14 was significantly higher than that at D7 (p < 0.05) (Figure 2a,b). The GSH content in PC group was significantly higher than that both in BA and NS group at D7 (p < 0.05), while it inhibited in all groups with no significant differences at D14 (p > 0.05) (Figure 2c). Additionally, the MDA content in both PC and BA groups were significantly higher than those in the NS group at D7 (p < 0.05), and the MDA content in the BA group was significantly reduced to a level comparable to that of the NS group at D14 (p < 0.05), while no significant changes were observed in PC group (Figure 2d).
FIGURE 2.
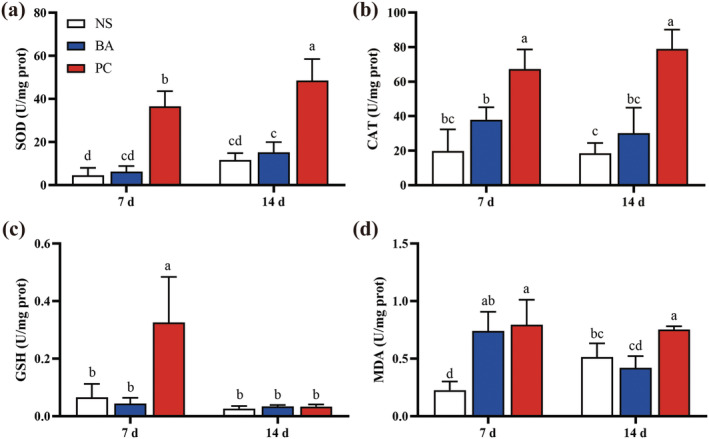
Enzymatic activity and lipid peroxidation of L. hoffmeisteri after different treatments. (a) SOD; (b) CAT; (c) GSH; (d) MDA.
3.3. Effects of the P. canaliculata on the Intestinal Microbiota of L. hoffmeisteri
For L. hoffmeisteri , a total of 2929, 3666, 3735, 1561, 2637, and 2089 ASVs were observed in the NS7d, BA7d, PC7d, NS14d, BA14d, and PC14d groups, respectively. Furthermore, 1168 ASVs (39.88%) were identified in both NS7d and BA7d groups, while 813 ASVs (27.76%) were shared between the NS7d and PC7d groups. Additionally, 809 (51.826%) ASVs were shared between NS14d and BA14d groups, and 403 ASVs (25.82%) were observed in both NS14d and PC14d groups. Moreover, 485 ASVs were identified as common between NS7d and NS14d groups, whereas 1044 ASVs exhibited between BA7d and BA14d groups. Lastly, 547 ASVs were shared by both PC7d and PC14d groups.
Both P. canaliculata and B. aeruginosa had an impact on the intestinal microbial structure of L. hoffmeisteri , with the effect of P. canaliculata much greater than that of the native snail. The most prevalent bacterial groups in both NS7d and NS14d group were Proteobacteria, Firmicutes, and Bacteroidota at phylum level (Figure 3a). Furthermore, several genera exhibited changes in abundance following different durations of exposure to different snail species. Notably, Pseudomonas was found to be one of the dominant bacteria in the intestine of L. hoffmeisteri . At D7, the relative abundance of Pseudomonas was higher in the group exposed to P. canaliculata compared to other groups, while its relative abundance was lower than that of other groups at D14. Exposure to the two snails species resulted in an enrichment of Aeromonas in the intestine of L. hoffmeisteri , with the relative abundance of Aeromonas being particularly high in the groups exposed to P. canaliculata . The relative abundance of Lactobacillus in different groups remained stable at D7, but its relative abundance significantly decreased in PC14d group. Additionally, the relative abundance of Rhodococcus was found to be extremely low in the PC7d group and was not detected in the PC14d group (Figure 3b).
FIGURE 3.
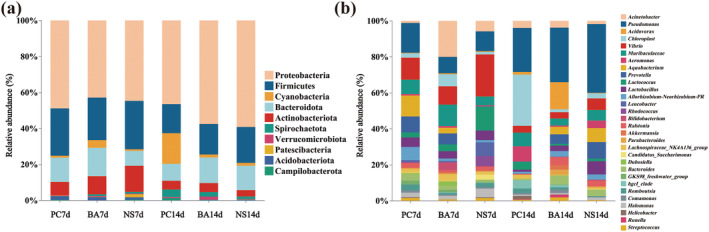
The composition of intestinal symbiotic bacteria of L. hoffmeisteri under different treatments. (a) phylum level; (b) genus level.
3.4. Effects of Aeromonas on the Survival Rate and the Behavior of L. hoffmeisteri
The survival rates of L. hoffmeisteri in AFPC group, As group, and IPC group at D7 was 100.0%, 81.2%, and 79.3%, respectively. With the extension of treatment duration, the survival rate of L. hoffmeisteri in As group and IPC group at D14 significantly decreased to 62.3% and 59.9%, respectively (Figure 4a).
