Abstract
Purpose
The purpose of this study is to examine the protective role of dietary vitamin B12 against depression, particularly as its prevalence is notably high among women experiencing urinary incontinence (UI). Nevertheless, the relationship between vitamin B12 intake, UI, and depression requires further clarification. This research aims to explore this association specifically among women.
Methods
This cross-sectional study analyzed data from 14,154 women sourced from the National Health and Nutrition Examination Survey (NHANES) conducted between 2007 and 2018. Depression was measured using the Personal Health Questionnaire (PHQ-9). Vitamin B12 intake was assessed through 24-h dietary recall interviews. Weighted univariate and multivariate logistic regression models assessed the relationships between UI, vitamin B12 intake, and depression in women, with additional analyses conducted across different age, body mass index (BMI), and vaginal delivery groups. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs).
Results
The study included 14,154 women, averaging 48.18±0.27 years in age, of whom 1609 (11.37%) exhibited depressive symptoms. Women with stress UI (OR=1.55, 95% CI: 1.26–1.91), urgency UI (OR=1.92, 95% CI: 1.52–2.44), and mixed UI (OR=2.60, 95% CI: 2.13–3.19) showed significantly greater odds of depression compared to those without UI. Vitamin B12 intake of ≥2.4 mcg/day was associated with lower odds of depression (OR=0.95, 95% CI: 0.080–1.14). In patients with UI, the odds of depression gradually decreased with a vitamin B12 intake of ≥2.4 mcg/day. A moderating role of vitamin B12 intake was observed among women with UI aged ≤ 64 years, with BMI ≥ 25 kg/m2, and those with or without vaginal deliveries.
Conclusion
UI is linked to elevated depression odds, while vitamin B12 intake appears to moderate this relationship. It is essential for healthcare providers to prioritize early depression assessments in women with UI and to offer timely interventions to enhance their quality of life.
Keywords: urinary incontinence, depression, vitamin B12 intake, NHANES
Background
Urinary incontinence (UI), denoting the involuntary or unintentional loss of urine, occurs in both genders but is more prevalent among females.1 The prevalence of UI ranged from 18.1% to 45.9% among women in the United States.2 There are three primary subtypes of UI: stress UI (SUI), more common in young women; urgency UI (UUI), increasingly prevalent with age; and mixed UI (MUI), more frequent in older women.1 UI may lead to physical and psychological consequences, imposing burdens on society and the economy. Negative psychological effects associated with UI include anxiety, depression, and reduced self-confidence. Moreover, UI is linked to an elevated risk of depression in women.3,4 Approximately 18.1% to 45.9% of women with UI experience depression.2 Depression could impact quality of life and increase mortality, monitoring depression in women with UI and identifying potential contributing factors holds significant clinical value.
Nutritional intervention has emerged as a crucial strategy for prevention and managing depression.5 Adequate nutrient intake also correlates closely with the quality of life in women affected by UI.6 Vitamins, essential for sustaining life, are pivotal in maintaining health. Vitamin B is crucial for the methylation cycle, phospholipid repair, and maintenance, with vitamin B12 particularly essential for nervous system development and function.7 Insufficient vitamin B12 levels can lead to oxidative stress, calcium imbalance, and altered energy metabolism, resulting in neuronal atrophy, cell death, and impaired neurotransmitter signaling, all contributing to depression.5,8 Additionally, oxidative stress and abnormal neurological function are also associated with UI development.9,10 The inverse relationship between vitamin B12 levels and UI has been reported in the elderly population.11 Furthermore, women with SUI exhibit lower vitamin B12 levels compared to healthy individuals.12 Women who have higher vitamin B12 intake were associated with reduced depression risk.13 Consequently, we hypothesize that an increased dietary vitamin B12 intake could ameliorate the depression risk associated with UI in women.
This study aimed to investigate the moderating effect of vitamin B12 intake on the relationship between UI and depression in women. And further investigate the association in different subgroups.
Methods
Study Design and Participants
The data were obtained from the National Health and Nutrition Examination Survey (NHANES), a nationally representative series of surveys designed to assess the health and nutritional status of the US population. The ethical approval for this was waived by the Institutional Review Board of Peking University People’s Hospital, because the data was accessed from NHANES (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of Peking University People’s Hospital due to the retrospective nature of the study.
Data on participants in this cross-sectional study were derived from six cycles of NAHNES conducted between 2007–2018. Participants were included based on the following criteria: (1) aged 20 years or older, (2) complete information on vitamin B12 intake, (3) an assessment of depression, and (4) data on UI. Exclusion criteria encompassed individuals with abnormal energy intake (<500 kcal/day or >5000 kcal/day) and those missing covariates data, including UI, depression, body mass index (BMI), and hysterectomy.
UI Assessment
UI was assessed using standardized questions during the mobile examination center interview.2 Each question had a binary response option (‘yes’ or “no”). SUI was defined as responding “Yes” to the question “During the past 12 months, have you leaked or lost control of even a small amount of urine with an activity like coughing, lifting, or exercise?”. UUI was defined as responding “Yes” to the question During the past 12 months, have you leaked or lost control of even a small amount of urine with an urge or pressure to urinate and you couldn’t get to the toilet fast enough?. Mixed UI was defined as a positive response to both the SUI and UUI.
