Abstract
Purpose
Net ultrafiltration (UFNET) during continuous renal replacement therapy (CRRT) can control fluid balance (FB), but is usually 0 ml·h−1 in patients with vasopressors due to the risk of hemodynamic instability associated with CRRT (HIRRT). We evaluated a UFNET strategy adjusted by functional hemodynamics to control the FB of patients with vasopressors, compared to the standard of care.
Methods
In this randomized, controlled, open-label, parallel-group, multicenter, proof-of-concept trial, adults receiving vasopressors, CRRT since ≤ 24 h and cardiac output monitoring were randomized (ratio 1:1) to receive during 72 h a UFNET ≥ 100 ml·h−1, adjusted using a functional hemodynamic protocol (intervention), or a UFNET ≤ 25 ml·h−1 (control). The primary outcome was the cumulative FB at 72 h and was analyzed in patients alive at 72 h and in whom monitoring and CRRT were continuously provided (modified intention-to-treat population [mITT]). Secondary outcomes were analyzed in the intention-to-treat (ITT) population.
Results
Between June 2021 and April 2023, 55 patients (age 69 [interquartile range, IQR: 62; 74], 35% female, Sequential Organ Failure Assessment (SOFA) 13 [11; 15]) were randomized (25 interventions, 30 controls). In the mITT population, (21 interventions, 24 controls), the 72 h FB was −2650 [−4574; −309] ml in the intervention arm, and 1841 [821; 5327] ml in controls (difference: 4942 [95% confidence interval: 2736–6902] ml, P < 0.01). Hemodynamics, oxygenation and the number of HIRRT at 72 h, and day-90 mortality did not statistically differ between arms.
Conclusion
In patients with vasopressors, a UFNET fluid removal strategy secured by a hemodynamic protocol allowed active fluid balance control, compared to the standard of care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-024-07676-1.
Keywords: Continuous renal replacement therapy, Fluid balance, Functional hemodynamics, Acute circulatory failure, Pulse contour, Transpulmonary thermodilution
Take-home message
| In patients with acute circulatory failure and severe acute kidney injury receiving continuous renal replacement therapy, an active fluid removal strategy with net ultrafiltration adjusted by an advanced hemodynamic protocol allowed early fluid balance control, compared to the standard of care (i.e. no fluid removal), without any detectable increase in hemodynamic instability episodes or severe adverse events. |
Introduction
Fluid overload may participate in multi-organ failure and is associated with delayed mechanical ventilation weaning, longer intensive care unit (ICU) stay, and increased mortality [1–6]. Yet, strategies aiming to control fluid balance (FB) without individualization have shown mixed results [7–10].
Patients with severe acute kidney injury (AKI) demonstrate the highest risk of developing fluid overload [11–15]. During continuous renal replacement therapy (CRRT), net ultrafiltration (UFNET) allows FB control if set appropriately to the patient hemodynamic status [11]. In practice, patients with acute circulatory failure do not receive active fluid removal by UFNET over the first days of CRRT [16–18]. Indeed, excessive UFNET may cause CRRT-related hemodynamic instability (HIRRT), leading to worsening organ failure [6, 19–21]. However, HIRRT is associated with preload dependence (i.e., a proxy of hypovolemia) in only 50% of cases [22], while preload dependence outside an HIRRT episode is associated with an increased risk of ulterior HIRRT [23, 24].
We hypothesized that FB control using UFNET during CRRT could be secured by functional hemodynamic monitoring. We aimed to assess the impact of an active fluid removal strategy by UFNET secured by a functional hemodynamic protocol on the cumulative FB measured over the first 72 h following inclusion in ICU patients requiring vasopressors, as compared to standard of care.
Methods
Study design
The GO NEUTRAL trial was a randomized controlled, open-label, parallel-group, multicenter, proof-of-concept trial, conducted in four ICUs in France. The study was registered at ClinicalTrials.gov (NCT04801784) before the first patient was enrolled. The trial protocol has been previously published [25] and was approved by a human research ethics committee (Comité de Protection des Personnes Sud Méditerrannée I, IDCRB 2021-A00692-39) on April 21, 2021. An amended version was approved on December 4, 2022 to increase the total sample size (see below). The trial was not monitored by a data and safety monitoring board, but the trial steering committee planned an interim analysis after the inclusion of 20 patients to identify a potential excess in mortality at 72 h. After this analysis was performed, enrollment was continued. This report follows the CONSORT guidelines (electronic supplementary material, ESM1) [26].
Trial participants
Eligible patients were ICU patients aged 18 years or older, with stage 3 AKI (kidney disease—improving global outcomes guidelines), treated with CRRT initiated within less than 24 h, receiving a continuous infusion of vasopressor for acute circulatory failure, and already equipped with a calibrated continuous cardiac output (CO) device (PiCCO®, Pulsion Medical, Feldkirch, Germany) [27]. Exclusion criteria are listed in ESM2.
Written informed consent was obtained from all participants, or their next-of-kin if the patient was unable to consent. The protocol allowed a consent waiver procedure for emergent inclusion under the investigator’s responsibility if the patient was unable to consent and if his next-of-kin was unavailable at time of screening. Consent to pursue trial participation was subsequently looked for as soon as possible [28].
Randomization
Inclusion and randomization of participants were performed by site investigators using a web-based platform (Ennov EDC, Ennov, France). Participants were randomly assigned (1:1 ratio) to receive the intervention or control strategy. The allocation sequence was computer-generated by the study’s statistician, with a stratification based on the participating center and fluid overload at inclusion (defined as a ≥ 10% increase in body weight between ICU admission and inclusion), with fixed-block size (n = 2) only known to the statistician [5].
Blinding of healthcare providers was not feasible due to the nature of interventions. Blinding of outcome assessors and data analysts was not feasible as the primary outcome incorporated cumulative UFNET for its computation.
Study populations
The intention-to-treat population (ITT) consisted of all enrolled participants fulfilling eligibility criteria, analyzed as per their allocation group, regardless of their adherence to the protocol and in whom consent to participate was obtained (i.e., patients randomized following the consent waiver procedure who did not consent to pursue participation were excluded from the ITT analysis).
Causes of early cessation of study procedures before 72 h were: death, CRRT suspension for a continuous period of 8 h or more, permanent dysfunction of the CO monitoring device, impossibility to perform postural maneuvers, active hemorrhage, ischemic or hemorrhagic stroke, or transfer to a non-participating ICU. The modified ITT (mITT) population was a subgroup of the ITT population consisting of patients alive at 72 h and in which CRRT and CO monitoring were continuously provided from inclusion to 72 h.
