Abstract
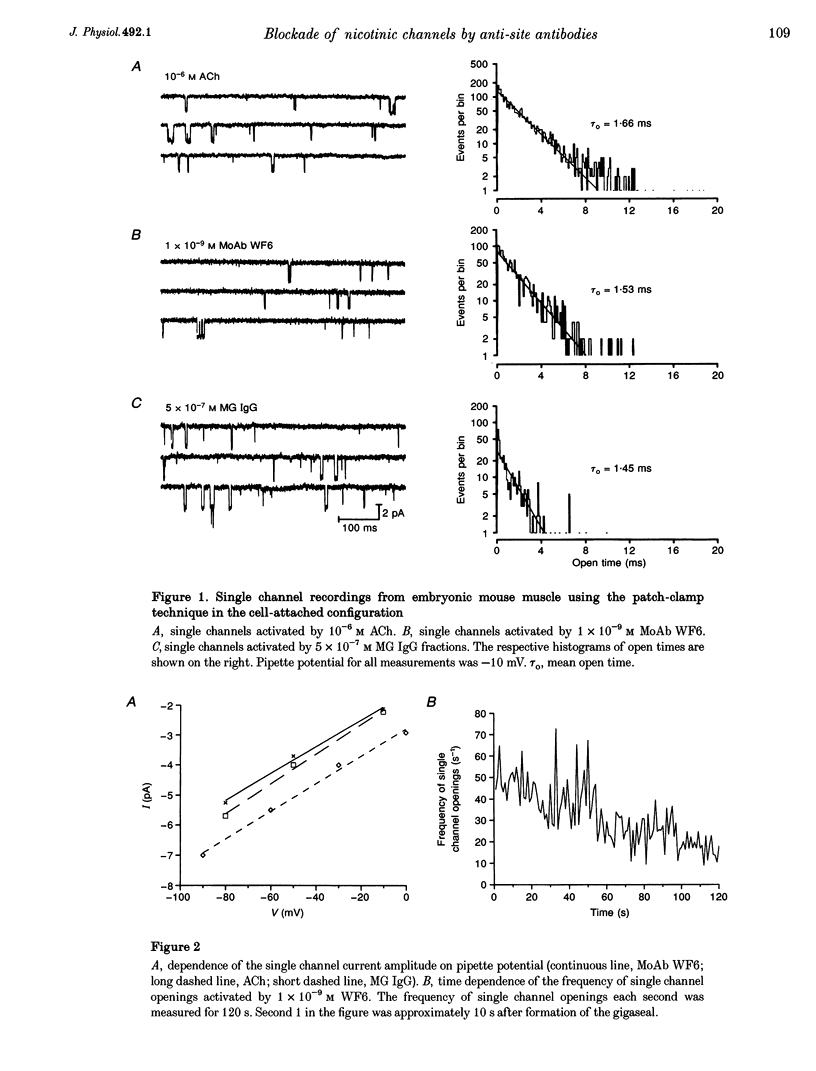
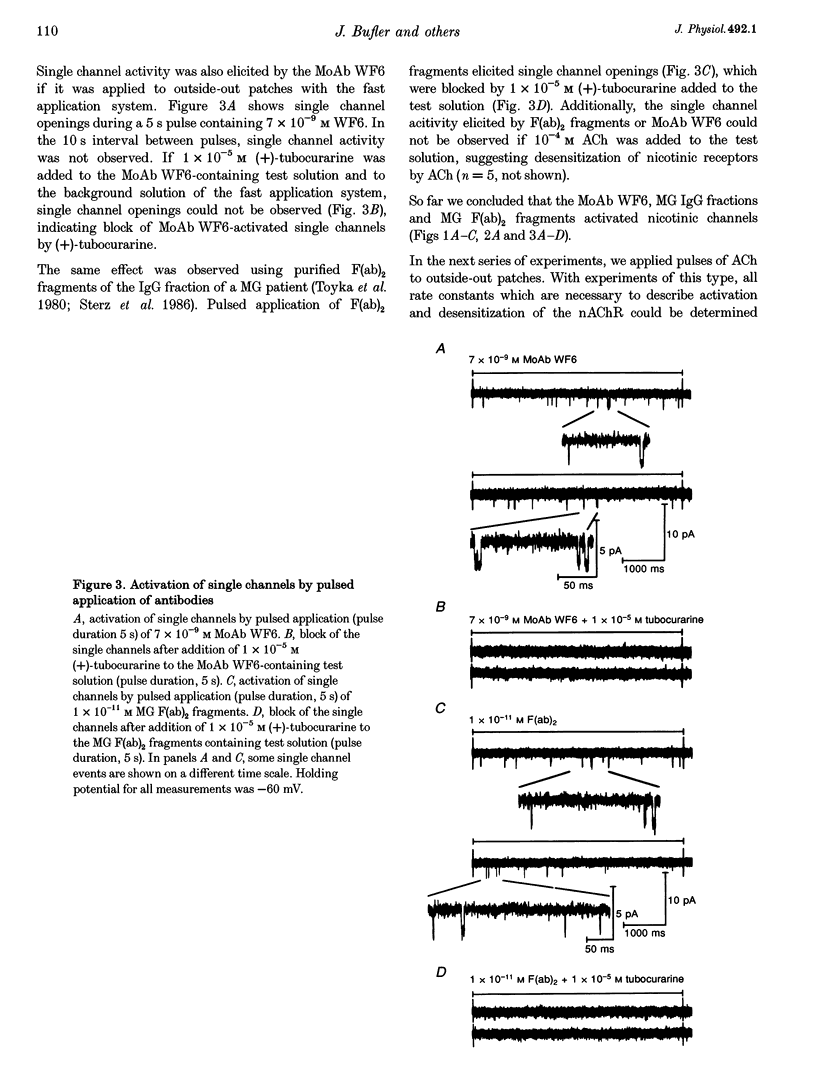
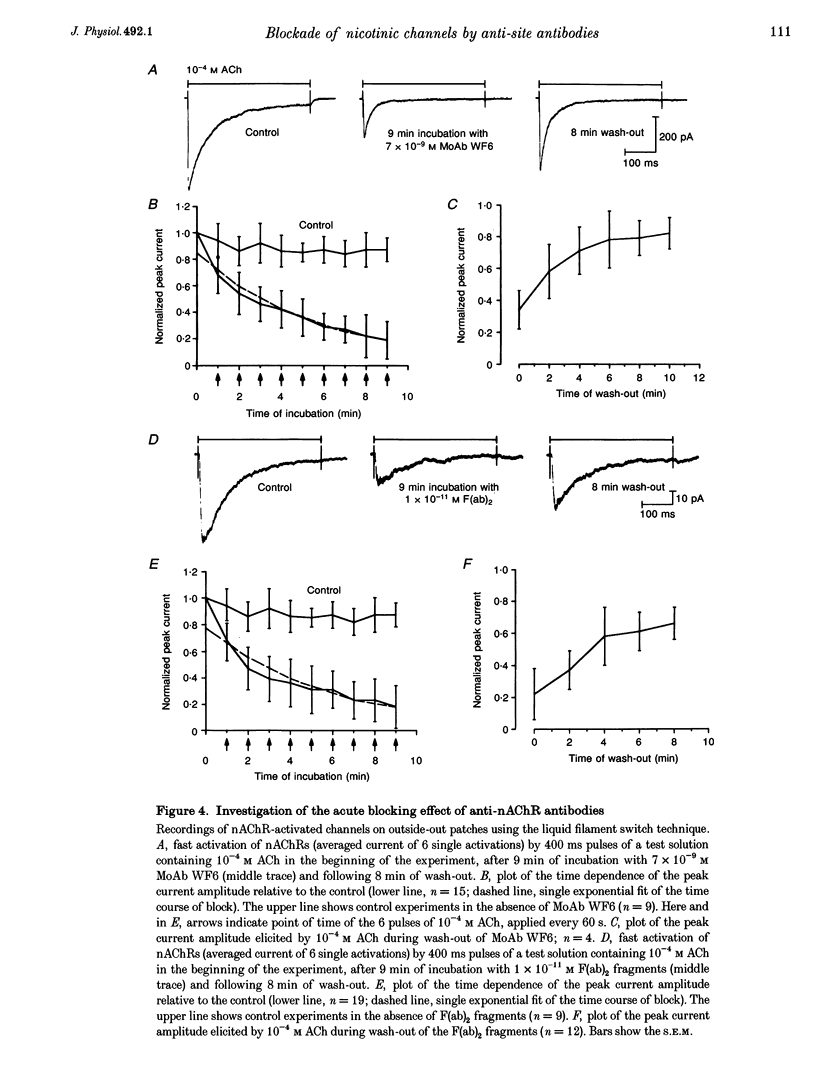
1. Using the patch-clamp technique, we have found that mouse muscle nicotinic acetylcholine receptor (nAChR) channels can be activated by low concentrations of a monoclonal antibody (MoAb), referred to as WF6, which is directed against the acetylcholine (ACh) binding site. Similar effects were seen using IgG or F(ab)2 fragments from the sera of patients with myasthenia gravis (MG), which contain polyclonal anti-nAChR antibodies. 2. The mean open times of MoAb and the slope conductance of single WF6-activated single channels were similar to those of ACh-activated channels under the same experimental conditions. 3. On outside-out patches, single channel activity was elicited by MoAb WF6 and MG F(ab)2 fragments, and was blocked by (+)-tubocurarine. We therefore concluded that MoAb WF6 and the MG F(ab)2 fragments activate the nAChR. 4. MoAb WF6 and MG F(ab)2 fragments blocked the current activated by pulsed application of 10(-4) M ACh to a significant extent. The block was partly reversible. The rate constants for the binding and dissociation of MoAb WF6 from the receptor were determined quantitatively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burges J., Wray D. W., Pizzighella S., Hall Z., Vincent A. A myasthenia gravis plasma immunoglobulin reduces miniature endplate potentials at human endplates in vitro. Muscle Nerve. 1990 May;13(5):407–413. doi: 10.1002/mus.880130507. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Tang F., Diethelm B. M., Spencer S. R., Reinhardt-Maelicke S., Maelicke A. Mapping of a cholinergic binding site by means of synthetic peptides, monoclonal antibodies, and alpha-bungarotoxin. Biochemistry. 1990 Jul 3;29(26):6221–6230. doi: 10.1021/bi00478a016. [DOI] [PubMed] [Google Scholar]