Depression Assessment
Depression was assessed using the Personal Health Questionnaire (PHQ-9) during face-to-face interviews. The PHQ-9 is a validated nine-item questionnaire used to evaluate symptoms of depression within the last two weeks, with a 0 to 3 score of each item indicating the frequency of the depression symptoms.14 The symptoms of depression include anhedonia, depressed mood, sleep disturbance, fatigue, appetite changes, low self-esteem, concentration problems, psychomotor disturbances, and suicidal ideation. A clinical cut-off score of 10 or higher on the PHQ-9, which had a sensitivity and specificity of 88% in the detection of major depressive.15
Dietary Vitamin B12 Intake Assessment
Trained dietary interviewers conducted two 24-hour dietary recalls using standardized measurement guides to assess total dietary intake. The first dietary recall occurred during face-to-face interviews at the Mobile Examination Center, whereas the second recall was collected via telephone after 3–10 days. Dietary vitamin B12 intake was calculated by averaging the data from both recalls. Vitamin B12 intake was categorized into two groups based on the Recommended Dietary Allowance.16 Adequate vitamin B12 intake was defined as a consumption of ≥ 2.4 mcg/day, whereas inadequate intake was considered < 2.4 mcg/day.
Assessment of Potential Covariates
Several important covariates were selected to account for potential confounding factors, including age, race, educational status, marital status, smoking status, drinking status, poverty-to-income ratio (PIR), physical activity level, menopausal status, cardiovascular disease (CVD), pelvic infection, use of female hormones, use of antidepressants, use of antipsychotics, sleep duration, cesarean deliveries, prior hysterectomy, and caffeine intake. The covariates above were collected using a standardized questionnaire. Information on Hypertension, diabetes, and dyslipidemia was obtained through physical examination, self-reported, and medication usage. BMI was defined as the weight (kg) divided by the height squared (m2).
Statistical Analysis
Continuous variables were described as mean and standard error (S.E), and the weighted t-test was used to compare groups with and without depression. Categorical variables were presented as frequencies and percentages, and differences between groups with and without depression were measured using the chi-square test. The moderating effect of vitamin B12 intake on the relationship between UI and depression was examined using the weighted univariate and multivariate logistic regression models. All results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). Model 1 represents a crude model, while Model 2 was adjusted for age, race, educational status, marital status, BMI, RIP, drinking status, smoking status, physical activity, menopausal status, hypertension, diabetes, dyslipidemia, CVD, pelvic infection, female hormone use, antidepressants, antipsychotics, sleep hours, cesarean deliveries, prior hysterectomy, and caffeine. The association between vitamin B12 levels and depression was further investigated in different age, BMI, and vaginal delivery groups. All analyses were performed using SAS 9.4, and P<0.05.
Results
Characteristic of Participants
Initially, 17907 women aged ≥20 years were included in this study. Women without UI data (n=2647), abnormal energy intake (n=821), missing depression data (n=128), missing body BMI data (n=112), or missing hysterectomy data (n=45) were subsequently excluded. Finally, 14154 women were included in the final analysis. The details of the selection process are illustrated in Figure 1. The mean age of participants was 48.18±0.27 years. A total of 1609 (11.37%) participants exhibited depressive symptoms. Significant differences were observed regarding age, race, educational status, marital status, BMI, PIR, drinking status, smoking status, physical activity, menopausal status, hypertension, diabetes, dyslipidemia, CVD, pelvic infection, female hormone use, antidepressants, antipsychotics, sleep duration, vaginal delivery, cesarean delivery, prior hysterectomy, caffeine, vitamin B12, and type of UI. Individuals with depression had higher BMI, increased caffeine intake, rates of vaginal deliveries, hypertension, dyslipidemia, and lower energy intake (all P <0.05). Table 1 presents the characteristics.
Figure 1.
The selection process of included participants.
Table 1.
Characteristics of Included Women
| Variables | Total (n=14154) | Depression | P | |
|---|---|---|---|---|
| No (n=12545) | Yes (n=1609) | |||
| Age, years, Mean (S.E) | 48.18 (0.27) | 48.33 (0.29) | 46.83 (0.51) | 0.009* |
| Race, n (%) | 0.002# | |||
| White | 5924 (68.06) | 5262 (68.57) | 662 (63.38) | |
| Black | 3026 (11.54) | 2684 (11.32) | 342 (13.53) | |
| Other | 5204 (20.41) | 4599 (20.11) | 605 (23.09) | |
| Education status, n (%) | <0.001# | |||
