Abstract
Treatment of relapsed/refractory multiple myeloma (RRMM) is challenging as patients exhaust all available therapies and the disease becomes refractory to standard drug classes. Here we report the final results of LocoMMotion, the first prospective study of real-world clinical practice (RWCP) in triple-class exposed (TCE) patients with RRMM, with a median follow-up of 26.4 months (range, 0.1–35.0). Patients (N = 248) had received median 4 prior LOT (range, 2–13) at enrollment. 91 unique regimens were used in index LOT. Overall response rate was 31.9% (95% CI, 26.1–38.0), median progression-free survival (PFS) was 4.6 months (95% CI, 3.9–5.6) and median overall survival was 13.8 months (95% CI, 10.8–17.0). 152 patients (61.3%) had subsequent LOTs with 134 unique regimens, of which 78 were used in first subsequent LOT. Median PFS2 (from start of study through first subsequent LOT) was 10.8 months (95% CI, 8.4–13.0). 158 patients died on study, 67.7% due to progressive disease. Additional subgroup analyses and long-term safety summaries are reported. The high number of RWCP treatment regimens utilized and poor clinical outcomes confirm a lack of standardized treatment for TCE patients with RRMM, highlighting the need for new treatments with novel mechanisms.
Subject terms: Clinical trials, Cancer therapy
Introduction
With recent advances in multiple myeloma (MM) treatment, patients with MM are living longer [1–3]. However, because most patients relapse and/or their disease becomes refractory to treatment [4, 5], they cycle through standard drug classes, including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), anti-CD38 monoclonal antibodies, and others. Despite a wide array of conventional treatment options for relapse/refractory MM (RRMM), periods of remission are generally short in real-world clinical practice (RWCP) [6] and outcomes worsen with each subsequent line of therapy (LOT) [5, 6], making treatment selection progressively more challenging [4, 5, 7].
LocoMMotion (ClinicalTrials.gov identifier: NCT04035226) was the first multinational, prospective, observational study to examine effectiveness and safety of RWCP therapies in triple-class exposed patients with MM. Its prospective study design allowed for collection of patient-level baseline characteristics and outcome parameters that are not commonly collected during routine clinical practice. Previous results from LocoMMotion, reported at 16.1 months median study follow-up, showed a lack of clear standard of care for treatment of triple-class exposed patients and poor outcomes [8]. Ninety-one unique regimens were used in the index LOT (first treatment after enrollment), and the overall response rate (ORR) was 31.5%, with median progression-free survival (PFS) and overall survival (OS) of 4.6 months and 13.8 months, respectively. Treatment-emergent adverse events (TEAEs) were reported in 85.9% of patients, with 56.5% reporting grade 3/4 TEAEs. Here, we report results from the final analysis of LocoMMotion, including mature survival data, additional subgroup analyses, subsequent therapies, and their outcomes, as well as long term safety data, including second primary malignancies (SPM), and TEAEs.
Patients and methods
Study design and treatment
LocoMMotion was a prospective, observational study that explored the use of RWCP therapies in the management of triple-class exposed patients with RRMM (i.e., had received a PI, an IMiD, and an anti-CD38 monoclonal antibody [mAb]). Eligible patients had received ≥3 prior LOT or their disease was double refractory to a PI and an IMiD, were triple-class exposed, and had documented disease progression during or after their last LOT. Further eligibility criteria included age ≥18 years with a documented diagnosis of MM per International Myeloma Working Group (IMWG) criteria [9–11], measurable disease assessed by serum free light chain (≥10 mg/dL and abnormal ratio) or M-protein (≥1.0 g/dL [serum] or ≥200 mg/24 h [urine]), and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Patients were enrolled across 75 sites in Europe (Belgium, France, Germany, Italy, Netherlands, Poland, Russia, Spain, and the United Kingdom) and the United States.
LocoMMotion included 3 phases: 1) a screening phase (the 28-day period prior to enrollment) in which baseline patient and disease characteristics, diagnosis, and medication history were collected; 2) an index LOT phase (time from the first day of on-study RWCP treatment until initiation of subsequent antimyeloma therapy) in which treatment type, effectiveness data, and safety data were collected; and 3) a follow-up phase until study completion wherein patients were followed for documentation of subsequent LOT, PFS2, and OS. Patients could receive ≥1 regimen during a LOT (e.g., if they started with triplet therapy and were de-escalated to a doublet therapy due to toxicity). Patients were allowed to enroll in other trials and/or use experimental therapies in subsequent LOT. End of study was defined as 24 months after the first dosing of the last patient enrolled. RWCP treatments were defined as those approved and used in local clinical practice for treatment of adult patients with RRMM. During index LOT, a response review committee (RRC) comprising three MM hematologists reassessed responses and evaluated disease progression in accordance with IMWG criteria, in a blinded manner, to ensure consistency of the assessments. Responses with subsequent LOT were subject to investigator assessment.
This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was provided by all patients, and the study protocol was approved by an independent ethics committee/institutional review board at each center (Supplementary Table S1).
Endpoints and assessments
The primary effectiveness endpoint of the LocoMMotion study was ORR during treatment with index LOT, as assessed by the RRC. ORR was defined as the proportion of patients achieving partial response or better according to the IMWG criteria. Secondary effectiveness endpoints included rates of stringent complete response, complete response (CR), very good partial response (VGPR), duration of response (DOR), PFS, time to first response, and time to best response. These were assessed by RRC during treatment with index LOT. Investigator assessments were also collected for secondary effectiveness endpoints: time to next treatment, ORR, PFS, PFS2, and OS.
