Abstract
Purpose
Although preoperative C-Reactive Protein to Albumin Ratio(CAR) is one of the important indicators for surgical risk assessment, the relationship between preoperative CAR and postoperative outcomes in older patients undergoing non-cardiac surgery is still unclear. Therefore, the purpose of this study is to explore the relationship between preoperative CAR and adverse postoperative outcomes in older patients undergoing non-cardiac surgery.
Patients and Methods
We conducted a secondary analysis of data from the multicenter, prospective, longitudinal study called Early-Warning model of Perioperative Adverse Events for Elderly Patients (EPAE). A total of 2511 individuals from seven centers in Guangdong province were included in this study. The CAR was the latest blood counts measured within 3 days prior to surgery. The primary outcome of interest in this study was Clavien–Dindo grade III (CD3) complications. Secondary outcomes included: overall morbidity, reoperation and readmission. This cohort compared baseline characteristics and clinical data between different groups based on the quartile of CAR. Multivariate logistic regression and restricted cubic spline analysis (RCS) were used to explore the relationship between CAR and adverse postoperative outcomes. Further, the subgroup analyses were also conducted.
Results
Among the 2511 older patients enrolled in the study, 1524 individuals (60.7%) were females and the median age at admission was 69.0 years (65.0, 73.0). Multivariate logistic regression analysis and sensitivity analysis both revealed that high CAR is associated with a high incidence of CD3 complications, overall morbidity, and reoperation (P < 0.05). Furthermore, the restricted cubic spline analysis shows a non-linear relationship between CAR and overall morbidity (cut-off value = 0.034, P for nonlinear < 0.001). No significant interaction was found in the subgroup analyses (P for interaction >0.05).
Conclusion
In older patients with non-cardiac surgery, high CAR was significantly associated with adverse postoperative outcomes, including CD3 complications, overall morbidity and reoperation.
Keywords: C-reactive protein to albumin ratio, older patients, adverse postoperative outcomes
Introduction
With the rapid progression of global aging and advancements in surgical methods, the number of older patients undergoing general anesthesia and surgery has substantially increased, accounting for around one-third of surgical patients aged 65 years and over.1 Compared with other age groups, older patients are often characterized by lower nutrition status, functional decline and various chronic diseases,2–4 which leads to a higher risk of adverse postoperative outcomes, including postoperative complications, reoperation, and mortality.5–7 Consequently, preoperative risk assessment is crucial for predicting postoperative adverse outcomes in older patients.8,9
The systemic inflammatory response and nutritional status are critical determinants of postoperative recovery. Previous studies have indicated that serum albumin (ALB) and C-reactive protein (CRP) reflect patients’ nutritional and inflammation status.10–12 However, relying on a single indicator may lack reliability, as it is often influenced by confounding factors. The C-Reactive Protein to Albumin Ratio (CAR), developed by Fairclough et al,13 combines CRP and ALB to provide a comprehensive assessment of both systemic inflammation and nutritional status. Previous studies have demonstrated the prognostic value of CAR in various patient cohorts, including those with cancer, fractures, as well as intensive care units (ICU).14–20 However, most research has concentrated on patients across all age categories, with limited studies specifically addressing the elderly population, primarily in orthopedic and oncology surgeries. Existing research indicates that preoperative CAR may serve as an independent risk factor for postoperative delirium and adverse surgical outcomes in elderly patients undergoing hip fracture surgery.21 Additionally, studies have found that elevated preoperative CAR in elderly individuals undergoing radical gastrectomy or colorectal cancer resection is associated with a higher incidence of postoperative complications and poorer OS and RFS.22,23
Although the precise biological mechanisms by how CAR influences surgical outcomes remain unclear, evidence suggests two primary pathways: First, inflammation may suppress immune function and delay recovery by inhibiting protein synthesis, promoting protein degradation, and reducing plasma albumin levels. Elevated CRP levels signal heightened inflammation, which can compromise multiple organ functions and increase complication risks;24 Second, serum albumin levels reflect nutritional status, with low ALB levels indicating poor nutrition, impairing immune response and tissue repair, and leading to a higher incidence of complications and adverse outcomes.25,26 In summary, inflammation and malnutrition together create a harmful cycle, impacting postoperative recovery.27
Given the unique physiological characteristics of older adults, such as chronic low-grade inflammation and suboptimal nutritional status,28,29 the predictive value of CAR in this population requires further validation. Therefore, we aimed to explore the association between preoperative CAR and adverse postoperative outcomes in elderly patients undergoing non-cardiac surgery, with the intention of providing a novel potential biomarker for preoperative risk assessment in the elderly.
Material and Methods
Study Design and Data Source
This research is a secondary analysis of data from the completed Early-Warning model of Perioperative Adverse Events for Elderly Patients (EPAE), a prospective, multicenter, longitudinal study conducted in China. The EPAE aimed to build risk prediction models and identify risk variables for perioperative adverse events in older patients. The trial was registered in the Chinese Clinical Trial Registry (ChiCTR2300071535) and approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (BE2022-165-01). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. All participants provided written informed consent prior to enrollment.
Study Population
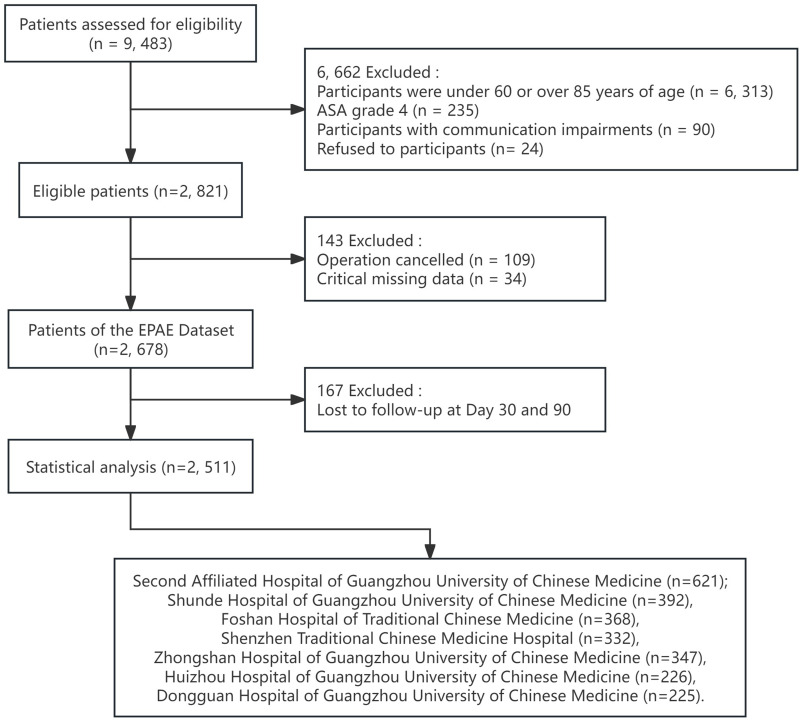
The EPAE study enrolled older patients who underwent elective non-cardiac surgery. The selection criteria were as follows: 1) age 60 to 85 years; 2) patients were scheduled for elective non-cardiac surgery; 3) American Society of Anesthesiologists (ASA) classification is I–III; 4) have good language expression ability, communicate with the investigators without obstacles; 5) voluntary participation in the study and signed informed consent. The exclusion criteria were as follows: 1) unable to participate in the study due to reasons such as severe neurological or psychiatric disease; 2) cancellation of surgery for any reason. 3) unable to cooperate with completing the relevant assessment. 4) critical missing data: Personal data was missing by more than 50%.30 A total of 2678 elderly patients undergoing elective surgeries from seven tertiary hospitals in Guangdong Province of China were enrolled in the EPAE. All patients were followed up during hospitalization, with 190 (7.09%) patients lost to follow-up at 30 days, and 508 (18.97%) patients lost to follow-up at 90 days after surgery. In this study, 167 patients who were lost to follow-up at both 30 and 90 days were excluded.