| Less Than 9th Grade | 1273 (4.35) | 1045 (3.99) | 228 (7.63) | |
| 9–11th Grade | 1869 (9.58) | 1540 (8.75) | 329 (17.08) | |
| High School Grad | 3107 (22.00) | 2739 (21.52) | 368 (26.30) | |
| Some College or AA degree | 4595 (33.83) | 4087 (33.72) | 508 (34.80) | |
| College Graduate or above | 3310 (30.25) | 3134 (32.02) | 176 (14.19) | |
| Marital status, n (%) | <0.001# | |||
| Married | 6565 (52.65) | 6035 (54.42) | 530 (36.62) | |
| Widowed | 1581 (8.34) | 1395 (8.26) | 186 (9.13) | |
| Divorced | 1842 (12.15) | 1545 (11.52) | 297 (17.92) | |
| Separated | 563 (2.65) | 434 (2.28) | 129 (6.10) | |
| Never married | 2516 (16.46) | 2205 (16.14) | 311 (19.31) | |
| Living with partner | 1087 (7.74) | 931 (7.39) | 156 (10.92) | |
| BMI, n (%) | <0.001# | |||
| <25 | 4095 (32.18) | 3754 (33.26) | 341 (22.41) | |
| ≥25 | 10,059 (67.82) | 8791 (66.74) | 1268 (77.59) | |
| PIR, n (%) | <0.001# | |||
| <1.3 | 4272 (20.78) | 3486 (18.74) | 786 (39.27) | |
| ≥1.3 | 8649 (72.40) | 7966 (74.35) | 683 (54.67) | |
| Unknown | 1233 (6.83) | 1093 (6.91) | 140 (6.06) | |
| Drinking status, n (%) | <0.001# | |||
| No | 4624 (25.29) | 4141 (25.51) | 483 (23.26) | |
| <1 time/week | 2675 (24.18) | 2425 (24.83) | 250 (18.22) | |
| ≥1 time/week | 4935 (34.81) | 4269 (33.98) | 666 (42.36) | |
| Unknown | 1920 (15.72) | 1710 (15.67) | 210 (16.17) | |
| Smoking status, n (%) | <0.001# | |||
| No | 9091 (61.94) | 8347 (64.03) | 744 (43.00) | |
| Current smoking | 2409 (17.24) | 1846 (14.92) | 563 (38.27) | |
| Quit smoking | 2654 (20.82) | 2352 (21.05) | 302 (18.73) | |
| Physical activity, (MET· min/week), n (%) | <0.001# | |||
| <450 | 5978 (37.25) | 5146 (35.84) | 832 (49.99) | |
| ≥450 | 8176 (62.75) | 7399 (64.16) | 777 (50.01) | |
| Menopausal status, n (%) | <0.001# | |||
| No | 6606 (49.29) | 5896 (49.43) | 710 (48.02) | |
| Yes | 5761 (37.46) | 5116 (37.82) | 645 (34.13) | |
| Unknown | 1787 (13.26) | 1533 (12.75) | 254 (17.86) | |
| Hypertension, n (%) | <0.001# | |||
| No | 6416 (49.83) | 5804 (50.75) | 612 (41.46) | |
| Yes | 7738 (50.17) | 6741 (49.25) | 997 (58.54) | |
| Diabetes, n (%) | <0.001# | |||
| No | 11739 (87.39) | 10,531 (88.21) | 1208 (79.95) | |
| Yes | 2415 (12.61) | 2014 (11.79) | 401 (20.05) | |
| Dyslipidemia, n (%) | 0.002# | |||
| No | 4796 (34.76) | 4352 (35.32) | 444 (29.67) | |
| Yes | 9358 (65.24) | 8193 (64.68) | 1165 (70.33) | |
| CVD, n (%) | <0.001# | |||
| No | 12918 (92.90) | 11,587 (93.80) | 1331 (84.69) | |
| Yes | 1236 (7.10) | 958 (6.20) | 278 (15.31) | |
| Pelvic infection, n (%) | <0.001# | |||
| No | 4331 (34.36) | 3905 (34.64) | 426 (31.86) | |
| Yes | 270 (1.96) | 203 (1.56) | 67 (5.62) | |
| Unknown | 9553 (63.68) | 8437 (63.81) | 1116 (62.51) | |
| Female hormones use, n (%) | 0.020# | |||
| No | 10831 (71.91) | 9571 (71.60) | 1260 (74.72) | |
| Yes | 3323 (28.09) | 2974 (28.40) | 349 (25.28) | |
| Antidepressants, n (%) | <0.001# | |||
| No | 12097 (82.15) | 11,071 (84.66) | 1026 (59.36) | |
| Yes | 2057 (17.85) | 1474 (15.34) | 583 (40.64) | |
| Antipsychotics, n (%) | <0.001# | |||
| No | 13905 (98.43) | 12,389 (98.92) | 1516 (94.00) | |
| Yes | 249 (1.57) | 156 (1.08) | 93 (6.00) | |
| Sleep hours, n (%) | <0.001# | |||
| <6h | 1831 (10.76) | 1391 (9.15) | 440 (25.34) | |
| 6h-9h | 10392 (75.83) | 9466 (77.67) | 926 (59.21) | |
| ≥9h | 1931 (13.41) | 1688 (13.19) | 243 (15.45) | |
| Vaginal deliveries, n (%) | <0.001# | |||
| No | 2242 (16.22) | 1987 (16.33) | 255 (15.24) | |
| Yes | 9620 (64.81) | 8457 (64.19) | 1163 (70.42) | |
| Unknown | 2292 (18.97) | 2101 (19.48) | 191 (14.34) | |
| Cesarean deliveries, n (%) | <0.001# | |||
| No | 3780 (25.86) | 3258 (25.24) | 522 (31.51) | |
| Yes | 2856 (19.03) | 2484 (18.92) | 372 (20.05) | |
| Unknown | 7518 (55.11) | 6803 (55.84) | 715 (48.45) | |
| Prior hysterectomy, n (%) | <0.001# | |||
| No | 10935 (78.30) | 9786 (79.16) | 1149 (70.48) | |
| Yes | 3219 (21.70) | 2759 (20.84) | 460 (29.52) | |
| Energy, kcal, Mean (S.E) | 1846.79 (8.08) | 1850.09 (8.24) | 1816.76 (25.23) | 0.199* |
| Caffeine, mg, Mean (S.E) | 159.82 (2.99) | 156.29 (3.09) | 191.81 (8.59) | <0.001* |
| Alcohol, gm, Mean (S.E) | 7.15 (0.25) | 7.22 (0.26) | 6.47 (0.67) | 0.292* |
| Vitamin B12, mcg, Mean (S.E) | 4.20 (0.04) | 4.18 (0.04) | 4.36 (0.20) | 0.386* |
| Vitamin B12, n (%) | 0.005# | |||
| <2.4 mcg/day | 5194 (33.97) | 4551 (33.49) | 643 (38.32) | |
| ≥2.4 mcg/day | 8960 (66.03) | 7994 (66.51) | 966 (61.68) | |
| Urinary incontinence, n (%) | <0.001# | |||
| No | 6568 (46.04) | 6077 (47.70) | 491 (30.97) | |
| Yes | 7586 (53.96) | 6468 (52.30) | 1118 (69.03) | |
| Type of UI, n (%) | <0.001# | |||
| No | 6568 (46.04) | 6077 (47.70) | 491 (30.97) | |
| Stress UI | 3339 (26.26) | 2982 (26.37) | 357 (25.21) | |
| Urgency UI | 1741 (11.10) | 1526 (10.91) | 215 (12.82) | |
| Mixed UI | 2506 (16.60) | 1960 (15.02) | 546 (30.99) | |
Notes: #Chi-square test, *t tests.