FIGURE 4.
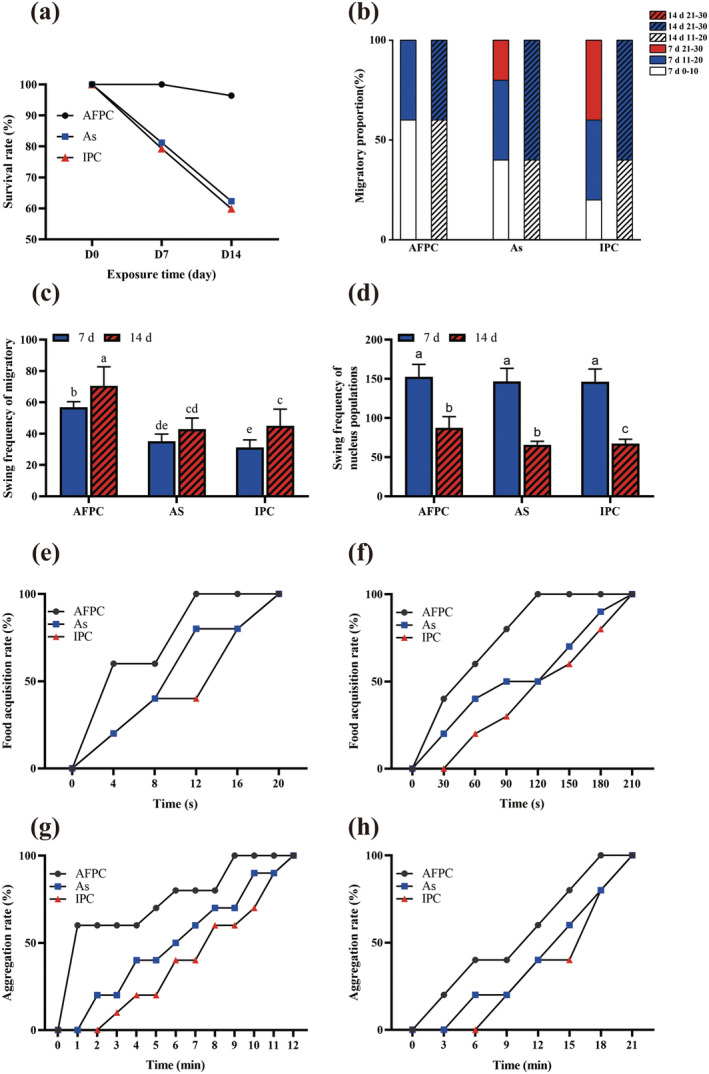
The survival rate and behavior of L. hoffmeisteri after different treatments (AFPC, As or IPC). (a) The survival rate of L. hoffmeisteri ; (b) Proportion of the migratory numbers of L. hoffmeisteri ; (c) Swing frequency of migratory L. hoffmeisteri ; (d) Swing frequency of nucleus populations L. hoffmeisteri ; (e) Successful food acquisition rate of L. hoffmeisteri at D7; (f) Successful food acquisition rate of L. hoffmeisteri at D14; (g) Aggregation rate of L. hoffmeisteri at D7; (h) Aggregation rate of L. hoffmeisteri at D14. Different superscript letters indicate significant difference between groups (p < 0.05).
At D7, the majority of AFPC groups exhibited low migratory activity with 0–10 individuals, while significant migrations were observed in the As and IPC groups (Figure 4b). Specifically, 20% of the experimental units in the As group had 21–30 migratory individuals, and 40% of the experimental units in the IPC group showed a similar trend with high numbers of migratory individuals. This finding suggests that the infection of Aeromonas or P. canaliculata with Aeromonas was more likely to cause alert or hyperactive behavior in L. hoffmeisteri . Besides, the proportion of high migratory individuals of L. hoffmeisteri in the As and IPC groups decreased at D14. This finding is consistent with previous experimental results in PC group, indicating that with the extension of exposure time, the activity of L. hoffmeisteri decreased The swing frequency of migratory L. hoffmeisteri individuals in the As and IPC groups was significantly lower compared to the AFPC group at D7 or D14 (p < 0.05) (Figure 4c). However, there was no significant difference in the swing frequency of the L. hoffmeisteri nucleus population at D7 (p > 0.05), but the swing frequency was significantly lower in the IPC group compared to the As and AFPC groups (p < 0.05) (Figure 4d). These findings suggest that P. canaliculata can cause a decrease in swing frequency and activity of L. hoffmeisteri through the secreting of Aeromonas.