Finally, 2511 patients were included in this study (Figure 1). Of these, 621 from the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, 392 from the Shunde Hospital of Guangzhou University of Chinese Medicine, 368 from the Foshan Hospital of Traditional Chinese Medicine, 332 from the Shenzhen Traditional Chinese Medicine Hospital, 347 from the Zhongshan Hospital of Guangzhou University of Chinese Medicine, 226 from the Huizhou Hospital of Guangzhou University of Chinese Medicine, 225 from the Dongguan Hospital of Guangzhou University of Chinese Medicine.
Figure 1.
Flow diagram of patient inclusion.
Sample Size Calculation
The sample size for this research was calculated using the formula 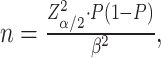 where the significance level α was set at 0.05, corresponding to Zα/2 = 1.96. The estimated probability P was based on previous studies reporting a 5.2% incidence of CD3 complications in older patients undergoing elective non-cardiac surgery.31 The margin of error β was set at 0.01. Considering a potential 20% attrition rate, the minimum required sample size was calculated to be 2273 cases. In this study, a total of 2511 cases were successfully collected, surpassing the necessary sample size.
where the significance level α was set at 0.05, corresponding to Zα/2 = 1.96. The estimated probability P was based on previous studies reporting a 5.2% incidence of CD3 complications in older patients undergoing elective non-cardiac surgery.31 The margin of error β was set at 0.01. Considering a potential 20% attrition rate, the minimum required sample size was calculated to be 2273 cases. In this study, a total of 2511 cases were successfully collected, surpassing the necessary sample size.
Measures and Data Collection
Demographic and Surgical Data
Selection of covariates was informed by existing literature and clinical expertise. Demographic variables included sex, age, marital status, educational level, pre-retirement occupation, smoking, drinking, family per capita monthly income. Clinical and physical variables included body mass index(BMI), multiple medication (defined as the concurrent use of five or more drugs), the Age-Adjusted Charlson Comorbidity Index (aCCI),32 Nutritional Risk Screening 2002 (NRS2002),33 Numerical Rating Scale (NRS),34 Athens Insomnia Scale (AIS),35 Activities of Daily Living (ADL),36 Morse Fall Scale (MFS),37 depression (measured by Patient Health Questionnaire-9),38 cognitive function (measured by Mini-Cog)39 and Social Supporting Rating Scale(SSRS).40 Surgery-related variables included ASA grade, surgery type, surgical site, mode of anesthesia, surgery time (min), intraoperative blood loss (mL).
Immune Inflammation Indexes
Each center conducted operational training for the nurses responsible for collecting blood samples according to standard operating procedures (SOPs). Only nurses who passed the assessment were allowed to collect blood samples. Fasting blood samples were required to be collected within three days prior to surgery, with an overnight fasting period of 8–10 hours. All blood samples were processed within one hour of collection according to established laboratory protocols to minimize variability. Relevant data were recorded in electronic medical records within 24 hours postoperatively, following a standardized data recording and reporting format. The CAR was calculated for all patients as the ratio of C-Reactive Protein (mg/L) to Albumin (g/L).13
Postoperative Outcomes
The primary outcome of interest in this study was CD3 complications, defined according to the Clavien-Dindo classification system (CD) as Clavien grade ≥ 3.41 These complications mainly include heart failure, arrhythmia, myocardial infarction, cardiac arrest, angina pectoris, respiratory failure, stroke, cerebral hemorrhage, deep vein thrombosis, pulmonary embolism, ICU admission and death.
The secondary outcomes included: (1) Overall morbidity, defined as the proportion of patients with one or more postoperative complications. (2) Minor complications, defined as Clavien grade < 3. These include postoperative infection, hypoalbuminemia, electrolyte disturbance (including hypernatremia, hyponatremia, hypokalemia, hyperkalemia), postoperative hemorrhage, and postoperative anemia. (3) Unplanned reoperation, defined as any reoperation occurring within 90 days after surgery. (4) Unplanned readmission, defined as any readmission occurring within 90 days postoperatively.
Date Collection
Before data collection, systematic training was conducted for the research nurses at the seven hospitals. This training encompassed interviewing techniques, data collection, extraction, and recording, as well as the requirements and operational assessments for blood sample collection, ensuring that all participants possessed consistent skills and knowledge. Those who successfully completed the training were designated as the primary data collectors to maintain consistency and accuracy in the data. Additionally, leaders at each center received specialized training to ensure they could effectively guide and supervise their respective teams.
Trained research nurses collected data through face-to-face interviews in the wards. Demographic characteristics, clinical data, and physical variables were gathered within 24 hours of patient admission to ensure timeliness. Surgical variables were obtained from electronic medical records within 24 hours postoperatively. Data on complications were recorded during the hospitalization period through handovers with charge nurses and from electronic medical records. Data on reoperation and readmission within 30 and 90 days post-surgery were obtained by trained researchers via telephone contacts.
Once data collection was completed, research nurses uniformly entered the data from the questionnaires into a specialized website developed by engineers for recording the EPAE dataset. Furthermore, to enhance data quality, we conducted monthly random checks of the questionnaires collected by each center, allowing us to promptly identify and summarize any issues encountered during the data collection process, such as incomplete entries, logical errors, or deviations from research standards. Through regular audits and timely feedback, we aimed to ensure the smooth progression of the data collection process.
Statistical Analysis
Statistical analysis was performed using R (version 4.2.0) with various R packages, including “rcssci” and “pROC”. To handle missing data, multiple imputations were done under the missing-at-random assumption. In this study, all collected variables had missing values below this 20% threshold, making them eligible for multiple imputation. Predictive mean matching was employed for continuous variables to estimate missing values based on observed data, while multinomial regression for categorical variables and logistic regression was used for binary variables (adverse outcomes),42 Ultimately, five imputed datasets were generated, and we randomly selected one of these datasets for statistical analysis. Older patients were divided into four groups based on quartile of the preoperative CAR value. Baseline characteristics, laboratory tests, and postoperative outcomes were compared among these four groups. Continuous variables were expressed as mean ± standard deviation if normally distributed, with group differences analyzed using one-way ANOVA. If the variables were non-normally distributed, they were expressed as median (P25, P75), with differences assessed using the Kruskal–Wallis H-test. Categorical variables were expressed as numbers and percentages (%), with comparisons made using Fisher’s exact test for small sample sizes or Pearson’s chi-square test for larger samples.
The association between preoperative CAR and adverse postoperative outcomes was analyzed using multivariable logistic regression models. The variance inflation factor (VIF) was used to test for multicollinearity, and the Box-Tidwell test was performed to check the log-linearity assumption for continuous variables. Variables with P < 0.05 and clinically significant variables were included in the multivariable logistic regression analysis. Four models were constructed. Model 1 did not adjust for any variables. Model 2 was adjust for general characteristics, including sex, age, marriage, BMI, education level, pre-retirement occupation, smoking, drinking, family per capita monthly income, and multiple medication. Model 3 further adjusted for surgery-related indicators base on Model 2, such as ASA grade, surgery type, surgical site, operation time, anesthesia and intraoperative blood loss. In Model 4, We further adjusted for physical variables, including aCCI, NRS2002, NRS, SSRS, AIS, Mini-Cog, ADL, MFS, PHQ-9 base on Model 3. Additionally, a logistic regression analysis was conducted again by converting CAR from a continuous variable to a categorical variable (quartiles) for sensitivity analysis. Subsequently, the restricted cubic splines (RCS) analysis was conducted to examine the dose-response relationships between CAR and adverse outcomes using four knots (5th, 35th, 65th and 95th percentiles). The RCS plots were adjusted for all covariates.
