Abstract
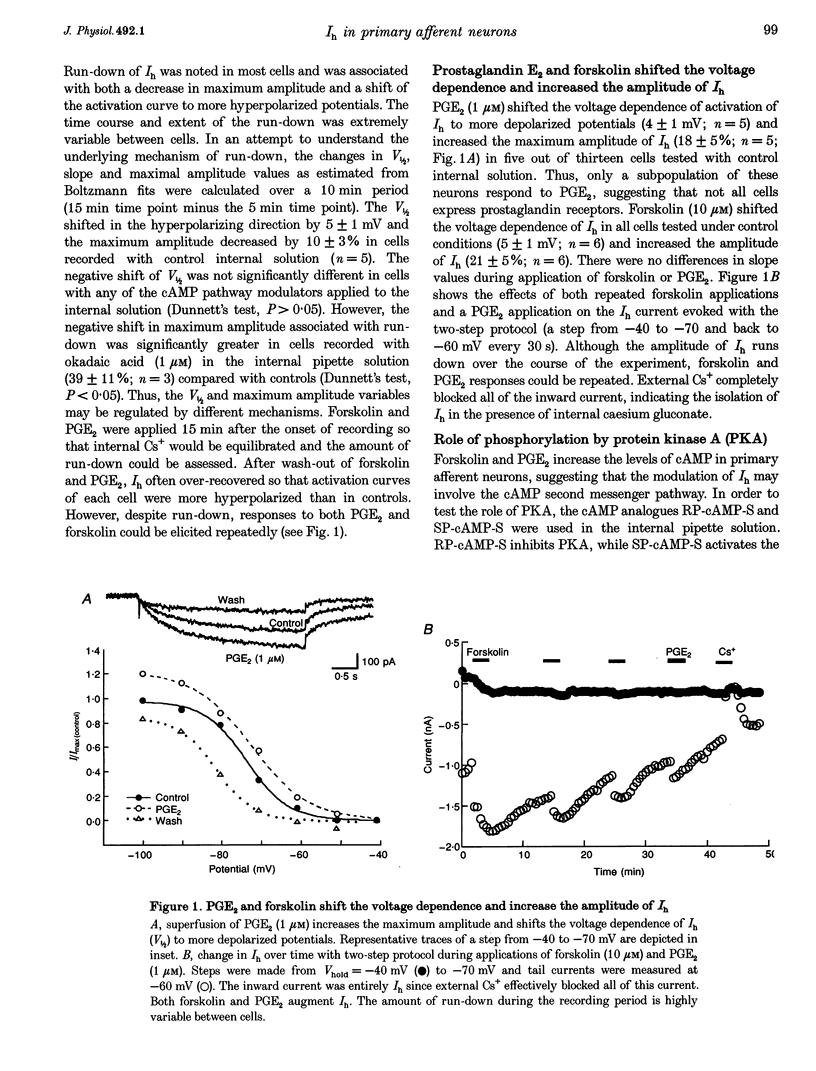
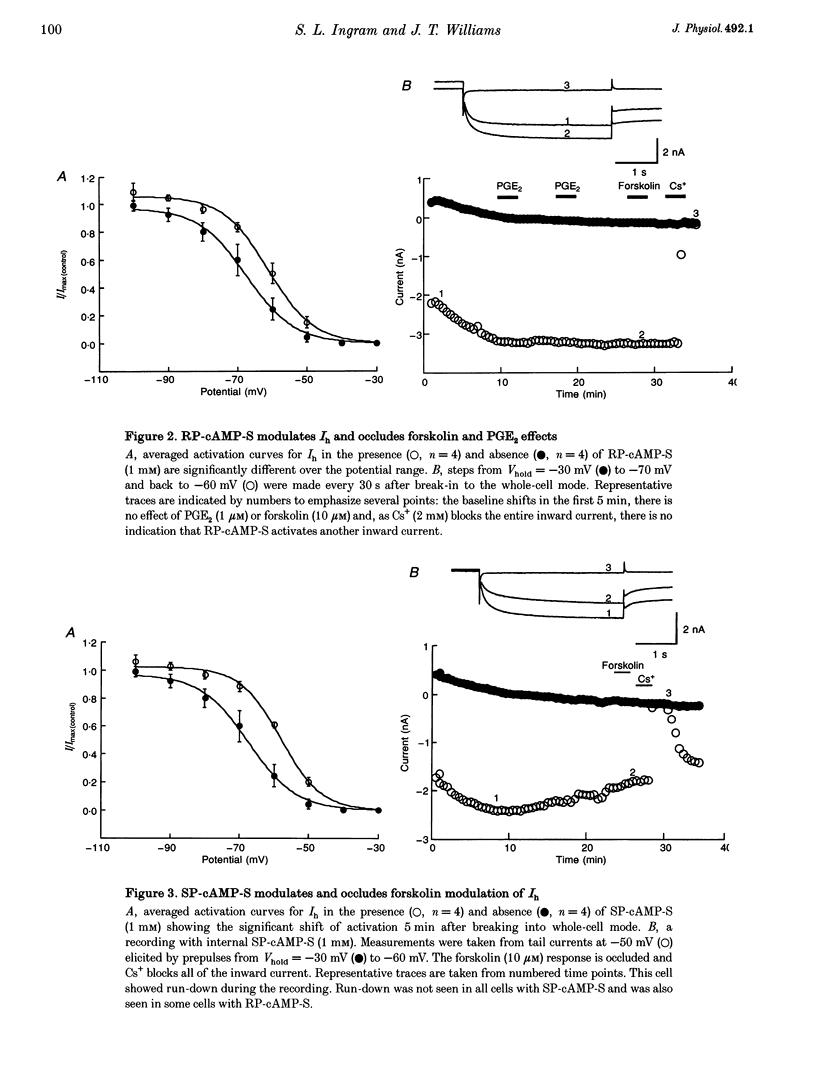
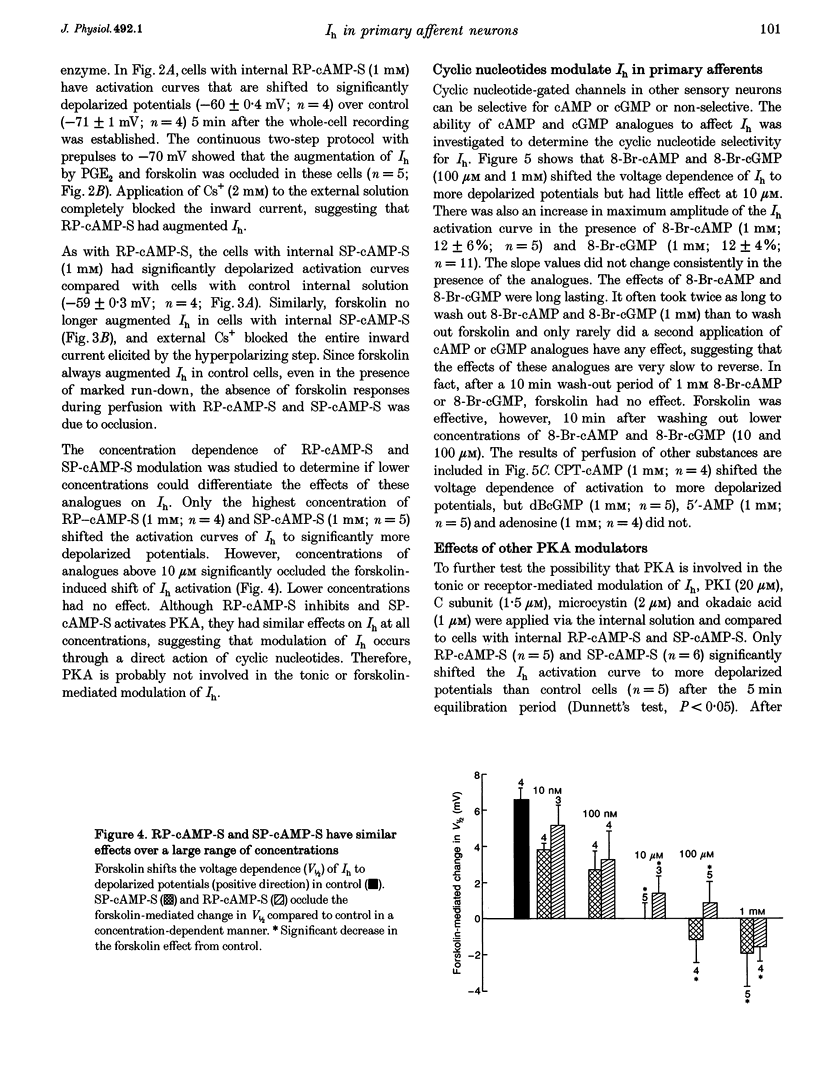
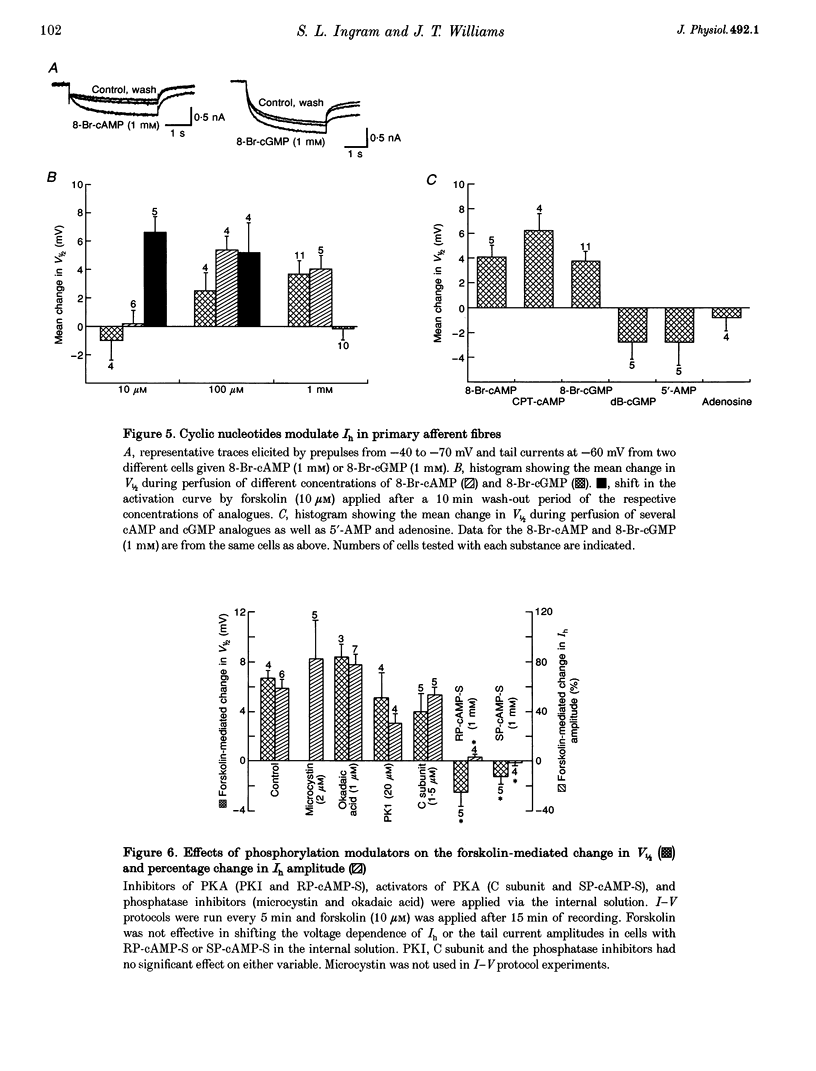
1. Whole-cell patch-clamp recordings were made from dissociated guinea-pig nodose and trigeminal ganglion neurons in culture to study second messenger mechanisms of the hyperpolarization-activated current (Ih) modulation. 2. Prostaglandin E2 (PGE2) and forskolin modulate Ih in primary afferents by shifting the activation curve in the depolarizing direction and increasing the maximum amplitude. 3. The cAMP analogues, RP-cAMP-S (an inhibitor of protein kinase A (PKA)) and SP-cAMP-S (an activator of PKA), both shifted the activation curve of Ih to more depolarized potentials and occluded the effects of forskolin. These results suggest that Ih is modulated by a direct action of the cAMP analogues. 4. Superfusion of other cyclic nucleotide analogues (8-Br-cAMP, 8-(4-chlorophenylthio)-cAMP and 8-Br-cGMP) mimicked the actions of forskolin and PGE2, but dibutyryl cGMP, 5'-AMP and adenosine had no effect on Ih. 8-Br-cAMP and 8-Br-cGMP had similar concentration response profiles, suggesting that Ih has little nucleotide selectivity. 5. The inhibitor peptide (PKI), the catalytic subunit of PKA (C subunit) and phosphatase inhibitors (microcystin and okadaic acid) had no effect on forskolin modulation of Ih. 6. These results indicate that Ih is regulated by cyclic nucleotides