DOR was defined as time from first documentation of partial response or better to first documented evidence of progressive disease (PD) as assessed by RRC or death, whichever occurred first, or start of subsequent therapy. PFS was defined as time from day 1 of index LOT to first documented evidence of PD by RRC, per IMWG response criteria, or death by any cause, whichever occurred first; data were censored at the last disease evaluation before the start of subsequent therapy. PFS2 was defined as the time between day 1 of index LOT and either the first date of documented PD by the study investigator after the start of the first subsequent LOT, or death from any cause from the start of the study. PFS2 included death events from the start of index LOT and PD events from the start of the first subsequent LOT (i.e., not PD events during index LOT). For patients who did not progress on index LOT and who were alive, data were censored prior to the start of the first subsequent LOT. Patients who withdrew before the first subsequent LOT were also censored. Time to next treatment was defined as the time from the first day of index LOT to initiation of subsequent antimyeloma therapy or death. For participants who did not receive subsequent antimyeloma therapy and are alive, data were censored at the last disease evaluation. OS was measured from day 1 of index LOT to the date of the patient’s death, with censoring for patients who were alive at study completion. Prespecified subgroup analysis was performed across a range of outcomes, including PFS, OS (reporting number of events, median time in months, and corresponding 95% confidence intervals [CIs]), and ORR.
Safety assessments included incidence and severity of adverse events (AEs), including TEAEs and second primary malignancies. TEAEs included all AEs that occurred between the start of and 30 days after the end of index LOT or the day prior to the first subsequent LOT, whichever occurred first. TEAEs also included AEs considered related to the study drug, regardless of start date, and AEs present at baseline that worsened.
Statistical analyses
As LocoMMotion was an observational study, no direct hypothesis was tested, and the sample size was based on the clinically acceptable precision of the 95% CI for the primary objective, as previously described [12]. For the primary endpoint, exact 95% CIs were calculated by the Clopper-Pearson method. Variables were summarized using descriptive statistics. Time-to-event data were summarized using the Kaplan–Meier method. Categorical values were summarized using the number of observations and percentages. Subgroup analysis was reported as descriptive summaries and graphically presented using forest plots. Additional measures applied to account for potential missing assessments in RWCP have been previously described [12].
Results
Patients
Of 313 patients screened for the LocoMMotion study, 248 patients were enrolled between August 2, 2019, and October 26, 2020 (all-treated analysis set). As previously reported, 225 (90.7%) patients were from Europe and 23 (9.3%) were from the United States. At the end of study in October 2022, median study follow-up was 26.4 months (range, 0.1–35.0), and 205 (82.7%) patients had completed the study, including 158 (63.7%) patients who died. Forty-three (17.3%) patients had discontinued, including 13 who were lost to follow-up (Fig. 1). Patient and disease baseline characteristics have been previously described [12]. Median age was 68 years (range, 41–89), 87 (40.1%) had creatinine clearance ≤60 mL/min, 81 (40.5%) had lactate dehydrogenase levels >245 U/L, and 135 patients (54.4%) were male. At baseline, patients had received a median of 4 prior LOT (range, 2–13), 25.0% had received 4 prior LOT, and 49.2% had received ≥5 prior LOT. One hundred eighty-two (73.4%) patients were triple-class refractory, 229 (92.3%) were refractory to the last LOT, and 160 (64.5%) had prior stem cell transplantation. Median time to triple-class exposure was 4.7 years (range, 0–20.4).
Fig. 1. Study disposition.
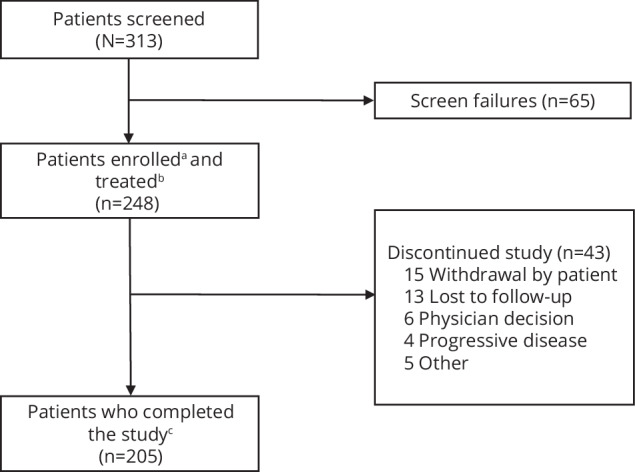
aEnrolled patients are those who signed informed consent and were formally enrolled into the study. bTreated patients are those who were enrolled into the study and received at least 1 real-world clinical practice treatment. cPatients who completed the study are those who either died or remained on study at the end of the study (24 months), whichever occurred first.
Index LOT
Ninety-one unique RWCP treatment regimens were used by patients in the index LOT, including a range of combinations of glucocorticoids (91.5%), PIs (54.4%), IMiDs (48.8%), and anti-CD38 monoclonal antibodies (9.7%) (Table 1). Six (2.4%) patients received stem cell transplantation (all autologous), and 7 patients (2.8%) received BCMA-targeted therapy. In total, 162 (65.3%) patients received a combination of ≥3 drugs. The most commonly received index regimens were carfilzomib-dexamethasone (Kd, 14.1%), pomalidomide-cyclophosphamide-dexamethasone (PCd, 14.1%), and pomalidomide-dexamethasone (Pd, 11.7%); most other regimens were used by fewer than 5 patients (15 regimens were used by only 2 patients and 46 were used by only 1 patient each). Patients received a median of 4 (range, 1–37) cycles of index treatment; median duration of treatment was 4.0 months (range, 0.1–33.6). Two hundred twenty-one patients (89.1%) discontinued index treatment, most commonly due to PD (54.8%), death (8.5%), and physician decision (8.5%). Twenty-seven patients completed the study.