To further explore the association between CAR and CD3 complications among different kinds of older adults, we conducted subgroup analyses and interaction tests to account for confounding factors, such as comorbidity, nutritional status, and surgical factors, that may influence the relationship between CAR and postoperative adverse outcomes. We categorized comorbidity based on the aCCI into three groups: low (0–2), moderate (3–4), and severe (≥5). Nutritional status was classified into three groups according to NRS2002 scores: low (0) and high (≥1). For surgical factors, we selected surgical type and ASA grade for the subgroup analyses. The statistical significance threshold was set at P < 0.05 for all tests.
Results
Baseline Characteristics and Outcomes
A total of 2511 older patients were enrolled in this study. Of all the patients, 60.6% were females, and the median (P25, P75) age at admission was 69.0 years (65.0, 73.0). The cohort was stratified into four groups based on the quartiles of CAR. No significant differences were observed in marital status, multiple medication, surgery time or intraoperative blood loss between the four groups. However, sex, age, BMI, education level, pre-retirement occupation, drinking, smoking, family per capita monthly income, cognitive function, depression, aCCI, NRS2002, NRS, AIS, ADL, MFS, SSRS, ASA grade, surgery type, surgical site and mode of anesthesia distribution differed significantly (P < 0.05). Demographic, clinical and surgical characteristics are shown in Tables 1 and 2.
Table 1.
The Demographic and Clinical Characteristics of Included Patients
| Variables | Total (n = 2511) | Q1 (n = 628) | Q2 (n = 627) | Q3 (n = 628) | Q4 (n = 628) | p |
|---|---|---|---|---|---|---|
| Sex, n(%) | < 0.001 | |||||
| Male | 987 (39.3) | 240 (38.2) | 201 (32.1) | 246 (39.2) | 300 (47.8) | |
| Female | 1524 (60.7) | 388 (61.8) | 426 (67.9) | 382 (60.8) | 328 (52.2) | |
| Age (years) | 69.0 (65.0, 73.0) | 68.0 (64.0, 73.0) | 68.0 (64.0, 73.0) | 69.0 (65.0, 74.0) | 69.0 (65.0, 74.0) | 0.003 |
| Marital status, n(%) | 0.32 | |||||
| Marriage | 2251 (89.6) | 569 (90.6) | 570 (90.9) | 554 (88.2) | 558 (88.9) | |
| Divorced or widowed | 260 (10.4) | 59 (9.4) | 57 (9.1) | 74 (11.8) | 70 (11.1) | |
| BMI (kg/m²) | 23.5 (21.4, 26.0) | 23.6 (21.5, 25.9) | 24.0 (22.0, 26.6) | 23.4 (21.4, 26.3) | 22.8 (20.6, 25.3) | < 0.001 |
| Education level, n(%) | 0.015 | |||||
| Below Primary | 1398 (55.7) | 339 (54) | 391 (62.4) | 335 (53.3) | 333 (53) | |
| Middle | 626 (24.9) | 165 (26.3) | 136 (21.7) | 160 (25.5) | 165 (26.3) | |
| Above high school | 487 (19.4) | 124 (19.7) | 100 (15.9) | 133 (21.2) | 130 (20.7) | |
| Pre-retirement occupation, n(%) | 0.006 | |||||
| Physical strength | 1564 (62.3) | 368 (58.6) | 433 (69.1) | 374 (59.6) | 389 (61.9) | |
| Both mental and physical strength | 555 (22.1) | 153 (24.4) | 117 (18.7) | 147 (23.4) | 138 (22) | |
| Mental strength | 392 (15.6) | 107 (17) | 77 (12.3) | 107 (17) | 101 (16.1) | |
| Smoking, n(%) | < 0.001 | |||||
| No | 1984 (79.0) | 519 (82.6) | 510 (81.3) | 498 (79.3) | 457 (72.8) | |
| Quit smoking | 183 (7.3) | 32 (5.1) | 42 (6.7) | 46 (7.3) | 63 (10) | |
| Yes | 344 (13.7) | 77 (12.3) | 75 (12) | 84 (13.4) | 108 (17.2) | |
| Drinking, n(%) | 0.009 | |||||
| No | 2100 (83.6) | 524 (83.4) | 533 (85) | 538 (85.7) | 505 (80.4) | |
| Quit drinking | 148 (5.9) | 27 (4.3) | 35 (5.6) | 43 (6.8) | 43 (6.8) | |
| Yes | 263 (10.5) | 77 (12.3) | 59 (9.4) | 47 (7.5) | 80 (12.7) | |
| Family per capita monthly income, n(%) | < 0.001 | |||||
| Below 5000 | 1450 (57.7) | 304 (48.4) | 382 (60.9) | 429 (68.3) | 335 (53.3) | |
| Above 5000 | 1061 (42.3) | 324 (51.6) | 245 (39.1) | 199 (31.7) | 293 (46.7) | |
| Multiple medication, n(%) | 0.085 | |||||
| No | 2118 (84.3) | 512 (81.5) | 536 (85.5) | 543 (86.5) | 527 (83.9) | |
| Yes | 393 (15.7) | 116 (18.5) | 91 (14.5) | 85 (13.5) | 101 (16.1) | |
| aCCI | 3.3 ± 1.4 | 3.2 ± 1.4 | 3.0 ± 1.2 | 3.3 ± 1.4 | 3.6 ± 1.6 | < 0.001 |
| NRS2002 | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 1.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | < 0.001 |
| NRS | 2.0 (0.0, 4.0) | 2.0 (0.0, 4.0) | 2.0 (0.5, 3.0) | 2.0 (0.0, 3.0) | 2.0 (0.0, 4.0) | < 0.001 |
| AIS | 2.0 (0.0, 6.0) | 1.0 (0.0, 6.0) | 2.0 (0.0, 6.0) | 2.0 (0.0, 6.0) | 1.5 (0.0, 6.0) | 0.008 |
| Cognitive function | 4.0 (2.0, 5.0) | 5.0 (3.0, 5.0) | 5.0 (2.0, 5.0) | 4.0 (2.0, 5.0) | 4.0 (2.0, 5.0) | < 0.001 |
| ADL | 95.0 (80.0, 100.0) | 100.0 (88.8, 100.0) | 95.0 (80.0, 100.0) | 95.0 (80.0, 100.0) | 95.0 (65.0, 100.0) | < 0.001 |
| MFS | 25.0 (15.0, 40.0) | 20.0 (15.0, 35.0) | 25.0 (15.0, 40.0) | 25.0 (15.0, 40.0) | 35.0 (15.0, 45.0) | < 0.001 |
| Depression | 1.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 1.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | < 0.001 |
| SSRS | 38.0 ± 6.5 | 38.8 ± 5.8 | 38.7 ± 6.7 | 36.7 ± 6.7 | 37.7 ± 6.6 | < 0.001 |
Notes: CAR: Q1 (≤ 0.024); Q2 (> 0.024, ≤ 0.244); Q3 (> 0.244, ≤ 0.7708); Q4 (> 0.7708).
Abbreviations: BMI, body mass index; aCCI, the Age-Adjusted Charlson comorbidity index; NRS2002, Nutritional Risk Screening 2002; NRS, Numerical Rating Scale; AIS, Athens Insomnia Scale; ADL, Activities of Daily Living; MFS, Morse Fall Scale; SSRS, Social Supporting Rating Scale.