Trial procedures
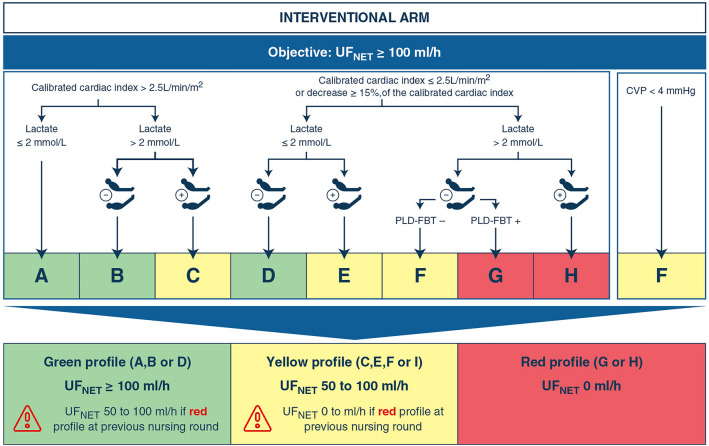
After enrollment, the strategy allocated by randomization had to be initiated within 2 h. In the intervention arm, the UFNET flow rate (i.e., the flow rate of fluid volume removed by the CRRT monitor [29]) was set to 100 ml·h−1 or more (based on the clinician decision) and was maintained for 72 h. The target UFNET aimed to neutralize the expected fluid input in this population [16, 30]. This intervention was combined to a hemodynamic monitoring protocol which allowed transitory UFNET decrease or suspension in case of a threatening hemodynamic profile evocative of hypovolemia. The protocol relied on the conjunct assessment of cardiac index (measured by transpulmonary thermodilution), arterial lactate concentration, postural maneuvers evaluating preload dependence using continuous cardiac index assessed by pulse contour analysis and central venous pressure, performed every 4 h by the treating nurse (without additional nursing staff) between inclusion and 72 h (Fig. 1) [31, 32]. The protocol was designed to identify macrocirculatory failure (low cardiac index) with tissue hypoxia (high arterial lactate) related to potential hypovolemia (identified by preload dependence during a postural maneuver). Using these parameters, the protocol identified three hemodynamic profiles (green, orange, red), which respectively recommended pursuing, decreasing or ceasing UFNET (Fig. 1).
Fig. 1.
Hemodynamic protocol applied 4-hourly in the intervention arm. The protocol was applied by the nursing staff every 4 h between inclusion (H0) and 72 h, and relied on the evaluation of cardiac indices measured by transpulmonary thermodilution, arterial lactate concentrations measured at least every 8 h, central venous pressure, and the result of a postural maneuver (either passive leg raising or Trendelenburg maneuver, represented in the diagram by the silhouettes) evaluating preload dependence. Based on a 3-steps approach, the staff categorized the hemodynamic profile of the patient using the 9 letters and the 3 profile colors, which subsequently led to the adjustment of the UFNET on the CRRT monitor. Of note, a CVP < 4 mmHg led to a decrease in UFNET whatever the value of the other parameters. The evaluation took into account the results of the previous evaluation performed 4 h earlier. CVP central venous pressure, PLD-FBT preload dependence evaluated by a fluid bolus challenge, UFNET net ultrafiltration
Furthermore, in case of the onset of HIRRT (tachycardia, hypotension, mottling or drop in cardiac index, supplemental Fig. 1—ESM3), a simplified hemodynamic assessment relying on postural maneuvers, central venous pressure or the clinician’s judgment, allowed decreasing or suspending UFNET until the next 4-hourly routine evaluation (supplemental Fig. 1—ESM3). The hemodynamic profile was re-evaluated every 4 h to restore UFNET ≥ 100 ml·h−1 in case of hemodynamic improvement.
Postural maneuvers and fluid responsiveness assessment were part of the hemodynamic protocol of the intervention arm. These procedures are described in supplemental Methods—ESM4.
In the 2-h following inclusion, UFNET was set to ≤ 25 ml·h−1 in the control arm and was maintained for 72 h. This aimed to mimic routine practice in ICU patients with vasopressors, as reported in previous studies [16–18, 33] and to limit contamination between study’s arms. No hemodynamic protocol was applied in this group, and the management of HIRRT episodes were left at the discretion of the treating team. UFNET could be transiently increased > 25 ml·h−1 in case of respiratory failure due to hydrostatic pulmonary edema (supplemental Methods—ESM5). Once the episode had resolved, the UFNET strategy had to be restored.
In both arms, after 72 h of inclusion, UFNET setting was left at the discretion of the treating team.
Study primary and secondary outcomes
The study primary outcome was the cumulative FB measured between inclusion (H0) and 72 h after inclusion (H72). The primary outcome (in ml) consisted of the difference of cumulative fluid inputs (intermittent and continuous intravenous (IV) medications [including electrolytes and vitamins], fluid boluses, blood products, enteral and parenteral nutrition, and maintenance fluids) and cumulative fluid outputs (urine output, UFNET and surgical drains), each item being quantified, collected and reported 4-hourly into the electronic ICU charts of participating units. The procedure regarding collection of fluid input and output components, and the procedure in case of missing values are detailed in the statistical analysis plan (supplemental Methods—ESM6) and in supplemental Table 3 (ESM7).
Secondary safety outcomes evaluated the number of HIRRT episodes per patient in each study group from H0 to H72 [20], the prevalence of preload-dependent HIRRT (i.e., a HIRRT episode associated with an increase in cardiac index above predefined thresholds during a postural maneuver performed at time of HIRRT) in the intervention arm [31, 34], the values of several hemodynamic measurements (cardiac index, mean arterial pressure, central venous pressure, norepinephrine dose and arterial lactate) from H0 to H72, the number of days alive without vasopressor at day 28, the daily Sepsis-related Organ Failure Assessment scores (SOFA) between inclusion and H72 [35], and the vital status at H72, day 28 and day 90 after inclusion.
Secondary efficacy outcomes evaluated the cumulative FB and UFNET volumes, normalized using the duration of follow-up from H0 up to H24 and H72 or end of participation whichever occurred first (in ml·h−1), the extravascular lung water index and the ratio of arterial O2 partial pressure to the inspired O2 fraction (PaO2/FiO2) using either measured FiO2 on the ventilator in ventilated patients or estimated FiO2 in non-ventilated patients [36] (evaluated daily between inclusion and H72), the number of ventilator-free days at day 28, the rate of Major Adverse Kidney Events at day 90 (MAKE90) and on the hospital and ICU lengths of stay [5, 37–40]. Secondary outcomes’ definitions are provided in supplemental Methods—ESM8.
CRRT settings and co-interventions
CRRT indications followed national and international recommendations [27, 41]. Recommended CRRT settings are described in supplemental Methods—ESM9. Fluid bolus therapy, vasopressor and diuretic management and hemodynamic monitoring procedures are described in supplemental Methods—ESM10.
