Abstract
The nucleocapsid (NC) protein of retroviruses is a small nucleic acid-binding protein important in virion assembly and in the encapsidation of the viral RNA genome into the virion particle. Multiple single-amino-acid substitutions were introduced into the NC of Moloney murine leukemia virus to examine further its role in viral replication. Two residues were shown to play important roles in the early events of replication. Unlike viruses with previously characterized NC mutations, these viruses showed no impairment in the late events of replication. Viruses containing the substitutions L21A and K30A expressed the normal complement of properly processed viral Gag proteins. Analysis of the RNA content of mutant virions revealed normal levels of unspliced and spliced viral RNA, and the tRNAPro primer was properly annealed to the primer binding site on the viral genome. The virions demonstrated no defect in initiation of reverse transcription using the endogenous tRNA primer or in the synthesis of long viral DNA products in vitro. Nonetheless, viruses possessing these NC mutations demonstrated significant defects in the synthesis and accumulation of viral DNA products in vivo.
The retroviral Gag polyprotein plays both structural and catalytic roles in viral replication. The virion particle, containing the viral RNA genome, is composed principally of a large array of Gag multimers (reviewed in reference 61). After assembly, Gag is processed by the virally encoded protease into several smaller products with specific functions. The nucleocapsid (NC) protein is a small, basic, carboxy-terminal portion of the Gag protein of most retroviruses. With the exception of the spumaviruses, all retroviral NC proteins possess either one or two highly conserved motifs called Cys-His boxes, which form a complex three-dimensional structure called a zinc finger, in which the conserved cysteines and histidines coordinate zinc (5, 37, 57, 59). In addition, all NC proteins, including spumavirus NCs, contain numerous basic residues. Many of the functions of NC are dependent on its ability to interact with viral RNA, and nuclear magnetic resonance structures of both human immunodeficiency virus type 1 (HIV-1) and Moloney murine leukemia virus (MoMuLV) NCs complexed with nucleic acid have clearly demonstrated the importance of the zinc finger (or fingers) in mediating this interaction (18, 55).
Both Gag and NC exhibit specific and nonspecific RNA binding activity in a wide variety of assays (4, 6, 14, 38, 54). In the context of the carboxy-terminal portion of the Gag polyprotein, the NC proteins of avian and mammalian retroviruses have been shown to be responsible for mediating the specific interaction between the Gag polyprotein and cis-acting viral RNA sequence Ψ, leading to the selective packaging of the viral RNA genome into assembling virions (reviewed in reference 5). Disruption of the conserved basic residues or residues in the Cys-His box of NC often drastically impairs RNA packaging (1, 31, 32, 40, 41). NC has also been shown to play a crucial role in the assembly of virions by mediating the multimerization of Gag proteins through protein-protein and nonspecific interactions with RNA (3, 9, 10, 25, 43, 65, 67). Deletion of multiple basic residues impairs viral assembly, with a consequent severe inhibition in the release of particles (8, 9, 35).
In addition to its roles in packaging and assembly, NC has been demonstrated to perform a wide variety of functions in vitro. Because of its ability to facilitate the temporary breakage and reformation of nucleic acid base pairs to allow the establishment of the most stable conformation, NC has been described as a nucleic acid chaperone (50). NC facilitates the annealing of the tRNA primer to the viral genome and the annealing of the dimerization initiation site of the genomic RNA that leads to the linking of the two strands of the viral genome to be packaged into virions (15, 19, 20, 35, 46, 47). Furthermore, NC associates nonspecifically along the length of the genomic RNA and promotes a conformational maturation of the RNA in the virion that leads to a greater thermostability of the RNA dimer (16, 26, 27, 42).
By virtue of its ability to promote both melting and annealing of RNA structures, NC has also been shown to facilitate many processes involved in reverse transcription of the viral genome (33, 62, 63). HIV-1 NC has been shown to facilitate processive polymerization by decreasing pausing at sites of secondary structure (52, 62). The complete process of reverse transcription involves several strand transfer reactions, demonstrated in HIV-1 to be facilitated by NC, where nucleic acid base pairs are broken and DNA strands are reannealed to terminally redundant complementary sequences in the genome (63). Furthermore, NC has been shown to prevent TAR-dependent self-priming in HIV-1 (33).
Because of the critical role of NC in the late events of replication, it has been difficult to study the effects of NC mutations on the early events of replication in vivo. However, a few mutations that caused a decrease in infectivity orders of magnitude greater than the defect observed in RNA packaging have been generated. Mutation of the conserved aromatic residue (Y28) immediately preceding the second Cys in MoMuLV caused an approximately threefold reduction in packaging efficiency, as well as a decrease in the specificity of RNA packaging, but rendered these viruses completely noninfectious (31, 40, 64). Less DNA was made from RNA packaged by this virus. However, it remains unclear if tRNA packaging, annealing, and initiation of reverse transcription are normal in this virus (64). Alteration of the MoMuLV CCHC zinc finger to CCHH or CCCC had no effect on the ability of these NC proteins to package RNA but caused greatly decreased synthesis of proviral DNA (29). Further analysis revealed that the ends of DNA generated by these viruses seem to be partially degraded, implicating NC in protection of the ends of newly synthesized viral DNA prior to integration (30). Supporting evidence for this role of NC in the protection of viral DNA from cellular nucleases was provided by the observation that HIV-1 mutants with replacement of basic residues in NC generated early DNA products that were soon degraded (8). To further study the role of MoMuLV NC in retroviral replication, we sought to create subtle alterations in NC that might result in a less drastic impairment of replication than did previously studied mutations, such that the poorly understood postentry functions of NC could be examined.
MATERIALS AND METHODS
MoMuLV plasmid construction.
Plasmid pNCA contains an infectious molecular clone of MoMuLV (12). MoMuLV and mutant versions of MoMuLV were expressed from derivative plasmid pNCA BstBI, which contains a silent BstBI restriction site in the pro sequence. To make these constructs, an intermediate plasmid, pNCA BstBI 3-1, was first generated. pNCA BstBI 3-1 was created by ligating two PCR fragments: one extending from the NruI site in NC to the BstBI site (using oligonucleotides NruIfor [5′AAGGAGGTCCCAACTCGATCGCGACCA3′] and BstBIrev [5′CCCATAACCTGAGCTCCTGATCCTTCGAAGTGGATTTGG3′]) and another extending from the BstBI site to the BclI site in pol (using oligonucleotides BstBIfor [5′CTAAAAGCCCAAATCCACTTCGAAGAATCAGG3′] and BclIrev [5′AGAGGTTGCTTTCAGAGGTATGATCAGAGG3′]). The DNAs were inserted into vector TOPO2.1 using the TOPO TA kit (Invitrogen). The complete amplified DNA was excised with NruI and BclI and ligated into plasmid pNCA using the same restriction sites, generating a two-base deletion in the NruI site. To create plasmid pNCA BstBI, a DNA fragment of pNCA from the XhoI site in CA to the BstBI site in pro was amplified using oligonucleotides XhoIfor (5′TTCCCCTCGAGCGCCCAGACTGG3′) and BstBIrev (5′CCCATAACCTGAGCTCCTGATCCTTCGAAGTGGATTTGG3′) and used to replace the XhoI-BstBI fragment from the pNCA BstBI 3-1 plasmid. All PCR was performed using the Expand High Fidelity PCR system (Boehringer Mannheim) according to the manufacturer's protocol.
Point mutations were introduced into the NC region of pNCA BstBI by two-step overlapping PCR with pNCA BstBI as the template. In the first step, the mutations were introduced into partial-length DNA fragments by PCR using mutation-specific primers and fixed primers MG4 (5′TCGCAGGGATCCCCCTCCGCGCAGGA3′) and BstBIrev (described above). In the second step, the entire CANC-BstBI fragment was generated using the two overlapping PCR products from the first step as the template and oligonucleotides MG4 and BstBIrev as outer primers. The mutant CANC-BstBI PCR products were cloned into the TOPO2.1 shuttle vector as described above. The NC mutations were then introduced in the full-length viral construct by digesting the TOPO2.1-CANC BstBI constructs that contain the mutant NC sequences with XhoI (which cuts within the CA sequence) plus BstBI, and ligating the fragments into the XhoI and BstBI sites of plasmid pNCA BstBI 3-1. The oligonucleotides used for mutagenesis are listed in Table 1.
TABLE 1.