in sensory neurons. Positive regulation of Ih by prostaglandins produced during inflammation may lead to depolarization and facilitation of repetitive activity, and thus contribute to sensitization to painful stimuli.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks M. I., Pearce R. A., Smith P. H. Hyperpolarization-activated cation current (Ih) in neurons of the medial nucleus of the trapezoid body: voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brain stem. J Neurophysiol. 1993 Oct;70(4):1420–1432. doi: 10.1152/jn.1993.70.4.1420. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bernheim L., Mathie A., Hille B. Intracellular Ca2+ buffers disrupt muscarinic suppression of Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Denbigh J. S., Lang R. J. Inward rectification in freshly isolated single smooth muscle cells of the rabbit jejunum. J Physiol. 1987 Feb;383:461–476. doi: 10.1113/jphysiol.1987.sp016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Cohen I. S. Mechanism of acetylcholine action on pacemaker current (i(f)) in canine Purkinje fibers. Pflugers Arch. 1992 Mar;420(3-4):389–392. doi: 10.1007/BF00374474. [DOI] [PubMed] [Google Scholar]
- Cui M., Nicol G. D. Cyclic AMP mediates the prostaglandin E2-induced potentiation of bradykinin excitation in rat sensory neurons. Neuroscience. 1995 May;66(2):459–466. doi: 10.1016/0306-4522(94)00567-o. [DOI] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current if. J Physiol. 