- Dudel J., Franke C. Single glutamate-gated synaptic channels at the crayfish neuromuscular junction. II. Dependence of channel open time on glutamate concentration. Pflugers Arch. 1987 Mar;408(3):307–314. doi: 10.1007/BF02181474. [DOI] [PubMed] [Google Scholar]
- Fels G., Plümer-Wilk R., Schreiber M., Maelicke A. A monoclonal antibody interfering with binding and response of the acetylcholine receptor. J Biol Chem. 1986 Nov 25;261(33):15746–15754. [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett. 1987 Jun 15;77(2):199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- Franke C., Költgen D., Hatt H., Dudel J. Activation and desensitization of embryonic-like receptor channels in mouse muscle by acetylcholine concentration steps. J Physiol. 1992;451:145–158. doi: 10.1113/jphysiol.1992.sp019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Parnas H., Hovav G., Dudel J. A molecular scheme for the reaction between acetylcholine and nicotinic channels. Biophys J. 1993 Feb;64(2):339–356. doi: 10.1016/S0006-3495(93)81374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlepp M. J., Kay P. H., Dawkins R. L., Bucknall R. C., Kemp A. Cross-reactivity of anti-acetylcholine receptor autoantibodies. Muscle Nerve. 1981 Jul-Aug;4(4):282–288. doi: 10.1002/mus.880040404. [DOI] [PubMed] [Google Scholar]