Oligonucleotide primers used in two-step overlapping PCR mutagenesisa
| Construct | Oligonucleotide |
|---|---|
| G6A | G6AFspI for: 5′CCACTGTCGTTAGTGCGCAGAAACAGGATAGACAGG3′ |
| G6AFspI rev: 5′TGCGCACTAACGACAGTGG3′ | |
| K8A | K8A for: 5′GTCGTTAGTGGACAGGCACAGGATAGACAGGG3′ |
| K8A rev B: 5′GTGCCTGTCCACTAACGAC3′ | |
| Q9A | Q9A for 2-1: 5′GGACAGAAAGCGGATAGACAGGGAGGAGAACG3′ |
| Q9A revC2-1: 5′CTGTCTATCCGCTTTCTGTCCACTAACGACAGTGG3′ | |
| Q12A | Q12A NaeIfor: 5′CGTTAGTGGACAGAAACAGGATAGAGCCGGCGGAGAACGAAGGAGGTCCCAACTCG3′ |
| Q12A NaeIrev: 5′GCCGGCTCTATCCTGTTTCTGTCCACTAACG3′ | |
| R17A | R17A for2-1: 5′GGAGAACGAGCGAGGTCCCAACTCGATCGC3′ |
| R17A rev2-1: 5′TTGGGACCTCGCTCGTTCTCCTCCCTGTCTATCC3′ | |
| Q20A | Q20A XbaIfor: 5′CAGGGAGGAGAACGAAGGAGGTCCGCTCTAGATCGCGACCAGTGTGCCTACTGC3′ |
| Q20A XbaIrev: 5′TAGAGCGGACCTCCTTCGTTCTCCTCC3′ | |
| L21A | L21A for2-1: 5′AGGTCCCAAGCCGATCGCGACCAGTGTGCC3′ |
| L21A rev2-1: 5′GTCGCGATCGGCTTGGGACCTCCTTCGTTCTCC3′ | |
| Q25A | Q25A SphIfor: 5′CGAAGGAGGTCCCAACTCGATCGCGACGCATGCGCCTATGCAAAGAAAAGGGGCACTGG3′ |
| Q25A SphIrev: 5′GCATGCGTCGCGATCGAGTTGGGACC3′ | |
| K30A | K30A for2-1: 5′GCCTACTGCGCAGAAAAGGGGCACTGGGC3′ |
| K30A rev2-1: 5′CCCCTTTTCTGCGCAGTAGGCACACTGGTCG3′ | |
| E31K | E31K forB: 5′GCCTACTGCAAAAAAAAGGGGCACTG3′ |
| E31K rev: 5′CAGTGCCCCTTTTTTTTGCAGTAGGC3′ | |
| K32A | K32A for2-1: 5′TGCAAAGAAGCGGGGCACTGGGCTAAAGATTGTCC3′ |
| K32A rev2-1: 5′CCAGTGCCCCGCTTCTTTGCAGTAGGCACACTGG3′ | |
| G33V | G33V forB: 5′AGAAAAGGTGCACTGGGCTAAAGATTGTCC3′ |
| G33V revB: 5′CAGTGCACCTTTTCTTTGCAGTAGG3′ | |
| W35G | W35G for: 5′GCAAAGAAAAGGTGCACTGGGC3′ |
| W35G revB: 5′CTTTAGCCCCGTGCCCCTTTTCTTTGC3′ | |
| K37A | K37A for2-1: 5′CACTGGGCTGCAGATTGTCCCAAGAAACCACG3′ |
| K37A rev2-1: 5′GGGACAATCTGCAGCCCAGTGCCCCTTTTCTTTGC3′ | |
| C39H | C39H forD: 5′CACTGGGCTAAAGATCATCCCAAGAAACCACG3′ |
| C39H revD: 5′GGTTTCTTGGGATGATCTTTAGCCCAGTGCC3′ | |
| K41A | K41A SacIIfor: 5′TGTCCCGCGAAACCACGAGGACCGCGGGGACCAAGACCC3′ |
| K41A SacIIrev: 5′TCCCCGCGGTCCTCGTGGTTTCGCGGGACAATCTTTAGC3′ | |
| K42A | K42A AvrIIfor: 5′AAGGCACCACGAGGACCTAGGGGACCAAGACC3′ |
| K42A AvrIIrev: 5′TCCCCTAGGTCCTCGTGGTGCCTTGGGACAATCTTTAGC3′ | |
| R44A | R44A EagIfor: 5′AAGAAACCGGCCGGACCTCGGGGACCAAGACCC3′ |
| R44A EagIrev: 5′TCTTGGTCCCCGAGGTCCGGCCGGTTTCTTGGG3′ | |
| C39H/E31K | C39H/E31K forB: 5′GCAAAAAAAAGCGGCACTGGGCTAAAGATCATCCCAAGAAACCACG3′ |
| C39H/E31K revB: 5′CTTGGGATGATCTTTAGCCCAGTGCCCCTTTTTTTTGCAGTAGGCACACTGG3′ |
Oligonucleotides named “for” match sense strand DNA, and oligonucleotides named “rev” match negative-strand DNA. Bases in boldface indicate changes in DNA sequence. All first-step PCRs were carried out using the specific “for” mutagenesis oligonucleotide paired with oligonucleotide BstBI rev and the specific “rev” oligonucleotide paired with oligonucleotide MG4. Second-step reactions were carried out using oligonucleotide pair BstBI rev and MG4 with the first-step PCR products as the template.
Cell culture.
NIH 3T3 cells and Rat 2-2 cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% bovine calf serum, l-glutamine, and penicillin-streptomycin at 37°C and 5% CO2. 293T cells were maintained under the same conditions in DMEM supplemented with 10% fetal calf serum, l-glutamine, and penicillin-streptomycin.
Transformation of mammalian cells.
293T cells were transfected using calcium phosphate (44). Gene expression was analyzed 2 days after transfection. NIH 3T3 cells were transfected using a standard calcium phosphate–HEPES-buffered saline protocol (53). Rat 2-2 cells were transfected using DEAE dextran (Pharmacia). Briefly, 2 × 105 Rat 2-2 cells washed with phosphate-buffered saline (PBS) containing Mg2+ and Ca2+ (PBS+). One microgram of DNA, 20 μl of 10-mg/ml DEAE dextran, and 380 μl of PBS+ were mixed and added to cells. Cells were incubated for 20 to 40 min at 37°C, with occasional rocking. Cells were rinsed once with PBS+, and medium was added.
Infection of mammalian cells.
Culture supernatants were normalized for virus by reverse transcriptase (RT) assays, and Polybrene was added to a final concentration of 5 μg/ml. Approximately 2 ml of these supernatants was then used to infect naive NIH 3T3 cells (2 × 105 cells in 60-mm-diameter dishes) for 2 h.
Western blot analysis and antibodies.
Western blot analyses were performed using 7.5 to 10% polyacrylamide gels, with proteins electrotransferred to an Immobilon-P membrane (Millipore) in transfer buffer containing 20% methanol. Peroxidase-conjugated secondary antibodies were detected by staining the membrane with ECL Western blotting detection reagent (Amersham Pharmacia Biotech) according to the manufacturer's protocol. Polyclonal anti-CA antiserum, raised in goats against AKV (79S-804), was used at a 1:5,000 dilution. Peroxidase-conjugated polyclonal antiserum, raised against goat immunoglobulin G (Boehringer Mannheim), was used at a 1:10,000 dilution.
Exogenous RT assay.
Exogenous RT assays were performed as described previously (60), and the radioactivity of the DNA product was quantitated by PhosphorImager analysis (28). The relative RT activity was calculated from the slope of the graph of radioactivity in DNA (in arbitrary pixel units) plotted against the reaction time (in minutes).
Endogenous RT assay.
Assays were performed as described previously (60) using virions prepared by centrifugation through sucrose step gradients. Short DNA products were separated on 8% acrylamide (19:1 acrylamide/bisacrylamide ratio)–7 M urea Tris-borate-EDTA gels and exposed overnight to film at −80°C or in a PhosphorImager cassette. To analyze longer RT products, samples were separated on 1% alkaline agarose gels.
tRNA tagging assay.
This assay is a modification of the endogenous RT assay in which only the first two nucleotides encoded by the viral RNA (both A) are added to the 3′ end of the tRNA primer. The reaction mixture for this assay contained 50 mM NaCl, 50 mM Tris-HCl (pH 8), 6 mM MgCl2, 1 mM dithiothreitol, 2.5 μM cold dATP, 0.1% NP-40, 5 μl of [α-32P]dATP (10 mCi/ml, 800 Ci/mmol). Approximately 25 μl of virions and 2 μl of RNase inhibitor (Boehringer Mannheim) were added to 50 μl of reaction mixture, and the samples were incubated for 5 min at 37°C. Following the reaction and proteinase K treatment and phenol extraction as described above, the pellets were immediately resuspended in 20 μl of formamide loading dye without digesting the tRNA. The samples were then analyzed as described above.