Abbreviations: S.E, standard error; BMI, body mass index; PIR, poverty income ratio; CVD, cardiovascular disease; UI, urinary incontinence.
Associations of UI, Vitamin B12 Intake, with Depression in Women
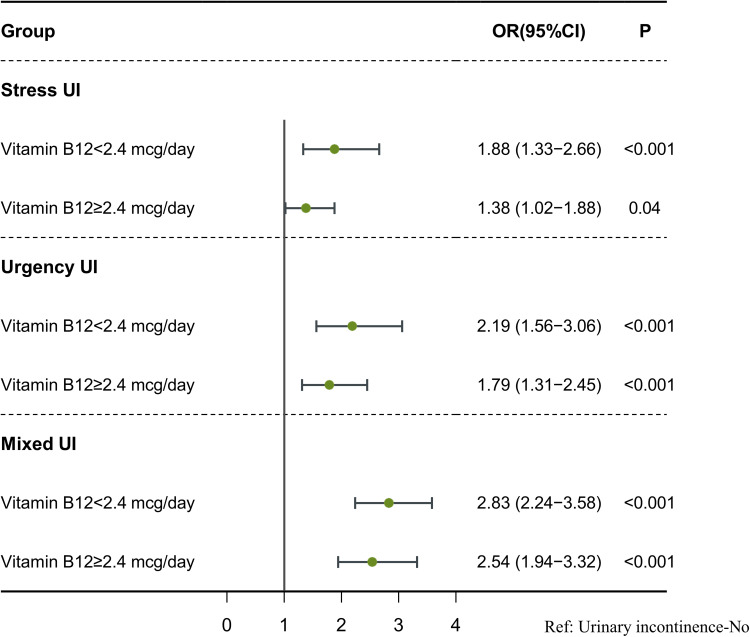
The associations between UI, vitamin B12 intake, and depression are shown in Table 2. Women with SUI (OR=1.55, 95% CI: 1.26–1.91), UUI (OR=1.92, 95% CI: 1.52–2.44), and mixed UI (OR=2.60, 95% CI: 2.13–3.19) were associated with higher odds of depression. Conversely, a vitamin B12 intake of ≥2.4 mcg/day was associated with lower odds of depression (OR=0.95, 95% CI: 0.80–1.14). Vitamin B12 intake affects the association between UI and depression, presented in Table 3 and Figure 2. Among women with a vitamin B12 intake <2.4 mcg/day, SUI (OR=1.88, 95% CI: 1.33–2.66), UUI (OR=2.19, 95% CI: 1.56–3.06), and mixed UI (OR=2.83, 95% CI: 2.24–3.58) were associated with significantly elevated odds of depression. In contrast, for those consuming vitamin B12 at ≥2.4 mcg/day, the odds of depression progressively decreased in association with SUI (OR=1.38, 95% CI: 1.02–1.88), UUI (OR=1.79, 95% CI: 1.31–2.45), and mixed UI (OR=2.54, 95% CI: 1.94–3.32).
Table 2.
Associations Between UI, Vitamin B12 Intake and Depression in Women
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Type of UI | ||||
| No | Ref | Ref | ||
| Stress UI | 1.47 (1.22–1.78) | <0.001 | 1.55 (1.26–1.91) | <0.001 |
| Urgency UI | 1.81 (1.44–2.28) | <0.001 | 1.92 (1.52–2.44) | <0.001 |
| Mixed UI | 3.18 (2.62–3.85) | <0.001 | 2.60 (2.13–3.19) | <0.001 |
| Vitamin B12 | ||||
| <2.4 mcg/day | Ref | Ref | ||
| ≥2.4 mcg/day | 0.81 (0.70–0.94) | 0.006 | 0.95 (0.80–1.14) | 0.588 |
Notes: Model 1: crude model. Model 2 was adjusted for age, race, educational status, marital status, BMI, PIR, drinking status, smoking status, physical activity, menopausal status, hypertension, diabetes, dyslipidemia, CVD, pelvic infection, female hormone use, antidepressants, antipsychotics, sleep duration, cesarean deliveries, prior hysterectomy, and caffeine use.
Abbreviations: OR, odds ratio; CI, confidence interval; Ref, reference; UI, urinary incontinence.
Table 3.
Moderating Effect of Vitamin B12 Intake on the Association Between UI and Depression
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Vitamin B12: <2.4 mcg/day (n=5194) | ||||
| Type of UI | ||||
| No | Ref | Ref | ||
| Stress UI | 1.71 (1.28–2.28) | <0.001 | 1.88 (1.33–2.66) | <0.001 |
| Urgency UI | 2.03 (1.47–2.79) | <0.001 | 2.19 (1.56–3.06) | <0.001 |
| Mixed UI | 3.18 (2.57–3.93) | <0.001 | 2.83 (2.24–3.58) | <0.001 |
| Vitamin B12: ≥2.4 mcg/day (n=8960) | ||||
| Type of UI | ||||
| No | Ref | Ref | ||
| Stress UI | 1.35 (1.02–1.78) | 0.035 | 1.38 (1.02–1.88) | 0.040 |
| Urgency UI | 1.68 (1.26–2.23) | <0.001 | 1.79 (1.31–2.45) | <0.001 |
| Mixed UI | 3.19 (2.47–4.11) | <0.001 | 2.54 (1.94–3.32) | <0.001 |
Notes: Model 1: crude model. Model 2 was adjusted for age, race, educational status, marital status, BMI, PIR, drinking status, smoking status, physical activity, hypertension, diabetes, dyslipidemia, CVD, pelvic infection, female hormone use, antidepressants, antipsychotics, sleep duration, prior hysterectomy, and caffeine use.