- Goldberg G., Mochly-Rosen D., Fuchs S., Lass Y. Monoclonal antibodies modify acetylcholine-induced ionic channel properties in cultured chick myoballs. J Membr Biol. 1983;76(2):123–128. doi: 10.1007/BF02000612. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kang S., Maelicke A. Fluorescein isothiocyanate-labeled alpha-cobratoxin. Biochemical characterization and interaction with acetylcholine receptor from Electrophorus electricus. J Biol Chem. 1980 Aug 10;255(15):7326–7332. [PubMed] [Google Scholar]
- Maelicke A., Fulpius B. W., Klett R. P., Reich E. Acetylcholine receptor. Responses to drug binding. J Biol Chem. 1977 Jul 25;252(14):4811–4830. [PubMed] [Google Scholar]
- Maselli R. A., Nelson D. J., Richman D. P. Effects of a monoclonal anti-acetylcholine receptor antibody on the avian end-plate. J Physiol. 1989 Apr;411:271–283. doi: 10.1113/jphysiol.1989.sp017573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa E. L., Chattopadhyay A., McNamee M. G. Desensitization of the nicotinic acetylcholine receptor: molecular mechanisms and effect of modulators. Cell Mol Neurobiol. 1989 Jun;9(2):141–178. doi: 10.1007/BF00713026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder B., Reinhardt-Maelicke S., Schrattenholz A., McLane K. E., Kretschmer A., Conti-Tronconi B. M., Maelicke A. Monoclonal antibodies FK1 and WF6 define two neighboring ligand binding sites on Torpedo acetylcholine receptor alpha-polypeptide. J Biol Chem. 1994 Apr 8;269(14):10407–10416. [PubMed] [Google Scholar]
- Sterz R., Hohlfeld R., Rajki K., Kaul M., Heininger K., Peper K., Toyka K. V. Effector mechanisms in myasthenia gravis: end-plate function after passive transfer of IgG, Fab, and F(ab')2 hybrid molecules. Muscle Nerve. 1986 May;9(4):306–312. doi: 10.1002/mus.880090404. [DOI] [PubMed] [Google Scholar]