The presence of Aeromonas from P. canaliculata 's intestinal, as well as the carriage of Aeromonas by P. canaliculata , greatly impacts the food exploration and acquisition of L. hoffmeisteri . Only 40% of experimental units in the IPC group successfully obtained food within 12 s at D7. In contrast, all experimental units from both the IPC and As groups were able to obtain food within 20 s, while all experimental units in AFPC group 12 s (Figure 4e). With the extension of treatment time, the time required for each group to successfully obtain food increased. In the As and IPC groups, the food acquisition rate reached 100% after a period of 210 s at D14 (Figure 4f).
Furthermore, it was observed that L. hoffmeisteri in the As and IPC groups had difficulty in identifying and aggregating with each other. All experimental units in the As and IPC groups successfully aggregated within 12 min, while all units in the AFPC group were identified and aggregated within 9 min (Figure 4g). With the extension of experimental exposure time, the aggregation speed between L. hoffmeisteri populations under different treatments decreased. All the experimental units in the As and IPC groups successfully aggregated at 21 min at D14 (Figure 4h), which is consistent with previous experimental results. These results suggest that both the presence of Aeromonas from the intestinal of P. canaliculata and the presence of P. canaliculata carrying Aeromonas significantly impact the population identification and aggregation of L. hoffmeisteri .
3.5. Effects of the Aeromonas on the Oxidation, and Antioxidant Responses of L. hoffmeisteri
The SOD activity of L. hoffmeisteri in As and IPC groups were significantly higher than that in the AFPC group (p < 0.05) both at D7 and D14, with further increased levels observed at D14 (p < 0.05) (Figure 5a). The CAT activity of L. hoffmeisteri in the IPC group was higher than the As group, and significantly elevated when compared to that in AFPC group at D7 and D14 (Figure 5b). The GSH content in the AFPC group was significantly higher compared to the As and IPC groups at D14 (p < 0.05). Furthermore, the content of GSH in IPC group decreased significantly as the experimental duration extended (p < 0.05) (Figure 5c). The MDA content in IPC group was significantly higher compared to the AFPC group (p < 0.05) at D7 (Figure 5d). Moreover, the MDA content in the IPC group was significantly higher when compared to both the As and AFPC groups at D14 (p < 0.05).
FIGURE 5.
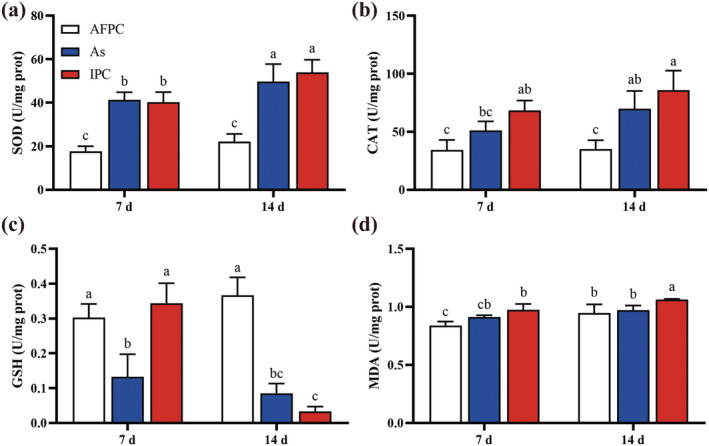
The oxidation and antioxidant responses of L. hoffmeisteri after different treatments. (a) SOD; (b) CAT; (c) GSH; (d) MDA.
3.6. Effects of Aeromonas on the Cumulative Mortality Rate of L. hoffmeisteri
The 24‐h cumulative mortality rate of L. hoffmeisteri with the treatment of Aeromonas sp., Aeromonas hydrophila , Aeromonas media , and Aeromonas veronii and mixed Aeromonas (As) was 28.3%, 38.3%, 61.7%, 51.7%, and 93.3%, respectively (Figure 6). In contrast, the cumulative mortality rate of L. hoffmeisteri in the control group without any Aeromonas was 0%. Notably, the mortality rate of L. hoffmeisteri exposed with the mixed Aeromonas was higher than other groups, which climbed to 60% at 1 h. The interactions among various Aeromonas strains originating from the intestinal environment of P. canaliculata may result in complex and synergistic effects.
FIGURE 6.
