Abstract
Mycotic intracranial aneurysms (MIAs) are rare but can cause significant morbidity and mortality due to rupture. Most patients have additional systemic medical comorbidities making endovascular treatment a vital modality in the treatment of these aneurysms. We aimed to share our institutional experience with the role of endovascular therapy in the treatment of mycotic aneurysms with a literature review. We conducted a retrospective review of our patient database to identify individuals diagnosed with MIAs who underwent endovascular intervention at our institution between January 2002 and December 2021. We have found three patients with ruptured MIAs. All three patients had a heart disease with infective endocarditis. Two patients presented with subarachnoid hemorrhage (SAH) in which, one had a rebleed resulting in intracerebral hemorrhage (ICH), the third patient initially presented with ICH. Distal anterior cerebral artery (ACA) was the site of MIA in two cases and distal middle cerebral artery (MCA) in one patient. Two patients were treated with simple coiling and one patient was treated by glue (n-butyl cyanoacrylate [NBCAs]) injection within the aneurysm. There was no periprocedural complication with complete obliteration of the aneurysm and preservation of the parent artery. All the patients had good outcomes on follow-up. Two patients had a modified Rankin scale (mRS) score of 0 at 6 months and one patient had an mRS score of 3 at the end of 3 months whose preprocedure mRS score was 5. Endovascular embolization of MIAs with coils or liquid embolic agents can be performed in critically ill patients and is an excellent treatment modality with high occlusion rates and low procedural complications.
Keywords: mycotic aneurysm, endovascular, subarachnoid hemorrhage, intracranial
Introduction
Mycotic intracranial aneurysms (MIAs) are very rare and represent less than 5% 1 (0.7–6.5%) 2 of all intracranial aneurysms, but are associated with high morbidity and mortality (mortality as high as 80% in ruptured cases and 30% in unruptured cases). 3 It was first described by Osler in the preantibiotic 19th century. 4 5 6 Mycotic aneurysms occur due to infectious etiology. They are also referred to as “infectious intracranial aneurysms (IIAs),” “intracranial microbial aneurysms,” “infectious aneurysms,” and “cerebral mycotic aneurysms (CMAs).” This pathology in the postantibiotic era results chiefly from left-sided cardiac bacterial endocarditis. Specifically, cardiac bacterial endocarditis/infective endocarditis (IE) is the primary source in greater than 80% of intracranial mycotic aneurysms. 6 7 Up to 30% of patients with IE develop neurological signs and symptoms and 2 to 4% of them develop mycotic MIA. 7 Infective causes include mainly septic emboli from left-sided cardiac bacterial endocarditis ( Staphylococcus aureus and viridans group streptococci) along with extravascular sources of infection like meningitis, postneurosurgical infections, cerebritis, and paranasal sinuses (PNS) infection. 8 They pose a significant challenge to surgical treatment due to systemic comorbidity and their occurrence in small vessels. Endovascular treatment is associated with good outcomes.
Materials and Methods
We retrospectively reviewed our imaging repository Radiology Information Systems and Picture Archiving and Communications System (RIS-PACS) data at our institute from January 2012 to December 2021 to identify individuals with MIAs who underwent endovascular treatment at our institute with follow-up. Demographic and radiological clinical data including patient age, gender, presentation, aneurysm location, types of endovascular treatment method, periprocedural complications, and follow-up were recorded. We excluded patients with extracranial mycotic aneurysms (e.g., cervical ICA) and MIAs who were treated conservatively or surgically. All patients underwent pre- and postembolization computed tomography (CT) and digital subtraction angiography (DSA; biplane, Phillips) therapeutic embolization under septic precautions. Treatment outcomes were evaluated 3 and 6 months after the procedure using the modified Rankin scale (mRS).
Results
We found three MIAs ranging in size from 3 to 11 mm in three patients who underwent endovascular treatment ( Table 1 ). All of our patients presented after aneurysmal rupture. Two patients were treated with endovascular coiling and one with glue injections. Besides this, we have also found nine cases of MIAs, of which six were treated with open surgical treatment and three with conservative management.