1990 Oct;429:401–409. doi: 10.1113/jphysiol.1990.sp018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingtgen C. M., Waite K. J., Vasko M. R. Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3',5'-cyclic monophosphate transduction cascade. J Neurosci. 1995 Jul;15(7 Pt 2):5411–5419. doi: 10.1523/JNEUROSCI.15-07-05411.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamondi A., Reiner P. B. Hyperpolarization-activated inward current in histaminergic tuberomammillary neurons of the rat hypothalamus. J Neurophysiol. 1991 Dec;66(6):1902–1911. doi: 10.1152/jn.1991.66.6.1902. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B. The cyclic nucleotide-gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 1991 Apr;14(4):150–157. doi: 10.1016/0166-2236(91)90087-b. [DOI] [PubMed] [Google Scholar]
- Khasar S. G., Ouseph A. K., Chou B., Ho T., Green P. G., Levine J. D. Is there more than one prostaglandin E receptor subtype mediating hyperalgesia in the rat hindpaw? Neuroscience. 1995 Feb;64(4):1161–1165. doi: 10.1016/0306-4522(94)00423-3. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990 Dec;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T., Nishihara I., Uda R., Ito S., Hyodo M., Hayaishi O. Characterization of EP-receptor subtypes involved in allodynia and hyperalgesia induced by intrathecal administration of prostaglandin E2 to mice. Br J Pharmacol. 