Sample size
We hypothesized that H72 cumulative FB would be 4000 ± 4000 ml in the control group based on the results of the IDEAL-ICU trial, and 0 ± 4000 ml in the intervention group based on the study’s aim to keep FB neutral, and estimated that the required sample size to identify a significant difference between arms (with an α- and a β-risk < 0.05 and 0.20, respectively) would be at least 16 patients per arm [16]. Since we expected a mortality rate of 25% at H72, this number was increased to 21 patients per arm [16]. A further increase of 25% was justified due to the potentially non-normal distribution of the primary outcome, resulting in a required sample size of 58 patients. The protocol was amended to further increase this number to 66, due to a mortality rate of 22% at H72 after the inclusion of the first 49 patients. Inclusions were stopped once the required 21 patients per arm was reached.
Statistics
The primary outcome measure was compared between groups in the mITT population. All secondary outcomes and severe adverse events were assessed in the ITT population.
A statistical analysis plan was prepared prior to database lock (supplemental Methods—ESM6). Statistical analysis was performed using the R software (version 4.1.3) with packages lme4 and lmerTest [42–44]. A P value < 0.05 was chosen for statistical significance. Data were expressed as median [1st quartile; 3rd quartile] for quantitative variables and counts (percentages) for categorical variables.
Comparison between groups regarding the primary outcome was performed using the Wilcoxon–Mann–Whitney test, and the location shift using the Hodges–Lehmann method. Statistical analyses are described in supplemental Methods—ESM11.
Results
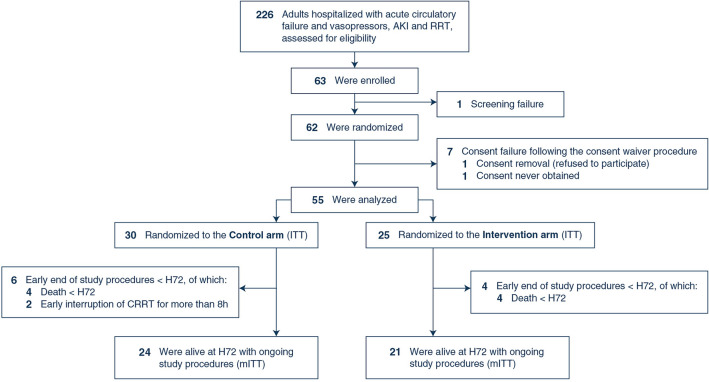
Between June 2021 and April 2023, 62 patients were randomized (Fig. 2 and supplemental Table 1—ESM12). Consent to participate could not be obtained in seven patients enrolled with the consent waiver procedure, leaving 55 patients in the ITT population (30 controls, 25 interventions). At inclusion, norepinephrine dose (tartrate formulation) was 0.45 [0.21; 1.05] µg·kg−1·min−1, SOFA score was 13 [11; 15] and the cumulative FB between ICU admission and inclusion was 2 [−3; 5] kg (Table 1).
Fig. 2.
Study flowchart. The total number of patients who met eligibility criteria was estimated using the medical information system of participating ICUs to identify patients receiving vasopressors and CRRT, as exact and continuous screening was rendered difficult due to the coronavirus disease 2019 (COVID-19) pandemic. Hence, reason for non-inclusion could not be prospectively collected and reported. AKI acute kidney injury, CRRT continuous renal replacement therapy, ITT intention-to-treat, mITT modified intention-to-treat, RRT renal replacement therapy
Table 1.
Patients’ characteristics at inclusion (ITT population)
| Whole population | Control | Intervention | |
|---|---|---|---|
| N = 55 | N = 30 | N = 25 | |
| Demographics | |||
| Age, years | 69 [62; 74] | 68 [61; 74] | 71 [66; 74] |
| Sex, male | 36 (65) | 21 (70) | 15 (60) |
| Body weight at ICU admission, kg | 76 [69; 92] | 80 [70; 102] | 76 [69; 86] |
| Body mass index, kg·m−2 | 28 [24; 34] | 28 [23; 34] | 28 [25; 34] |
| Comorbidities | |||
| Hypertension, N (%) | 23 (42) | 12 (40) | 11 (44) |
| Diabetes, N (%) | 28 (51) | 16 (53) | 12 (48) |
| Peripheral artery disease, N (%) | 6 (11) | 5 (17) | 1 (4) |
| Prior stroke, N (%) | 4 (7) | 3 (10) | 1 (4) |
| Chronic heart failure, N (%)a | 14 (25) | 9 (30) | 5 (20) |
| Chronic respiratory failure, N (%)b | 8 (15) | 6 (20) | 2 (8) |
| Chronic kidney disease, N (%)c | 10 (18) | 5 (17) | 5 (20) |
| Premorbid creatinine, µmol·L−1d e | 91 [74; 93] | 90 [72; 93] | 91 [87; 93] |
| Cirrhosis, N (%) | 8 (15) | 4 (13) | 4 (16) |
| Immunodepression, N (%)f | 12 (22) | 5 (17) | 7 (28) |
| Admission category | |||
| Medical, N (%) | 54 (98) | 29 (97) | 25 (100) |
| Post-operative, emergent, N (%) | 1 (2) | 1 (3) | 0 (0) |
| Post-operative, scheduled, N (%) | 0 (0) | 0 (0) | |
| Admission diagnosis | |||
| Cardiovascular, N (%) | 7 (13) | 3 (10) | 4 (16) |
| Respiratory, N (%) | 28 (51) | 14 (47) | 14 (56) |
| Gastro-intestinal, N (%) | 4 (7) | 2 (7) | 2 (8) |
| Other, N (%) | 16 (29) | 11 (37) | 5 (20) |
| Severity of disease at inclusion | |||
| SAPS-2 score | 60 [50; 72] | 62 [49; 73] | 57 [51; 63] |
| SOFA score | 13 [11; 15] | 14 [12; 16] | 12 [11; 13] |
| Sepsis, N (%)g | 48 (87) | 28 (93) | 20 (80) |
| Septic shock, N (%)g | 19 (35) | 10 (33) | 9 (36) |
| Hemodynamics at inclusion | |||
| Heart rate, min−1 | 91 [84; 108] | 90 [85; 108] | 91 [83; 107] |