Abbreviations: OR, odds ratio; CI, confidence interval; Ref, reference; UI, urinary incontinence.
Figure 2.
The moderating effect of dietary vitamin B12 intake on the association between UI and depression.
The Moderating Effect of Vitamin B12 Intake in Different Age, BMI, and Vaginal Deliveries Groups
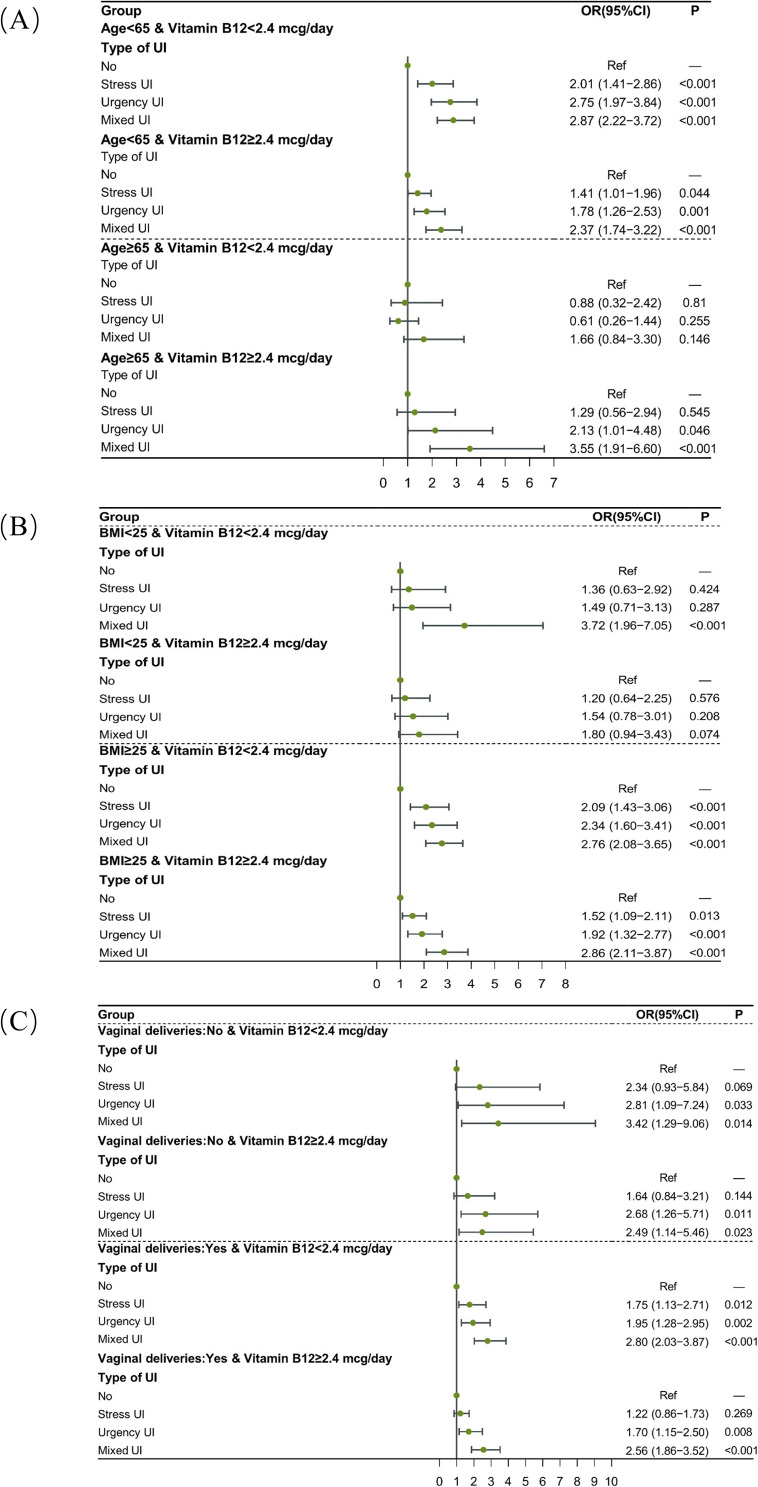
The moderating effect of vitamin B12 intake on the association between UI and depression was further investigated in different age, BMI, and vaginal delivery groups. The results are presented in Tables 4 and Figure 3, respectively. Compared to participants without UI, UI patients aged <65 years who have vitamin B12 intake ≥2.4 mcg/day were related to higher odds of depression (all P<0.05), and vitamin B12 intake moderated the relationship between UUI (OR=1.78, 95% CI: 1.26–2.53), mixed UI (OR=2.37, 95% CI: 1.74–3.22) and depression. A moderating effect of vitamin B12 intake was also found in UI patients with a BMI ≥25 (all P<0.05). In women with and without vaginal delivery, vitamin B12 intake affected the association between UUI, mixed UI, and depression (all P<0.05).
Table 4.