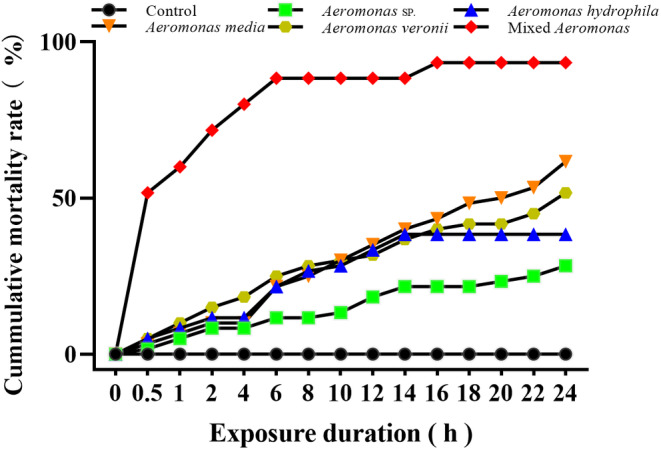
The cumulative mortality rate L. hoffmeisteri after exposed to the Aeromonas strains isolated from the intestinal tract of P. canaliculata.
4. Discussion
The impact of invasive species on the native ecosystem is multifaceted and profound (Parras and Casadío 2006). The secretions and excreta of P. canaliculata contain diverse symbiotic bacteria, among which Aeromonas represents a prominent group colonizing the intestinal tract (Chen et al. 2021; Li et al. 2019, 2022). Recent research has demonstrated that the secretions of P. canaliculata can release diverse pathogenic bacteria and threat the safety of native aquatic animals (Liu et al. 2024; Sui et al. 2024). L. hoffmeisteri is sensitivity to environmental microorganisms, the pathogens released by P. canaliculata into their ecosystem are likely a significant factor in the reduction of their populations (Liu et al. 2024). In present study, exposure to non‐free moving P. canaliculata has lasting and harmful impacts on L. hoffmeisteri . The survival rate of L. hoffmeisteri progressively declined within 14 days (Figure 1a), and it was no longer influenced after the removal of Aeromonas from the intestinal tract of P. canaliculata . Simultaneously, both the mixed strains of Aeromonas and P. canaliculata significantly reduced the survival rate of L. hoffmeisteri (Figure 4a), implying that the release of Aeromonas is the primary causative factor in the mortality of L. hoffmeisteri induced by P. canaliculata .
Exposure to P. canaliculata induced alterations in the behavior of migration, swinging, food acquisition, and population identification of L. hoffmeisteri . In contrast to the native snail B. aeruginosa, exposure to P. canaliculata elicited an intensified alarm response to L. hoffmeisteri , leading to considerable dispersal from the core population, as well as the diminished motility (Figure 1b–d). Furthermore, exposure to P. canaliculata affected the food recognition and population dynamics of L. hoffmeisteri , leading to prolonged feeding and integration into larger populations (Figure 1e–h). It has been demonstrated that infection with pathogenic bacteria can alter animal behavior (Swanson et al. 2002), and Aeromonas infection can alter fish swimming behavior (Sun et al. 2016). Consistently, AFPC did not affect the behavior of L. hoffmeisteri , while L. hoffmeisteri from the As and IPC groups displayed similar behavioral patterns to those of the PC group (Figure 4b–h). This finding suggests that Aeromonas plays a significant role in shaping the behavioral pattern of L. hoffmeisteri . The decline in survival rates and alterations in behavior patterns greatly affects the bioturbation ability of L. hoffmeisteri , which may pose the adverse effects on aquatic environment (Nogaro and Burgin 2014; Stief 2013).
Environmental pollution, interspecies interactions, and pathogen infections may induce the generation of reactive oxygen species (ROS) in aquatic animal, inducing to oxidative stress. Invasive species serve as stresses to native populations, leading to extensive oxidative damage in these communities (Leza et al. 2019). Similarly, the current study revealed that the superoxide dismutase (SOD) activity, catalase (CAT) activity, and Malondialdehyde (MDA) content in L. hoffmeisteri from the PC group were significantly elevated compared to other groups after 14 days of exposure (Figure 2a,b,d), suggesting that L. hoffmeisteri in the PC group has undergone strongly oxidative damage, with the immune system actively engaged in the removal of cellular ROS (Birben et al. 2012; Chen et al. 2017). Excessive ROS can exert cytotoxic effects on cells and lead to damage of proteins, DNA, and lipids, and infection by Aeromonas has been associated with the activation of antioxidant enzyme systems and detoxifying processes (Chen et al. 2020). L. hoffmeisteri in both the As and IPC groups showed elevated oxidative stress levels, similar to the PC group. However, the AFPC group displayed no signs of oxidative stress, closely resembling the NS group (Figure 5). This suggests that Aeromonas infection and exposure to P. canaliculata may potentially induce oxidative stress in L. hoffmeisteri , thereby influencing their survival and behavior patterns. Furthermore, oxidative stress has a profound impact on the intestinal microbial composition, function and metabolic pathways of the host.