Table 1. Demographic and clinical characteristics of patients.
| Patient no. | Age group | Presentation | Location of MIAs | Preceding fever or IE | Endovascular procedure | Complications | Outcome (mRS) |
|---|---|---|---|---|---|---|---|
| 1 | Late 50s | Severe headache, vomiting, ICH, and SAH, hemiparesis | Left distal MCA (M3–M4 junction) | IE: (+) | Coiling of aneurysm | None | 3 (at 3 mo) |
| 2 | Mid-20s | Severe headache, vomiting | Right distal ACA (callosomarginal artery) | Fever: (+) IE: (+) |
Coiling of aneurysm | None | 0 (at 6 mo) |
| 3 | Early 20s | Severe headache, vomiting | Left distal ACA (pericallosal artery) | IE: (+) | Glue injection in aneurysm | None | 0 (at 6 mo) |
Abbreviations: ACA, anterior cerebral artery; ICH, intracerebral hemorrhage; IE, infective endocarditis; MCA, middle cerebral artery; MIA, mycotic intracranial aneurysm; mRS, modified Rankin scale; SAH, subarachnoid hemorrhage.
Case Presentations
Case 1
A patient in their late 50s presented with complaints of severe headaches associated with vomiting for the past 3 days ( Figs. 1 and 2 ). The patient underwent double valve replacement in 2002 at our institute for rheumatic heart disease (RHD) with thickened calcified mitral and aortic valves and stopped taking all medications (was on anticoagulants before) recently. On examination, the patient was E4V5M6 with intact power. Noncontrast CT (NCCT) revealed subarachnoid hemorrhage (SAH; modified Fischer grade 1) in the left sylvian fissure. CT angiography revealed a mycotic aneurysm in the left distal middle cerebral artery (MCA; M3–M4 territory). Cardiac echo was positive for IE (presence of vegetations). The patient was started on conservative medications, antibiotics and nimodipine. The condition of the patient suddenly deteriorated on the sixth day: E4V1M6, drowsiness with right hemiparesis (power of 0/5 in the right upper limb and 1/5 in the right lower limb). On repeat CT, there was a rebleed (intracerebral hemorrhage [ICH]) in the left frontal lobe along with SAH. The patient was taken up for endovascular therapy (EVT) under general anesthesia (GA). An initial diagnostic angiogram revealed a lobulated saccular aneurysm (3 mm × 4 mm) involving the branch arising from the superior division of the left MCA (at the M3–M4 junction). Initially, we could navigate the microcatheter–microwire combination up to the left M3 MCA of the superior division, after which the microwire was not tracking due to the tortuous anatomy. We then put the coils through the catheter to reach the site of the aneurysm and the microcatheter was navigated over the coil till the neck of the aneurysm. Initially, soft three-dimensional (3D) Axium Prime coils were deployed, followed by finishing coils. The control angiogram showed complete obliteration of the aneurysm with patent parent vessels and normal antegrade distal flow. The patient was extubated on the table with no new deficits. The patient was started on anticoagulants the next day (E4V1M6) and was discharged after 1 week. At the time of discharge, power had improved by one grade in the right upper limb (1/5) and lower limb (2/5) with some speech improvement (V2). The patient came for a follow-up after 15 days and had more improvement in power (2/5 in the right upper limb and 3/5 in the right lower limb). The patient's preprocedure mRS score was 5 and the postprocedure mRS score at 3 months was 3.
Fig. 1.