Analysis of viral DNA from infected cells.
Culture supernatants were collected, buffered with HEPES, filtered, normalized by quantitative exogenous RT assays, and used to infect fresh naive subconfluent Rat 2-2 cells or NIH 3T3 cells (approximately 2 × 106 cells per 10-cm-diameter dish). Infections were performed with 8 ml of normalized viral supernatant containing 8 μg of Polybrene per milliliter for 3 to 5 h, after which the virus was aspirated and replaced with fresh medium. After 20 h (5 h for one experiment), low-molecular-weight DNA was prepared by the method of Hirt (34). In one experiment, a 1:10 dilution of virus was used to infect naive cells. Equal amounts of all DNA preparations were added to an agarose gel, and viral DNA levels were assessed by Southern blots using a complete viral genomic DNA as the probe. DNA recovery was monitored by probing the filters with a random-hexamer-primed 524-base fragment of rat mitochondrial DNA generated by PCR (described below). Quantitation of viral DNA was determined using Image Quant software to measure the intensity of signals on a PhosphorImager screen. Quantitation of mitochondrial DNA was determined using Image Quant software on a densitometer-scanned film exposure. Mitochondrial and viral DNA intensities were determined separately. Relative viral band intensities were adjusted for mitochondrial DNA levels.
PCR analysis of Hirt DNA.
Three pairs of primers were used to analyze viral DNA intermediates. The first pair amplified an approximately 600-bp fragment present in 2-long-terminal-repeat (2-LTR) circles (MR4091 [5′CTCTTTTATTGAGCTCGGG3′] and MR5784 [5′AGTCCTCCGATTGACTGAG3′]). The second pair amplified a 150-bp fragment representing minus-strand strong-stop DNA (−SSS) (−SSS-Sp [5′GCGCCAGTCCTCCGATTGACTG3′] and −SSS-As [5′CGGGTAGTCAATCACTGAG3′]).
The third pair of primers amplified a 524-bp fragment of rat mitochondrial DNA (mtDNA for [5′GTTAATGTAGCTTATAATAAAGC3′] and mtDNArev [5′GTTTAGGGCTAAGCATAGTGGG3′]). In experiments where NIH 3T3 cells were infected, mouse mitochondrial primers (mouse mtDNAfor [5′CACCACTAACAGGATTCTTACC3′] and mouse mtDNArev [5′CTTTGAAGGCTCGCGGACTAG3′]) were used to amplify a 300-bp fragment.
Preparation of viral and cellular RNA.
RNA was prepared from purified virions produced by stably infected Rat 2-2 cells or transiently transfected 293T cells. Virions were normalized by quantitative RT assays. Ninety microliters of buffer containing 10 μg of yeast RNA were then added to 10 μl of purified virions, followed by 1 ml of RNAzolB (Tel-Test) and 100 μl of chloroform. The manufacturer's protocol was then followed. For purification of cellular RNA, 2 ml of RNAzolB was added to the 10-cm-diameter plates from which the virions were harvested, followed by 100 μl of chloroform. The manufacturer's protocol was then followed.
RNase protection assays.
A 572-base XbaI-to-EagI fragment from MoMuLV, spanning the splice donor site (7), was cloned in plasmid pBluescript SK and used as a template for synthesis of a riboprobe. The plasmid DNA was linearized by digestion with HindIII, and the riboprobe was transcribed with T3 RNA polymerase using the Ambion MAXIscript in vitro transcription kit according to the manufacturer's protocol. RNA was labeled with [32P]UTP at a specific activity of 3,000 Ci/mmol. The riboprobe was purified on a 5% polyacrylamide (19:1 acrylamide/bisacrylamide ratio)–7 M urea Tris-borate-EDTA gel, excised, and eluted overnight at room temperature in elution buffer (0.5 M ammonium acetate, 1 mM EDTA, 0.1% sodium dodecyl sulfate). RNase protections were performed using an Ambion RPA III kit according to the manufacturer's protocol. Approximately 10 μg of cellular RNA and 100 μl of the viral RNA preparations were used in each reaction. Products were separated on 5% polyacylamide–7 M urea gel and exposed to X-ray film overnight.
RESULTS
Construction of mutant viruses.
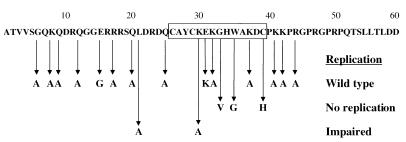
To study the effects of single point mutations on the functions of MoMuLV NC, we replaced conserved and less-conserved amino acids within and outside the Cys-His box with alanine (Fig. 1). Additionally, several other previously characterized substitutions, including G33V, W35G (40), and C39H (29), were made as controls. Finally, the substitution mutation E31K, which affects interactions of NC with a host helicase (unpublished observations), and the double substitution C39H E31K were also introduced into the NC region. All these mutations were cloned into a full-length infectious viral clone.
FIG. 1.
Point mutations in the NC region of gag. Viruses harboring the mutations L21A and K30A replicated slowly in culture (15- and 6-day delays, respectively). G33V, W35G, and C39H viruses did not replicate, and all others replicated like the wild type.
Analysis of replication kinetics of mutated viruses.
To examine the effects of the NC mutations on viral replication, Rat 2-2 cells were transiently transfected with full-length viral constructs containing the NC mutations. The cells were passaged when near confluence (approximately every 3 days), and aliquots of culture supernatants were collected daily and assayed for RT activity as a measure of viral spread. Most of the point mutations in NC had no effect on the replication rates of mutant viruses in cells grown in culture. G6A, K8A, Q9A, Q12A, R17A, Q20A, Q25A, K32A, K37A, K41A, K42A, R44A, and E31K mutants all exhibited a detectable RT signal at the same time posttransfection as wild-type virus (Fig. 1). In agreement with previously published reports (29, 40), mutations G33V, W35G, and C39H, as well as the C39H E31K double mutation, were lethal to the virus (Fig. 1).
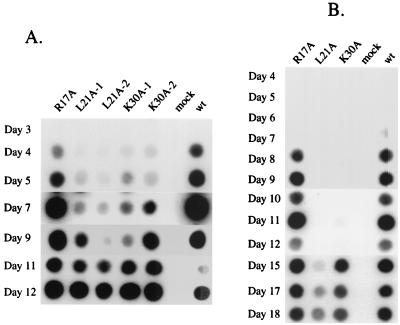
Although most point mutations in NC had no apparent effect on viral replication, replacement of a leucine residue preceding the Cys-His box (L21A) or a lysine within the Cys-His box (K30A) significantly delayed retroviral replication. For cells transfected by wild-type DNA, a detectable RT signal first appeared at day 4 posttransfection, and the signal reached maximum plateau levels by day 7. In contrast, an RT signal was not detectable in cultures transfected with the K30A mutant until day 5 or 6 and was not maximal until days 9 to 11 posttransfection. Even slower replication was observed for the L21A mutant, for which only a very faint RT signal was detected at days 5 to 7, with saturating signals not reached until day 11 or 12 (see Fig. 2A). Similar results were obtained in repeated experiments.
FIG. 2.
Viruses harboring NC mutations L21A and K30A replicate slowly in culture. (A) Rat 2-2 cells were transfected with DNAs of wild-type (wt) or mutant viruses using DEAE-dextran. Mutants were transfected in duplicate. Culture supernatants were collected daily, and RT assays were performed as a measure of virus production. (B) Kinetics of virus replication after initiating infection in Rat 2-2 cells with normalized levels of virus.
The late appearance of the RT signal in the cultures that were transfected by the L21A or K30A viruses could be the result of the slow replication of these viruses or could be due to the appearance of revertants during replication in the Rat 2-2 cells. To distinguish between these possibilities, cell cultures were transfected with mutant viruses and grown for 2 weeks and viral supernatants were collected, normalized by exogenous RT activity, and used to infect naive Rat 2-2 cells. Cultures infected with the K30A or the L21A mutant viruses still showed significant delays in the appearance of RT activity, indicating that the late appearance of virus in culture probably represents slow replication rather than reversion or suppression of the original mutations. K30A virus-infected cells showed a 6-day delay while L21A virus-infected cells showed an 11- to 15-day delay compared to cultures infected with wild-type or R17A control viruses (Fig. 2B and 3B).