1994 Jul;112(3):735–740. doi: 10.1111/j.1476-5381.1994.tb13139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Shigemoto R., Namba T., Negishi M., Mizuno N., Narumiya S., Ichikawa A. Distribution of the messenger RNA for the prostaglandin E receptor subtype EP3 in the mouse nervous system. Neuroscience. 1994 Oct;62(3):919–928. doi: 10.1016/0306-4522(94)90483-9. [DOI] [PubMed] [Google Scholar]
- Taiwo Y. O., Bjerknes L. K., Goetzl E. J., Levine J. D. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32(3):577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Taiwo Y. O., Levine J. D. Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res. 1989 Jul 17;492(1-2):397–399. doi: 10.1016/0006-8993(89)90928-1. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. Cyclic AMP regulates an inward rectifying sodium-potassium current in dissociated bull-frog sympathetic neurones. J Physiol. 1990 Jan;420:409–429. doi: 10.1113/jphysiol.1990.sp017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Shiraishi M., Akasu T. Morphological and electrophysiological properties of C-cells in bullfrog dorsal root ganglia. Neurosci Lett. 1990 Aug 24;116(3):304–308. doi: 10.1016/0304-3940(90)90091-m. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yatani A., Okabe K., Codina J., Birnbaumer L., Brown A. M. Heart rate regulation by G proteins acting on the cardiac pacemaker channel. Science. 1990 Sep 7;249(4973):1163–1166. doi: 10.1126/science.1697697. [DOI] [PubMed] [Google Scholar]