Table 1.
Antimyeloma RWCP treatments during index and subsequent LOT.
| Treatment, n (%)a | Index LOT (N = 248) | 1st Subsequent LOT (n = 152) | 2nd Subsequent LOT (n = 74) | 3rd Subsequent LOT (n = 42) | ≥4th Subsequent LOT (n = 17) |
|---|---|---|---|---|---|
| Glucocorticoids | 227 (91.5) | 107 (70.4) | 47 (63.5) | 23 (54.8) | 9 (52.9) |
| PI | 135 (54.4) | 53 (34.9) | 24 (32.4) | 9 (21.4) | 8 (47.1) |
| Carfilzomib | 63 (25.4) | 22 (14.5) | 11 (14.9) | 3 (7.1) | 3 (17.6) |
| Bortezomib | 50 (20.2) | 24 (15.8) | 10 (13.5) | 5 (11.9) | 6 (35.3) |
| Ixazomib | 22 (8.9) | 7 (4.6) | 3 (4.1) | 1 (2.4) | 1 (5.9) |
| IMiD | 121 (48.8) | 51 (33.6) | 17 (23) | 10 (23.8) | 7 (41.2) |
| Pomalidomide | 74 (29.8) | 30 (19.7) | 7 (9.5) | 6 (14.3) | 4 (23.5) |
| Lenalidomide | 36 (14.5) | 9 (5.9) | 5 (6.8) | 3 (7.1) | 5 (29.4) |
| Thalidomide | 11 (4.4) | 11 (7.2) | 5 (6.8) | 1 (2.4) | 1 (5.9) |
| Iberdomide | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) |
| Alkylating agents | 108 (43.5) | 68 (44.7) | 29 (39.2) | 13 (31) | 5 (29.4) |
| Cyclophosphamide | 80 (32.3) | 43 (28.3) | 17 (23) | 7 (16.7) | 4 (23.5) |
| Bendamustine | 16 (6.5) | 14 (9.2) | 6 (8.1) | 2 (4.8) | 0 (0) |
| Melphalan | 14 (5.6) | 13 (8.6) | 6 (8.1) | 4 (9.5) | 0 (0) |
| Carmustine | 1 (0.4) | 1 (0.7) | 1 (1.4) | 1 (2.4) | 0 (0) |
| Other | 1 (0.4) | 0 (0) | 0 (0) | 1 (2.4) | 0 (0) |
| Melflufen | 0 (0) | 0 (0) | 1 (1.4) | 0 (0) | 1 (5.9) |
| Anti-CD38 mAb | 24 (9.7) | 16 (10.5) | 5 (6.8) | 11 (26.2) | 7 (41.2) |
| Daratumumab | 23 (9.3) | 13 (8.6) | 4 (5.4) | 5 (11.9) | 6 (35.3) |
| Isatuximab | 1 (0.4) | 3 (2) | 1 (1.4) | 6 (14.3) | 2 (11.8) |
| BCMA-targeted therapies | 7 (2.8) | 24 (15.8) | 21 (28.4) | 10 (23.8) | 7 (41.2) |
| CAR-T | 0 (0) | 0 (0) | 2 (2.7) | 0 (0) | 1 (5.9) |
| Bispecific antibody | 0 (0) | 5 (3.3) | 2 (2.7) | 0 (0) | 4 (23.5) |
| Antibody-drug conjugate | 7 (2.8) | 19 (12.5) | 17 (23) | 10 (23.8) | 3 (17.6) |
| Anthracyclines | 21 (8.5) | 8 (5.3) | 7 (9.5) | 4 (9.5) | 2 (11.8) |
| Topoisomerase inhibitor | 17 (6.9) | 6 (3.9) | 3 (4.1) | 1 (2.4) | 0 (0) |
| Histone deacetylase inhibitor | 12 (4.8) | 6 (3.9) | 0 (0) | 0 (0) | 0 (0) |
| Anti-SLAMF7 mAb | 9 (3.6) | 7 (4.6) | 0 (0) | 1 (2.4) | 2 (11.8) |
| BCL-2 inhibitor | 6 (2.4) | 3 (2) | 2 (2.7) | 2 (4.8) | 1 (5.9) |
| Selective inhibitor of nuclear export | 2 (0.8) | 11 (7.2) | 3 (4.1) | 3 (7.1) | 4 (23.5) |
| Other | 19 (7.67) | 9 (5.9) | 7 (9.5) | 4 (9.5) | 4 (23.5) |
BCMA B-cell maturation antigen, Bcl B-cell lymphoma, CAR chimeric antigen receptor, IMiD immunomodulatory drug, LOT line of therapy, mAb monoclonal antibody, RWCP real-world clinical practice, PI proteasome inhibitor, SLAM signaling lymphocytic activation molecule.
aPatients can be counted in >1 drug group.