The Moderating Effect of Vitamin B12 on the Association of UI and Depression in Different Age, BMI, and Vaginal Deliveries Groups
| Variables | Vitamin B12: <2.4 mcg/day | Vitamin B12: ≥2.4 mcg/day | Vitamin B12: <2.4 mcg/day | Vitamin B12: ≥2.4 mcg/day | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Type of UI | Age <65 years | Age ≥65 years | ||||||
| No | Ref | Ref | Ref | Ref | ||||
| Stress UI | 2.01 (1.41–2.86) | <0.001 | 1.41 (1.01–1.96) | 0.044 | 0.88 (0.32–2.42) | 0.810 | 1.29 (0.56–2.94) | 0.545 |
| Urgency UI | 2.75 (1.97–3.84) | <0.001 | 1.78 (1.26–2.53) | 0.001 | 0.61 (0.26–1.44) | 0.255 | 2.13 (1.01–4.48) | 0.046 |
| Mixed UI | 2.87 (2.22–3.72) | <0.001 | 2.37 (1.74–3.22) | <0.001 | 1.66 (0.84–3.30) | 0.146 | 3.55 (1.91–6.60) | <0.001 |
| Type of UI | BMI <25 kg/m2 | BMI ≥25 kg/m2 | ||||||
| No | Ref | Ref | Ref | Ref | ||||
| Stress UI | 1.36 (0.63–2.92) | 0.424 | 1.20 (0.64–2.25) | 0.576 | 2.09 (1.43–3.06) | <0.001 | 1.52 (1.09–2.11) | 0.013 |
| Urgency UI | 1.49 (0.71–3.13) | 0.287 | 1.54 (0.78–3.01) | 0.208 | 2.34 (1.60–3.41) | <0.001 | 1.92 (1.32–2.77) | <0.001 |
| Mixed UI | 3.72 (1.96–7.05) | <0.001 | 1.80 (0.94–3.43) | 0.074 | 2.76 (2.08–3.65) | <0.001 | 2.86 (2.11–3.87) | <0.001 |
| Type of UI | Vaginal deliveries: No | Vaginal deliveries: Yes | ||||||
| No | Ref | Ref | Ref | Ref | ||||
| Stress UI | 2.34 (0.93–5.84) | 0.069 | 1.64 (0.84–3.21) | 0.144 | 1.75 (1.13–2.71) | 0.012 | 1.22 (0.86–1.73) | 0.269 |
| Urgency UI | 2.81 (1.09–7.24) | 0.033 | 2.68 (1.26–5.71) | 0.011 | 1.95 (1.28–2.95) | 0.002 | 1.70 (1.15–2.50) | 0.008 |
| Mixed UI | 3.42 (1.29–9.06) | 0.014 | 2.49 (1.14–5.46) | 0.023 | 2.80 (2.03–3.87) | <0.001 | 2.56 (1.86–3.52) | <0.001 |
Notes: Age subgroups were adjusted for race, educational status, marital status, BMI, PIR, drinking status, smoking status, physical activity, hypertension, diabetes, dyslipidemia, CVD, pelvic infection, female hormone use, antidepressants, antipsychotics, sleep hours, prior hysterectomy, and caffeine. The age subgroups were adjusted for age, race, educational status, marital status, PIR, drinking status, smoking status, physical activity, hypertension, diabetes, dyslipidemia, CVD, pelvic infection, female hormone use, antidepressants, antipsychotics, sleep hours, prior hysterectomy, and caffeine. The vaginal delivery subgroups included age, race, educational status, marital status, BMI, PIR, drinking status, smoking status, physical activity, hypertension, diabetes, dyslipidemia, CVD, pelvic infection, female hormone use, antidepressants, antipsychotics, sleep hours, prior hysterectomy, and caffeine.
Abbreviations: OR, odds ratio; CI, confidence interval; Ref, reference; UI, urinary incontinence; BMI, body mass index.
Figure 3.
Dietary vitamin B12 intake affects the association of UI with depression in different age (A), BMI (B), and vaginal deliveries (C) groups.
Discussion
In this study, we explored the moderating effect of vitamin B12 intake on the relationship between UI and depression in women. The findings indicated a significant association between UI and higher odds of depression, with vitamin B12 playing a moderating role. In women aged <65 years, with BMI ≥ 25 kg/m2, and having vaginal deliveries or not, vitamin B12 also had a moderating effect.
Our study confirms that women affected by UI were more prone to experiencing depressive symptoms compared to those without UI.3,17,18 A study involving elderly Korean women discovered that individuals with UI not only reported elevated levels of depression but also increased stress and reduced self-esteem.19 Furthermore, UI adversely affects women’s mental health, leading to social isolation, restricted social engagements, and eventually diminishing their quality of life.20,21 Uterine aging may also contribute to the development of anxiety and depression in perimenopausal women.22 Our study identified the moderating effect of vitamin B12 intake on the relationship between UI and depression, representing a novel contribution to the existing literature. Collagen, a vital component of pelvic floor muscle, plays a crucial role in preventing UI, with vitamin B12 influencing collagen formation.12 A review of 35 studies highlighted an association between lower vitamin B12 levels and an increased risk of developing depression.8 Interestingly, women with higher BMI exhibit an increased likelihood of developing UI and overactive bladder.23 One potential explanation is the elevated pelvic floor pressure resulting from excess weight, which can weaken pelvic floor muscles and contribute to UI and overactive bladder. Ospemifene presents as a promising therapy for postmenopausal women with vulvovaginal atrophy, improving overactive bladder symptoms and quality of life, weight reduction may serve as an additional intervention.23–25 Subgroup analyses indicated that the moderating effect of vitamin B12 varied across different types of UI, suggesting diverse underlying mechanisms.