Table 2.
The Surgical Characteristics of Included Patients
| Variables | Total (n = 2511) | Q1 (n = 628) | Q2 (n = 627) | Q3 (n = 628) | Q4 (n = 628) | p |
|---|---|---|---|---|---|---|
| ASA grade, n(%) | < 0.001 | |||||
| Level 1 | 214 (8.5) | 63 (10) | 43 (6.9) | 43 (6.8) | 65 (10.4) | |
| Level 2 | 1463 (58.3) | 393 (62.6) | 354 (56.5) | 383 (61) | 333 (53) | |
| Level 3 | 834 (33.2) | 172 (27.4) | 230 (36.7) | 202 (32.2) | 230 (36.6) | |
| Surgery type, n(%) | < 0.001 | |||||
| Open operation | 791 (31.5) | 192 (30.6) | 88 (14) | 309 (49.2) | 202 (32.2) | |
| Minimally invasive surgery | 1720 (68.5) | 436 (69.4) | 539 (86) | 319 (50.8) | 426 (67.8) | |
| Surgical site, n(%) | < 0.001 | |||||
| Limbs | 1111 (44.2) | 234 (37.3) | 335 (53.4) | 289 (46) | 253 (40.3) | |
| Abdomen | 575 (22.9) | 162 (25.8) | 72 (11.5) | 129 (20.5) | 212 (33.8) | |
| Head and neck | 126 (5.0) | 46 (7.3) | 27 (4.3) | 24 (3.8) | 29 (4.6) | |
| Thorax | 68 (2.7) | 23 (3.7) | 11 (1.8) | 8 (1.3) | 26 (4.1) | |
| Back | 185 (7.4) | 36 (5.7) | 48 (7.7) | 54 (8.6) | 47 (7.5) | |
| Hip | 446 (17.8) | 127 (20.2) | 134 (21.4) | 124 (19.7) | 61 (9.7) | |
| Mode of anesthesia, n(%) | < 0.001 | |||||
| Local anesthesia | 911 (36.3) | 234 (37.3) | 246 (39.2) | 181 (28.8) | 250 (39.8) | |
| General anesthesia | 1600 (63.7) | 394 (62.7) | 381 (60.8) | 447 (71.2) | 378 (60.2) | |
| Surgery time (min) | 120.0 (85.0, 175.0) | 120.0 (80.0, 180.0) | 115.0 (80.0, 151.0) | 120.0 (90.0, 176.0) | 120.0 (80.0, 180.0) | 0.066 |
| Intraoperative blood loss (mL) | 50.0 (35.0, 100.0) | 50.0 (35.0, 100.0) | 50.0 (35.0, 100.0) | 50.0 (35.0, 100.0) | 50.0 (35.0, 100.0) | 0.233 |
Notes: CAR: Q1 (≤ 0.024); Q2 (> 0.024, ≤ 0.244); Q3 (> 0.244, ≤ 0.7708); Q4 (> 0.7708).
Table 3 presents the postoperative outcomes of four groups. Among all the outcomes, significant differences were observed between the four groups in overall morbidity, minor complications, CD3 complications, reoperation and readmission (P < 0.05).
Table 3.
The Postoperative Outcomes of Included Patients
| Variables | Total (n = 2511) | Q1 (n = 628) | Q2 (n = 627) | Q3 (n = 628) | Q4 (n = 628) | p |
|---|---|---|---|---|---|---|
| Overall morbidity, n(%) | < 0.001 | |||||
| No | 1633 (65.0) | 404 (64.3) | 465 (74.2) | 395 (62.9) | 369 (58.8) | |
| Yes | 878 (35.0) | 224 (35.7) | 162 (25.8) | 233 (37.1) | 259 (41.2) | |
| Minor Complications, n(%) | < 0.001 | |||||
| No | 1741 (69.3) | 425 (67.7) | 492 (78.5) | 410 (65.3) | 414 (65.9) | |
| Yes | 770 (30.7) | 203 (32.3) | 135 (21.5) | 218 (34.7) | 214 (34.1) | |
| CD3 complication, n(%) | < 0.001 | |||||
| No | 2261 (90.0) | 570 (90.8) | 566 (90.3) | 587 (93.5) | 538 (85.7) | |
| Yes | 250 (10.0) | 58 (9.2) | 61 (9.7) | 41 (6.5) | 90 (14.3) | |
| Reoperation, n(%) | < 0.001 | |||||
| No | 2376 (94.6) | 604 (96.2) | 582 (92.8) | 609 (97) | 581 (92.5) | |
| Yes | 135 (5.4) | 24 (3.8) | 45 (7.2) | 19 (3) | 47 (7.5) | |
| Readmission, n(%) | < 0.001 | |||||
| No | 2288 (91.1) | 571 (90.9) | 599 (95.5) | 570 (90.8) | 548 (87.3) | |
| Yes | 223 (8.9) | 57 (9.1) | 28 (4.5) | 58 (9.2) | 80 (12.7) |
Notes: CD3, Clavien-Dindo grade III. CAR: Q1 (≤ 0.024); Q2 (> 0.024, ≤ 0.244); Q3 (> 0.244, ≤ 0.7708); Q4 (> 0.7708).
The Multivariate Logistic Regression of Preoperative CAR Level and Adverse Postoperative Outcomes
The Box-Tidwell test indicated that all the continuous covariates have a linear relationship with the dependent variable (P > 0.0017) (Table S1). Subsequently, we ran a multicollinearity analysis, which showed no collinearity among the covariates variance inflation factors (VIF < 5) (Table S2).
The univariate logistic regression analysis showed that CAR is an independent risk factor for postoperative CD3 complications, reoperation, and readmission in elderly patients undergoing elective non-cardiac surgery. After adjusting for all covariates, the results of the multivariate logistic regression analysis indicated that a higher CAR level was significantly linked to an increased risk of overall morbidity (OR=1.17, 95% CI: 1.04–1.32, P < 0.01), CD3 complications (OR=1.33, 95% CI: 1.15–1.53, P < 0.001) and reoperation (OR=1.59, 95% CI: 1.33–1.89, P < 0.001) in older patients following non-cardiac surgery (Table 4).
Table 4.
Multivariate Logistic Regression of the Association Between CAR and Adverse Postoperative Outcomes
| Indexs | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| OR (95% CI) P-Value | OR (95% CI) P-Value | OR (95% CI) P-Value | OR (95% CI) P-Value | |
| Overall morbidity | 1.08 (0.99, 1.17) | 1.07 (0.98, 1.17) | 1.13 (1.03, 1.24)** | 1.17 (1.04–1.32)** |
| CD3 Complications | 1.24 (1.11, 1.39)*** | 1.25 (1.11, 1.39)*** | 1.30 (1.15, 1.46)*** | 1.33 (1.15, 1.53)*** |
| reoperation | 1.29 (1.13, 1.47)*** | 1.29 (1.12, 1.48)*** | 1.51 (1.30, 1.76)*** | 1.59 (1.33, 1.89)*** |
| Readmission | 1.21 (1.08, 1.36)*** | 1.20 (1.06, 1.35)** | 1.22 (1.08, 1.39)*** | 1.05 (0.87, 1.27) |
Notes: *** P < 0.001; ** P < 0.01. Model 1: no covariates were adjusted.; Model 2: sex, age, marriage, BMI, education, Pre-retirement occupation, smoking, drinking, Family per capita monthly income; multiple medication. Model 3: Model 2 + ASA grade, surgery type, surgical site, operation time, anesthesia and intraoperative blood loss. Model 4: Model 3 + aCCI, NRS2002, NRS, SSRS, AIS, MiniCog, ADL, MFS, PHQ-9.