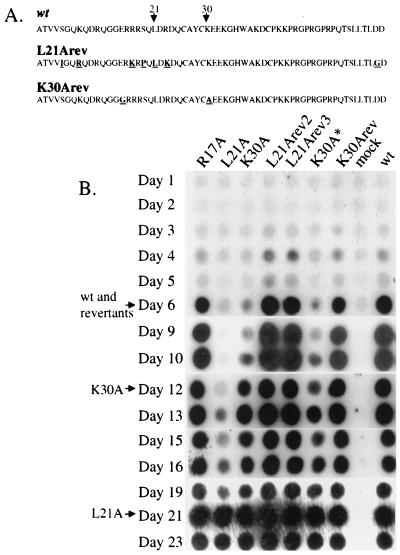
FIG. 3.
Revertant viruses replicate with wild-type kinetics. (A) Amino acid sequences of the L21A revertant (both L21 Arev2 and -3 possess the same sequence), K30A revertant (K30Arev), and wild-type (wt) NC. Altered residues are underlined. (B) Viral supernatants were harvested from chronically infected Rat 2-2 cells, normalized by exogenous RT assays, and used to infect naive Rat 2-2 cells. Culture supernatants were collected daily, and RT assays were performed as a measure of virus production. L21Arev2 and L21Arev3 possess the same sequence. K30A∗ possesses only the K30A mutation. Arrows indicate times at which maximal RT signals are detected in cultures.
Isolation of revertants of L21A and K30A viruses that replicate with wild-type kinetics.
To select for revertant viruses with accelerated replication kinetics, 293T cells were transfected with full-length viral constructs harboring the L21A or the K30A mutations, and culture supernatants were harvested 60 h later. The filtered virus preparations were diluted 1:1,000 and added to naive NIH 3T3 cells. Infected cells were passaged, and, as soon as RT activity was detected in the culture medium, the supernatants were used to initiate a new round of infection in naive NIH 3T3 cells. Following four such rounds of infection, all of the tested viruses appeared to spread in the cell cultures with wild-type kinetics (data not shown). To identify changes in the sequences of these viruses, virus from the fourth round of infection was used to acutely infect naive NIH 3T3 cells and DNA preparations were made 20 h postinfection. PCR was performed with primers designed to amplify a region from the central portion of the capsid region of gag to the middle of pro, and the resulting DNA was cloned. For each virus, the sequence of six clones was determined. For the K30A virus, three of the six sequenced PCR clones retained the original K30A mutation and possessed no other mutations. The other three, named K30Arev (for reversion to wild-type kinetics), retained the original K30A and possessed an additional missense mutation changing a glutamate residue at position 15 of NC to glycine (E15G) (Fig. 3A). For the L21A virus, all six clones possessed the same sequence, which was evidently the result of a recombination event with an endogenous retroviral sequence. The recombined sequence in the virus, named L21Arev, included the entire NC and at least part of CA. The sequence of the reverted virus, although very different at the nucleotide level, possessed a limited amino acid variance from that of MoMuLV (Fig. 3A). Most importantly, amino acid 21 was leucine as in wild-type MoMuLV. Three of the additional six mutations were conservative (K8R, R17K, and R23K), but the other three were not (S5I, S19P, and D59G).
To evaluate the effect of these changes and to rule out effects of other mutations that might be present in the viral genome outside the sequenced region, the XhoI-BstBI fragments from the reverted viruses were used to replace the corresponding fragment of a wild-type DNA. The original L21A and K30A viruses, as well as a K30A virus reconstructed by replacing sequence from one of the six clones that retained the original K30A sequence (K30A∗), were used as controls. Rat 2-2 cells were transfected with viral DNAs, and cells were passaged for several weeks until virus production reached maximal levels. At this time, viral supernatants were harvested from these chronically infected Rat 2-2 cells, normalized by exogenous RT assays, and used to infect naive Rat 2-2 cells (Fig. 3B). The parental mutants recapitulated the slow replication seen previously, while the revertant viruses replicated with the same kinetics as the wild type (Fig. 3B). The wild-type replication kinetics of K30Arev3 indicates that E15G is a second-site compensatory mutation for the K30A substitution. The coexistence of the parental K30A virus with the K30A/E15G virus in the reverting culture probably represents the ability of the parent virus to be efficiently encapsidated and spread by the revertant acting as a helper.
L21A and K30A mutant viruses assemble and release normal amounts of viral particles.
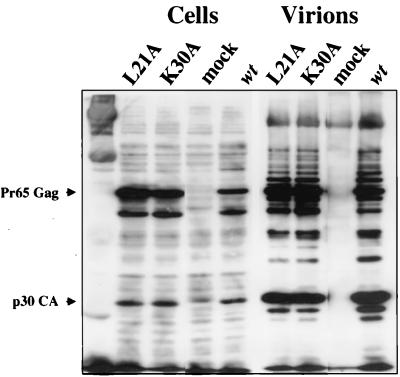
We next investigated the stages of replication in which the L21A and K30A mutant viruses are defective. The NC domain of Gag has an important role in virus assembly: truncations and point mutations in NC can drastically reduce the amount of virions released (9, 43, 67). In addition, such Gag mutants tend to exhibit poor processing and assembly (8, 65). We tested whether the L21A and K30A mutations cause an effect on the synthesis, processing, or release of Gag from cells. The L21A and K30A mutations were cloned into the pNCA BstBI plasmid, and 293T cells were transiently transfected with these constructs. After 48 h, culture supernatants were collected and filtered, viral particles were purified through a 25% sucrose cushion, and cells were lysed. Cell extracts and virion pellets were analyzed by Western blotting with antiserum specific for Gag. Cells expressing wild-type, L21A, and K30A viruses all released equal numbers of viral particles, and there was no significant difference in the yield or migration of the processed or unprocessed Gag products within the transfected cells or in the virions (Fig. 4).
FIG. 4.
Mutant viruses assemble and release normal amounts of virions containing properly processed Gag proteins. 293T cells were transfected with viral DNAs. Culture supernatants were collected, and virions were purified. Virions and transfected cells were lysed and analyzed by Western blotting with antiserum that recognizes MoMulV Gag. The positions of Gag and CA are indicated.
L21A and K30A mutant viruses encapsidate their genomic RNA as efficiently as their revertant viruses or the wild type.
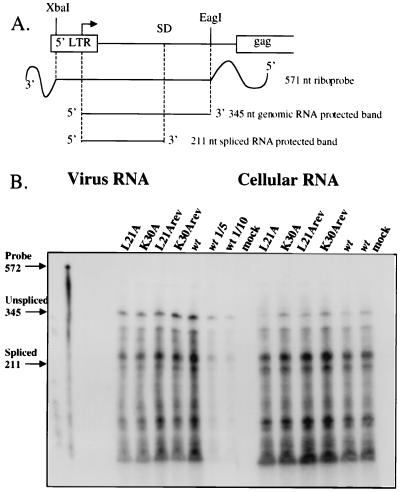
Interactions between the RNA encapsidation signal and the NC domain of Gag mediate the encapsidation of the viral genomic RNA into the budding virion particle (reviewed in reference 5). To examine the ability of the mutant viruses to package viral RNA, viral RNA levels were determined in producer cells and in purified virions of wild-type, L21A, K30A, L21Arev, and K30Arev viruses. Equal amounts of total RNA extracted from producer cells and RNA extracted from equal amounts of purified virions, normalized by RT activity, were analyzed by an RNase protection assay using a virus-specific riboprobe. This probe spans the splice donor site in the viral genome and allows the detection of both spliced and unspliced genomic RNA (Fig. 5A) (7). The source of the viruses was either transiently transfected 293T cells (Fig. 5B) or virus-containing supernatants of chronically infected Rat 2-2 cells that had been transfected several weeks earlier (data not shown). In these experiments, both spliced and unspliced RNA of L21A and K30A viruses was expressed in cells at the same level as that for wild-type, L21Arev, or K30Arev virus and there was no significant difference in the amounts of the genomic RNA packaged by these viruses. In addition, all the viruses exhibited the same ratio of spliced to unspliced viral RNA in the producer cells and in the virions (Fig. 5B). Thus, the L21A and K30A mutations do not affect the efficiency or specificity of viral RNA packaging.
FIG. 5.
Mutant virions show no impairment in specific viral RNA encapsidation in an RNase protection assay. (A) Map of antisense RNA probe and expected protected RNA fragments. SD, splice donor site. (B) 293T cells were transfected with DNA of wild-type (wt) or mutant viruses, and virions were purified. Cellular RNA was prepared and normalized by absorbance at 260 nm, and viral RNA was prepared from equivalent amounts of virions. The levels of viral RNA were assessed by annealing to a radiolabeled antisense RNA probe followed by RNase digestion. The positions of the undigested probe and the bands corresponding to protection by unspliced and spliced viral RNA are indicated. As a quantitation control, reactions on 1:5 and 1:10 dilutions of wild-type RNA were also performed.
The products of reverse transcription are reduced in cells infected by mutant viruses.