Subsequent LOT
One hundred fifty-two patients (61.3%) received at least 1 subsequent LOT after the index LOT (Supplementary Table S2). One hundred thirty-four unique regimens were used; 19 regimens were used by only 2 patients and 89 by only 1 patient. Overall, the most commonly used first subsequent LOTs were belantamab mafodotin (10.5% of those who received subsequent LOT) and PCd (6.6%; Table 2). A range of doublet and triplet therapies were also used during subsequent LOT, including daratumumab-carfilzomib-dexamethasone, isatuximab-carfilzomib-dexamethasone, Kd, Pd, and isatuximab-pomalidomide-dexamethasone. Compared to index LOT, more patients received BCMA-targeted therapy as subsequent LOT (2.8% during index vs 39.5% of those who received subsequent LOT). Overall, patients spent a median 4.5 months (range, 0.03–29.70) on subsequent LOT. Seventy-eight patients (31.5% of enrolled patients) had only 1 subsequent LOT, and 74 (29.8%) had ≥2 subsequent LOT (Fig. 2).
Table 2.
Most common regimens used across index and subsequent LOT.
| LOT | Number of unique regimens | Most common regimens,a (%) |
|---|---|---|
| Index LOT | 91 | Kd (14.1), CPd (14.1), Pd (11.7) |
| 1st Subsequent LOT (n = 152) | 78 | blmf (10.5), PCd (6.6) |
| 2nd Subsequent LOT (n = 74) | 47 | blmf (18.9), KCd (5.4), Kd (5.4) |
| 3rd Subsequent LOT (n = 42) | 30 | blmf (19.0), IsaPd (11.9), DKd (4.8) |
| ≥4th Subsequent LOT (n = 17) | 34 | blmf (17.6) |
blmf belantamab mafodotin, PCd pomalidomide, cyclophosphamide, and dexamethasone, DKd daratumumab, carfilzomib, and dexamethasone, IsaPd isatuximab, pomalidomide, and dexamethasone, KCd carfilzomib, cyclophosphamide, and dexamethasone, Kd carfilzomib and dexamethasone, LOT line of therapy, Pd pomalidomide and dexamethasone.
aPatients can be counted in >1 regimen per LOT.
Fig. 2. Patients receiving subsequent LOT.
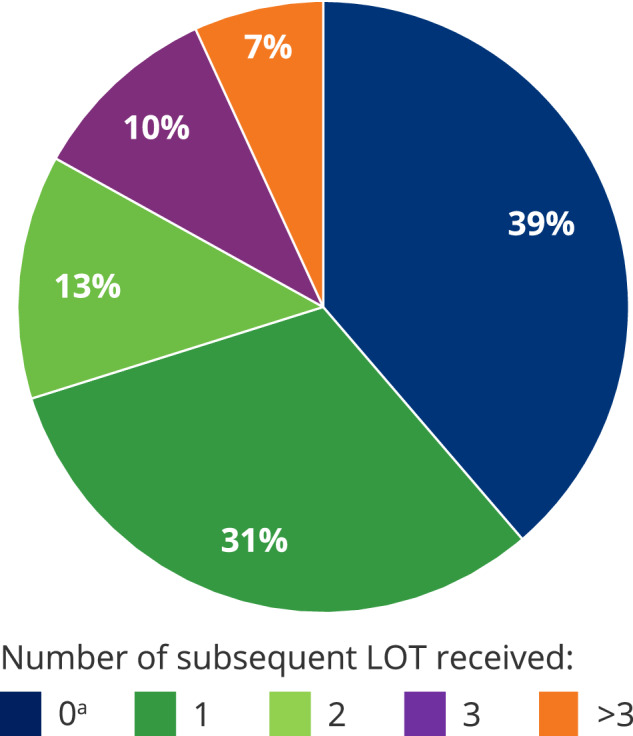
LOT line of therapy. aIncludes 36 (14.5%) patients who remained on index LOT.
Effectiveness: Index LOT
ORR with index treatment, assessed by RRC, for patients treated with RWCP therapies was 31.9% (95% CI, 26.1–38.0), median duration of treatment was 4.0 months (range, 0.1–33.6), and median DOR was 7.4 months (95% CI, 4.9–11.1). Overall best response for patients was generally unchanged from the previous data cut at 16.1 months median study follow-up [8]. No patients achieved stringent complete response, 1 patient (0.4%) achieved CR, 32 (12.9%) achieved VGPR, 46 (18.5%) achieved PR, 14 (5.6%) had minimal response, 78 (31.5%) had stable disease, and 43 (17.3%) had PD. Thirty-four patients (13.7%) were not evaluable by RRC (mainly due to death, AEs, or rapid disease progression requiring a switch to another treatment, occurring before confirmation of response). Among the 34 RRC-unevaluable patients, investigators assessed 3 as responders and 31 as not evaluable, PD, stable disease, or minimal response.
In RRC-evaluable responders (n = 79), median time to first response was 1.9 months (range, 0.7–25.8), and median time to best response was 2.4 months (range, 0.7–25.8). ORR results as assessed by investigators (34.7% [95% CI, 28.8–41.0%]) were similar (85.9% concordant). Median PFS by RRC was 4.6 months and median OS was 13.8 months (Fig. 3). Twelve- and 24-month PFS rates were 21.0% (95% CI, 15.3–27.3) and 10.5% (95% CI, 6.1–16.3); 12- and 24-month OS rates were 53.4% (95% CI, 46.7–59.6) and 33.7% (95% CI, 27.3–40.2), respectively.
Fig. 3. Survival outcomes with RWCP therapies.