Abbreviations: CI, confidence index; OR, odds ratio.
Additionally, a sensitivity analysis was conducted with CAR as a categorical variable. After adjusting for all covariates, it was found that compared to the lowest CAR group (Q1 ≤ 0.0247), the risk of overall morbidity (OR=0.71, 95% CI: 0.51–0.98, P < 0.05) and readmission (OR=0.53, 95% CI: 0.28–0.99, P < 0.05) were lower in the Q2 group (> 0.0247, ≤ 0.2467), whereas the Q4 group (> 0.7708) had an increased risk of overall morbidity (OR=1.44, 95% CI: 1.04–2.01, P<0.05), CD3 complications (OR=2.07, 95% CI: 1.27–3.37, P < 0.01), and reoperation (OR=4.01, 95% CI: 1.92–8.38, P < 0.001). (Table 5)
Table 5.
Sensitivity Analysis of CAR with Overall Morbidity, CD3 Complications, Reoperation and Readmission
| Indexs | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| OR (95% CI) P-Value | OR (95% CI) P-Value | OR (95% CI) P-Value | OR (95% CI) P-Value | |
| Overall morbidity | ||||
| Q1 | Ref | Ref | Ref | Ref |
| Q2 | 0.61 (0.48, 0.78)*** | 0.62 (0.48–0.79)*** | 0.66 (0.5–0.85)*** | 0.71 (0.51, 0.98)* |
| Q3 | 1.06 (0.85–1.34) | 1.09 (0.86–1.38) | 1.14 (0.89–1.48) | 1.18 (0.85, 1.63) |
| Q4 | 1.27 (1.01–1.59)* | 1.22 (0.97–1.54) | 1.39 (1.08–1.78)** | 1.44 (1.04, 2.01)* |
| CD3 Complications | ||||
| Q1 | Ref | Ref | Ref | Ref |
| Q2 | 1.06 (0.72–1.56) | 1 (0.68–1.49) | 0.95 (0.64–1.43) | 1.29 (0.78–2.13) |
| Q3 | 0.69 (0.45–1.04) | 0.58 (0.37–0.91)* | 0.69 (0.44–1.09) | 0.76 (0.42–1.36) |
| Q4 | 1.64 (1.16–2.33)** | 1.45 (1.1–2.1)* | 1.54 (1.05–2.26)* | 2.07 (1.27–3.37)** |
| Reoperation | ||||
| Q1 | Ref | Ref | Ref | Ref |
| Q2 | 1.95 (1.17–3.24)* | 1.84 (1.09–3.09)* | 1.54 (0.89–2.67) | 2.19 (1.05, 4.58)* |
| Q3 | 0.79 (0.43–1.45) | 0.58 (0.31–1.08) | 0.84 (0.44–1.61) | 1.22 (0.52, 2.85) |
| Q4 | 2.04 (1.23–3.37)** | 2.03 (1.21–3.42)** | 2.52 (1.46–4.34)*** | 4.01 (1.92, 8.38)*** |
| Readmission | ||||
| Q1 (ref) | Ref | Ref | Ref | Ref |
| Q2 | 0.47 (0.29–0.75)*** | 0.49 (0.3–0.78)** | 0.56 (0.35–0.91)* | 0.53 (0.28–0.99)* |
| Q3 | 1.02 (0.69–1.5) | 1.01 (0.68–1.49) | 0.89 (0.6–1.34) | 0.8 (0.46–1.38) |
| Q4 | 1.46 (1.02–2.09)** | 1.41 (0.98–2.04) | 1.42 (0.97–2.06) | 0.9 (0.52–1.56) |
Notes: CAR: Q1 (≤ 0.024); Q2 (> 0.024, ≤ 0.244); Q3 (> 0.244, ≤ 0.7708); Q4 (> 0.7708), *** P < 0.001; ** P < 0.01; * P < 0.05. Model 1: no covariates were adjusted.; Model 2: sex, age, marriage, BMI, education, Pre-retirement occupation, smoking, drinking, Family per capita monthly income; multiple medication. Model 3: Model 2 + ASA grade, surgery type, surgical site, operation time, anesthesia and intraoperative blood loss. Model 4: Model 3 + aCCI, NRS2002, NRS, SSRS, AIS, MiniCog, ADL, MFS, PHQ-9.
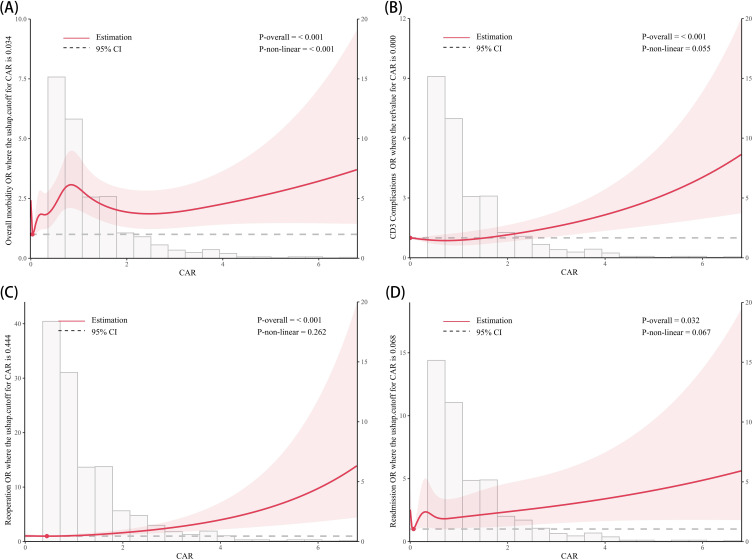
Restricted cubic splines (RCS) analyses were employed to assess the dose-response relationship between CAR and adverse postoperative outcomes, the result is presented in Figure 2. After adjusting for all covariates, we found a U-shaped association between CAR and overall morbidity (cut-off value = 0.034, P for nonlinear < 0.001), suggesting that intermediate level of CAR may be associated with a lower risk of overall morbidity. Besides, no nonlinear relationships were observed between CAR and the other three postoperative adverse outcomes (P for nonlinear > 0.05).
Figure 2.
Multivariable restricted cubic spline of CAR.
Notes: The threshold of significance is P-overall < 0.05. The model was conducted with 4 knots at the 5th, 35th, 65th, 95th percentiles. Solid lines indicate ORs, and shadow shape indicate 95% CIs. (A) Overall morbidity; (B) CD3 complications; (C) Re-operation; (D) Readmission.
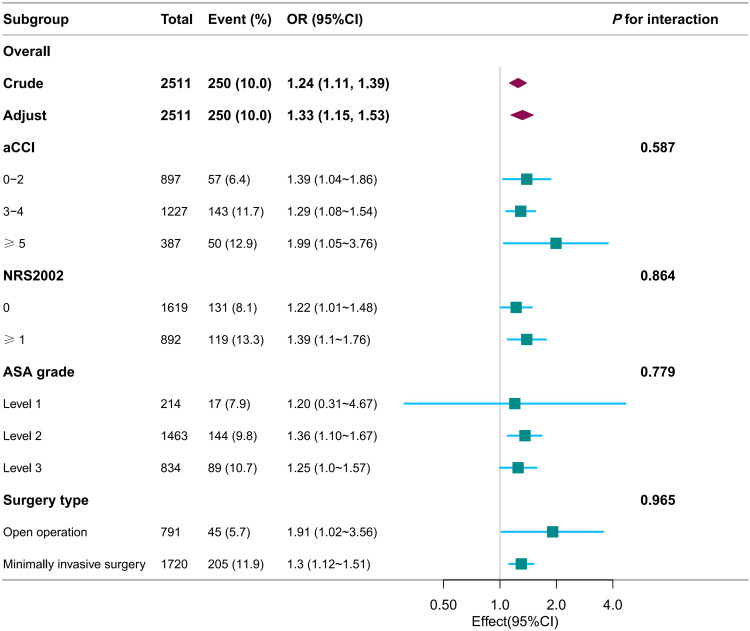
Subgroup Analysis for the Relationship Between CAR and CD3 Complications
Interaction tests revealed that the relationship between CAR and CD3 complications was not statistically different across strata, indicating that aCCI, NRS2002, ASA grade, and surgery type did not significantly impact this positive correlation. (Figure 3, P for interaction > 0.05)
Figure 3.