Because the mutant viruses spread slowly in culture but were not defective in production, assembly, or release of particles, we suspected that the replication defect might be manifest in a postentry step. To analyze the products of reverse transcription, naive NIH 3T3 cells were infected with equal numbers of viral particles, as determined by quantitative RT assay. The source of virus for these experiments was supernatants of chronically infected Rat 2-2 cells that had been transfected 6 weeks earlier, ensuring that no transfected DNA would remain undegraded in the culture supernatant. Twenty hours after infection, low-molecular-weight DNA was extracted and newly reverse transcribed viral DNA was analyzed by semiquantitative PCR and by Southern blotting. PCR was performed with primers designed to amplify early reverse transcription products (−SSS DNA) and late products (2-LTR circles). Amplification of mitochondrial DNA, which copurifies with viral DNA, was used as an internal control for extraction efficiency. The number of cycles was controlled to produce PCR products in the linear range of detection, as determined by analysis of 5- and 10-fold dilutions. DNA preparations made from cells infected by the L21A mutant yielded approximately 25-fold-less −SSS DNA than those from cells infected by the wild type, while those from K30A-infected cells produced approximately 15-fold less than those from cells infected by the wild type (Fig. 6A). There was a similar, but less drastic, reduction in 2-LTR circular DNA (Fig. 6A). However, as over 30 cycles of PCR were required to detect 2-LTR circular DNA, quantification of these DNA products was less reliable.
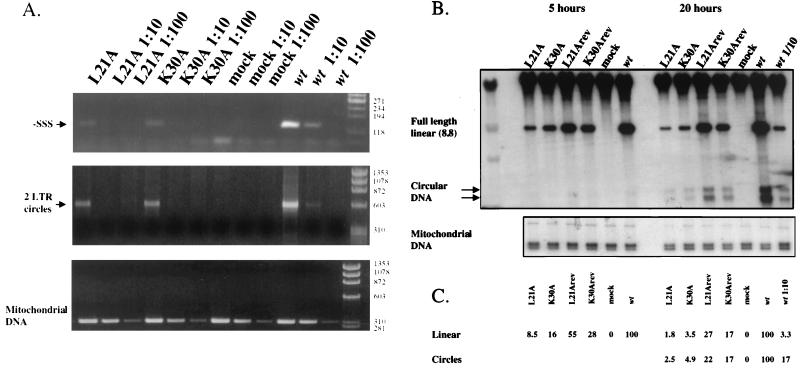
FIG. 6.
Mutant viruses are impaired in viral DNA synthesis in vivo. Viral supernatants were harvested from chronically infected Rat 2-2 cells, normalized by exogenous RT assays, and used to infect naive NIH 3T3 cells (PCR) or Rat 2-2 cells (Southern blots). (A) Twenty hours after infection, low-molecular-weight DNA was prepared and PCR was performed on 10-fold and 100-fold dilutions of DNA preparations to amplify early DNA products (−SSS) and late DNA products (2-LTR circles). Mitochondrial DNA was amplified as a control for DNA recovery. Wt, wild type. (B) Southern blots of low-molecular-weight DNA were performed with full-length viral DNA as a probe. The positions of full-length linear DNA and 1- and 2-LTR circle DNA are indicated. To control for the amount of input virus, a 1:10 dilution of wild-type virus was also used to infect cells in the 20-h experiment. To control for DNA recovery, filters were probed with a 524-base fragment of rat mitochondrial DNA. (C) Quantitation of the DNA bands detected by Southern hybridization.
Southern blots were also used to measure the levels of the reverse-transcribed viral genomic DNA. Rat 2-2 cells were infected by equal amounts of mutant virus, revertants, or wild-type viruses for 5 or 20 h. DNA was prepared from infected cells and analyzed by Southern blotting using the full viral genome as a probe. Viral DNA levels were determined by PhosphorImager and normalized to the levels of mitochondrial DNA. Like the PCR analysis, Southern blot analysis revealed a strong reduction in the levels of the full-length DNA of the L21A and K30A mutant viruses compared to those of wild-type or revertant viruses at each of the two time points (Fig. 6B). At 5 h postinfection, the levels of full-length linear viral DNA of L21A and K30A virus-infected cells were reduced approximately 10- and 5-fold, respectively, compared to those of the wild type. At 20 h postinfection these reductions were 50- and 20-fold, respectively. At this time point, the 1- and 2-LTR circle forms of viral DNA could also be detected, showing reductions in the mutant viruses similar to that seen in the linear viral DNA.
The revertant viruses produced higher levels of DNA than the parental mutants. There were still modest reductions in viral DNA synthesized by the L21Arev (fourfold) and K30Arev (five-fold) viruses compared to that synthesized by the wild type, despite the fact that these viruses replicate with wild-type kinetics in culture.
In vitro initiation of DNA synthesis and DNA elongation in the mutant viruses are identical to the wild type.
The reduced levels of the reverse-transcribed products of L21A and K30A viruses might be caused, in part, by impaired initiation or elongation of reverse transcription. We first examined the ability of L21A and K30A mutant viruses to initiate reverse transcription from the natural tRNA that is annealed to the genomic viral RNA in an endogenous assay. Virions were purified and normalized as described above, partly disrupted with detergent, provided with labeled dATP as the sole nucleotide, and allowed to extend the tRNA primer two bases. The products of this reaction were separated by electrophoresis, and the labeled tRNA was quantified. With the efficiency of wild-type virus to extend tRNA set as 100%, L21A, K30A, L21Arev, and K30Arev viruses extended their tRNA with efficiencies of 96, 113, 83, and 61%, respectively (Fig. 7A). Thus, the L21A and K30A mutations did not affect the initiation of reverse transcription from the endogenous tRNA primer.
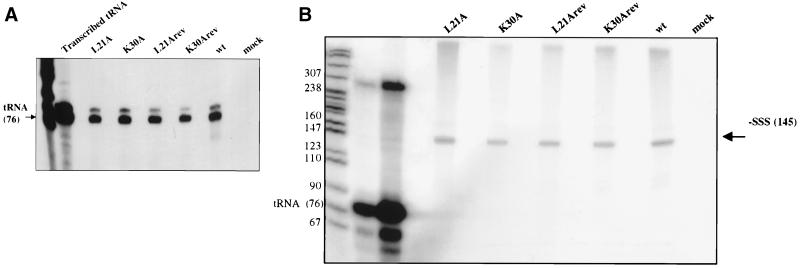
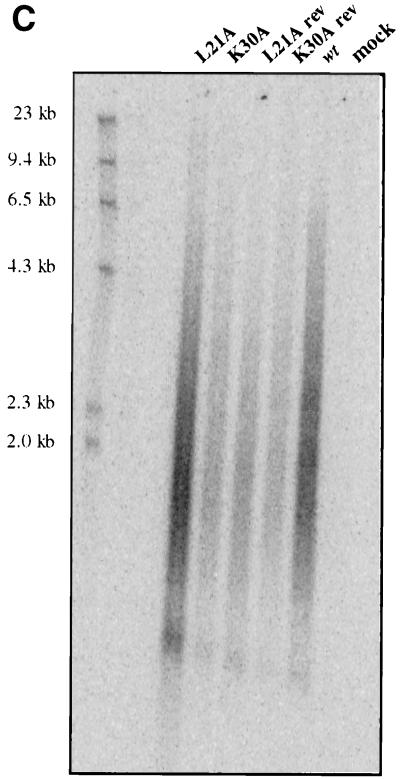
FIG. 7.
Viruses harboring L21A and K30A mutations exhibit no impairment in the initiation of reverse transcription or in processive DNA synthesis in vitro. Viral supernatants were collected from chronically infected Rat 2-2 cells, purified by two-step sucrose step cushions, and normalized by quantitative exogenous RT activity. (A) Initiation of reverse transcription was evaluated by disrupting virions with detergent and allowing RT to extend the tRNA primer two bases. The products were separated by electrophoresis. The labeled tRNA was quantitated and compared to that of wild-type (wt) virions. (B) Endogenous RT reactions were performed using the endogenous viral RNA template and tRNA primer. Fifteen-minute reactions were performed, allowing the synthesis of the entire 145-base −SSS DNA product. Prior to separation by 8% polyacrylamide gel electrophoresis, the tRNA primer was degraded by NaOH. (C) Endogenous RT reaction mixtures were incubated for 9 h, and the products were separated on a 1% alkaline agarose gel. L21A and K30A mutant viruses are not impaired in the synthesis of long viral DNA. Size markers are indicated.