| Mean arterial pressure, mmHg | 75 [69; 82] | 76 [71; 89] | 72 [69; 77] |
| Central venous pressure, mmHg | 9 [6; 13] | 10 [7; 14] | 9 [5; 12] |
| Central venous pressure < 4 mmHg, N (%) | 5 (9) | 1 (3) | 4 (16) |
| Cardiac index, L·min−1·m−2h | 2.7 [2.2; 3.5] | 2.8 [2.4; 3.5] | 2.5 [2.1; 2.9] |
| Cardiac index ≤ 2.5 L·min−1·m−2, N (%) | 22 (40) | 10 (33) | 12 (48) |
| Extravascular lung water index, ml·kg−1 PBW | 11 [9; 14] | 11 [9; 14] | 11 [8; 12] |
| Pulmonary vascular permeability index | 2 [1.7; 2.6] | 2 [1.8; 2.6] | 2.3 [1.7; 2.8] |
| Preload dependence identified by the postural maneuver, N (%)i | 17 (31) | 9 (30) | 8 (32) |
| Arterial lactate, mmol·L−1 | 1.8 [1.3; 3.2] | 1.8 [1.3; 3.8] | 1.9 [1.4; 3.2] |
| Arterial lactate > 2 mmol·L−1, N (%) | 21 (38) | 10 (33) | 11 (44) |
| Norepinephrine dose (tartrate), µg·kg−1·min−1 | 0.45 [0.21; 1.05] | 0.40 [0.21; 0.83] | 0.51 [0.33; 1.5] |
| Additional vasopressor, N (%) | 5 (9) | 3 (10) | 2 (8) |
| Inotropic drug support | 4 (7) | 2 (7) | 2 (8) |
| Respiratory characteristics at inclusion | |||
| Invasive mechanical ventilation, N (%) | 50 (91) | 26 (87) | 24 (96) |
| PaO2/FiO2, mmHg | 203 [138; 318] | 209 [135; 307] | 203 [148; 326] |
| Renal and CRRT settings at inclusion | |||
| Urine output during the preceding 24H, ml·h−1·kg−1 | 0.3 [0.1; 0.4] | 0.3 [0.1; 0.4] | 0.2 [0.1; 0.4] |
| Diuretics use, N (%) | 0 (0) | 0 (0) | 0 (0) |
| CRRT modality | |||
| CVVH, N (%) | 16 (29) | 10 (33) | 6 (24) |
| CVVHD, N (%) | 28 (51) | 14 (47) | 14 (56) |
| CVVHDF, N (%) | 6 (11) | 3 (10) | 3 (12) |
| 24 h-SLED, N (%) | 5 (9) | 3 (10) | 2 (8) |
| Replacement fluid or dialysate flow rate, ml·h−1·kg−1 | 25 [23; 30] | 25 [23; 32] | 26 [23; 30] |
| UFNET flow rate, ml·h−1 | 80 [0; 200] | 140 [0; 240] | 50 [0; 125] |
| UFNET flow rate, ml·h−1·kg−1 | 1.1 [0; 2.5] | 1.2 [0; 2.5] | 0.7 [0; 2.2] |
| Fluid balance at inclusion | |||
| Weight at inclusion, kg | 79 [72; 98] | 83 [75; 103] | 74 [71; 92] |
| Fluid balance between ICU admission and inclusion, L | 2 [−3; 5] | 3 [−2; 7] | 2 [−3; 3] |
| Fluid overload, N (%)j | 9 (16) | 7 (23) | 2 (10) |
| Delay between ICU admission and CRRT, days | 4 [2; 7] | 4 [2; 7] | 4 [2; 7] |
| Delay between CRRT initiation and inclusion, hours | 15 [5; 20] | 16 [7; 22] | 13 [4; 18] |
Data is presented with median [interquartile range] or count N (percentage)
CRRT continuous renal replacement therapy, CVVH continuous veno-venous hemofiltration, CVVHD continuous veno-venous hemodialysis, CVVHDF continuous veno-venous hemodiafiltration, ICU intensive care unit, PaO2/FiO2 ratio of the oxygen arterial partial pressure over the inspired fraction of oxygen, PBW predicted body weight, SAPS-2 simplified acute physiology score-2, SLED sustained low-efficiency dialysis, SOFA sepsis-related organ failure assessment, UFNET net ultrafiltration
aDefined as a history of hospital admission for congestive heart failure in the previous 12 months or a left ventricle ejection fraction < 45% before ICU admission
bDefined as a history of home non-invasive ventilation, home oxygen therapy, known GOLD stage IV chronic obstructive pulmonary disease, or an arterial partial pressure in O2 < 60 mmHg at room air
cDefined a glomerular filtration rate < 60 ml·min−1.1.73 m−2 prior to ICU admission
dIn case of a missing baseline creatinine, it was estimated using backward calculation by the Modified diet in renal disease formula with glomerular filtration rate of 75 ml·min−1·1.73 m−2
eNumber of missing baseline creatinine: 36/55
fDefined as a history of human immunodeficiency infection, hematological malignancies under treatment, chemotherapy in the previous 6 months, immunotherapy in the previous 6 months, or chronic steroid treatment > 7.5 mg of prednisone equivalent per day
gDefined as per the Sepsis-III consensus definition
hOne missing cardiac index value at baseline
i13/55 missing preload dependence evaluations at inclusion
jDefined as an increased in body weight > 10% between ICU admission and study inclusion
Primary outcome (mITT population)
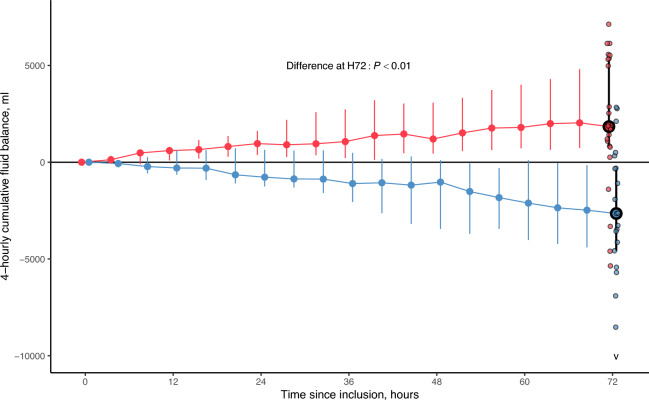
The mITT population comprised 24 patients in the control group and 21 patients in the intervention arm (Fig. 2). The characteristics of the mITT population and that of the ITT excluded from mITT are given in supplemental Table 2 (ESM13). The two reasons for exclusion from the mITT population were death before H72 (N = 8) and CRRT suspension for more than 8 h (N = 2, both patients being definitely weaned from renal replacement therapy). H72 cumulative FB was significantly higher in controls, as compared to the intervention (1841 [821; 5327] ml vs. −2650 [−4574; −309] ml, P < 0.01, Table 2 and Fig. 3).
Table 2.