Forest plot for subgroup analysis of association between CAR and CD3 complications.
Discussion
This prospective cohort study conducted a secondary analysis of EPAE data to investigate the association between preoperative CAR levels and postoperative adverse outcomes in 2511 elderly patients undergoing non-cardiac surgery. Our research demonstrated that elevated preoperative CAR is independently associated with an increased risk of overall morbidity, CD3 complications, and reoperation. The robustness of these associations was confirmed through sensitivity and subgroup analyses. Moreover, the study identified a nonlinear relationship between preoperative CAR and overall morbidity.
In recent years, numerous studies have established a link between CAR and adverse outcomes across various diseases, including different cancers,14–17,19,20,23 critically ill patients,18 COVID-19,43 and myocardial infarction.44 However, these studies mainly focus on patients across all age groups, with relatively few focusing specially on the elderly. For instance, Takemoto et al conducted a retrospective cohort study of 379 elderly patients with gastric cancer, finding that a high preoperative CAR (≥ 0.024) was associated with a higher incidence of complications within 30 days post-surgery (OR=1.62, 95% CI: 1.01–2.62).22 Another retrospective study involving 629 elderly patients undergoing hip fracture surgery also found that a high CAR (≥ 1.5) was significantly associated with an increased risk of postoperative delirium (OR = 2.11, 95% CI: 1.40–3.18) and overall mortality (HR = 1.44, 95% CI: 1.07–1.93).21 In contrast to previous studies that focused on specific surgeries, our research included a diverse range of surgical procedures and conducted a comprehensive preoperative evaluation of elderly patients. After adjusting for demographic, surgical, and disease-related factors, the multivariate logistic regression and sensitivity analyses revealed that a high preoperative CAR (> 0.7708) was significantly associated with increased risks of overall morbidity, CD3 complications, and reoperation in elderly patients undergoing non-cardiac surgery. These findings suggest that CAR may serve as a valuable biomarker for predicting prognosis in elderly patients, facilitating the identification of those at higher risk for complications and mortality, and enabling timely preventive interventions.
The specific mechanisms by which CAR may predict adverse postoperative outcomes in elderly patients remain unclear. We hypothesize that CAR serves as a comprehensive indicator reflecting both systemic inflammation and nutritional status. CRP, an acute-phase reactive protein produced by the liver, increasing during inflammation or infection.45 ALB, another liver-synthesized protein, is an important nutritional indicator, with low levels typically indicating malnutrition.46 Aging and comorbidities commonly result in immune function decline in the elderly,47 along with chronic low-grade inflammation,28 which elevates CRP levels. These inflammatory mediators can damage vascular endothelial and tissue cells, exacerbating tissue damage and organ dysfunction, thereby increasing the risk of complications.48,49 Moreover, malnutrition, prevalent in the elderly,29 impairs tissue healing and exacerbates the production of inflammatory mediators, further increasing the likelihood of adverse postoperative outcomes.50–52
Another interesting finding in our study is the dose-dependent relationship between preoperative CAR and overall morbidity. Sensitivity analysis revealed that when CAR was divided into quartiles, the risk of overall morbidity was lower in the Q2 group compared to Q1, but increased in the Q4 group. This finding is consistent with the RCS analysis, which suggests a U-shaped relationship between CAR and overall morbidity (P for non-linearity < 0.001, cutoff value: 0.034). These findings suggest that both excessively low and high CAR levels may elevate the risk of overall morbidity, possibly due to moderate inflammatory responses could support infection control, whereas extreme levels may signal immune dysregulation in response to surgical trauma.53,54 Low CAR levels may be linked to reduced immune activity, potentially impairing the body’s adaptive response to postoperative infections and increasing complication risk.54 Conversely, high CAR levels may indicate excessive inflammation or poor nutritional status, leading to immune imbalance and compromised tissue healing, thereby reducing the body’s resilience to surgical trauma.48–52 However, in both our study and previous studies on elderly populations, only a direct positive association was found between higher CAR levels and increased risk of adverse postoperative outcomes, with no evidence of a non-linear relationship.21–23 This discrepancy may arise because overall morbidity encompasses a wide range of complications, and a larger sample size could increase statistical power to detect non-linear relationships. Moreover, unlike most previous studies that categorized CAR into only two groups for logistic regression,21–23 our quartile-based method and RCS analysis provide a more nuanced approach to risk stratification. In conclusion, further research is needed to validate this non-linear relationship between CAR and overall morbidity across various populations and with larger sample sizes. Investigating the underlying mechanisms will also be essential to determine CAR’s potential utility in preoperative risk assessment.
Elevated CAR levels have been associated with poor prognosis in elderly patients. Given that CRP and ALB are commonly used blood markers in clinical practice, offering simplicity, cost-effectiveness, and ease of use, preoperative CAR is highly suitable for evaluating older patients. However, the definition of “high CAR” in this population often varies across studies,21,22 typically based on sample distribution and specific clinical characteristics. This variation may affect the comparability of results and limit the generalizability of clinical applications. Therefore, future research should focus on standardizing the cut-off value of CAR to ensure its applicability and reliability across different clinical settings. In addition, the interventions that could improve CAR levels and patient outcomes remain unclear. Previous study has shown that intraoperative administration of NSAIDs may improve outcomes in patients with breast, kidney, and lung cancer.55 Yasui et al also found that normalization of CAR before and after surgery improved prognosis in colorectal cancer patients.56 Future research could explore targeted preoperative interventions, such as anti-inflammatory or nutritional therapies, to assess their effects on CAR levels and determine whether reducing CAR contributes to improved surgical outcomes.
However, our study has several limitations. Firstly, our study only collected blood samples at a single time point, limiting insight into dynamic changes in the CAR. Future studies should consider repeated measurements to capture fluctuations in CAR. Secondly, the dataset used for secondary analysis includes a variety of surgery types. Although we controlled for surgical factors as covariates and conducted subgroup analyses based on surgical type and ASA classification, the heterogeneity across surgical types may still influence the results and limit the generalizability of our conclusions. Thus, further studies are warranted to explore the relationship between preoperative CAR and postoperative adverse outcomes in elderly patients across different surgical types. Thirdly, data on adverse outcomes were only collected only up to 90 days postoperatively, restricting the ability to establish associations with long-term prognostic outcomes. Future research should include extended follow-up periods to fully understand the implications of preoperative CAR levels. Fourthly, due to the observational nature of this study, causality cannot be established based on the observed associations. Fifthly, although we controlled for numerous potential confounding factors in our study, unmeasured confounders, such as blood sugar levels, may still influence the robustness of the results. Future research should aim to include a broader range of data on these factors to enhance the validity of the findings. Finally, our study population was limited to older patients in China, which may affect the generalizability of the findings to other countries. Further studies in different populations or settings could help validate and extend these conclusions.