The mechanisms underlying the moderating role of vitamin B12 intake vary across different UI types. For SUI, vitamin B12 potentially enhances pelvic floor muscle function and strengthens the urethral sphincter, possibly alleviating symptom severity.12 Moreover, vitamin B12 plays a role in synthesizing neurotransmitters like serotonin and dopamine, which are crucial for regulating mood and emotional well-being.26 Heightened sympathetic nervous system activity related to depression may elevate cortisol and catecholamine circulation levels, leading to physiological changes in bladder function and UI.4 Hence, adequate vitamin B12 intake may mitigate depressive symptoms associated with SUI. For UUI, the underlying mechanisms are less clear. Nonetheless, we hypothesized that vitamin B12 could aid in modulating bladder function and reducing overactive bladder symptoms. UUI, stemming from the bladder’s inability to comfortably and appropriately store urine, was frequently associated with neurological conditions.27,28 Vitamin B12 contributes to nerve function and myelin synthesis, which protects nerve fibers.29 Therefore, maintaining adequate vitamin B12 levels may regulate bladder contractions and decrease the occurrence of UUI, thereby reducing the risk of depression. For MUI, the moderating effect of vitamin B12 intake likely involves a combination of the mechanisms observed in SUI and UUI. The presence of both stress and urge components in MUI suggests the involvement of multiple mechanisms. Further investigations are needed to elucidate the specific pathways of the moderating role of vitamin B12.
Effective management of UI has been shown to improve patient’s quality of life and may significantly reduce depressive symptoms. Current treatment modalities for UI, include lifestyle modifications (weight loss, timed voids, fluid restriction, and pelvic floor exercises), pessaries, pharmacotherapy, botox and neuromodulation, and surgery.30 Moreover, the psychological implications of urinary incontinence cannot be overlooked, as they may exacerbate depressive symptoms, creating a bidirectional relationship. Understanding that UI is associated with higher odds of depression underscores the importance of comprehensive screening protocols. Clinicians should consider routine assessment of mental health in women presenting with UI symptoms, as early identification and management of depression could potentially improve overall quality of life and treatment outcomes. Moreover, the moderating role of dietary vitamin B12 intake suggests a potential avenue for intervention. Clinicians might consider dietary counseling or supplementation strategies for women with UI, particularly those at risk for or already experiencing depression. This personalized approach could complement existing treatment modalities for UI and depression, potentially enhancing therapeutic efficacy and patient well-being. Future research exploring longitudinal outcomes and interventional studies focusing on optimizing vitamin B12 intake in this population could further elucidate these findings and guide evidence-based.
The strength of our study lies in the utilization of a nationally representative sample of 14154 individuals, ensuring robust statistical power and minimizing selection bias. To the best of our knowledge, our study represents the first attempt to explore the moderating effect of dietary vitamin B12 intake on the association between different UI types and depression. Despite these strengths, our study has limitations. Firstly, due to its cross-sectional design, causality could not be identified and only correlations could be reached. The study is limited by its exclusive focus on vitamin B12 without investigating other micronutrients, such as vitamin D, which may influence the relationship between UI and depression. Future studies could explore these additional factors. Therefore, further long-term, prospective studies are essential to confirm our findings. Secondly, the study relied on self-reported assessment of UI and depression symptoms, potentially introducing recall bias. Future research could employ more objective assessment tools to enhance data accuracy. Lastly, despite adjusting for covariates, the study did not account for residual or unmeasured confounders, which may have introduced bias.
Conclusion
Our study suggested that UI was associated with increased odds of depression in women, with dietary vitamin B12 intake moderating this relationship. Our findings provide new directions for investigating the role of vitamin B12 in neurologic function and mental health, offering new strategies for UI prevention and intervention.
Funding Statement
This study was supported by the Research and Development Fund of Peking University People’s Hospital (RDX2021-03), Research and Development Fund of Peking University People’s Hospital (RDJP2022-72), and Special Research Fund of the Chinese Association of Plastics and Aesthetics (FRPR2020-nxxt-01).
Ethics Approval and Informed Consent
The requirement of ethical approval for this was waived by the Institutional Review Board of Peking University People’s Hospital, because the data was accessed from NHANES (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of Peking University People’s Hospital due to the retrospective nature of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author reported no conflicts of interest in this work.
References
- 1.Vaughan CP, Markland AD. Urinary incontinence in women. Ann Intern Med. 2020;172:ITC17–ITC32. doi: 10.7326/AITC202002040 [DOI] [PubMed] [Google Scholar]
- 2.Abufaraj M, Xu T, Cao C, et al. Prevalence and trends in urinary incontinence among women in the United States, 2005-2018. Am J Obstet Gynecol. 2021;225:166.e1–166.e12. doi: 10.1016/j.ajog.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 3.Park G-R, Park S, Kim J. Urinary incontinence and depressive symptoms: the mediating role of physical activity and social engagement. J Gerontol B Psychol Sci Soc Sci. 2022;77:1250–1258. doi: 10.1093/geronb/gbab212 [DOI] [PubMed] [Google Scholar]
