Abstract
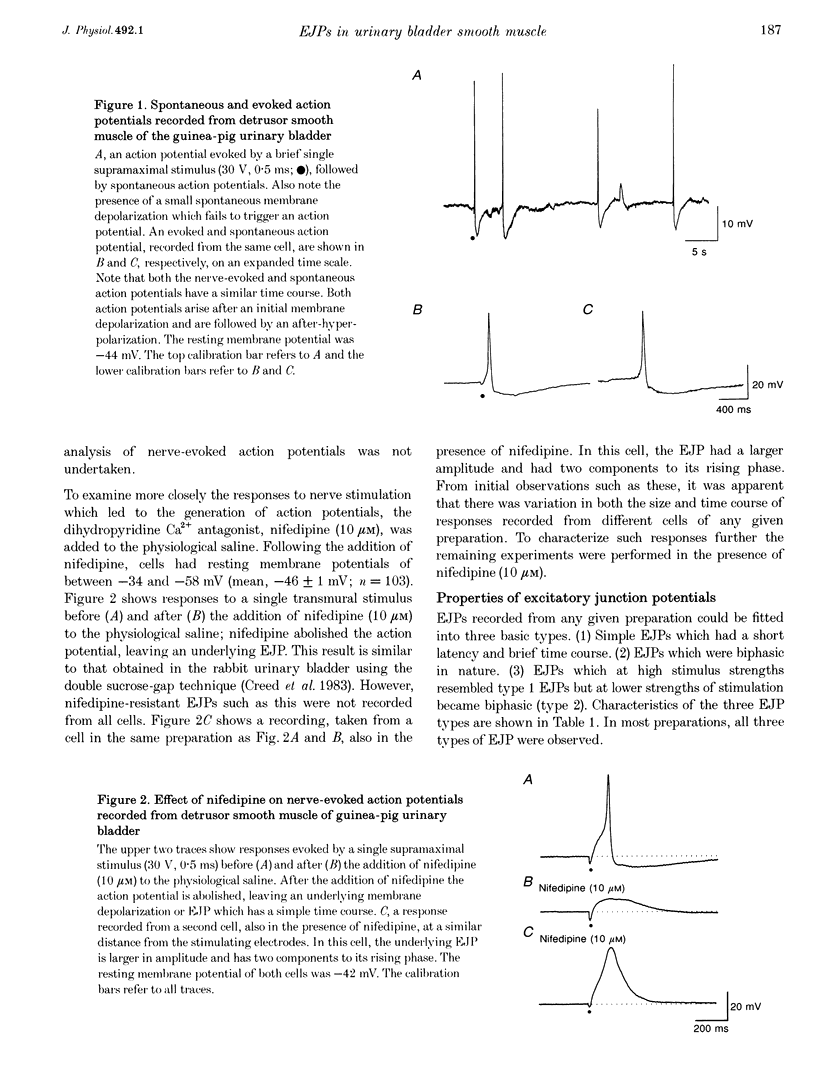
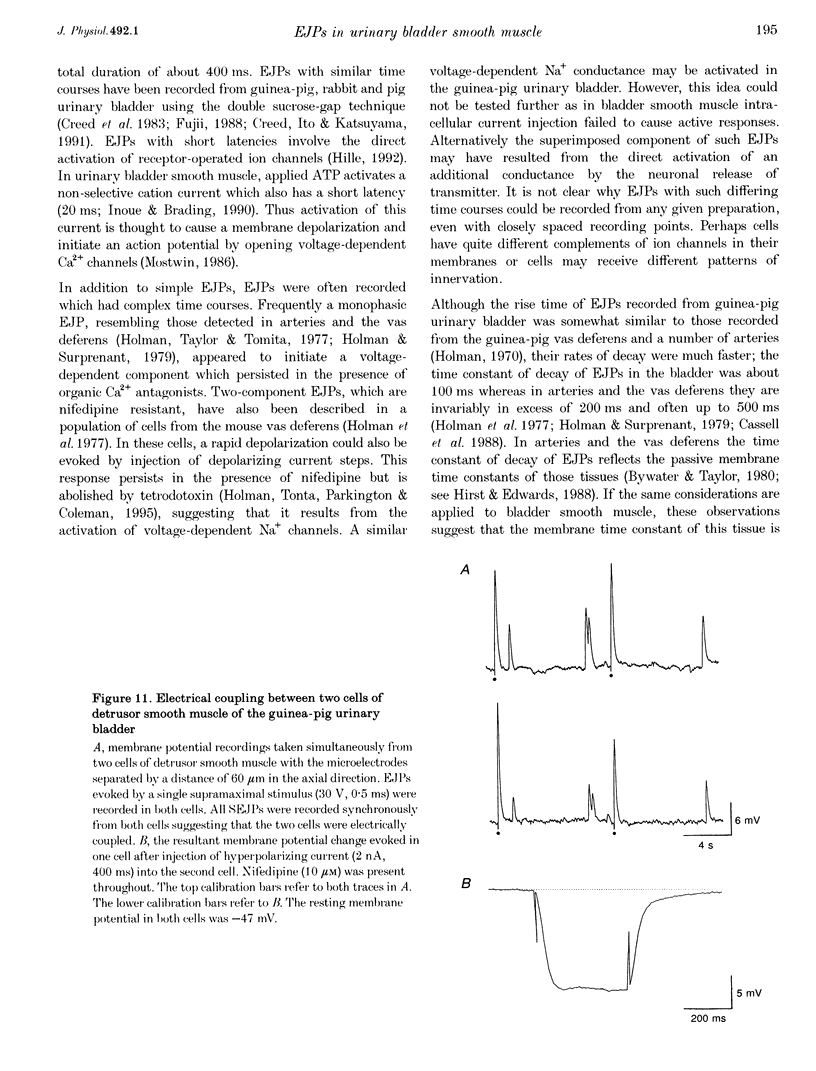
1. The effects of transmural nerve stimulation were examined on preparations of detrusor smooth muscle from guinea-pig urinary bladder using intracellular recording techniques. Most recordings were made from preparations in which spontaneous and evoked action potentials had been inhibited by nifedipine (10 microM), a dihydropyridine that blocks L-type Ca2+ channels. 2. Supramaximal stimuli evoked excitatory junction potentials (EJPs) which could be divided into three basic types. Type 1 EJPs had short latencies (< 30 ms) and fast rise times (< 60 ms). Type 2 EJPs consisted of two components: a small depolarization that was followed by a second depolarization with a faster rise time. In a third type of cell, at high strengths of stimulation, EJPs resembled type 1 EJPs but at lower strengths of stimulation were similar in time course to type 2 EJPs. 3. All EJPs were abolished by tetrodotoxin (1 microM) and reduced by omega-conotoxin (0.1 microM), but were unaffected by hexamethonium (0.1 mM), suggesting that they result from the release of transmitter from post-ganglionic nerve fibres. All responses persisted in the presence of atropine (1 microM) but were abolished following the desensitization of P2-purinoceptors with alpha, beta-methylene ATP (m-ATP; 10 microM). 4. Spontaneous excitatory junction potentials (SEJPs) were also recorded from most cells. SEJPs were similar in appearance to fast single-component EJPs; however, in general they had a briefer time course. SEJPs persisted in the presence of tetrodotoxin (1 microM). 5. The electrical properties of urinary bladder smooth muscle were also examined. Voltage changes induced by point current injection into cells had fast rates of rise and decay (time constant, 5-20 ms). The input resistance of cells ranged between 12 and 108 M omega. When recordings were taken from cells near the point of current injection, resultant electrotonic potentials could be detected in only a small proportions of cells. 6. The results are discussed in relation to the idea that transmural nerve stimulation in the guinea-pig urinary bladder causes the activation of at least two different membrane conductances. Cells appear to be electrically coupled with one another. However, it is likely that coupling exists within discrete bundles of the smooth muscle.