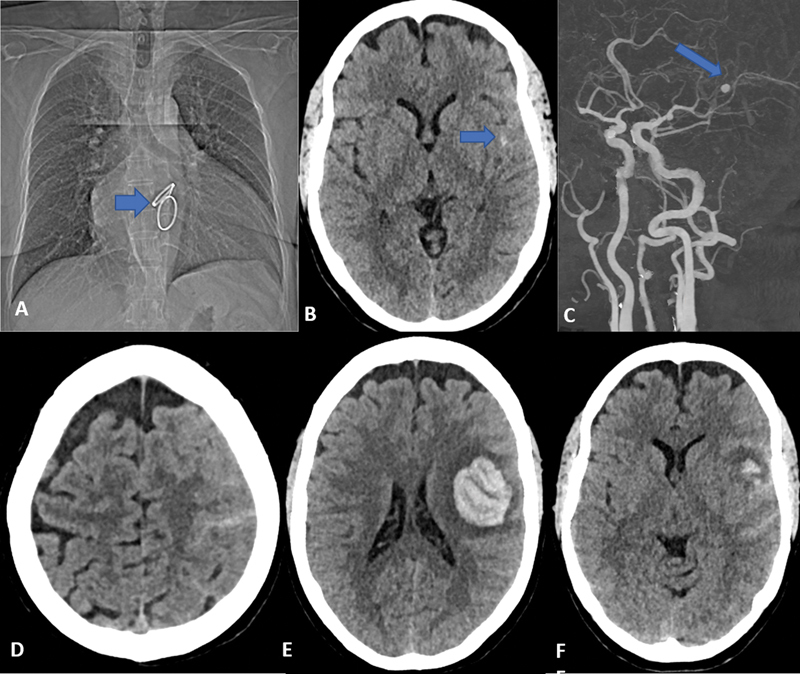
Case 1. ( A ) Chest computed tomography (CT) topogram showing the double artificial valve ( arrow ). ( B ) Noncontrast CT (NCCT) of the head done 3 days after ictus at the time of presentation in casualty showing minimal subarachnoid hemorrhage (SAH) in the left sylvian fissure with ( C ) CT angiography at the same time showing a small well-defined aneurysm in the left distal middle cerebral artery (MCA) near the M3–M4 junction ( arrow ). ( D–F ) Repeat NCCT done after 6 days of the presentation shows a rebleed of the aneurysm with intracerebral hemorrhage (ICH) and SAH.
Fig. 2.
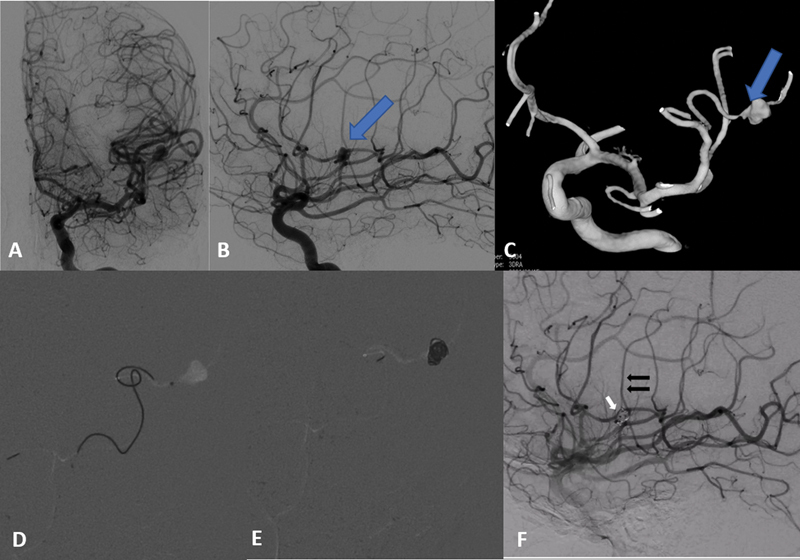
Case-1 (contd.). ( A ) Anteroposterior (AP) and ( b ) lateral digital subtraction angiography (DSA) images of left internal carotid artery (ICA) injection show a lobulated saccular aneurysm arising from the superior division of the left M3 middle cerebral artery (MCA). Note the change in morphology of the aneurysm compared to as seen on computed tomography (CT) angiography ( Fig. 1C ). ( C ) Three-dimensional (3D) rotational angiography reconstructed image showing the distal location of the aneurysm with a tortuous anatomy of the parent artery. Fluoroscopic fade lateral DSA image showing ( D ) the navigated microcatheter tip at the neck of the aneurysm and ( E ) coiled aneurysm. ( F ) The coiled mass ( white arrow ) with preserved distal parent artery ( black arrows ).