Clinical outcomes
| Control | Intervention | Between group difference (95% confidence interval) | P value | |
|---|---|---|---|---|
| Primary outcome (mITT population) | N = 24 | N = 21 | ||
| Cumulative fluid balance at H72, ml | 1841 [821; 5327] | −2650 [−4574; -309] | 4942 (2736 to 6902) | < 0.01 |
| Safety analysis (ITT population) | N = 30 | N = 25 | ||
| Cumulative number of HIRRT episodes per patient, N (%) | 1 [0; 3] | 2 [1; 4] | −1 (−2 to 0) | 0.23 |
| Cumulative number of HIRRT episodes associated with preload dependence, N (%)a | NA | 28/63 (44%) | NA | NA |
| Vasopressor-free days at day 28, daysb | 0 [0; 17] | 0 [0; 0] | 0 (0 to 0) | 0.27 |
| Vital status | ||||
| Death at H72, N (%) | 4/30 (13%) | 4/25 (16%) | −3% (−22% to 16%) | > 0.99 |
| Death at day 28, N (%)c | 17/30 (57%) | 16/25 (64%) | −7% (−32% to 18%) | > 0.99 |
| Death at day 90, N (%)c | 18/30 (60%) | 17/25 (68%) | −8% (−32% to 17%) | > 0.99 |
| Efficacy analysis (ITT population) | N = 30 | N = 25 | ||
| Normalized cumulative fluid balance, ml·h−1d | ||||
| At H24e | 48 [15; 79] | −16 [−51; 32] | 60 (17–97) | 0.01 |
| At H72f | 26 [8; 69] | −27 [−59; 29] | 51 (18–82) | 0.01 |
| Normalized cumulative UFNET, ml·h−1,d | ||||
| At H24e | 12 [3; 20] | 90 [44; 120] | −58 (−86 to −33) | < 0.01 |
| At H72f | 17 [7; 36] | 78 [47; 107] | −50 (−76 to −29) | < 0.01 |
| Ventilator-free days at day 28, daysb | 0 [0; 17] | 0 [0; 9] | 0 (0 to 0) | 0.43 |
| MAKE-90, N (%)g,h | 22/30 (73%) | 22/25 (88%) | −15% (−34% to 7%) | 0.31 |
| RRT dependence at day 90, N (%) | 1/30 (3%) | 4/25 (16%) | −13% (−29% to 5%) | 0.17 |
| Persistent renal dysfunction at day 90, N (%)i | 3/30 (10%) | 1/25 (4%) | −6% (−10 to 20%) | 0.62 |
| ICU length of stay, daysj | 15 [8; 22] | 9 [6; 18] | 2 (−3 to 8) | 0.46 |
| ICU length of stay in alive patients at time of ICU discharge (N = 25), days | 16 [9; 24] | 10 [8; 21] | 3 (−9 to 12) | 0.58 |
| Hospital length of stay, daysk | 19 [9; 25] | 15 [7; 34] | −1 (−10 to 8) | 0.90 |
| Hospital length of stay in alive patients at time of hospital discharge (N = 25), days | 26 [16; 44] | 34 [23; 63] | −9 (−40 to 16) | 0.42 |
Data is presented with median [interquartile range] or count N/N total (percentage)
AKI acute kidney injury, HIRRT hemodynamic instability related to renal replacement therapy, ICU intensive care unit, ITT intention-to-treat, MAKE major adverse kidney events, mITT modified intention-to-treat, NA not applicable, RRT renal replacement therapy, UFNET net ultrafiltration
a4/63 missing preload dependence evaluations
bMissing value in two patients
cMissing vital status at day 28 and day 90 in two patients of the control group
dThe cumulative fluid balance and UFNET were normalized to the duration of follow-up up to the time point or death or end of follow-up, whichever occurred first
eOne patient died before H24
fEight patients died before H72
gMAKE-90 consisted in the occurrence of either death at day 90, RRT dependence at day 90 or persistence of renal dysfunction (100% increase in baseline serum creatinine) at day 90
hMissing value in two patients
iRenal function at day 90 was evaluated using a serum creatinine measurement performed at day 90 ± 7. In case of a missing value, the serum creatinine measured at time of ICU discharge in alive patients weaned from RRT was used (estimated creatinine values N = 8/13)
jMissing value in three patients
kMissing value in four patients
Fig. 3.
Cumulative fluid balance measured between H0 and H72 in each study group of the mITT population. The figure shows the cumulative fluid balance in the two groups (controls in red, intervention in blue) over time, between inclusion (H0) up to H72 in the modified ITT population (N = 45). Data is presented using the median value (large dots) of the cumulative fluid balance at each time point, with its interquartile range (vertical bars). At H72, the primary outcome measure of the trial is identified with the large dots circled in black. Individual values of the H72 fluid balance in each study group are also shown (small dots). The “v” symbol at H72 in the intervention arm indicates an outlier value not represented in the figure (H72 fluid balance in this patient = −23,636 ml). The P value evaluates the difference in the cumulative fluid balance at H72 (identified with large data points circled in black) using the Wilcoxon–Mann–Whitney rank-sum test. mITT modified intention-to-treat
Efficacy secondary outcomes (ITT population)
Over the 72 h after inclusion, UFNET flow rate was significantly lower in the control arm, compared to the intervention (supplemental Fig. 2—ESM14). In the intervention arm, UFNET was decreased or suspended in 94/378 4-hourly routine observations (25%), due to orange or red hemodynamic profiles (supplemental Fig. 3—ESM15). The description of 4-hourly hemodynamics is given in supplemental Table 3 (ESM16), and hemodynamic protocol’s observed components and profile letters in supplemental Fig. 4 (ESM17).
The cumulative FB and cumulative fluid inputs and outputs are shown in supplemental Fig. 5 (ESM18) and supplemental Fig. 6 (ESM19). Normalized cumulative FB was significantly higher, and UFNET significantly lower in controls at H24 and H72, compared to the intervention (Table 2). Supplemental Table 4 (ESM20) shows the normalized FB at H72 in survivors and non-survivors, based on their allocation group.
PaO2/FiO2 ratio, extravascular lung water, ventilator-free days at day 90, MAKE90 and ICU and hospital lengths of stay did not significantly differ between arms (Table 2 and supplemental Fig. 7—ESM21).
Subgroup analyses did not identify subgroups with significant differences regarding the primary outcome (supplemental Fig. 8—ESM22).
Safety secondary outcomes (ITT population)
The number of HIRRT episodes did not significantly differ between groups (1 [0; 3] vs. 2 [1; 4] episodes per patient, Table 2 and supplemental Fig. 9—ESM23). The prevalence of preload-dependent HIRRT was 44% (95% confidence interval: 39%; 66%) in the intervention arm (Table 2, supplemental Table 5—ESM24 for the description of HIRRT episodes). HIRRT led to a substantial increase in red profiles’ rate at the following 4-hourly observation (supplemental Fig. 10—ESM25).
Longitudinal hemodynamics, vasopressor dose, arterial lactate, and vasopressor-free days did not differ between arms (supplemental Fig. 11—ESM26, supplemental Table 6—ESM27), as did the mortality at H72, day 28 and day 90 (day-90 mortality: 60% vs. 68%, Table 2). Total SOFA was lower in controls at H48 and H72, compared to the intervention, with no difference in its cardiovascular component (supplemental Fig. 12—ESM28). Severe adverse events are reported in supplemental Table 7—ESM29.
Discussion
In this multicenter, randomized, controlled, proof-of-concept trial, a UFNET strategy secured by a hemodynamic protocol using dynamic indices of preload dependence allowed a significant decrease in H72 cumulative FB in patients with vasopressors, compared to standard of care, without significant increase in HIRRT episodes.