Intestinal microbiota plays a crucial role in the metabolism, immunity, and detoxification of the host, influencing the overall health and normal physiological activities (Dong et al. 2013; Nie et al. 2017; Tremaroli and Bäckhed 2012; Wen et al. 2014). As the primary defense mechanism, the functionality of intestinal microbiota plays a crucial role in the immunity of host (Dong et al. 2013; Nie et al. 2017; Wen et al. 2014). The exposure to P. canaliculata resulted in a decrease in the diversity of intestinal microbiota, and leading to infiltration of opportunistic pathogens into the intestine. Exposed to non‐free P. canaliculata has been shown to cause a sustained increase in Aeromonas of L. hoffmeisteri , Aeromonas is a common opportunistic pathogen in aquatic environment recognized for eliciting diverse clinical responses in numerous animals (Awan et al. 2018; Parker and Shaw 2011). The PC7d group had a comparatively elevated presence of Bacteroides while the abundance in the PC14d group diminished. The bacteria of this genus are closely linked to host intestinal immunity, homeostasis and immune system development (Lanning et al. 2000; Rhee et al. 2004). The PC7d group enhanced the proliferation of Bacteroides in L. hoffmeisteri , whereas prolonged exposure resulted in the replacement of Bacteroides by other microbiota. Exposed to non‐free P. canaliculata resulted in a reduction in intestinal microbes associated with nitrogen and phosphate pollution and the treatment of hazardous chemicals, including P. seudomonas and Lactobacillus (Ramani et al. 2012). This will likely hinder the capacity of bioturbators to eliminate toxins and intensify pollution within the ecosystem. In addition, Rhodococcus and Microbacteriaceae, which play a crucial role in host intestinal metabolism by facilitating organic matter degradation, vitamin B synthesis, and pathogen colonization resistance (Hu et al. 2020; Sassera et al. 2013), exhibited extremely low relative abundance in the group exposed to P. canaliculata . Consequently, exposure to P. canaliculata leads to the colonization of pathogens in the intestine of L. hoffmeisteri and a reduction in the abundance of beneficial functional microbiota, potentially altering the overall function of the intestinal microbiota.
5. Conclusion
Exposure to Pomacea canaliculata induces considerable mortality and behavioral alterations in Limnodrilus hoffmeisteri . These alterations include a notable reduction in successful food acquisition and aggregation rates, an elevated migration rate, and a diminished swing frequency. These alterations are linked to increased oxidative stress levels in L. hoffmeisteri when exposed to P. canaliculata . Furthermore, this exposure affects the intestinal microbiota of L. hoffmeisteri , leading to a rise in pathogenic colonization, such as Aeromonas. Aeromonas strains isolated from the intestinal tract of P. canaliculata and Aeromonas‐reinfected AFPC (IPC) demonstrated analogous patterns in survival, behavior, and oxidative stress in L. hoffmeisteri , consistent with the PC group. P. canaliculata , of which Aeromonas was eliminated from its gut (AFPC), lost the capacity to impact L. hoffmeisteri . Consequently, the release of Aeromonas by P. canaliculata may significantly and extensively harm native benthic organisms (Figure 7). This study elucidates the effects of P. canaliculata invasion on native organisms and ecosystems, establishing a theoretical framework for the assessment and restoration of ecosystems invaded by P. canaliculata .
FIGURE 7.
Flow chart of experiment.
Author Contributions
Mingyuan Liu: conceptualization (lead), formal analysis (lead), methodology (lead), project administration (equal), validation (equal), visualization (lead), writing – original draft (lead). Changrun Sui: data curation (equal), validation (lead), writing – original draft (equal). Baolong Wang: formal analysis (equal), investigation (equal). Pengfei Ma: formal analysis (equal), investigation (equal), validation (equal). Weixiao Zhang: formal analysis (equal), investigation (equal). Ruipin Huang: formal analysis (equal), investigation (equal). Yuqing Wang: formal analysis (equal), investigation (equal). Zhujun Qiu: formal analysis (equal), investigation (equal). Wenyu Zhao: formal analysis (equal), investigation (equal). Tao Zhang: formal analysis (equal), investigation (equal). Qian Zhang: conceptualization (equal), data curation (equal), funding acquisition (lead), methodology (equal), project administration (lead), resources (lead), supervision (lead), validation (equal), visualization (equal), writing – review and editing (lead). Ying Liu: funding acquisition (lead), methodology (equal), project administration (lead), resources (lead), validation (equal), visualization (equal), writing – review and editing (equal).
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Ms. Li Na for her help during the experiment and Dr. Song Jing for her assistance to the experimental facilities.
Funding: This study was funded by Joint Fund of General Research Project of Liaoning Province (2023‐MSLH‐007), Basic scientific research project of Educational Department of Liaoning province (LJKMZ20221107), the Earmarked found for China Agriculture Research System (CARS‐49).