Case 2
A patient in their mid-20s presented with sudden onset severe headache, and vomiting, followed by loss of consciousness for the past 5 days ( Fig. 3 ). The patient had a known case of congenital heart disease (CHD; tetralogy of Fallot) and had a history of fever for 1 week. NCCT showed an acute small hematoma in the interhemispheric region with edema on the right frontal lobe. Magnetic resonance (MR) angiography showed a saccular aneurysm (6 mm × 7 mm) at the origin of the right pericallosal artery. A cardiac echo showed vegetation consistent with IE. The patient was put on antibiotics as per the cardiac protocol. Initial diagnostic angiogram showed a bilobed saccular mycotic aneurysm in the right pericallosal artery with stenosis in the right proximal anterior cerebral artery (ACA). Simple coiling of the aneurysm was done under GA in the same sitting using an echelon microcatheter in combination with a 0.014-inch microwire. Postcoiling NCCT after 2 days revealed no fresh infarct or acute hematoma. The hospital course of the patient was uneventful, with no residual deficit and the patient was discharged after 2 days. On follow-up, he had a 6-month mRS score of 0.
Fig. 3.
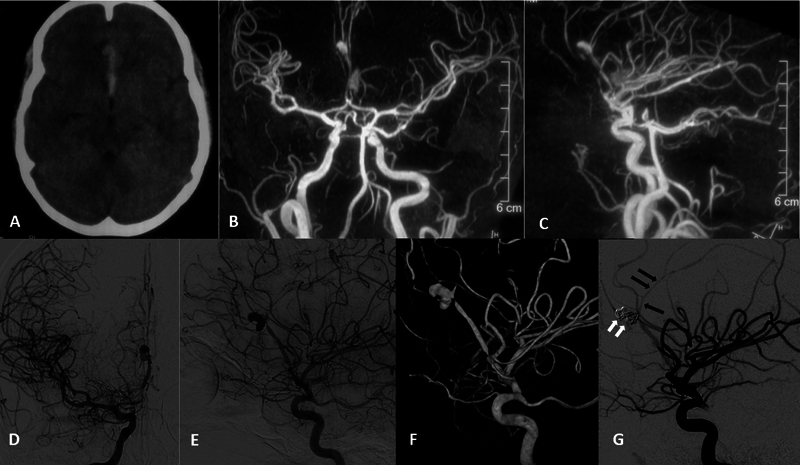
Case-2. ( A ) Initial noncontrast computed tomography (NCCT) done outside showing interhemispheric bleed. Magnetic resonance angiography (MRA) done outside. ( B ) Anteroposterior (AP) and ( C ) lateral projection images show a saccular aneurysm at the right pericallosal artery. ( D ) AP and ( E ) lateral and ( F ) three-dimensional (3D) reconstructed DSA images of right internal carotid artery (ICA) injection show bilobed saccular right pericallosal artery aneurysm near its origin. ( G ) Postcoiling lateral DSA image shows complete occlusion of the aneurysm and coil mass ( white arrows ) with preservation of the parent vessel ( black arrows ). Note the proximal stenosis of the right anterior cerebral artery (ACA) with spasm in the distal ACA in pre- and postcoiling images.
Case 3
A patient in their early 20s (a known case of ventricular septal defect [VSD]), presented with sudden onset severe headache, and vomiting, followed by loss of consciousness for the past 7 days ( Fig. 4 ). Power was normal in all four limbs. The patient was diagnosed as having IE on echo. On examinations, the patient was E4V5M6. Initial NCCT showed acute hematoma with edema involving the left frontal lobe near the midline without any mass effect. A six-vessel cerebral angiogram revealed lobulated dissecting mycotic aneurysm (9 mm × 11 mm) in the left callosomarginal artery. Using the triaxial system, the microcatheter tip was placed distally into the fundus of the sac and a 50% glue mixture was injected into the obliterated sac with deliberate sparing of the neck of the aneurysm to preserve normal branches. The hospital course was uneventful with no periprocedural complications. The patient was discharged and referred to the cardiology department for further management. His mRS score was 0 at the end of 6 months. He has no new neurological deficit to date.
Fig. 4.