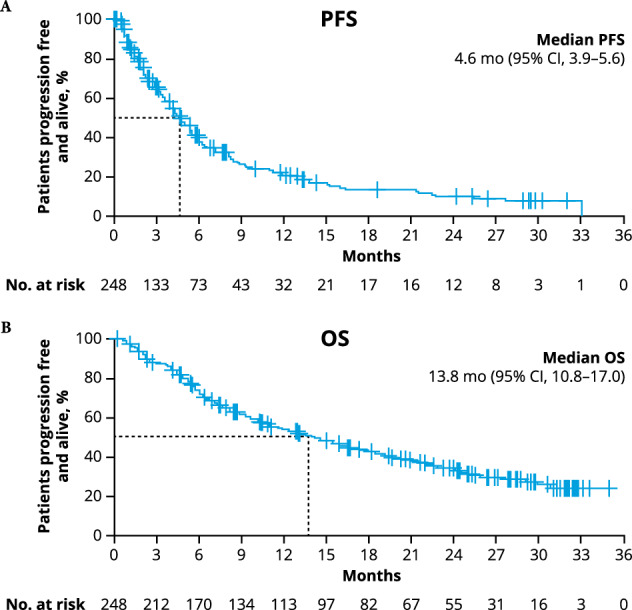
Kaplan–Meier plots showing (A) progression-free survival and B overall survival based on RRC assessment at median study follow-up 26.4 months in all patients. OS overall survival, PFS progression-free survival, RRC response review committee, VGPR very good partial response.
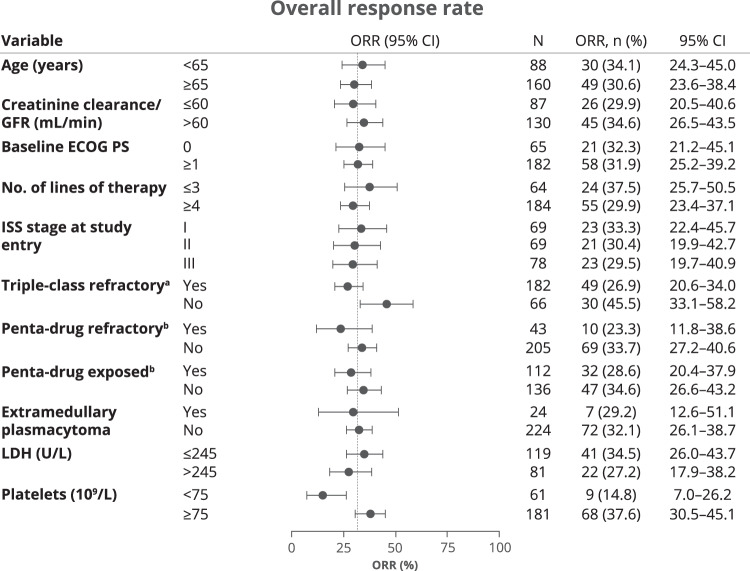
Prespecified subgroup analysis showed that ORR was minimally impacted by high-risk disease characteristics at baseline, including ≥4 prior LOT (29.9%), International Staging System (ISS) stage III at study entry (29.5%), extramedullary plasmacytomas (29.2%), penta-drug refractory MM (23.3%), and penta-drug exposed MM (28.6%) (Fig. 4). ORRs were lower in patients who had a low level of thrombocytes (<75 × 109/L) at baseline (14.8% vs 37.6% in those with thrombocyte levels ≥75 × 109/L) and those who had triple-class refractory MM (26.9% vs 45.5% in patients who did not have triple-class refractory MM) than in patients without these risk factors. Missing data prevented interpretation of data for subgroups by cytogenetic risk and percentage of bone marrow plasma cells. Several risk factors also impacted PFS outcomes, including extramedullary plasmacytomas (median 2.7 months with plasmacytomas vs 5.1 months without plasmacytomas), triple-class refractoriness (4.1 vs 8.2 months in patients who did not have triple-class refractory MM), penta-refractoriness (3.4 vs 5.5 months in patients who did not have penta-drug refractory MM), low thrombocyte levels (3.1 vs 5.6 months in patients with ≥75 × 109/L thrombocytes), and lactate dehydrogenase (LDH) > 245 U/L (3.4 vs 5.6 months in patients with LDH ≤ 245 U/L) (Supplementary Fig. S1). OS outcomes were worse in patients who had baseline ECOG PS ≥ 1 (median 11.1 months vs 24.1 months in those with ECOG PS 0), ISS stages II or III (stage II: 9.7 months and stage III: 12.4 months vs stage I: 24.0 months), LDH > 245 U/L (7.4 vs 17.0 months in patients with LDH ≤ 245 U/L), low thrombocyte levels (5.8 vs 18.1 months in patients with ≥75 × 109/L thrombocytes), and those who had penta-drug refractory MM (8.2 vs 15.3 months in patients who did not have penta-drug refractory MM).
Fig. 4. Forest plot of subgroup analyses of overall response rate by RRC.
ECOG Eastern Cooperative Oncology Group, GFR glomerular filtration rate, ISS International Staging System, LDH lactate dehydrogenase, PS performance status, RRC response review committee. aTriple-class exposed/refractory is defined as exposed/refractory to a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and an anti-CD38 antibody. bPenta-drug exposed/refractory is defined as exposed/refractory to at least 2 PIs, 2 IMiDs, and 1 anti-CD38 antibody (includes triple-class exposed/refractory).
Outcomes were worse for patients who did not (n = 215) versus those who did achieve (n = 33) ≥ VGPR. Median PFS was 3.9 months in patients who did not achieve ≥VGPR versus 15.2 months in patients who did achieve ≥VGPR, and median OS was 10.9 months versus not estimable. Patients who achieved ≥VGPR showed significantly longer median DOR versus those who achieved PR (13.1 months vs 4.7 months).