Since the mutant viruses encapsidated wild-type levels of viral RNA and initiated reverse transcription normally, we next assayed their ability to synthesize longer DNA products using their endogenous template and primer. All four deoxynucleoside triphosphates were provided in an assay similar to the tRNA extension assay described above, and virions were allowed to reverse transcribe for 15 min, sufficient time for the synthesis of the approximately 150-base-long −SSS DNA. The products were then analyzed by electrophoresis. Again, the mutant viruses synthesized −SSS DNA with the same efficiency as wild-type virus (Fig. 7B). The average of two experiments showed that L21A, K30A, L21Arev, and K30Arev viruses synthesized 118, 93, 125, and 76% of the level of wild-type −SSS DNA, respectively. To test the ability of the mutant viruses to synthesize longer DNA products, which requires translocation of DNA products from one end of the viral genome to the other, the endogenous reaction was carried out for a longer period of time (9 h) and the DNA products were analyzed on an alkaline agarose gel. Wild-type, L21A, K30A, L21Arev, and K30Arev viruses all produced similar smears on the gel, resulting from the formation of heterogeneous-length DNA products (Fig. 7C). While the absolute amounts of DNA in the various lanes in Fig. 7C differ, these differences were not uniformly seen and could be attributed to variability in yields during purification. Importantly, all of the viruses exhibited the same size distribution of these DNA products on the gel, indicating their ability to synthesize long DNA products. Thus, this assay revealed that the mutant viruses were as efficient as the wild-type or the revertant viruses in synthesizing long strands of DNA, suggesting that elongation and “jumping” during reverse transcription are not impaired. Overall, these results suggest that the L21A and K30A mutations do not reduce the formation of viral DNA in vitro but reduce its levels in vivo in infected cells.
DISCUSSION
The experiments presented above suggest that NC plays an important role in viral DNA synthesis in vivo. Two separate mutations in the NC protein, changing a leucine preceding the Cys-His box (L21A) or a lysine within the Cys-His box (K30A), impair the early steps of viral replication and reduce the level of viral genomic DNA synthesized in infected cells. The reduction in the levels of viral DNA could be observed in a relatively short time (5 h) postinfection. Interestingly, the initiation and elongation processes of reverse transcription in these viruses appeared to be as efficient as those in wild-type virus when tested in vitro.
Most NC mutations have been found to impair the late events of viral replication. The basic residues of NC have been demonstrated to be necessary for efficient assembly and release of MoMuLV, HIV-1, and Rous sarcoma virus (RSV) virion particles (8, 9, 17), perhaps by mediating interactions between assembling Gag multimers and RNA (11, 65, 67). Other mutations in the conserved residues of the MoMuLV Cys-His box have been demonstrated to severely impair the efficiency of specific viral RNA packaging (31, 40, 49, 66). Similar results have been found with RSV (23) and HIV-1 (21, 32, 45). In contrast to these findings, the L21A and K30A mutations caused no significant reduction in the assembly of virions or the levels of RNA packaging. The first obvious manifestation of the mutations is a reduced ability of the virus to synthesize viral DNA upon infection.
What is the mechanism by which these NC mutations impair or retard virus infection? NC has been shown to have important functions as an RNA chaperone. It promotes the dimerization and maturation of genomic RNA in assembled virions (15, 20, 24, 27, 46; reviewed in reference 50), promotes the annealing of the tRNA primer to the primer binding site (47, 51), and facilitates reverse transcription both by reducing pausing at sites of secondary structure and by promoting strand transfer reactions (33, 52, 62, 63). Thus, it is possible that the L21A and K30A mutations reduce the ability of NC to act as an RNA chaperone, leading to inappropriately folded viral RNA in the virion and in turn to inefficient reverse transcription. However, the in vitro experiments performed with mutant virions do not support this explanation: the genomic RNA of these viruses was associated with normal levels of properly annealed tRNA that could be extended with wild-type efficiency in vitro. In addition, these viruses showed normal levels of short and long products of reverse-transcribed DNA in endogenous reactions, indicating normal RT processivity and strand transfer. There are various scenarios by which a misfolded RNA might only cause problems with replication in vivo. For example, an unfolded viral RNA in these virions might be rendered more susceptible to degradation by host nucleases upon entry into a cell. However, this possibility seems unlikely, as degradation of the viral genome would be expected to cause a greater defect in the synthesis of full-length 8.8-kb viral DNA than in that of the 150-base-long −SSS DNA, and our experiments demonstrated similar diminutions in both.
The L21A and K30A mutations might have a direct effect on the interaction of NC with RT during DNA synthesis. Recent work has provided further support for a role of NC in reverse transcription. Multiple methods both in vitro and in vivo have demonstrated a direct physical interaction of HIV-1 NC with HIV-1 RT (22, 36), which may explain how mutations in NC affect reverse transcription and promotion of strand transfer (33). Although an interaction of MoMuLV NC with RT has yet to be demonstrated, it is plausible that the L21A and K30A mutant NC proteins may be impaired in their interactions with RT. As before, a difficulty with this notion is that the mutations showed no effect on reverse transcription in vitro but rather only impaired reverse transcription in vivo. It is possible that the mutations modify some specific interaction that is only important for efficient viral DNA synthesis in vivo. For example, the mutations in NC may lead to inefficient initiation of reverse transcription in vivo yet have no effect on processivity. This notion is consistent with our observation that, at 20 h postinfection, cells infected by the L21A and K30A mutants show similar diminutions in both short DNA (−SSS) and long DNA products (2-LTR circles) compared with cells infected by the wild-type virus.
An alternative explanation is that reverse transcription by the L21A and K30A mutant viruses is normal but that their mutant NC proteins fail to protect the viral DNA from cellular nucleases in infected cells. Indeed, degradation of viral DNA has been demonstrated in HIV-1 mutants with substitutions in the basic residues preceding the first Cys-His box (8). Other NC mutations in the Cys-His box (C39H and H34C [29]) have been shown to reduce DNA synthesis dramatically, and detailed analysis revealed that the small amount of viral DNA produced had heterogeneous LTR ends, consistent with their degradation (30). This notion is supported by the increasing magnitude of the reduction in viral DNA with time after infection. At early times (5 h postinfection), the reduction in DNA levels compared to wild-type levels is significant, but at later times (20 h postinfection) the reduction is even greater. While this result is consistent with degradation of viral DNA in the mutants, it should be noted that the wild-type and revertant viruses serving as controls for these experiments may be capable of a second round of infection by 20 h, and thus of synthesizing higher levels of DNA than the mutants. For this reason we cannot argue strongly for specific degradation of viral DNA in the mutants.
Perhaps the most likely mechanism whereby the L21A and K30A NC mutations may cause at least the initial reduction in viral DNA synthesis in vivo is by impairing the process of virion uncoating, or other steps occurring before the initiation of reverse transcription. Many viruses with mutations in gag that failed to synthesize viral DNA in infected cells have been described (2, 13, 39, 48, 56, 58). Although NC is contained in the viral core, it has not previously been implicated in the uncoating of the virus upon entry. However, the NC proteins may in fact be involved in opening the virion core and exposing the viral RNA to permit efficient reverse transcription. This function of NC may be irrelevant in the in vitro reverse transcription assays, as the detergent used to permeabilize the virion cores in vitro would overcome a block to uncoating manifested by the mutant NC proteins in vivo. This model suggests that the levels of even the first intermediate of DNA synthesis, the −SSS DNA, would be affected, as was indeed observed. Once the particles were opened and DNA synthesis was initiated, there would be no additional reduction in later DNA intermediates if the course of reverse transcription were not directly affected. The results of our analyses of −SSS and 2-LTR circle DNA at 20 h are consistent with these predictions.
What is known about the positions of the L21 and K30 residues in the structure of the NC protein? The recently determined nuclear magnetic resonance structure of MoMuLV NC complexed with pentanucleotide d(ACGCC) makes several predictions regarding the structural roles of many NC residues (55). Residue L21 was suggested to provide important interactions that stabilize the protein-nucleic acid complex. The authors suggest that the methyl group of L21 interacts with both the side chains of A27 and A36 and with the protons of C2 in the pentanucleotide (55). Additionally, complex formation also appears to alter the structure of residues 31 to 35, compared with that for the unbound NC molecule, such that residues K30, K32, and K41 are positioned on the same side as the complex, allowing their participation in binding. These predictions are consistent with our observations that L21A and K30A mutant viruses are impaired in replication. It is interesting that the suppression of the K30A mutant involved the alteration of an acidic residue (glutamate 15) to a neutral one (glycine). It is possible that the loss of the basic residue in the K30A mutant is merely compensated by the loss of the acidic residue. However, it is also possible that the two residues structurally interact in such a way that the positioning of residue K30 is dependent on the size or charge of residue E15.