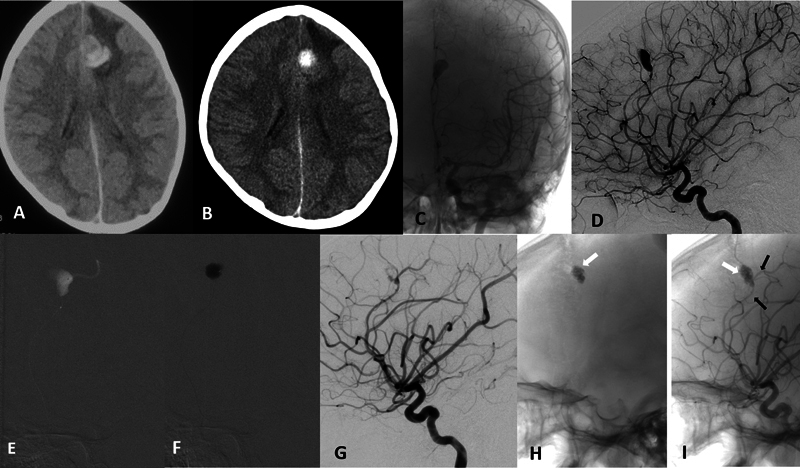
Case-3. ( A ) Initial axial noncontrast computed tomography (NCCT) of the brain showing intracerebral hemorrhage (ICH) in the left frontal lobe in a para-alpine location without any mass effect. ( B ) Repeat NCCT done at the time of admission showed minimal resolution of hematoma. ( C ) Native anteroposterior (AP) and ( D ) subtracted digital subtraction angiography (DSA) images of the left internal carotid artery (ICA) injection show a dissecting aneurysm of the left distal pericallosal artery. ( E ) Fluoroscopic image of microcatheter injection in the left pericallosal artery proximal to the aneurysm showing the aneurysm and distal artery. ( F ) Fluoroscopic image showing the glue injected within the aneurysm sac with sparing of the neck to preserve the parent artery. ( G ) Immediate postglue DSA run showing near complete obliteration of aneurysm with minimal staining of the wall. Delayed native DSA image ( H ) without contrast and ( I ) after contrast showing the glue cast ( white arrow ) with complete aneurysm occlusion and preserved parent artery ( black arrows ).
Discussion
Mycotic aneurysms can develop from either intravascular sources of infection (septic emboli from IE) or direct extension of extravascular sources (meningitis). The underlying mechanism is the development of arteritis, which leads to subsequent inflammation and weakening of the vessel wall and ultimately ruptures, resulting in ICH or SAH. 9 Risk factors include intravenous (IV) drug use, cardiac valvular abnormalities, biologic or mechanical valve implants, postneurosurgical infections, immunocompromised state, meningitis, incomplete treatment of prior intracranial abscesses, and paranasal sinus infections. 10 Also, these patients have added hemodynamic stress due to cardiac valvular disturbance, leading to more pressure on the intracranial vessels. MIAs are commonly detected after SAH or/and ICH due to aneurysmal rupture 7 despite constitutional neurological symptoms. Common locations include the distal vasculature, and 20% of cases may have multiple aneurysms. The most frequent locations of aneurysms are MCA > PCA > ACA > others. 7 8 All our patients presented after aneurysmal rupture with feature of SAH, with one patient having MIA in the distal MCA and two patients in distal ACAs. Early diagnosis of a ruptured MIA is essential to initiate aggressive medical management and to secure the aneurysm primarily with EVT. 11 12 DSA remains the gold standard for both diagnosis and management of mycotic aneurysms given their predilection to form in the distal vasculature. 7 Other diagnostic workup includes echo for probable bacterial endocarditis to look for the development of infective vegetation on the cardiac valves. 13 All our patients had IE with cardiac disease.
Management : No definitive guidelines are present to dictate treatment protocols for mycotic aneurysms. Treatment varies for ruptured and unruptured MIA. Unruptured MIAs may have spontaneous obliteration in many cases. 14 The management of ruptured MIAs depends on the presence or absence of hemorrhage, anatomic location, and clinical course. Usually antibiotic with or without EVT is the treatment of choice. 15 For unruptured MIAs less than 10 mm in size, the best medical management with regular angiographic follow-up is the first line of treatment. 7 The presence of bilateral/multiple aneurysms favors the use of antibiotics alone. 16 Larger unruptured MIAs, MIAs with an increase in size or no change in size despite adequate medical management, and ruptured MIAs should be treated with operative procedure (preferably) EVT with or without decompressive craniectomy depending on the mass effect. 17
Surgery: Open surgical management is feasible (1) in stable patients if endovascular treatment of the mycotic aneurysm fails to completely occlude the aneurysm and (2) in patients with large concurrent intracerebral hematomas wherein a craniotomy to evacuate the clot and directly secure the aneurysm can be achieved in one procedure. 15 Vessel wall and aneurysm wall friability, prior use of anticoagulants (as most patients have valve replacement done or have other cardiac conditions requiring anticoagulation), lack of definitely identifiable aneurysm neck, and lack of normal vessels for re-implantation (for bypass) make open surgery less preferable.