Data Availability Statement
The datasets presented in this study can be found in the online data repositories: https://doi.org/10.6084/m9.figshare.27283269.
References
- Alpert, P. , Bone E., and Holzapfel C.. 2000. “Invasiveness, Invasibility and the Role of Environmental Stress in the Spread of Non‐Native Plants.” Urban & Fischer 3: 52–66. [Google Scholar]
- Arthur, J. C. , Perez‐Chanona E., Mühlbauer M., et al. 2013. “Intestinal Inflammation Targets Cancer‐Inducing Activity of the Microbiota.” Science 338: 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan, F. , Dong Y., Wang N., Liu J., Ma K., and Liu Y.. 2018. “The Fight for Invincibility: Environmental Stress Response Mechanisms and Aeromonas hydrophila .” Microbial Pathogenesis 116: 135–145. [DOI] [PubMed] [Google Scholar]
- Birben, E. , Sahiner U. M., Sackesen C., Erzurum S., and Kalayci O.. 2012. “Oxidative Stress and Antioxidant Defense.” World Allergy Organization Journal 5: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson, N. O. , Brönmark C., and Hansson L.‐A.. 2004. “Invading Herbivory: The Golden Apple Snail Alters Ecosystem Functioning in Asian Wetlands.” Ecology 85: 1575–1580. [Google Scholar]
- Chen, J. , Liu N., Zhang H., Zhao Y., and Cao X.. 2020. “The Effects of Aeromonas hydrophila Infection on Oxidative Stress, Nonspecific Immunity, Autophagy, and Apoptosis in the Common Carp.” Developmental and Comparative Immunology 105: 103587. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Li S., Xiao Q., Lin Y., and Li H.. 2021. “Composition and Diversity of Gut Microbiota in Pomacea canaliculata in Sexes and Between Developmental Stages.” BMC Microbiology 21: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Yan Q., Wang K., Zhuang Z., and Wang X.. 2008. “Portal of Entry for Pathogenic Vibrio alginolyticus Into Large Yellow Croaker Pseudosciaena crocea, and Characteristics of Bacterial Adhesion to Mucus.” Diseases of Aquatic Organisms 80: 181–188. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Huang X., Wang J., and Li C.. 2017. “Effect of Pure Microcystin‐LR on Activity and Transcript Level of Immune‐Related Enzymes in the White Shrimp ( Litopenaeus vannamei ).” Ecotoxicology 26: 702–710. [DOI] [PubMed] [Google Scholar]
- Didham, R. K. , Tylianakis J. M., Gemmell N. J., Rand T. A., and Ewers R. M.. 2007. “Interactive Effects of Habitat Modification and Species Invasion on Native Species Decline.” Trends in Ecology & Evolution 22, no. 9: 489–496. [DOI] [PubMed] [Google Scholar]
- Didham, R. K. , Tylianakis J. M., Hutchison M. A., Ewers R. M., and Gemmell N. J.. 2005. “Are Invasive Species the Drivers of Ecological Change?” Trends in Ecology & Evolution 20, no. 9: 470–474. [DOI] [PubMed] [Google Scholar]
- Dong, M. , Feng L., Kuang S. Y., et al. 2013. “Growth, Body Composition, Intestinal Enzyme Activities and Microflora of Juvenile Jian Carp ( Cyprinus carpio Var. Jian) Fed Graded Levels of Dietary Valine.” Aquaculture Nutrition 19: 1–14. [Google Scholar]
- Escalas, A. , Avouac A., Belmaker J., et al. 2022. “An Invasive Herbivorous Fish ( Siganus rivulatus ) Influences Both Benthic and Planktonic Microbes Through Defecation and Nutrient Excretion.” Science of the Total Environment 838: 156207. [DOI] [PubMed] [Google Scholar]
- Fassarella, M. , Blaak E. E., Penders J., Nauta A., and Zoetendal E. G.. 2021. “Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health.” Gut 70: 595–605. [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Xie H., Gao M., et al. 2020. “Dynamic of Composition and Diversity of Gut Microbiota in Triatoma rubrofasciata in Different Developmental Stages and Environmental Conditions.” Frontiers in Cellular and Infection Microbiology 10: 587708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King‐Lun, K. , Chan R. K. Y., and Qiu J.‐W.. 2009. “The Potential of the Invasive Snail Pomacea canaliculata as a Predator of Various Life‐Stages of Five Species of Freshwater Snails.” Malacologia 51: 343–356. [Google Scholar]
- Lanning, D. , Sethupathi P., Rhee K. J., Zhai S. K., and Knight K. L.. 2000. “Intestinal Microflora and Diversification of the Rabbit Antibody Repertoire.” Journal of Immunology 165: 2012–2019. [DOI] [PubMed] [Google Scholar]