Effectiveness: subsequent LOT
Estimated median time from start of index LOT to next treatment was 5.2 months (95% CI, 4.4–6.0). Median duration of treatment with first subsequent LOT was 2.8 months (range, <1–29.7). Median PFS2 by investigator assessment was 10.8 months (95% CI, 8.4–13.0).
Safety
During index LOT, 86.7% of patients experienced an AE; grade 3/4 TEAEs occurred in 144 (58.1%) patients (Table 3). The most common TEAEs were hematologic, occurring in 50% of patients, and included thrombocytopenia (any grade 26.2%, grade 3/4 19.4%), anemia (any grade 25.8%, grade 3/4 10.9%), and neutropenia (any grade 20.2%, grade 3/4 17.3%) (Supplementary Table S3). The most common non-hematologic TEAEs (all grades; reported in ≥15% patients) were general disorders and administration site conditions (40.7%), gastrointestinal disorders (33.5%), and infections and infestations (33.1%). No grade 3/4 non-hematological TEAEs occurred in ≥15% of patients. SPMs were reported in 13 (5.2%) patients; 5 were reported during index LOT and 8 were reported after index LOT. Cases of SPMs included squamous cell carcinoma (n = 4), basal cell carcinoma (n = 2), secondary acute myeloid leukemia (n = 2), cancer of the lung/bronchus (n = 2), and 1 case each of high grade suspected cholangio cellular carcinoma, plasma cell leukemia, and an SPM described as multiple destructive fluorodeoxyglucose (FDG)-positive lesions throughout the skeleton and skull. Of note, the case of plasma cell leukemia and of SPM with multiple destructive FDG-positive lesions may have been MM; however, they were reported as SPM by the investigators. Only 1 SPM case (plasma cell leukemia) was reported as fatal.
Table 3.
Severity of TEAEs during index LOT.
| TEAE, n (%) | N = 248 |
|---|---|
| Any TEAE | 215 (86.7) |
| Any serious TEAE | 91 (36.7) |
| Maximum severity of TEAE | |
| Grade 1 | 17 (6.9) |
| Grade 2 | 46 (18.5) |
| Grade 3 | 84 (33.9) |
| Grade 4 | 46 (18.5) |
| Grade 5 | 22 (8.9) |
| TEAE with outcome death | 21 (8.5)a |
LOT line of therapy, PD progressive disease, TEAE treatment-emergent adverse event.
a1 patient with unresolved grade 5 TEAE died from PD.
Overall, 158 (63.7%) patients died during the study. Most deaths were due to PD (67.7%), 15.8% were due to AEs, and 16.5% were due to other causes (Fig. 5). Sixty deaths occurred during or after index LOT (but before subsequent LOT) and 98 occurred after the start of subsequent LOT. During index LOT, 21 (8.5%) patients died from TEAEs, most commonly infection (13 patients, 5.2%), and 1 patient had a grade 5 TEAE that did not resolve before the patient died from PD.
Fig. 5. Deaths and causes of death.
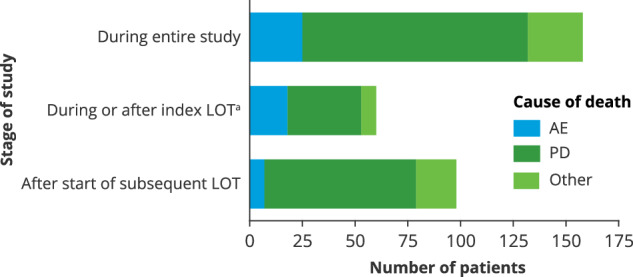
AE adverse event, PD progressive disease, LOT line of therapy. aThese patients did not receive subsequent LOT.
Discussion
LocoMMotion was the first prospective, observational study to explore the effect of RWCP treatments in triple-class exposed patients with RRMM. The high number of unique regimens in the index and subsequent LOTs, including that more than two-thirds of unique regimens were used by fewer than 5 patients, demonstrates a lack of established standard-of-care therapy in this population. Triple-class exposed patients showed rapid disease progression on index LOT and with first subsequent LOT and poor survival despite the use of a wide range of treatments during the study. Response rate to index LOT was low (79 patients, 31.9%), and responses were neither deep nor durable. OS, PFS, and DOR were longer in patients who achieved ≥VGPR; however, only 1 in 10 were able to achieve this level of response with the observed treatments. Median PFS and median OS were short at 4.6 months and 13.8 months, respectively. Similar to other studies [13, 14], OS outcomes were worse in patients who had higher baseline ECOG PS, ISS stage II or III, high LDH, penta-drug refractory disease, and low levels of thrombocytes. ORR was less sensitive to these factors, as it was impacted only by low levels of thrombocytes and triple-refractoriness. However, greater depth of response did correspond to improved PFS and OS outcomes, suggesting that achievement of ≥VGPR is an important treatment goal in RWCP.
Across first subsequent LOTs, median PFS2 by investigator assessment was 10.8 months, which includes a median of 4.6 months for index LOT. Thus, PFS after subsequent LOT was similar to that after index LOT. This highlights the continuous short cycles of remission and subsequent relapses, and the increasing challenge that patients and clinicians face in determining the next treatment choice.