EVT: With the advancement in endovascular therapies of MIAs, open surgical management is rarely required. There are many advantages of EVT: lower risk of anesthesia and cardiac risk due to less procedure time as these patients have many additional comorbidities, less intracranial manipulation of vessels, and lower risk to surrounding inflamed tissues, and anticoagulation can be started very early (in fact, there are reports of the institution of anticoagulation after 24 hours of EVT management for cardiac surgery). 18 The success rate is very high (from 80% to as high as 95.3–100% 19 20 21 22 ). Case reports have shown that MIAs located deep in the brain or on the peripheral MCA that are lying on the eloquent cortex 23 can also be safely treated with EVT. Current endovascular treatment includes parent artery occlusion (coils or liquid embolic agents) if the aneurysm has a complex morphology and is located in a noneloquent area, and the reconstructive approach by an aneurysm using coils, 22 stent-assisted coiling, flow diverters, and liquid embolic agents 24 such as onyx 25 and glue. 26
Endovascular interventions can be a permutation and combination of various strategies. The main aim is to obliterate the MIA with coils/liquid embolic agent to secure the aneurysm and prevent recurrent bleeding and additional neurologic injury. Other endovascular options include vessel sacrifice (parent artery occlusion) with coils or embolic agents to cause hemodynamic occlusion of the vessels feeding the aneurysm. The risk of stroke related to occlusion of the parent vessel and ischemia to the eloquent brain can be assessed by super-selective Wada testing before the endovascular vessel though nowadays even MIA lying on the eloquent cortex can be safely treated. 21 24 Many times, the collateral vessels are good enough to compensate for the occlusion of blood supply by parent artery occlusion. Finally, endovascular devices requiring dual anticoagulation are generally avoided, although many case reports have shown success with stents/flow diverters. 27
Conclusion
Endovascular embolization of IIAs can be performed successfully in all age groups and critically ill patients requiring immediate open heart surgery and systemic anticoagulation. For cases with associated hematomas and mass effects, surgery may be offered; otherwise, endovascular modalities are generally preferred. Also, once an aneurysm is ruptured, there is a very high chance of rebleeding like in our first case, and operation (surgery/EVT) should be performed at the earliest without any delay. All our patients were successfully treated with endovascular techniques (like coiling or liquid embolic agent) and had good outcomes. A proposed simple algorithm for the management of MIA is shown in Fig. 5 .
Fig. 5.
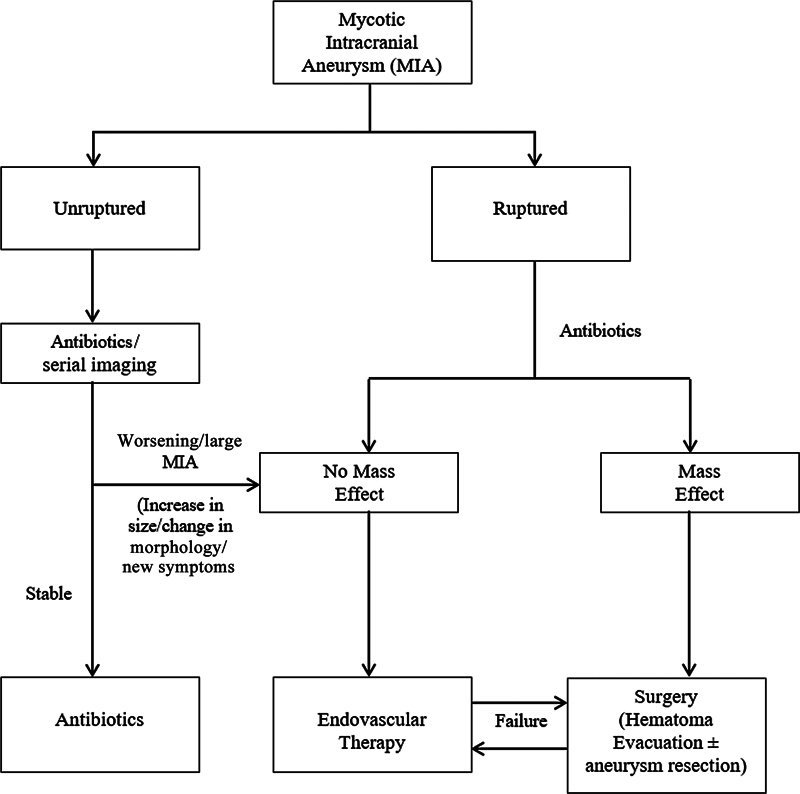
A proposed simple algorithm for the management of mycotic intracranial aneurysm.