Non-interventional studies exploring the effects of UFNET have shown mixed results on clinical outcomes [33, 45–47]. Murugan et al. reported conflicting effects of UFNET on mortality, with favorable outcome being associated with UFNET between 1.01 ml·kg−1·h−1 and 1.75 ml·kg−1·h−1 (i.e., within the UFNET range applied with the intervention) in patients with high cardiovascular SOFA [33, 45, 47].
Fluid removal within 24 h of CRRT might appear too early in hemodynamically unstable patients. Yet, the protocol allowed adjusting UFNET to their hemodynamic profile, in line with guidelines recommending to individualize deresuscitation to the circulatory status [48]. Furthermore, international surveys showed that physicians would choose a UFNET of 80 ml·h−1 in hemodynamically unstable patients, with hemodynamic status being the main criterion to adjust UFNET [21]. These elements support using advanced hemodynamic profiling to guide fluid removal.
We did not observe significant clinical benefits of the intervention although the difference in H72 FB was large (approx. 4L), lying between that of the CLASSIC trial (< 2 L) and the FACCT study (> 5 L) [7, 8]. We hypothesize that the absence of respiratory improvement may be due to moderately elevated extravascular lung water indices and/or limited fluid overload at inclusion (although similar to those reported in the STARRT-AKI and IDEAL-ICU trials) [16, 49].
Debate may arise from keeping UFNET flow rates ≤ 25 ml·h−1 in the control group, when clinicians declared in international surveys choosing non-zero UFNET in hemodynamically unstable patients although this is in shear contrast with near-zero UFNET applied to patients with vasopressors reported in recent studies [16–18, 21, 33, 49].
We report several strengths of this trial. First, the hemodynamic protocol seemed feasible, and was appropriately applied by nurses in charge, without supplemental staffing. Second, our data support the relevance of individualizing UFNET to the hemodynamic status (relevant in 25% of observations), using a limited number of parameters. Third, we used a protocolized control group which mandated near-zero UFNET to increase proof-of-concept and to limit contamination between arms [16].
We also acknowledge a number of limitations. First, hemodynamic monitoring required the full involvement of nursing staffs and their training in transpulmonary thermodilution and postural maneuvers. Also, we acknowledge the hemodynamic protocol was complex, and all of its components may not be relevant. Second, participating ICUs already had FB control strategies, implying that participants might have received a “restrictive” fluid strategy prior to enrollment (as suggested by limited fluid overload at inclusion) and during trial participation, which may explain negative H72 FB in the intervention arm, and a H72 FB in the control arm below that of our initial hypothesis. However, despite the absence of fluid overload, FB was efficiently decreased (within the range of the planned effect size), suggesting that fluid overload may have been underestimated. Third, participants represented a specific set of patients who were already monitored with CO monitoring, which delineates a potential selection bias, and limits the generalizability of our results. Fourth, staff was not blinded, which may question the primary outcome’s reliability. However, both groups received a similar amount of fluid input during participation, with a 100-ml difference between arms, demonstrating that the difference in H72 FB was intervention-related. Fifth, an intermediate primary outcome (i.e., H72 FB) was used to perform this proof-of-concept trial. However, the H72 time frame was previously identified as being the earliest FB associated with mortality [3]. Also, targeting a predicted FB to set UFNET might have been more patient-oriented, but is challenging in clinical routine and not the preferred criterion in a large international survey [21]. Finally, our study was not powered to identify an excess mortality associated with the intervention although the non-statistically higher numbers in this group, along with higher SOFA scores, stress the need for further investigations.
Conclusions
An early and active UFNET strategy secured by an advanced hemodynamic protocol using dynamic indices of preload dependence had the capacity to control H72 FB in an ICU population of patients with acute circulatory failure, compared to the standard of care.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to patients and families who allowed this study to be performed. The authors would also like to thank all ICU nursing staff and treating physicians and investigation teams of participating centers, without whom the study would not have been feasible, especially in the context of the COVID-19 pandemic. They are also grateful to Loredana Baboi for the trial coordination, the Direction de la Recherche en Santé of the Hospices Civils de Lyon, and especially Clémence Van-Boxsom and Cécile Gayet for supervising the trial, and the Centre de Recherche Clinique of the Croix Rousse hospital, especially Sylvie Thevenon for her precious help in designing the case report form and her vigilant data management.
Author contributions
LB and JCR designed the study, collected the data, interpreted the results and drafted the manuscript. LB accessed and verified the underlying data. LB and PP wrote the statistical analysis plan and performed the statistical analysis. PP also interpreted the results, and revised the manuscript for important intellectual content. CD, GD, KK, MM, LC, HY, JI and BS collected the data, interpreted the results, and revised the manuscript for important intellectual content. LB obtained the funding. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Funding
Open access funding provided by Hospices Civils de Lyon. Programme Hospitalier de Recherche Clinique Inter-régional (PHRC-I, 2019, recipient: LB) of the Direction Générale de l’Offre de Soin (French Ministry of Health). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data availability
The trial protocol was published prior to the end of participants’ enrollment [25], and the statistical analysis plan written prior to database lock and is available in the Online Supplement. Source datasets are not publicly available due to ethical reasons. Further enquiries can be directed to the corresponding author at laurent.bitker@chu-lyon.fr. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Declarations
Conflicts of interest
LB is the recipient of the study’s funding grant from the Programme Hospitalier de Recherche Clinique Inter-régional (PHRC-I 2019) from the Direction Générale de l’Offre de Soin (French Ministry of Health). JI declares registration and travel fees for congress attendance payed by Pfizer. J-CR declares registration and travel fees for congress attendance payed by Pfizer. All other authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and with local regulations. The study was registered at ClinicalTrials.gov (NCT04801784) before the first patient was enrolled. The study protocol was reviewed and approved by an ethics committee for human research (Comité de Protection des Personnes Sud Méditerranée I, IDCRB 2021-A00692-39).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schortgen F, Tabra Osorio C, Carpentier D, Henry M, Beuret P, Lacave G, Simon G, Blanchard PY, Gobe T, Guillon A, Bitker L, Duhommet G, Quenot JP, Le Meur M, Jochmans S, Dubouloz F, Mainguy N, Saletes J, Creutin T, Nicolas P, Senay J, Berthelot AL, Rizk D, Van Tran D, Riviere A, Heili-Frades SB, Nunes J, Robquin N, Lhotellier S, Ledochowski S, Guenegou-Arnoux A, Constan A (2023) Fluid intake in critically ill patients: the “save useless fluids for intensive resuscitation” multicenter prospective cohort study. Crit Care Med 52:258–267 [DOI] [PubMed] [Google Scholar]