- Leza, M. , Herrera C., Marques A., Roca P., Sastre‐Serra J., and Pons D. G.. 2019. “The Impact of the Invasive Species Vespa velutina on Honeybees: A New Approach Based on Oxidative Stress.” Science of the Total Environment 689: 709–715. [DOI] [PubMed] [Google Scholar]
- Li, L.‐H. , Lv S., Lu Y., et al. 2019. “Spatial Structure of the Microbiome in the Gut of Pomacea canaliculata .” BMC Microbiology 19: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Qian Z., Yang J., Lin Y., Li H., and Chen L.. 2022. “Seasonal Variation in Structure and Function of Gut Microbiota in Pomacea canaliculata .” Ecology and Evolution 12: e9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, F. , Wong P. K., Lin L. I., Lan C., and Qiu J. W.. 2010. “Impact of Invasive Apple Snails in Hong Kong on Wetland Macrophytes, Nutrients, Phytoplankton and Filamentous Algae.” Freshwater Biology 55: 1191–1204. [Google Scholar]
- Liu, M. , Sui C., Wang B., et al. 2024. “Effects of Short‐Term Exposure to Pomacea canaliculata Secretions on Limnodrilus hoffmeisteri and Propsilocerus akamusi: A Study Based on Behavior, Intestinal Microbiota, and Antioxidant System.” Ecology and Evolution 14: e11591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrer, A. M. , Thrush S. F., and Gibbs M. M.. 2004. “Bioturbators Enhance Ecosystem Function Through Complex Biogeochemical Interactions.” Nature 431: 1092–1095. [DOI] [PubMed] [Google Scholar]
- Maldonado, M. A. , and Martín P.. 2019. “Dealing With a Hyper‐Successful Neighbor: Effects of the Invasive Apple Snail Pomacea canaliculata on Exotic and Native Snails in South America.” Current Zoology 65: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura, K. , Izumi Y., Yoshida K., and Wada T.. 2016. “Cold Tolerance of Invasive Freshwater Snails, Pomacea canaliculata , P. maculata, and Their Hybrids Helps Explain Their Different Distributions.” Freshwater Biology 61: 80–87. [Google Scholar]
- Melotto, A. , Manenti R., and Ficetola G. F.. 2020. “Rapid Adaptation to Invasive Predators Overwhelms Natural Gradients of Intraspecific Variation.” Nature Communications 11: 3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud, E. , Desrosiers G., Mermillod‐Blondin F., Sundby B., and Stora G.. 2005. “The Functional Group Approach to Bioturbation: The Effects of Biodiffusers and Gallery‐Diffusers of the Macoma balthica Community on Sediment Oxygen Uptake.” Journal of Experimental Marine Biology and Ecology 326: 77–88. [Google Scholar]
- Michaud, E. , Desrosiers G., Mermillod‐Blondin F., Sundby B., and Stora G.. 2006. “The Functional Group Approach to Bioturbation: II. The Effects of the Macoma balthica Community on Fluxes of Nutrients and Dissolved Organic Carbon Across the Sediment‐Water Interface.” Journal of Experimental Marine Biology and Ecology 337: 178–189. [Google Scholar]
- Nie, L. , Zhou Q. J., Qiao Y., and Chen J.. 2017. “Interplay Between the Gut Microbiota and Immune Responses of Ayu ( Plecoglossus altivelis ) During Vibrio anguillarum Infection.” Fish & Shellfish Immunology 68: 479–487. [DOI] [PubMed] [Google Scholar]
- Nogaro, G. , and Burgin A. J.. 2014. “Influence of Bioturbation on Denitrification and Dissimilatory Nitrate Reduction to Ammonium (DNRA) in Freshwater Sediments.” Biogeochemistry 120: 279–294. [Google Scholar]
- Nunes, A. L. , Richter‐Boix A., Laurila A., and Rebelo R.. 2013. “Do Anuran Larvae Respond Behaviourally to Chemical Cues From an Invasive Crayfish Predator? A Community‐Wide Study.” Oecologia 171: 115–127. [DOI] [PubMed] [Google Scholar]
- O'Neil, C. M. , Guo Y., Pierre S., Boughton E. H., and Qiu J.. 2023. “Invasive Snails Alter Multiple Ecosystem Functions in Subtropical Wetlands.” Science of the Total Environment 864: 160939. [DOI] [PubMed] [Google Scholar]
- Oya, S. , Hirai Y., and Miyahara Y.. 1987. “Overwintering of the Apple Snail, Pomacea canaliculata LAMARCK, in North Kyushu.” Japanese Journal of Applied Entomology and Zoology 31: 206–212. [Google Scholar]