In summary, two residues of MoMuLV NC protein that are crucial for efficient synthesis of viral DNA in infected cells have been identified. The two mutations characterized here are unusual because they both cause a delay in virus replication at a postentry step that is not manifested as a deficiency in reverse transcription in vitro. Although neither mutation has an effect on assembly of virions, packaging of the genome, placement of the tRNA at the primer binding site, or reverse transcription of the endogenous template in vitro, both mutant viruses produce greatly reduced proviral DNA in infected cells and spread slowly in culture. Only under selective pressure for rapid replication did suppressors of these mutant viruses emerge. These mutations suggest a crucial role of the NC protein in the early events of viral replication in vivo that is not manifested in reverse transcription assays in vitro.
ACKNOWLEDGMENTS
We thank Guangxia Gao, Marion Dorsch, Marianna Orlova, and Amiela Kleinschmidt for helpful advice, technical assistance, and moral support.
J.G. is a Fellow of the Medical Scientist Training Program. E.B. is an Associate and S.P.G. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Aldovini A, Young R A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990;64:1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alin K, Goff S P. Amino acid substitutions in the CA protein of Moloney murine leukemia virus that block early events in infection. Virology. 1996;222:339–351. doi: 10.1006/viro.1996.0431. [DOI] [PubMed] [Google Scholar]
- 3.Alin K, Goff S P. Mutational analysis of interactions between the Gag precursor proteins of murine leukemia viruses. Virology. 1996;216:418–424. doi: 10.1006/viro.1996.0078. . (Erratum, 222:297.) [DOI] [PubMed] [Google Scholar]
- 4.Bacharach E, Goff S P. Binding of the human immunodeficiency virus type 1 Gag protein to the viral RNA encapsidation signal in the yeast three-hybrid system. J Virol. 1998;72:6944–6949. doi: 10.1128/jvi.72.8.6944-6949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkowitz R, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz R D, Luban J, Goff S P. Specific binding of human immunodeficiency virus type 1 Gag polyprotein and nucleocapsid protein to viral RNAs detected by RNA mobility shift assays. J Virol. 1993;67:7190–7200. doi: 10.1128/jvi.67.12.7190-7200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkowitz R D, Ohagen A, Hoglund S, Goff S P. Retroviral nucleocapsid domains mediate the specific recognition of genomic viral RNAs by chimeric Gag polyproteins during RNA packaging in vivo. J Virol. 1995;69:6445–6456. doi: 10.1128/jvi.69.10.6445-6456.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoux L, Pechoux C, Ottmann M, Morel G, Darlix J L. Mutations in the N-terminal domain of human immunodeficiency virus type 1 nucleocapsid protein affect virion core structure and proviral DNA synthesis. J Virol. 1997;71:6973–6981. doi: 10.1128/jvi.71.9.6973-6981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowzard J B, Bennett R P, Krishna N K, Ernst S M, Rein A, Wills J W. Importance of basic residues in the nucleocapsid sequence for retrovirus Gag assembly and complementation rescue. J Virol. 1998;72:9034–9044. doi: 10.1128/jvi.72.11.9034-9044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burniston M T, Cimarelli A, Colgan J, Curtis S P, Luban J. Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein. J Virol. 1999;73:8527–8540. doi: 10.1128/jvi.73.10.8527-8540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cimarelli A, Sandin S, Hoglund S, Luban J. Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J Virol. 2000;74:3046–3057. doi: 10.1128/jvi.74.7.3046-3057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colicelli J, Goff S P. Sequence and spacing requirements of a retrovirus integration site. J Mol Biol. 1988;199:47–59. doi: 10.1016/0022-2836(88)90378-6. [DOI] [PubMed] [Google Scholar]
- 13.Crawford S, Goff S P. Mutations in Gag proteins P12 and P15 of Moloney murine leukemia virus block early stages of infection. J Virol. 1984;49:909–917. doi: 10.1128/jvi.49.3.909-917.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dannull J, Surovoy A, Jung G, Moelling K. Specific binding of HIV-1 nucleocapsid protein to PSI RNA in vitro requires N-terminal zinc finger and flanking basic amino acid residues. EMBO J. 1994;13:1525–1533. doi: 10.1002/j.1460-2075.1994.tb06414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darlix J L, Gabus C, Nugeyre M T, Clavel F, Barre-Sinoussi F. Cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J Mol Biol. 1990;216:689–699. doi: 10.1016/0022-2836(90)90392-Y. [DOI] [PubMed] [Google Scholar]
- 16.Davis J, Scherer M, Tsai W P, Long C. Low-molecular-weight Rauscher leukemia virus protein with preferential binding for single-stranded RNA and DNA. J Virol. 1976;18:709–718. doi: 10.1128/jvi.18.2.709-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson L, Yu X F. The role of nucleocapsid of HIV-1 in virus assembly. Virology. 1998;251:141–157. doi: 10.1006/viro.1998.9374. [DOI] [PubMed] [Google Scholar]
- 18.De Guzman R N, Wu Z R, Stalling C C, Pappalardo L, Borer P N, Summers M F. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science. 1998;279:384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- 19.De Rocquigny H, Ficheux D, Gabus C, Allain B, Fournie-Zaluski M C, Darlix J L, Roques B P. Two short basic sequences surrounding the zinc finger of nucleocapsid protein NCp10 of Moloney murine leukemia virus are critical for RNA annealing activity. Nucleic Acids Res. 1993;21:823–829. doi: 10.1093/nar/21.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rocquigny H, Gabus C, Vincent A, Fournie-Zaluski M C, Roques B, Darlix J L. Viral RNA annealing activities of human immunodeficiency virus type 1 nucleocapsid protein require only peptide domains outside the zinc fingers. Proc Natl Acad Sci USA. 1992;89:6472–6476. doi: 10.1073/pnas.89.14.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorfman T, Luban J, Goff S P, Haseltine W A, Gottlinger H G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993;67:6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Druillennec S, Caneparo A, de Rocquigny H, Roques B P. Evidence of interactions between the nucleocapsid protein NCp7 and the reverse transcriptase of HIV-1. J Biol Chem. 1999;274:11283–11288. doi: 10.1074/jbc.274.16.11283. [DOI] [PubMed] [Google Scholar]
- 23.Dupraz P, Oertle S, Meric C, Damay P, Spahr P F. Point mutations in the proximal Cys-His box of Rous sarcoma virus nucleocapsid protein. J Virol. 1990;64:4978–4987. doi: 10.1128/jvi.64.10.4978-4987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y X, Copeland T D, Henderson L E, Gorelick R J, Bosche W J, Levin J G, Rein A. HIV-1 nucleocapsid protein induces “maturation” of dimeric retroviral RNA in vitro. Proc Natl Acad Sci USA. 1996;93:7577–7581. doi: 10.1073/pnas.93.15.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke E K, Yuan H E, Bossolt K L, Goff S P, Luban J. Specificity and sequence requirements for interactions between various retroviral Gag proteins. J Virol. 1994;68:5300–5305. doi: 10.1128/jvi.68.8.5300-5305.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu W, Gorelick R J, Rein A. Characterization of human immunodeficiency virus type 1 dimeric RNA from wild-type and protease-defective virions. J Virol. 1994;68:5013–5018. doi: 10.1128/jvi.68.8.5013-5018.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu W, Rein A. Maturation of dimeric viral RNA of Moloney murine leukemia virus. J Virol. 1993;67:5443–5449. doi: 10.1128/jvi.67.9.5443-5449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao G, Goff S P. Replication defect of Moloney murine leukemia virus with a mutant reverse transcriptase that can incorporate ribonucleotides and deoxyribonucleotides. J Virol. 1998;72:5905–5911. doi: 10.1128/jvi.72.7.5905-5911.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorelick R J, Chabot D J, Ott D E, Gagliardi T D, Rein A, Henderson L E, Arthur L O. Genetic analysis of the zinc finger in the Moloney murine leukemia virus nucleocapsid domain: replacement of zinc-coordinating residues with other zinc-coordinating residues yields noninfectious particles containing genomic RNA. J Virol. 1996;70:2593–2597. doi: 10.1128/jvi.70.4.2593-2597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorelick R J, Fu W, Gagliardi T D, Bosche W J, Rein A, Henderson L E, Arthur L O. Characterization of the block in replication of nucleocapsid protein zinc finger mutants from Moloney murine leukemia virus. J Virol. 1999;73:8185–8195. doi: 10.1128/jvi.73.10.8185-8195.