- 2.Claure-Del Granado R, Mehta RL (2016) Fluid overload in the ICU: evaluation and management. BMC Nephrol 17:109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messmer AS, Zingg C, Muller M, Gerber JL, Schefold JC, Pfortmueller CA (2020) Fluid overload and mortality in adult critical care patients-a systematic review and meta-analysis of observational studies. Crit Care Med 48:1862–1870 [DOI] [PubMed] [Google Scholar]
- 4.Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, Parviainen I, Pettila V, Group FS (2012) Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit care 16:R197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodward CW, Lambert J, Ortiz-Soriano V, Li Y, Ruiz-Conejo M, Bissell BD, Kelly A, Adams P, Yessayan L, Morris PE, Neyra JA (2019) Fluid overload associates with major adverse kidney events in critically ill patients with acute kidney injury requiring continuous renal replacement therapy. Crit Care Med 47:e753–e760 [DOI] [PubMed] [Google Scholar]
- 6.Silversides JA, Pinto R, Kuint R, Wald R, Hladunewich MA, Lapinsky SE, Adhikari NK (2014) Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Crit care 18:624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NHLB Institute, ARDSCT Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, de Boisblanc B, Connors AF Jr, Hite RD, Harabin AL (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 8.Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, Jakob SM, Cecconi M, Nalos M, Ostermann M, Malbrain M, Pettila V, Moller MH, Kjaer MN, Lange T, Overgaard-Steensen C, Brand BA, Winther-Olesen M, White JO, Quist L, Westergaard B, Jonsson AB, Hjortso CJS, Meier N, Jensen TS, Engstrom J, Nebrich L, Andersen-Ranberg NC, Jensen JV, Joseph NA, Poulsen LM, Herlov LS, Solling CG, Pedersen SK, Knudsen KK, Straarup TS, Vang ML, Bundgaard H, Rasmussen BS, Aagaard SR, Hildebrandt T, Russell L, Bestle MH, Schonemann-Lund M, Brochner AC, Elvander CF, Hoffmann SKL, Rasmussen ML, Martin YK, Friberg FF, Seter H, Aslam TN, Adnoy S, Seidel P, Strand K, Johnstad B, Joelsson-Alm E, Christensen J, Ahlstedt C, Pfortmueller CA, Siegemund M, Greco M, Radej J, Kriz M, Gould DW, Rowan KM, Mouncey PR, Perner A, Group CT (2022) Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med 386:2459–2470 [DOI] [PubMed] [Google Scholar]
- 9.Bollaert PE, Monnier A, Schneider F, Argaud L, Badie J, Charpentier C, Meziani F, Bemer M, Quenot JP, Buzzi M, Outin H, Bruel C, Ziegler L, Gibot S, Virion JM, Alleyrat C, Louis G, Agrinier N (2023) Fluid balance control in critically ill patients: results from POINCARE-2 stepped wedge cluster-randomized trial. Crit care 27:66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corl KA, Prodromou M, Merchant RC, Gareen I, Marks S, Banerjee D, Amass T, Abbasi A, Delcompare C, Palmisciano A, Aliotta J, Jay G, Levy MM (2019) The restrictive IV fluid trial in severe sepsis and septic shock (RIFTS): a randomized pilot study. Crit Care Med 47:951–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prowle J, Mehta R (2021) Fluid balance management during continuous renal replacement therapy. Semin Dial 34:440–448 [DOI] [PubMed] [Google Scholar]
- 12.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R (2010) Fluid balance and acute kidney injury. Nat Rev Nephrol 6:107–115 [DOI] [PubMed] [Google Scholar]
- 13.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL (2008) Sepsis occurrence in acutely ill patients I, a positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit care 12:R74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaara ST, Ostermann M, Bitker L, Schneider A, Poli E, Hoste E, Fierens J, Joannidis M, Zarbock A, van Haren F, Prowle J, Selander T, Bäcklund M, Pettilä V, Bellomo R (2021) Restrictive fluid management versus usual care in acute kidney injury (REVERSE-AKI): a pilot randomized controlled feasibility trial. Intensive Care Med 47:665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N, Jiang L, Zhu B, Wen Y, Xi XM (2015) Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit care 19:371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyere R, Lebert C, Bohe J, Badie J, Eraldi JP, Rigaud JP, Levy B, Siami S, Louis G, Bouadma L, Constantin JM, Mercier E, Klouche K, du Cheyron D, Piton G, Annane D, Jaber S, van der Linden T, Blasco G, Mira JP, Schwebel C, Chimot L, Guiot P, Nay MA, Meziani F, Helms J, Roger C, Louart B, Trusson R, Dargent A, Binquet C, Quenot JP, I-IT Investigators, the CTN (2018) Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 379:1431–144230304656 [Google Scholar]
- 17.Wald R, Kirkham B, daCosta BR, Ghamarian E, Adhikari NKJ, Beaubien-Souligny W, Bellomo R, Gallagher MP, Goldstein S, Hoste EAJ, Liu KD, Neyra JA, Ostermann M, Palevsky PM, Schneider A, Vaara ST, Bagshaw SM (2022) Fluid balance and renal replacement therapy initiation strategy: a secondary analysis of the STARRT-AKI trial. Crit care 26:360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White KC, Laupland KB, Ostermann M, Neto AS, Gatton ML, Hurford R, Clement P, Sanderson B, Bellomo R (2024) Current fluid management practice in critically ill adults on continuous renal replacement therapy: a binational, observational study. Blood Purif 53:624–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douvris A, Malhi G, Hiremath S, McIntyre L, Silver SA, Bagshaw SM, Wald R, Ronco C, Sikora L, Weber C, Clark EG (2018) Interventions to prevent hemodynamic instability during renal replacement therapy in critically ill patients: a systematic review. Crit care 22:41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douvris A, Zeid K, Hiremath S, Bagshaw SM, Wald R, Beaubien-Souligny W, Kong J, Ronco C, Clark EG (2019) Mechanisms for hemodynamic instability related to renal replacement therapy: a narrative review. Intensive Care Med 45:1333–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murugan R, Ostermann M, Peng Z, Kitamura K, Fujitani S, Romagnoli S, Di Lullo L, Srisawat N, Todi S, Ramakrishnan N, Hoste E, Puttarajappa CM, Bagshaw SM, Weisbord S, Palevsky PM, Kellum JA, Bellomo R, Ronco C (2020) Net ultrafiltration prescription and practice among critically ill patients receiving renal replacement therapy: a multinational survey of critical care practitioners. Crit Care Med 48:e87–e97 [DOI] [PubMed] [Google Scholar]