Limitations include the study enrollment period, which occurred between 2019 and 2020, and does not represent novel therapies approved since 2020, including chimeric antigen receptor T-cell therapies and bispecific antibodies. While the United States population (9.3%) was represented in this study, generalizability of the study to the United States might be limited by frontline treatment differences between the United States and Europe; however, inclusion criteria in LocoMMotion were uniform across countries in terms of number of prior LOT and triple-class exposure, minimizing the impact of differences in frontline treatment. Additionally, LocoMMotion was a single-arm, open-label, prospective study with no comparator group. In real-world studies, some baseline information and laboratory assessments required per IMWG criteria might be missing. However, a framework was implemented by RRC to avoid underestimation of response and to ensure unified evaluation across all study participants.
One limitation of the study may be possible selection bias for enrolling patients with less advanced disease or poorer overall fitness into this observational study versus into interventional trials. Unadjusted comparisons between the LocoMMotion population and populations in interventional trials like CARTITUDE-1 and MajesTEC-1 have shown some imbalances in patient characteristics, such as in refractory status, time to progression on prior LOT, and duration of prior LOT. Hence, indirect treatment comparisons using the LocoMMotion study population as an external control arm were adjusted for baseline differences, and major findings were published [15, 16].
Sample sizes of some subgroups were smaller, and the non-randomized design of the study may have led to unbalanced subgroups with respect to other characteristics. Also, PFS/OS analysis by achievement of VGPR is a post-baseline measurement that complicates interpretation because response is partly a consequence of PFS/OS.
Finally, the observational nature of LocoMMotion likely resulted in underreporting of TEAEs, and the reporting period for AEs was limited from the start of index LOT treatment to the end of index LOT. Therefore, safety of RWCP therapies should be interpreted with caution given that the median time on RWCP therapy was only 4 months.
In conclusion, the final (2-year) analysis of the LocoMMotion study confirms a lack of clear standard of care treatment and poor outcomes for triple-class exposed patients with RRMM. Hopefully, the emergence of novel therapies, including chimeric antigen receptor T-cell therapies and bispecific T-cell redirecting therapies, will improve outcomes for heavily pretreated patients with MM. Therapeutics in these treatment classes were approved for use in the United States [17–19] and in Europe [20–22] after the enrollment period for LocoMMotion. Data from the LocoMMotion study are a valuable benchmark for comparison with newly approved and emerging therapies [15]. The prospective design, comprehensive data collection, and the completeness of the baseline characteristics data allow for comparative analyses with other single-arm trials in this patient population.
Supplementary information
Table S1: Ethics committees/Institutional Review Boards in LocoMMotion
Table S2: Regimens received by ≥3 patient in any given LOT.
Table S3: TEAEs reported in ≥5% of patients in index LOT.
Figure S1: Forest plot of subgroup analyses of progression-free survival and overall survival by RRC.
Acknowledgements
This study (ClinicalTrials.gov identifier: NCT04035226) was funded by Janssen Research & Development, LLC and Legend Biotech USA Inc. Medical writing support was provided by Andrew Marson and Sarika Pathak Sharma, PhD, of Eloquent Scientific Solutions, and funded by Janssen Global Services, LLC. The authors thank the LocoMMotion study team, including Jonathan Squire, Kensa Hatch, Lorenzo Acciarri, Henrieke Bruin, and Jeannie Kearl.
Author contributions
SK conceived and/or designed the work that led to the submission. All authors acquired data, and/or played a significant role in interpreting the results. All authors drafted or revised the manuscript. All authors approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability
Although these data are not currently publicly available for sharing, requests for sharing can be sent to the Corresponding Author and will be evaluated on an individual basis.
Competing interests
M-VM has received honoraria from or served on the board of directors/advisory committees for AbbVie, Adaptive Biotechnologies, Amgen, BMS/Celgene, GSK, Janssen, Oncopeptides, Pfizer, Regeneron, Roche, Sanofi, Seagen, and Takeda. KW has served as a consultant and received honoraria from Adaptive Biotechnologies, Karyopharm, and Takeda; has received honoraria from Roche; and has received honoraria and served as a member on boards of directors and/or advisory committees for Amgen, BMS, Celgene, GSK, Janssen, Oncopeptides, and Sanofi. VDS has served on an advisory board or speakers’ bureaus and received honoraria from AbbVie, Alexion, AOP Health, argenx, BMS, GSK, Grifols, Leo Pharma, Novartis, Novo Nordisk, Sanofi, SOBI, and Takeda. HG has served as a consultant for Amgen, Novartis, and Takeda; has served as a consultant and received honoraria, grants, and/or provision of investigational medicinal product and research funding from Amgen, BMS, Celgene, Chugai, Janssen, and Sanofi; has received research funding from Incyte, Molecular Partners, MSD, Mundipharma, and Novartis; and has received other grants from Dietmer Hopp Foundation. MD has received honoraria from Amgen, BMS, GSK, and Janssen; and has served on the speakers’ bureau for Janssen. MM has received honoraria from Adaptive Biotech, Amgen, Astellas, BMS, Gilead, Novartis, Pfizer, and Takeda; and has received honoraria/research funding from Celgene and Sanofi. JL-H has no relationships to disclose. DD is an employee/consultant/honoraria/member of board of directors/advisory committees for Janssen Cilag; and a member of the board of directors/advisory committees/received research funding from Celgene. EA has no relationships to disclose. LV has served on the advisory board of BMS, Janssen, and Takeda. AP has received honoraria from or has served in a consulting/advisory role for AbbVie, Amgen, BMS, Janssen, Pfizer, Sanofi, and Takeda. RB has received honoraria/research funding from Allogene, BMS, Gilead, Janssen, and Servier. NWCJvdD has received research funding from AbbVie, Amgen, BMS, Celgene, Cellectis, Janssen Pharmaceuticals, and Novartis; and has served in a consulting/advisory role for Adaptive, Amgen, Bayer, BMS, Celgene, Janssen, Novartis, Pfizer, Roche, Sanofi, Servier, and Takeda. EMO has received honoraria from or has served in a consulting/advisory role for AbbVie, Amgen, BMS, GSK, Janssen, Karyopharm, Menarini, Oncopeptides, Pfizer, Sanofi, and Takeda. TR is a former Janssen employee. JMS is a Janssen employee. SK is a Janssen employee. IH is a Janssen employee. VS is a Janssen employee. LM is a Janssen employee. JB is a Janssen employee. OCF is an employee of Legend Biotech USA Inc. HE has received research funding or honoraria and has served in a consulting/advisory role for Amgen, BMS/Celgene, GSK, Janssen, and Sanofi. PM has received honoraria and/or served on advisory boards for AbbVie, Amgen, Celgene, Janssen, Sanofi, and Takeda.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-024-02404-6.