Funding Statement
Funding None.
Conflict of Interest None declared.
Authors' Contributions
B.D.C., S.B.G., and S.A. contributed to the acquisition, analysis, conception, design, and drafting of the work. B.D.C., S.B.G., S.J., and S.A. contributed to the final draft, revisions, upload, and submission of the final revised work. All the authors have agreed to both be personally accountable for their contributions and ensure that questions related to the accuracy or integrity of any part of the work, even ones in which one was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Ethical Approval
This work was performed in accordance with the guidelines of the declaration of Helsinki.
Patients' Consent
Informed consent was waived owing to the retrospective nature of the study.
References
- 1.Frazee J G, Cahan L D, Winter J. Bacterial intracranial aneurysms. J Neurosurg. 1980;53(05):633–641. doi: 10.3171/jns.1980.53.5.0633. [DOI] [PubMed] [Google Scholar]
- 2.Kannoth S, Thomas S V. Intracranial microbial aneurysm (infectious aneurysm): current options for diagnosis and management. Neurocrit Care. 2009;11(01):120–129. doi: 10.1007/s12028-009-9208-x. [DOI] [PubMed] [Google Scholar]
- 3.Koch P, Desal H A, Auffray-Calvier E, De Kersaint-Gilly A. Natural history and management of mycotic intracranial aneurysm. J Neuroradiol. 2005;32(04):258–265. doi: 10.1016/s0150-9861(05)83148-7. [DOI] [PubMed] [Google Scholar]
- 4.Osler W. Malignant endocarditis. Lecture I. Lancet. 1885;1:415–418. [Google Scholar]
- 5.Osler W. Malignant endocarditis. Lecture II. Lancet. 1885;1:459–464. [Google Scholar]
- 6.Osler W. Malignant endocarditis. Lecture III. Lancet. 1885;1:505–508. [Google Scholar]
- 7.Peters P J, Harrison T, Lennox J L. A dangerous dilemma: management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis. 2006;6(11):742–748. doi: 10.1016/S1473-3099(06)70631-4. [DOI] [PubMed] [Google Scholar]
- 8.Bohmfalk G L, Story J L, Wissinger J P, Brown W E., Jr Bacterial intracranial aneurysm. J Neurosurg. 1978;48(03):369–382. doi: 10.3171/jns.1978.48.3.0369. [DOI] [PubMed] [Google Scholar]
- 9.Barrow D L, Prats A R.Infectious intracranial aneurysms: comparison of groups with and without endocarditis Neurosurgery 19902704562–572., discussion 572–573 [PubMed] [Google Scholar]
- 10.Patir R, Mahapatra A K, Banerji A K.Risk factors in postoperative neurosurgical infection. A prospective study Acta Neurochir (Wien) 1992119(1-4):80–84. [DOI] [PubMed] [Google Scholar]
- 11.Karamessini M T, Kagadis G C, Petsas T et al. CT angiography with three-dimensional techniques for the early diagnosis of intracranial aneurysms. Comparison with intra-arterial DSA and the surgical findings. Eur J Radiol. 2004;49(03):212–223. doi: 10.1016/S0720-048X(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 12.Okahara M, Kiyosue H, Yamashita M et al. Diagnostic accuracy of magnetic resonance angiography for cerebral aneurysms in correlation with 3D-digital subtraction angiographic images: a study of 133 aneurysms. Stroke. 2002;33(07):1803–1808. doi: 10.1161/01.str.0000019510.32145.a9. [DOI] [PubMed] [Google Scholar]