- Parker, J. L. , and Shaw J. G.. 2011. “ Aeromonas Spp. Clinical Microbiology and Disease.” Journal of Infection 62: 109–118. [DOI] [PubMed] [Google Scholar]
- Parras, A. , and Casadío S.. 2006. “The Oyster Crassostrea? Hatcheri (Ortmann, 1897), a Physical Ecosystem Engineer From the Upper Oligocene—Lower Miocene of Patagonia, Southern Argentina.” PALAIOS 21: 168–186. [Google Scholar]
- Pratihary, A. K. , Naqvi S., Naik H., et al. 2009. “Benthic Fluxes in a Tropical Estuary and Their Role in the Ecosystem.” Estuarine, Coastal and Shelf Science 85: 387–398. [Google Scholar]
- Ramani, A. , Rein K., Shetty K. G., and Jayachandran K.. 2012. “Microbial Degradation of Microcystin in Florida's Freshwaters.” Biodegradation 23: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, K. J. , Sethupathi P., Driks A., Lanning D. K., and Knight K. L.. 2004. “Role of Commensal Bacteria in Development of Gut‐Associated Lymphoid Tissues and Preimmune Antibody Repertoire.” Journal of Immunology 172: 1118–1124. [DOI] [PubMed] [Google Scholar]
- Sassera, D. , Epis S., Pajoro M., and Bandi C.. 2013. “Microbial Symbiosis and the Control of Vector‐Borne Pathogens in Tsetse Flies, Human Lice, and Triatomine Bugs.” Pathogens and Global Health 107: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberloff, D. , Martin J. L., Genovesi P., et al. 2013. “Impacts of Biological Invasions: What's What and the Way Forward.” Trends in Ecology & Evolution 28: 58–66. [DOI] [PubMed] [Google Scholar]
- Stief, P. 2013. “Stimulation of Microbial Nitrogen Cycling in Aquatic Ecosystems by Benthic Macrofauna: Mechanisms and Environmental Implications.” Biogeosciences 10: 7829–7846. [Google Scholar]
- Sui, C. , Liu M., Chuan S., et al. 2024. “Responses of Survival, Antioxidant System and Intestinal Microbiota of Native Snail Bellamya purificata to the Invasive Snail Pomacea canaliculata .” Scientific Reports 14: 21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, G. , Mengmeng Y. I., Yishuai D. U., et al. 2016. “The Impact of Aeromonas salmonicida Infection on Behaviour and Physiology of Atlantic Salmon ( Salmo salar L.).” Aquaculture Research 47: 2287–2296. [Google Scholar]
- Susin, T. 2004. “Biology of the Golden Apple Snail, Pomacea canaliculata (Lamarck, 1822), with Emphasis on Responses to Certain Environmental Conditions in Sabah, Malaysia.” Molluscan Research 24: 139–148. [Google Scholar]
- Swanson, C. , Baxa D. V., Young P. S., Cech J. J. Jr., and Hedrick R. P.. 2002. “Reduced Swimming Performance in Delta Smelt Infected With Mycobacterium Spp.” Journal of Fish Biology 61: 1012–1020. [Google Scholar]
- Tremaroli, V. , and Bäckhed F.. 2012. “Functional Interactions Between the Gut Microbiota and Host Metabolism.” Nature 489: 242–249. [DOI] [PubMed] [Google Scholar]
- Wang, E. , ZihaoWang K. G., DongyaLiu Z. L., and Mark R.. 2019. “Consumption of Florfenicol‐Medicated Feed Alters the Composition of the Channel Catfish Intestinal Microbiota Including Enriching the Relative Abundance of Opportunistic Pathogens.” Aquaculture 501: 111–118. [Google Scholar]
- Wang, J. , Lu X., Zhang J., Wei G., and Xiong Y.. 2020. “Regulating Soil Bacterial Diversity, Community Structure and Enzyme Activity Using Residues From Golden Apple Snails.” Scientific Reports 10: 16302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, H. , Feng L., Jiang W., et al. 2014. “Dietary Tryptophan Modulates Intestinal Immune Response, Barrier Function, Antioxidant Status and Gene Expression of TOR and Nrf2 in Young Grass Carp ( Ctenopharyngodon idella ).” Fish & Shellfish Immunology 40: 275–287. [DOI] [PubMed] [Google Scholar]
- Yang, J. , Yang H., and Li Y.. 2022. “The Triple Interactions Between Gut Microbiota, Mycobiota and Host Immunity.” Critical Reviews in Food Science and Nutrition 63, no. 33: 1–21. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Gu X. Z., Wang Z. D., Shen Q. S., and Yin J. C.. 2010. “The Influence of Tubificid Worms Bioturbation on the Exchange of Phosphorus Across Sediment‐Water Interface in Lakes.” Journal of Lake Science 22: 666–674. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in the online data repositories: https://doi.org/10.6084/m9.figshare.27283269.