- Toyka K. V., Brachman D. B., Pestronk A., Kao I. Myasthenia gravis: passive transfer from man to mouse. Science. 1975 Oct 24;190(4212):397–399. doi: 10.1126/science.1179220. [DOI] [PubMed] [Google Scholar]
- Toyka K. V., Löwenadler B., Heininger K., Besinger U. A., Birnberger K. L., Fateh-Moghadam A., Heilbronn E. Passively transferred myasthenia gravis: protection of mouse endplates by Fab fragments from human myasthenic IgG. J Neurol Neurosurg Psychiatry. 1980 Sep;43(9):836–842. doi: 10.1136/jnnp.43.9.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Kokla A., Walgrave S. L., Conti-Tronconi B. M. Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67-76 of the alpha subunit. Proc Natl Acad Sci U S A. 1988 May;85(9):2899–2903. doi: 10.1073/pnas.85.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Remoundos M. S. Precise epitope mapping of monoclonal antibodies to the cytoplasmic side of the acetylcholine receptor alpha subunit. Dissecting a potentially myasthenogenic epitope. Eur J Biochem. 1992 Aug 1;207(3):915–922. doi: 10.1111/j.1432-1033.1992.tb17124.x. [DOI] [PubMed] [Google Scholar]
- Vincent A., Li Z., Hart A., Barrett-Jolley R., Yamamoto T., Burges J., Wray D., Byrne N., Molenaar P., Newsom-Davis J. Seronegative myasthenia gravis. Evidence for plasma factor(s) interfering with acetylcholine receptor function. Ann N Y Acad Sci. 1993 Jun 21;681:529–538. doi: 10.1111/j.1749-6632.1993.tb22936.x. [DOI] [PubMed] [Google Scholar]
- Watters D., Maelicke A. Organization of ligand binding sites at the acetylcholine receptor: a study with monoclonal antibodies. Biochemistry. 1983 Apr 12;22(8):1811–1819. doi: 10.1021/bi00277a011. [DOI] [PubMed] [Google Scholar]