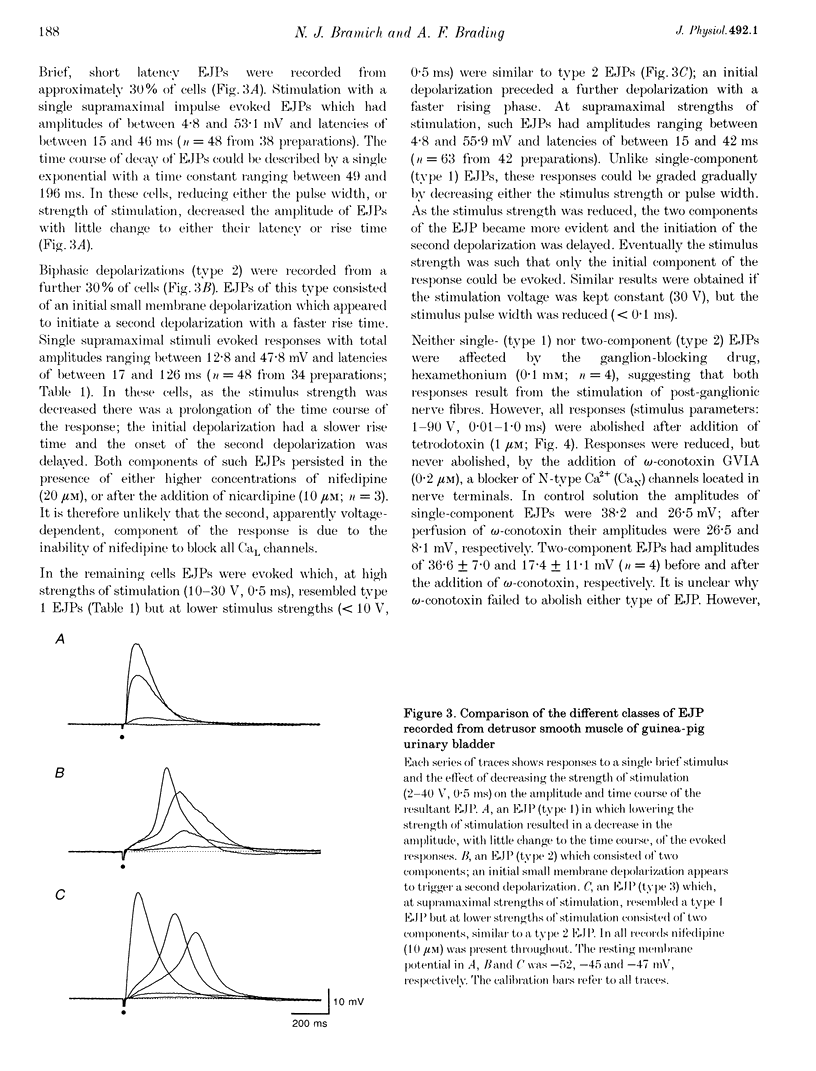
Full text
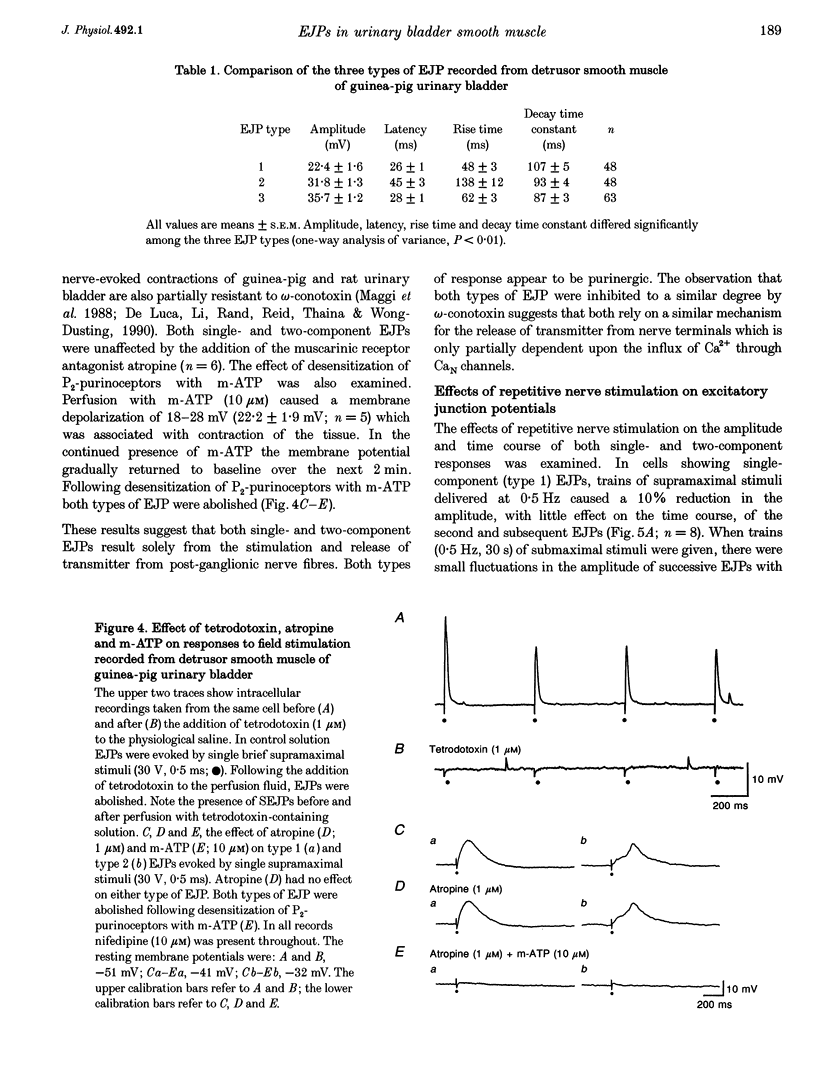
PDF

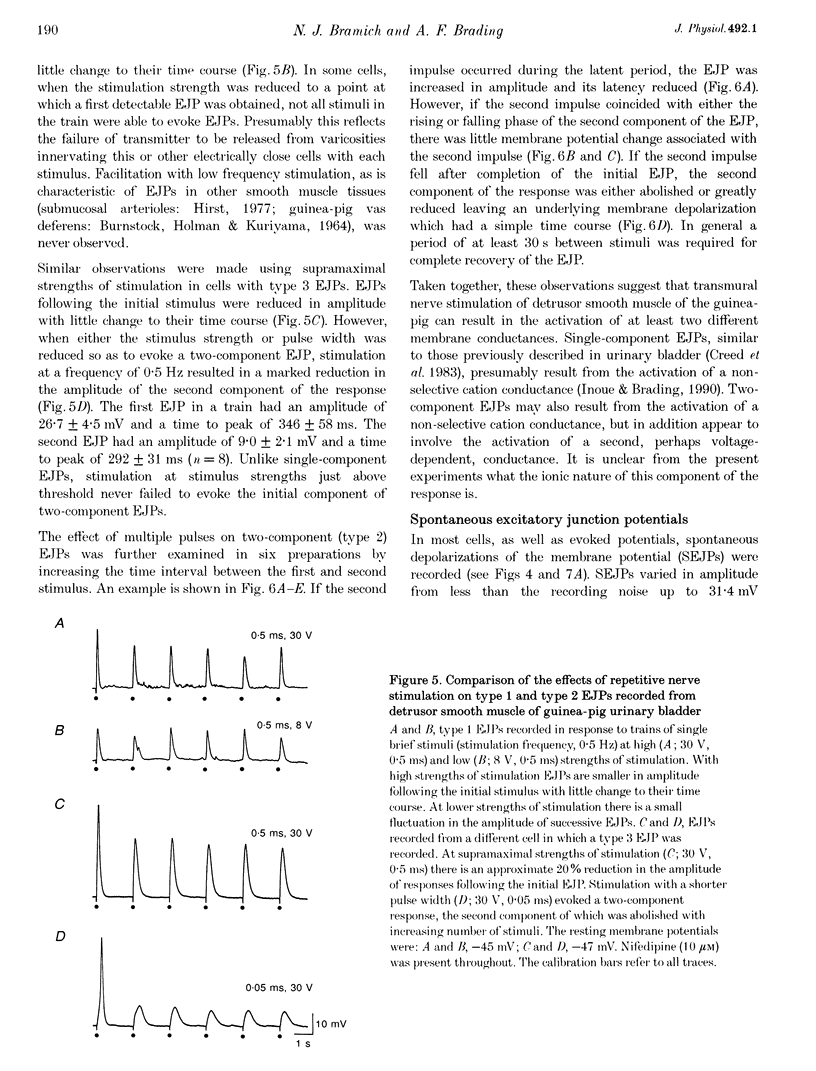



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Holman M. E., Taylor G. S., Tomita T. Some properties of the smooth muscle of mouse vas deferens. J Physiol. 1977 Apr;266(3):751–764. doi: 10.1113/jphysiol.1977.sp011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Tonta M. A., Parkington H. C., Coleman H. A. Tetrodotoxin-sensitive action potentials in smooth muscle of mouse vas deferens. J Auton Nerv Syst. 1995 Apr 8;52(2-3):237–240. doi: 10.1016/0165-1838(94)00157-f. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by alpha,beta-methylene ATP. Eur J Pharmacol. 1985 Aug 15;114(2):239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara M., Kitamura K., Kuriyama H. Neuromuscular transmission and smooth muscle membrane properties in the guinea-pig ear artery. J Physiol. 1981 Jun;315:283–302. doi: 10.1113/jphysiol.1981.sp013748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part II. The Bladder. Part III. The External Generative Organs. Part IV. The Internal Generative Organs. Part V. Position of the Nerve Cells on the Course of the Efferent Nerve Fibres. J Physiol. 1895 Dec 30;19(1-2):71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Santicioli P., Lippe I. T., Giuliani S., Geppetti P., Del Bianco E., Selleri S., Meli A. The effect of omega conotoxin GVIA, a peptide modulator of the N-type voltage sensitive calcium channels, on motor responses produced by activation of efferent and sensory nerves in mammalian smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1988 Aug;338(2):107–113. doi: 10.1007/BF00174856. [DOI] [PubMed] [Google Scholar]