- 22.Chazot G, Bitker L, Mezidi M, Chebib N, Chabert P, Chauvelot L, Folliet L, David G, Provoost J, Yonis H, Richard JC (2021) Prevalence and risk factors of hemodynamic instability associated with preload-dependence during continuous renal replacement therapy in a prospective observational cohort of critically ill patients. Ann Intensive Care 11:95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitker L, Bayle F, Yonis H, Gobert F, Leray V, Taponnier R, Debord S, Stoian-Cividjian A, Guérin C, Richard J-C (2016) Prevalence and risk factors of hypotension associated with preload-dependence during intermittent hemodialysis in critically ill patients. Crit Care 20:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monnet X, Cipriani F, Camous L, Sentenac P, Dres M, Krastinova E, Anguel N, Richard C, Teboul JL (2016) The passive leg raising test to guide fluid removal in critically ill patients. Ann Intensive Care 6:46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitker L, Pradat P, Dupuis C, Klouche K, Illinger J, Souweine B, Richard JC (2022) Fluid balance neutralization secured by hemodynamic monitoring versus protocolized standard of care in critically ill patients requiring continuous renal replacement therapy: study protocol of the GO NEUTRAL randomized controlled trial. Trials 23:798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D, Group C (2010) CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 11:32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khwaja A (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138 [Google Scholar]
- 28.Shakur H, Roberts I, Barnetson L, Coats T (2007) Clinical trials in emergency situations. BMJ 334:165–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostermann M, Ankawi G, Cantaluppi V, Madarasu R, Dolan K, Husain-Syed F, Kashani K, Mehta R, Prowle J, Reis T, Rimmele T, Zarbock A, Kellum JA, Ronco C, Nomenclature Standardization Faculty (2023) Nomenclature of extracorporeal blood purification therapies for acute indications: the nomenclature standardization conference. Blood Purif 53:358–372 [DOI] [PubMed] [Google Scholar]
- 30.Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG (2018) Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med 44:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL (2006) Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 34:1402–1407 [DOI] [PubMed] [Google Scholar]
- 32.Guerin L, Monnet X, Teboul JL (2013) Monitoring volume and fluid responsiveness: from static to dynamic indicators. Best Pract Res Clin Anaesthesiol 27:177–185 [DOI] [PubMed] [Google Scholar]
- 33.Serpa Neto A, Naorungroj T, Murugan R, Kellum JA, Gallagher M, Bellomo R (2021) Heterogeneity of effect of net ultrafiltration rate among critically ill adults receiving continuous renal replacement therapy. Blood Purif 50:336–346 [DOI] [PubMed] [Google Scholar]
- 34.Yonis H, Bitker L, Aublanc M, Perinel Ragey S, Riad Z, Lissonde F, Louf-Durier A, Debord S, Gobert F, Tapponnier R, Guérin C, Richard J-C (2017) Change in cardiac output during Trendelenburg maneuver is a reliable predictor of fluid responsiveness in patients with acute respiratory distress syndrome in the prone position under protective ventilation. Crit Care. [DOI] [PMC free article] [PubMed]
- 35.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med 22:707–710 [DOI] [PubMed] [Google Scholar]
- 36.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, Devaquet J, Nseir S, Razazi K, Mira J-P, Argaud L, Chakarian J-C, Ricard J-D, Wittebole X, Chevalier S, Herbland A, Fartoukh M, Constantin J-M, Tonnelier J-M, Pierrot M, Mathonnet A, Béduneau G, Delétage-Métreau C, Richard J-CM, Brochard L, Robert R (2015) High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 372:2185–2196 [DOI] [PubMed] [Google Scholar]
- 37.De Corte W, Dhondt A, Vanholder R, De Waele J, Decruyenaere J, Sergoyne V, Vanhalst J, Claus S, Hoste EA (2016) Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: a prospective cohort study. Crit care 20:256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meersch M, Kullmar M, Schmidt C, Gerss J, Weinhage T, Margraf A, Ermert T, Kellum JA, Zarbock A (2017) Long-term clinical outcomes after early initiation of RRT in critically ill patients with AKI. J Am Soc Nephrol 29:1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW (2019) Re-appraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med 200:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeda A, Inokuchi R, Bellomo R, Doi K (2024) Heterogeneity in the definition of major adverse kidney events: a scoping review. Intensive care med 50:1049–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vinsonneau C, Allain-Launay E, Blayau C, Darmon M, Ducheyron D, Gaillot T, Honore PM, Javouhey E, Krummel T, Lahoche A, Letacon S, Legrand M, Monchi M, Ridel C, Robert R, Schortgen F, Souweine B, Vaillant P, Velly L, Osman D, Van Vong L (2015) Renal replacement therapy in adult and pediatric intensive care: recommendations by an expert panel from the French Intensive Care Society (SRLF) with the French Society of Anesthesia Intensive Care (SFAR) French Group for Pediatric Intensive Care Emergencies (GFRUP) the French Dialysis Society (SFD). Ann Intensive Care 5:58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cnaan A, Laird NM, Slasor P (1997) Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 16:2349–2380 [DOI] [PubMed] [Google Scholar]
- 43.Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48 [Google Scholar]
- 44.R Development Core Team (2008) R: a language and environment for statistical computing. ISBN: 3-900051-07-0. http://www.R-project.org/
- 45.Murugan R, Balakumar V, Kerti SJ, Priyanka P, Chang CH, Clermont G, Bellomo R, Palevsky PM, Kellum JA (2018) Net ultrafiltration intensity and mortality in critically ill patients with fluid overload. Crit Care 22:223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murugan R, Hoste E, Mehta RL, Samoni S, Ding X, Rosner MH, Kellum JA, Ronco C, ADQI Consensus Group (2016) Precision fluid management in continuous renal replacement therapy. Blood Purif 42:266–278 [DOI] [PubMed] [Google Scholar]
- 47.Murugan R, Kerti SJ, Chang CH, Gallagher M, Clermont G, Palevsky PM, Kellum JA, Bellomo R (2019) Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a secondary analysis of the randomized evaluation of normal vs augmented level (RENAL) of renal replacement therapy trial. JAMA Netw Open 2:e195418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N (2014) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 46:361–380 [DOI] [PubMed] [Google Scholar]
- 49.Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R, da Costa BR, Dreyfuss D, Du B, Gallagher MP, Gaudry S, Hoste EA, Lamontagne F, Joannidis M, Landoni G, Liu KD, McAuley DF, McGuinness SP, Neyra JA, Nichol AD, Ostermann M, Palevsky PM, Pettila V, Quenot JP, Qiu H, Rochwerg B, Schneider AG, Smith OM, Thome F, Thorpe KE, Vaara S, Weir M, Wang AY, Young P, Zarbock A, STARRT-AKI Investigators, Canadian Critical Care Trials Group, Australian, New Zealand Intensive Care Society Clinical Trials Group, United Kingdom Critical Care Research Group, Canadian Nephrology Trials Network, Irish Critical Care Trials Group (2020) Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med 383:240–25132668114 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The trial protocol was published prior to the end of participants’ enrollment [25], and the statistical analysis plan written prior to database lock and is available in the Online Supplement. Source datasets are not publicly available due to ethical reasons. Further enquiries can be directed to the corresponding author at laurent.bitker@chu-lyon.fr. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.