- 13.ESC Committee for Practice Guidelines ; Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer . Habib G, Hoen B, Tornos P et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2009;30(19):2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 14.Barami K, Ko K. Ruptured mycotic aneurysm presenting as an intraparenchymal hemorrhage and nonadjacent acute subdural hematoma: case report and review of the literature. Surg Neurol. 1994;41(04):290–293. doi: 10.1016/0090-3019(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 15.Chun J Y, Smith W, Halbach V V, Higashida R T, Wilson C B, Lawton M T.Current multimodality management of infectious intracranial aneurysms Neurosurgery 200148061203–1213., discussion 1213–1214 [DOI] [PubMed] [Google Scholar]
- 16.Yen P S, Teo B T, Chen S C, Chiu T L. Endovascular treatment for bilateral mycotic intracavernous carotid aneurysms. Case report and review of the literature. J Neurosurg. 2007;107(04):868–872. doi: 10.3171/JNS-07/10/0868. [DOI] [PubMed] [Google Scholar]
- 17.Zanaty M, Chalouhi N, Starke R M et al. Endovascular treatment of cerebral mycotic aneurysm: a review of the literature and single center experience. BioMed Res Int. 2013;2013:151643. doi: 10.1155/2013/151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng-Ching E, John S, Bain Met al. Endovascular embolization of intracranial infectious aneurysms in patients undergoing open heart surgery using n-butyl cyanoacrylate Intervent Neurol 20176(1–2):82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esenkaya A, Duzgun F, Cinar C et al. Endovascular treatment of intracranial infectious aneurysms. Neuroradiology. 2016;58(03):277–284. doi: 10.1007/s00234-015-1633-2. [DOI] [PubMed] [Google Scholar]
- 20.Petr O, Brinjikji W, Burrows A M, Cloft H, Kallmes D F, Lanzino G. Safety and efficacy of endovascular treatment for intracranial infectious aneurysms: a systematic review and meta-analysis. J Neuroradiol. 2016;43(05):309–316. doi: 10.1016/j.neurad.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Gross B A, Puri A S.Endovascular treatment of infectious intracranial aneurysms Neurosurg Rev 2013360111–19., discussion 19 [DOI] [PubMed] [Google Scholar]
- 22.Chapot R, Houdart E, Saint-Maurice J P et al. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002;222(02):389–396. doi: 10.1148/radiol.2222010432. [DOI] [PubMed] [Google Scholar]
- 23.Nonaka S, Oishi H, Tsutsumi S et al. Endovascular therapy for infectious intracranial aneurysm: a report of four cases. J Stroke Cerebrovasc Dis. 2016;25(03):e33–e37. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 24.Eddleman C S, Surdell D, DiPatri A, Jr, Tomita T, Shaibani A. Infectious intracranial aneurysms in the pediatric population: endovascular treatment with onyx. Childs Nerv Syst. 2008;24(08):909–915. doi: 10.1007/s00381-008-0614-8. [DOI] [PubMed] [Google Scholar]
- 25.Jadhav A P, Pryor J C, Nogueira R G. Onyx embolization for the endovascular treatment of infectious and traumatic aneurysms involving the cranial and cerebral vasculature. J Neurointerv Surg. 2013;5(06):562–565. doi: 10.1136/neurintsurg-2012-010460. [DOI] [PubMed] [Google Scholar]
- 26.Katakura K, Kayama T, Kondo R et al. A case of multiple cerebral mycotic aneurysms treated with endovascular surgery. No Shinkei Geka. 1995;23(12):1127–1132. [PubMed] [Google Scholar]
- 27.Charan B D, Thanneru S, Sebastian L JD, Jain S. Reconstructive endovascular treatment of petrous ICA pseudoaneurysm in skull base osteomyelitis: a hidden catastrophe. BMJ Case Rep. 2024;17(02):e258539. doi: 10.1136/bcr-2023-258539. [DOI] [PMC free article] [PubMed] [Google Scholar]