- 4.Lim Y-M, Lee SR, Choi EJ, et al. Urinary incontinence is strongly associated with depression in middle-aged and older Korean women: data from the Korean longitudinal study of ageing. Eur J Obstet Gynecol Reprod Biol. 2018;220:69–73. doi: 10.1016/j.ejogrb.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 5.Ekinci GN, Sanlier N. The relationship between nutrition and depression in the life process: a mini-review. Exp Gerontol. 2023;172:112072. doi: 10.1016/j.exger.2022.112072 [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Lee HS. Nutrient intake and urinary incontinence in Korean women: a propensity score-matched analysis from the Korea national health and nutrition examination survey data. Int J Urol. 2017;24:793–797. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen K, Stojanovska L, Apostolopoulos V. The effects of vitamin B in depression. Curr Med Chem. 2016;23:4317–4337. doi: 10.2174/0929867323666160920110810 [DOI] [PubMed] [Google Scholar]
- 8.Sangle P, Sandhu O, Aftab Z, et al. Vitamin B12 supplementation: preventing onset and improving prognosis of depression. Cureus. 2020;12:e11169. doi: 10.7759/cureus.11169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Post WM, Widomska J, Grens H, et al. Molecular processes in stress urinary incontinence: a systematic review of human and animal studies. Int J Mol Sci. 2022;23:3401. doi: 10.3390/ijms23063401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Y, Tan W, Huang Y, et al. Association between oxidative balance score and urinary incontinence in females: results from the national health and nutrition examination survey in 2005-2018. Int Urol Nephrol. 2023;55:2145–2154. doi: 10.1007/s11255-023-03665-3 [DOI] [PubMed] [Google Scholar]
- 11.Rana S, D’Amico F, Merenstein JH. Relationship of vitamin B12 deficiency with incontinence in older people. J Am Geriatr Soc. 1998;46:931–932. doi: 10.1111/j.1532-5415.1998.tb02741.x [DOI] [PubMed] [Google Scholar]
- 12.Kesiktas N, Karan A, Erkan H, et al. Is there a relationship between vitamin B12 and stress urinary incontinence? Low Urin Tract Symptoms. 2012;4:55–58. doi: 10.1111/j.1757-5672.2011.00116.x [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Zhang L, Li S, et al. Associations of dietary vitamin B1, vitamin B2, vitamin B6, and vitamin B12 with the risk of depression: a systematic review and meta-analysis. Nutr Rev. 2022;80:351–366. doi: 10.1093/nutrit/nuab014 [DOI] [PubMed] [Google Scholar]
- 14.Patel JS, Oh Y, Rand KL, et al. Measurement invariance of the patient health questionnaire-9 (PHQ-9) depression screener in U.S. adults across sex, race/ethnicity, and education level: NHANES 2005-2016. Depress Anxiety. 2019;36:813–823. doi: 10.1002/da.22940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2015-2020 Dietary Guidelines | health.gov. Available from: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015. Accessed October 11, 2023.
- 17.Zhang Q, Wang Q, Liu Z, et al. Prevalence and clinical correlates for depression in women with urinary incontinence: a cross-sectional study. Int Urogynecol J. 2022;33:1303–1309. doi: 10.1007/s00192-022-05169-7 [DOI] [PubMed] [Google Scholar]
- 18.Kwon J, Lee HJ, Joo JH, et al. Urinary incontinence status changes and depressive symptoms among middle-aged and older women: using data from a survey of the Korean longitudinal study of aging. J Affect Disord. 2021;279:549–553. doi: 10.1016/j.jad.2020.10.039 [DOI] [PubMed] [Google Scholar]
- 19.Lee H-Y, Rhee Y, Choi KS. Urinary incontinence and the association with depression, stress, and self-esteem in older Korean women. Sci Rep. 2021;11:9054. doi: 10.1038/s41598-021-88740-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daneshpajooh A, Naghibzadeh-Tahami A, Najafipour H, et al. Prevalence and risk factors of urinary incontinence among Iranian women. Neurourol Urodyn. 2021;40:642–652. doi: 10.1002/nau.24597 [DOI] [PubMed] [Google Scholar]
- 21.Pizzol D, Demurtas J, Celotto S, et al. Urinary incontinence and quality of life: a systematic review and meta-analysis. Aging Clin Exp Res. 2021;33:25–35. doi: 10.1007/s40520-020-01712-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinelli A, Andjić M, Morciano A, et al. Uterine aging and reproduction: dealing with a puzzle biologic topic. Int J Mol Sci. 2023;25:322. doi: 10.3390/ijms25010322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsannan B, Laganà AS, Alhermi J, et al. Prevalence of overactive bladder among overweight and obese women: a prospective cross-sectional cohort study. Eur J Obstet Gynecol Reprod Biol. 2024;295:59–64. doi: 10.1016/j.ejogrb.2024.02.010 [DOI] [PubMed] [Google Scholar]
- 24.Schiavi MC, D’Oria O, Aleksa N, et al. Usefulness of Ospemifene in the treatment of urgency in menopausal patients affected by mixed urinary incontinence underwent mid-urethral slings surgery. Gynecol Endocrinol. 2019;35:155–159. doi: 10.1080/09513590.2018.1500534 [DOI] [PubMed] [Google Scholar]
- 25.Schiavi MC, Zullo MA, Faiano P, et al. Retrospective analysis in 46 women with vulvovaginal atrophy treated with ospemifene for 12 weeks: improvement in overactive bladder symptoms. Gynecol Endocrinol. 2017;33:942–945. doi: 10.1080/09513590.2017.1323859 [DOI] [PubMed] [Google Scholar]
- 26.Dhiman P, Pillai RR, Wilson AB, et al. Cross-sectional association between vitamin B12 status and probable postpartum depression in Indian women. BMC Pregnancy Childbirth. 2021;21:146. doi: 10.1186/s12884-021-03622-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AL.Understanding overactive bladder and urgency incontinence: what does the brain have to do with it? F1000Research.2018;7:F1000. doi: 10.12688/f1000research.16418.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bapir R, Bhatti KH, Eliwa A, et al. Efficacy of overactive neurogenic bladder treatment: a systematic review of randomized controlled trials. Arch Ital Urol Androl. 2022;94:492–506. doi: 10.4081/aiua.2022.4.492 [DOI] [PubMed] [Google Scholar]
- 29.Julian T, Syeed R, Glascow N, et al. B12 as a treatment for peripheral neuropathic pain: a systematic review. Nutrients. 2020;12:2221. doi: 10.3390/nu12082221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monti M, Fischetti M, Santangelo G, et al. Urinary incontinence in women: state of the art and medical treatment. Minerva Obstet Gynecol. 2021;73(2):135–139. doi: 10.23736/S2724-606X.20.04635-3 [DOI] [PubMed] [Google Scholar]