Conclusion
Through this multicenter, prospective, longitudinal analysis involving 2511 elderly patients across several hospitals in Guangdong, China, we found that elevated CAR levels are positively associated with an increased risk of CD3 complications, overall morbidity, and reoperation. These findings highlight the potential of CAR as a valuable biomarker for preoperative risk stratification. Future studies should investigate whether interventions to modify CAR levels could further reduce the risk of postoperative complications. Additionally, mechanistic research is needed to clarify the biological pathways linking elevated CAR to postoperative outcomes, which could refine its use as a predictive biomarker and identify therapeutic targets.
Funding Statement
This study was supported by the Institute of Science and Technology of the National Health Commission of the People’s Republic of China (No.2021KYSHX016010201) and the State Key Laboratory of Traditional Chinese Medicine Syndrome (No. SZ2022KF15). Commercial funding was not received.
Data Sharing Statement
The data collected in this study is not publicly available because the participants did not consent to the public release of their data. However, the corresponding author may provide further analytical information and supporting data upon reasonable request.
Declarations
Ethics approved has been received from the Ethics Committee of Guangdong Provincial Hospital of Chinese Medical (Ethics Document Batch number: BE2022‐165‐01). The study was carried out in compliance with the Helsinki Declaration, and all the participants provided written informed consent prior to enrolment. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Culley DJ, Flaherty D, Fahey MC. et al. Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology. 2017;127(5):765–774. doi: 10.1097/ALN.0000000000001859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh T, Newman AB. Inflammatory markers in population studies of aging. AGEING RES REV. 2011;10(3):319–329. doi: 10.1016/j.arr.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao S, Cao G, Han L, et al. Prevalence and Patterns of Multimorbidity in a Nationally Representative Sample of Older Chinese: results From the China Health and Retirement Longitudinal Study. J Gerontol a Biol Sci Med Sci. 2020;75(10):1974–1980. doi: 10.1093/gerona/glz185 [DOI] [PubMed] [Google Scholar]
- 4.Ofori-Asenso R, Chin KL, Mazidi M, et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: a Systematic Review and Meta-analysis. JAMA NETW OPEN. 2019;2(8):e198398. doi: 10.1001/jamanetworkopen.2019.8398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall DE, Arya S, Schmid KK, et al. Development and Initial Validation of the Risk Analysis Index for Measuring Frailty in Surgical Populations. JAMA SURG. 2017;152(2):175–182. doi: 10.1001/jamasurg.2016.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Ma F, Wang C, Zhao D, Zhang Y, Tian Y. Elderly patients had more severe postoperative complications after pancreatic resection: a retrospective analysis of 727 patients. World J Gastroenterol. 2018;24(7):844–851. doi: 10.3748/wjg.v24.i7.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J AM COLL SURGEONS. 2006;203(6):865–877. doi: 10.1016/j.jamcollsurg.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 8.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA. 2014;311(20):2110–2120. doi: 10.1001/jama.2014.4573 [DOI] [PubMed] [Google Scholar]
- 9.Partridge JSL, Harari D, Martin FC, Dhesi JK. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. ANAESTHESIA. 2014;69(Suppl 1):8–16. doi: 10.1111/anae.12494 [DOI] [PubMed] [Google Scholar]
- 10.Kudsk KA, Tolley EA, DeWitt RC, et al. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enteral Nutr. 2003;27(1):1–9. doi: 10.1177/014860710302700101 [DOI] [PubMed] [Google Scholar]
- 11.Xue W, Zhang Y, Wang H, Zhang Y, Hu X. Multicenter Study of Controlling Nutritional Status (CONUT) Score as a Prognostic Factor in Patients With HIV-Related Renal Cell Carcinoma. FRONT IMMUNOL. 2021;12:778746. doi: 10.3389/fimmu.2021.778746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurokawa Y, Yamashita K, Kawabata R, et al. Prognostic value of postoperative C-reactive protein elevation versus complication occurrence: a multicenter validation study. Gastric Cancer. 2020;23(5):937–943. doi: 10.1007/s10120-020-01073-5 [DOI] [PubMed] [Google Scholar]
- 13.Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med. 2009;9(1):30–33. doi: 10.7861/clinmedicine.9-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao Y, Yang J, Duan Y, Chen Y, Chen W, Sun D. The C-reactive protein to albumin ratio is an excellent prognostic predictor for gallbladder cancer. BIOSCI TRENDS. 2021;14(6):428–435. doi: 10.5582/bst.2020.03326 [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Xu J, Chen G, et al. Post-diagnostic C-reactive protein and albumin predict survival in Chinese patients with non-small cell lung cancer: a prospective cohort study. Sci Rep. 2019;9(1):8143. doi: 10.1038/s41598-019-44653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Y, Fan X, Chen G, Zhou D, Lin H, Cai X. Preoperative C-reactive protein/albumin ratio to predict mortality and recurrence of patients with hepatocellular carcinoma after curative resection. Med Clin. 2019;153(5):183–190. doi: 10.1016/j.medcli.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Tsujino T, Komura K, Hashimoto T, et al. C-reactive protein-albumin ratio as a prognostic factor in renal cell carcinoma - A data from multi-institutional study in Japan. Urol Oncol. 2019;37(11):811–812. doi: 10.1016/j.urolonc.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 18.Oh TK, Ji E, Na H, et al. C-Reactive Protein to Albumin Ratio Predicts 30-Day and 1-Year Mortality in Postoperative Patients after Admission to the Intensive Care Unit. J CLIN MED. 2018;7(3):39. doi: 10.3390/jcm7030039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Yang X, Li H, et al. Postoperative ratio of C-reactive protein to albumin is an independent prognostic factor for gastric cancer. EUR J MED RES. 2023;28(1):360. doi: 10.1186/s40001-023-01334-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajibandeh S, Hajibandeh S, Romman S, et al. Preoperative C-Reactive Protein-to-Albumin Ratio and Its Ability to Predict Outcomes of Pancreatic Cancer Resection: a Systematic Review. Biomedicines. 2023;11(7):1983. doi: 10.3390/biomedicines11071983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H, Lee S, Kim S, Lee S, Sim J, Ro Y. Association of C-reactive protein to albumin ratio with postoperative delirium and mortality in elderly patients undergoing Hip fracture surgery: a retrospective cohort study in a single large center. EXP GERONTOL. 2023;172:112068. doi: 10.1016/j.exger.2022.112068 [DOI] [PubMed] [Google Scholar]
- 22.Takemoto Y, Tanabe K, Chikuie E, et al. Preoperative High C-Reactive Protein to Albumin Ratio Predicts Short- and Long-Term Postoperative Outcomes in Elderly Gastric Cancer Patients. CANCERS. 2024;16(3):616. doi: 10.3390/cancers16030616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bekki T, Shimomura M, Hattori M, et al. C-Reactive Protein/Albumin Ratio Is an Independent Risk Factor for Recurrence and Survival Following Curative Resection of Stage I-III Colorectal Cancer in Older Patients. ANN SURG ONCOL. 2024;31(7):4812–4821. doi: 10.1245/s10434-024-14961-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Miao YC. Prognostic evaluation of preoperative systemic immune inflammatory index in patients with colorectal cancer. FRONT ONCOL. 2023;13:1260796. doi: 10.3389/fonc.2023.1260796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown KL, Phillips TJ. Nutrition and wound healing. CLIN DERMATOL. 2010;28(4):432–439. doi: 10.1016/j.clindermatol.2010.03.028 [DOI] [PubMed] [Google Scholar]