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorelick R J, Henderson L E, Hanser J P, Rein A. Point mutants of Moloney murine leukemia virus that fail to package viral RNA: evidence for specific RNA recognition by a “zinc finger-like” protein sequence. Proc Natl Acad Sci USA. 1988;85:8420–8424. doi: 10.1073/pnas.85.22.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorelick R J, Nigida S M, Jr, Bess J W, Jr, Arthur L O, Henderson L E, Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990;64:3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Henderson L E, Bess J, Kane B, Levin J G. Human immunodeficiency virus type 1 nucleocapsid protein promotes efficient strand transfer and specific viral DNA synthesis by inhibiting TAR-dependent self-priming from minus-strand strong-stop DNA. J Virol. 1997;71:5178–5188. doi: 10.1128/jvi.71.7.5178-5188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 35.Housset V, De Rocquigny H, Roques B P, Darlix J L. Basic amino acids flanking the zinc finger of Moloney murine leukemia virus nucleocapsid protein NCp10 are critical for virus infectivity. J Virol. 1993;67:2537–2545. doi: 10.1128/jvi.67.5.2537-2545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lener D, Tanchou V, Roques B P, Le Grice S F, Darlix J L. Involvement of HIV-I nucleocapsid protein in the recruitment of reverse transcriptase into nucleoprotein complexes formed in vitro. J Biol Chem. 1998;273:33781–33786. doi: 10.1074/jbc.273.50.33781. [DOI] [PubMed] [Google Scholar]
- 37.Linial M L. Foamy viruses are unconventional retroviruses. J Virol. 1999;73:1747–1755. doi: 10.1128/jvi.73.3.1747-1755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luban J, Goff S P. Binding of human immunodeficiency virus type 1 (HIV-1) RNA to recombinant HIV-1 Gag polyprotein. J Virol. 1991;65:3203–3212. doi: 10.1128/jvi.65.6.3203-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mammano F, Ohagen A, Hoglund S, Gottlinger H G. Role of the major homology region of human immunodeficiency virus type 1 in virion morphogenesis. J Virol. 1994;68:4927–4936. doi: 10.1128/jvi.68.8.4927-4936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meric C, Goff S P. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Virol. 1989;63:1558–1568. doi: 10.1128/jvi.63.4.1558-1568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meric C, Gouilloud E, Spahr P F. Mutations in Rous sarcoma virus nucleocapsid protein p12 (NC): deletions of Cys-His boxes. J Virol. 1988;62:3328–3333. doi: 10.1128/jvi.62.9.3328-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oertle S, Spahr P F. Role of the Gag polyprotein precursor in packaging and maturation of Rous sarcoma virus genomic RNA. J Virol. 1990;64:5757–5763. doi: 10.1128/jvi.64.12.5757-5763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parent L J, Bennett R P, Craven R C, Nelle T D, Krishna N K, Bowzard J B, Wilson C B, Puffer B A, Montelaro R C, Wills J W. Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol. 1995;69:5455–5460. doi: 10.1128/jvi.69.9.5455-5460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poon D T, Wu J, Aldovini A. Charged amino acid residues of human immunodeficiency virus type 1 nucleocapsid p7 protein involved in RNA packaging and infectivity. J Virol. 1996;70:6607–6616. doi: 10.1128/jvi.70.10.6607-6616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prats A C, Housset V, de Billy G, Cornille F, Prats H, Roques B, Darlix J L. Viral RNA annealing activities of the nucleocapsid protein of Moloney murine leukemia virus are zinc independent. Nucleic Acids Res. 1991;19:3533–3541. doi: 10.1093/nar/19.13.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prats A C, Sarih L, Gabus C, Litvak S, Keith G, Darlix J L. Small finger protein of avian and murine retroviruses has nucleic acid annealing activity and positions the replication primer tRNA onto genomic RNA. EMBO J. 1988;7:1777–1783. doi: 10.1002/j.1460-2075.1988.tb03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reicin A S, Ohagen A, Yin L, Hoglund S, Goff S P. The role of Gag in human immunodeficiency virus type 1 virion morphogenesis and early steps of the viral life cycle. J Virol. 1996;70:8645–8652. doi: 10.1128/jvi.70.12.8645-8652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rein A, Harvin D P, Mirro J, Ernst S M, Gorelick R J. Evidence that a central domain of nucleocapsid protein is required for RNA packaging in murine leukemia virus. J Virol. 1994;68:6124–6129. doi: 10.1128/jvi.68.9.6124-6129.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rein A, Henderson L E, Levin J G. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem Sci. 1998;23:297–301. doi: 10.1016/s0968-0004(98)01256-0. [DOI] [PubMed] [Google Scholar]
- 51.Remy E, De Rocquigny H, Petitjean P, Muriaux D, Theilleux V, Paoletti J, Roques B P. The annealing of tRNA3Lys to human immunodeficiency virus type 1 primer binding site is critically dependent on the NCp7 zinc fingers structure. J Biol Chem. 1998;273:4819–4822. doi: 10.1074/jbc.273.9.4819. [DOI] [PubMed] [Google Scholar]
- 52.Rong L, Liang C, Hsu M, Kleiman L, Petitjean P, De Rocquigny H, Roques B P, Wainberg M A. Roles of the human immunodeficiency virus type 1 nucleocapsid protein in annealing and initiation versus elongation in reverse transcription of viral negative-strand strong-stop DNA. J Virol. 1998;72:9353–9358. doi: 10.1128/jvi.72.11.9353-9358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 54.Schmalzbauer E, Strack B, Dannull J, Guehmann S, Moelling K. Mutations of basic amino acids of NCp7 of human immunodeficiency virus type 1 affect RNA binding in vitro. J Virol. 1996;70:771–777. doi: 10.1128/jvi.70.2.771-777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuler W, Dong C, Wecker K, Roques B P. NMR structure of the complex between the zinc finger protein NCp10 of Moloney murine leukemia virus and the single-stranded pentanucleotide d(ACGCC): comparison with HIV-NCp7 complexes. Biochemistry. 1999;38:12984–12994. doi: 10.1021/bi990378d. [DOI] [PubMed] [Google Scholar]
- 56.Schwartzberg P, Colicelli J, Gordon M L, Goff S P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984;49:918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.South T L, Blake P R, Sowder R C D, Arthur L O, Henderson L E, Summers M F. The nucleocapsid protein isolated from HIV-1 particles binds zinc and forms retroviral-type zinc fingers. Biochemistry. 1990;29:7786–7789. doi: 10.1021/bi00486a002. [DOI] [PubMed] [Google Scholar]
- 58.Strambio-de-Castillia C, Hunter E. Mutational analysis of the major homology region of Mason-Pfizer monkey virus by use of saturation mutagenesis. J Virol. 1992;66:7021–7032. doi: 10.1128/jvi.66.12.7021-7032.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Summers M F, Henderson L E, Chance M R, Bess J W, Jr, South T L, Blake P R, Sagi I, Perez-Alvarado G, Sowder R C D, Hare D R, et al. Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci. 1992;1:563–574. doi: 10.1002/pro.5560010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Telesnitsky A, Blain S, Goff S P. Assays for retroviral reverse transcriptase. Methods Enzymol. 1995;262:347–362. doi: 10.1016/0076-6879(95)62029-x. [DOI] [PubMed] [Google Scholar]
- 61.Wills J W, Craven R C. Form, function, and use of retroviral gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Wu W, Henderson L E, Copeland T D, Gorelick R J, Bosche W J, Rein A, Levin J G. Human immunodeficiency virus type 1 nucleocapsid protein reduces reverse transcriptase pausing at a secondary structure near the murine leukemia virus polypurine tract. J Virol. 1996;70:7132–7142. doi: 10.1128/jvi.70.10.7132-7142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You J C, McHenry C S. Human immunodeficiency virus nucleocapsid protein accelerates strand transfer of the terminally redundant sequences involved in reverse transcription. J Biol Chem. 1994;269:31491–31495. [PubMed] [Google Scholar]
- 64.Yu Q, Darlix J L. The zinc finger of nucleocapsid protein of Friend murine leukemia virus is critical for proviral DNA synthesis in vivo. J Virol. 1996;70:5791–5798. doi: 10.1128/jvi.70.9.5791-5798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Barklis E. Effects of nucleocapsid mutations on human immunodeficiency virus assembly and RNA encapsidation. J Virol. 1997;71:6765–6776. doi: 10.1128/jvi.71.9.6765-6776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Barklis E. Nucleocapsid protein effects on the specificity of retrovirus RNA encapsidation. J Virol. 1995;69:5716–5722. doi: 10.1128/jvi.69.9.5716-5722.1995. . (Erratum, 71:5712, 1997.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Qian H, Love Z, Barklis E. Analysis of the assembly function of the human immunodeficiency virus type 1 Gag protein nucleocapsid domain. J Virol. 1998;72:1782–1789. doi: 10.1128/jvi.72.3.1782-1789.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]