References
- 1.Rodriguez-Otero P, San-Miguel JF. Cellular therapy for multiple myeloma: what’s now and what’s next. Hematol Am Soc Hematol Educ Program. 2022;2022:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Arriba de la Fuente F, Montes Gaisán C, de la Rubia Comos J. How to manage patients with lenalidomide-refractory multiple myeloma. Cancers. 2022;15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-Lobato LG, Pereira A, Fernández de Larrea C, Cibeira MT, Tovar N, Jiménez-Segura R, et al. Real-world data on survival improvement in patients with multiple myeloma treated at a single institution over a 45-year period. Br J Haematol. 2022;196:649–59. [DOI] [PubMed] [Google Scholar]
- 4.Dima D, Ullah F, Mazzoni S, Williams L, Faiman B, Kurkowski A, et al. Management of relapsed-refractory multiple myeloma in the era of advanced therapies: evidence-based recommendations for routine clinical practice. Cancers. 2023;15:2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:1086–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yong K, Delforge M, Driessen C, Fink L, Flinois A, Gonzalez-McQuire S, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol. 2016;175:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Donk N. Sequencing multiple myeloma therapies with and after antibody therapies. Hematol Am Soc Hematol Educ Program. 2020;2020:248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreau PW, K. De Stefano, V et al. Updated Results from LocoMMotion: A Prospective, Nonintervational, Multinational Study of Real-Life Current Standards of Care in Heavily Pretreated Patients with Relapsed/Refractory Multiple Myeloma. In: 19th International Myeloma Society (IMS) Annual Meeting. Los Angeles, CA, USA: IMS; 2022.
- 9.Durie BG, Miguel JF, Blade J, Rajkumar SV. Clarification of the definition of complete response in multiple myeloma. Leukemia. 2015;29:2416–7. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46. [DOI] [PubMed] [Google Scholar]
- 11.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateos MV, Weisel K, De Stefano V, Goldschmidt H, Delforge M, Mohty M, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia. 2022;36:1371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usmani S, Ahmadi T, Ng Y, Lam A, Desai A, Potluri R, et al. Analysis of real-world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016;21:1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33:2266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateos MV, Weisel K, Martin T, Berdeja JG, Jakubowiak A, Stewart AK, et al. Adjusted comparison of outcomes between patients from CARTITUDE-1 versus multiple myeloma patients with prior exposure to proteasome inhibitors, immunomodulatory drugs and anti-CD38 antibody from the prospective, multinational LocoMMotion study of real-world clinical practice. Haematologica. 2023;108:2192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreau P, van de Donk N, Delforge M, Einsele H, De Stefano V, Perrot A, et al. Comparative efficacy of teclistamab versus current treatments in real-world clinical practice in the prospective LocoMMotion study in patients with triple-class-exposed relapsed and/or refractory multiple myeloma. Adv Ther. 2023;40:2412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celgene Corporation, a Bristol Myers Squibb Company: ABECMA® (idecabtagene vicleucel) prescribing information. 2024. https://packageinserts.bms.com/pi/pi_abecma.pdf.
- 18.Janssen Biotech, Inc and Legend Biotech: CARVYKTI® (ciltacabtagene autoleucel) prescribing information. 2024. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/CARVYKTI-pi.pdf.
- 19.Janssen Biotech, Inc: TECVAYLI® (teclistamabl) prescribing information. 2024. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TECVAYLI-pi.pdf.
- 20.Bristol Myers Squibb Pharma: ABECMA® (idecabtagene vicleucel) summary of product characteristics. 2024. https://www.ema.europa.eu/en/documents/product-information/abecma-epar-product-information_en.pdf.
- 21.Janssen Biotech, Inc and Legend Biotech: CARVYKTI® (ciltacabtagene autoleucel) summary of product characteristics. 2024. https://www.ema.europa.eu/en/documents/product-information/carvykti-epar-product-information_en.pdf.
- 22.Janssen Biotech, Inc: TECVAYLI® (teclistamab) summary of product characteristics. 2024. https://www.ema.europa.eu/en/documents/product-information/tecvayli-epar-product-information_en.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Ethics committees/Institutional Review Boards in LocoMMotion
Table S2: Regimens received by ≥3 patient in any given LOT.
Table S3: TEAEs reported in ≥5% of patients in index LOT.
Figure S1: Forest plot of subgroup analyses of progression-free survival and overall survival by RRC.
Data Availability Statement
Although these data are not currently publicly available for sharing, requests for sharing can be sent to the Corresponding Author and will be evaluated on an individual basis.