- 26.Schalk BWM, Visser M, Deeg DJH, Bouter LM. Lower levels of serum albumin and total cholesterol and future decline in functional performance in older persons: the Longitudinal Aging Study Amsterdam. AGE AGEING. 2004;33(3):266–272. doi: 10.1093/ageing/afh073 [DOI] [PubMed] [Google Scholar]
- 27.Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr. 1997;66(2):460S–463S. doi: 10.1093/ajcn/66.2.460S [DOI] [PubMed] [Google Scholar]
- 28.Dolin TG, Christensen IJ, Johansen AZ, et al. Pre- and Perioperative Inflammatory Biomarkers in Older Patients Resected for Localized Colorectal Cancer: associations with Complications and Prognosis. CANCERS. 2021;14(1):161. doi: 10.3390/cancers14010161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komici K, Vitale DF, Mancini A, et al. Impact of Malnutrition on Long-Term Mortality in Elderly Patients with Acute Myocardial Infarction. NUTRIENTS. 2019;11(2):224. doi: 10.3390/nu11020224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham JW. Missing data analysis: making it work in the real world. ANNU REV PSYCHOL. 2009;60(1):549–576. doi: 10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Zhao Y, Liu J, Teng Y, Ou M, Hao X. Predictive value of perioperative procalcitonin, C reactive protein and high-sensitivity C reactive protein for the risk of postoperative complications after non-cardiac surgery in elderly patients: a nested case-control study. BMJ OPEN. 2023;13(10):e71464. doi: 10.1136/bmjopen-2022-071464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Li J, He J, Zhang H, Liu M, Rong J. The Age-adjusted Charlson Comorbidity Index predicts post-operative delirium in the elderly following thoracic and abdominal surgery: a prospective observational cohort study. FRONT AGING NEUROSCI. 2022;14:979119. doi: 10.3389/fnagi.2022.979119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shamlan G, Albreiki M, Almasoudi HO, et al. Nutritional status of elderly patients previously ill with COVID-19: assessment with nutritional risk screening 2002 (NRS-2002) and mini nutritional assessment (MNA-sf). J INFECT PUBLIC HEAL. 2024;17(2):372–377. doi: 10.1016/j.jiph.2023.11.005 [DOI] [PubMed] [Google Scholar]
- 34.Wood BM, Nicholas MK, Blyth F, Asghari A, Gibson S. Assessing Pain in Older People With Persistent Pain: the NRS Is Valid But Only Provides Part of the Picture. J Pain. 2010;11(12):1259–1266. doi: 10.1016/j.jpain.2010.02.025 [DOI] [PubMed] [Google Scholar]
- 35.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J PSYCHOSOM RES. 2000;48(6):555–560. doi: 10.1016/S0022-3999(00)00095-7 [DOI] [PubMed] [Google Scholar]
- 36.Katz S. Akpom CA: 12. Index of ADL. MED CARE. 1976;14(5 Suppl):116–118. doi: 10.1097/00005650-197605001-00018 [DOI] [PubMed] [Google Scholar]
- 37.Baek S, Piao J, Jin Y, Lee S. Validity of the Morse Fall Scale implemented in an electronic medical record system. J CLIN NURS. 2014;23(17–18):2434–2440. doi: 10.1111/jocn.12359 [DOI] [PubMed] [Google Scholar]
- 38.Tuck AN, Scribani MB, Grainger SD, Johns CA, Knight RQ. The 9-Item Patient Health Questionnaire (PHQ-9): an aid to assessment of patient-reported functional outcomes after spinal surgery. Spine J. 2018;18(8):1398–1405. doi: 10.1016/j.spinee.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 39.Abayomi SN, Sritharan P, Yan E, et al. The diagnostic accuracy of the Mini-Cog screening tool for the detection of cognitive impairment-A systematic review and meta-analysis. PLoS One. 2024;19(3):e298686. doi: 10.1371/journal.pone.0298686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, Li H, Fan P, Rong C. Factors associated with the quality of life of Chinese parents who have lost their only child. Sci Rep. 2024;14(1):17296. doi: 10.1038/s41598-024-68225-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. ANN SURG. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polit DF. Statistics and Data Analysis for Nursing Research. 2009. [Google Scholar]
- 43.Kalabin A, Mani VR, Valdivieso SC, Donaldson B. Does C reactive protein/Albumin ratio have prognostic value in patients with COVID-19. J INFECT DEV COUNTR. 2021;15(8):1086–1093. doi: 10.3855/jidc.14826 [DOI] [PubMed] [Google Scholar]
- 44.Karabağ Y, Çağdaş M, Rencuzogullari I, et al. Usefulness of The C-Reactive Protein/Albumin Ratio for Predicting No-Reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. EUR J CLIN INVEST. 2018;48(6):e12928. doi: 10.1111/eci.12928 [DOI] [PubMed] [Google Scholar]
- 45.Geyer CE, Newling M, Sritharan L, et al. C-Reactive Protein Controls IL-23 Production by Human Monocytes. Int J Mol Sci. 2021;22(21):11638. doi: 10.3390/ijms222111638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nie L, Pan X, Zhang X, Zhang S, Rao J, Su Z. Research on the correlation of immunity in patients with chronic insomnia. FRONT PSYCHIATRY. 2022;13:1034405. doi: 10.3389/fpsyt.2022.1034405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim CM, Lee JB, Shin SJ, Ahn JB, Lee M, Kim HS. The efficacy of immune checkpoint inhibitors in elderly patients: a meta-analysis and meta-regression. ESMO OPEN. 2022;7(5):100577. doi: 10.1016/j.esmoop.2022.100577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulboaca AE, Boarescu P, Porfire AS, et al. The Effect of Nano-Epigallocatechin-Gallate on Oxidative Stress and Matrix Metalloproteinases in Experimental Diabetes Mellitus. Antioxidants. 2020;9(2):172. doi: 10.3390/antiox9020172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature reviews. Cardiology. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng H, Lu J, Li P, et al. Effects of Preoperative Malnutrition on Short- and Long-Term Outcomes of Patients with Gastric Cancer: can We Do Better? ANN SURG ONCOL. 2017;24(11):3376–3385. doi: 10.1245/s10434-017-5998-9 [DOI] [PubMed] [Google Scholar]
- 51.Fukuda Y, Yamamoto K, Hirao M, et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. ANN SURG ONCOL. 2015;22(Suppl 3):S778–S785. doi: 10.1245/s10434-015-4820-9 [DOI] [PubMed] [Google Scholar]
- 52.Lau S, Pek K, Chew J, et al. The Simplified Nutritional Appetite Questionnaire (SNAQ) as a Screening Tool for Risk of Malnutrition: optimal Cutoff, Factor Structure, and Validation in Healthy Community-Dwelling Older Adults. NUTRIENTS. 2020;12(9):2885. doi: 10.3390/nu12092885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caballero-Sánchez N, Alonso-Alonso S, Nagy L. Regenerative inflammation: when immune cells help to re-build tissues. FEBS J. 2024;291(8):1597–1614. doi: 10.1111/febs.16693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaudillière B, Fragiadakis GK, Bruggner RV, et al. Clinical recovery from surgery correlates with single-cell immune signatures. SCI TRANSL MED. 2014;6(255):255ra131. doi: 10.1126/scitranslmed.3009701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forget P, Machiels J, Coulie PG, et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. ANN SURG ONCOL. 2013;20(Suppl 3):S650–S660. doi: 10.1245/s10434-013-3136-x [DOI] [PubMed] [Google Scholar]
- 56.Yasui K, Shida D, Nakamura Y, Ahiko Y, Tsukamoto S, Kanemitsu Y. Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Br J Cancer. 2021;124(5):933–941. doi: 10.1038/s41416-020-01189-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data collected in this study is not publicly available because the participants did not consent to the public release of their data. However, the corresponding author may provide further analytical information and supporting data upon reasonable request.