Abstract
Background
Previously reported results from phase III KEYNOTE-048 demonstrated similar or improved overall survival (OS) with pembrolizumab or pembrolizumab-chemotherapy versus cetuximab-chemotherapy (EXTREME) in Japanese patients with recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). We report results in Japanese patients from KEYNOTE-048 after 5 years of follow-up.
Methods
Patients with R/M HNSCC of the oropharynx, oral cavity, hypopharynx, or larynx were randomly assigned 1:1:1 to pembrolizumab, pembrolizumab-chemotherapy, or EXTREME. Primary endpoints were OS and progression-free survival. Efficacy was evaluated in the programmed cell death ligand 1 (PD-L1) combined positive score (CPS) ≥ 20, PD-L1 CPS ≥ 1, and total Japanese populations.
Results
In Japan, 67 patients were enrolled (pembrolizumab, n = 23; pembrolizumab-chemotherapy, n = 25; EXTREME, n = 19). Median follow-up was 71.0 months (range, 61.2–81.5); data cutoff, February 21, 2022. 5-year OS rates with pembrolizumab versus EXTREME were 35.7% versus 12.5% (hazard ratio [HR] 0.38; 95% CI 0.13–1.05), 23.8% versus 12.5% (HR 0.70; 95% CI 0.34–1.45), and 30.4% versus 10.5% (HR 0.54; 95% CI 0.27–1.07) in the PD-L1 CPS ≥ 20, CPS ≥ 1, and total Japanese populations, respectively. 5-year OS rates with pembrolizumab-chemotherapy versus EXTREME were 20.0% versus 14.3% (HR 0.79; 95% CI 0.27–2.33), 10.5% versus 14.3% (HR 1.18; 95% CI 0.56–2.48), and 8.0% versus 12.5% (HR 1.11; 95% CI 0.57–2.16) in the PD-L1 CPS ≥ 20, CPS ≥ 1, and total Japanese populations, respectively.
Conclusion
After 5 years of follow-up, pembrolizumab and pembrolizumab-chemotherapy showed long-term clinical benefits; results further support these treatments as first-line options for Japanese patients with R/M HNSCC.
Clinical trial registration
Supplementary Information
The online version contains supplementary material available at 10.1007/s10147-024-02632-x.
Keywords: Recurrent/metastatic head and neck squamous cell carcinoma, Pembrolizumab, PD-L1, Immunotherapy, EXTREME
Introduction
Head and neck squamous cell carcinoma (HNSCC) accounts for the majority of malignant tumors of the head and neck and are prevalent in Eastern Asia [1–4]. The epidermal growth factor receptor (EGFR) inhibitor cetuximab in combination with chemotherapy (platinum and 5-fluorouracil; EXTREME) was, until recently, the first-line standard of care for patients with recurrent or metastatic (R/M) HNSCC in many countries, including Japan [5–9]. The emergence of immune checkpoint inhibitors, however, has resulted in a paradigm shift in the management of patients with R/M HNSCC in both the first- and second-line treatment settings [10–12].
KEYNOTE-048 was a global phase III study evaluating pembrolizumab monotherapy and with chemotherapy (pembrolizumab-chemotherapy) versus EXTREME in patients with R/M HNSCC [12]. Pembrolizumab monotherapy significantly improved overall survival (OS) compared with EXTREME in patients with programmed cell death ligand 1 (PD-L1) combined positive score (CPS) ≥ 20 and CPS ≥ 1 and was associated with non-inferior OS in the total population. Pembrolizumab-chemotherapy significantly prolonged OS compared with EXTREME in all PD-L1 populations [12]. Based on these results, pembrolizumab was approved in Japan as a first-line treatment option as monotherapy or in combination with platinum and 5-fluorouracil for all patients with R/M HNSCC, regardless of PD-L1 CPS [13].
Given the high incidence of HNSCC in Japan [1, 3], it was important to investigate the efficacy and safety of pembrolizumab with or without chemotherapy in patients enrolled in KEYNOTE-048 from Japanese clinical centers [13]. With a follow-up of approximately 3 years, pembrolizumab improved median OS versus EXTREME in the PD-L1 CPS ≥ 20 population (28.2 versus 13.3 months; hazard ratio [HR], 0.29 [95% confidence interval (CI), 0.09–0.89]) and had similar OS in the PD-L1 CPS ≥ 1 and total Japanese populations [13]. Treatment with pembrolizumab-chemotherapy resulted in similar OS versus EXTREME in all populations.
Longer-term follow-up data from KEYNOTE-048 remain of interest to determine the durability of the OS benefit in patients with R/M HNSCC enrolled in Japan. Here, we report survival results after 5 years of follow-up in the Japanese population of KEYNOTE-048.
Materials and methods
Study design and participants
The design and methods of the open-label phase III KEYNOTE-048 study (ClinicalTrials.gov identifier NCT02358031) have been reported previously [12, 13]. Eligible patients were aged ≥ 18 years with previously untreated R/M squamous cell carcinoma of the oropharynx, oral cavity, hypopharynx, or larynx that was incurable by local therapy; had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; had measurable disease per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1); and had known p16 expression for oropharyngeal cancers (non-oropharyngeal cancers were considered human papillomavirus [HPV] negative). Patients were randomly assigned 1:1:1 to pembrolizumab alone, pembrolizumab-chemotherapy, or EXTREME and stratified by percentage of tumor cells expressing PD-L1 (≥ 50% vs < 50%), HPV status for oropharyngeal cancers (p16 positive vs. p16 negative), and ECOG PS (0 vs 1).
The study protocol and amendments were approved by the appropriate institutional review boards or independent ethics committees at each center. The study was conducted in accordance with the protocol and Good Clinical Practice guidelines. All patients provided written informed consent.
Procedures
In the pembrolizumab monotherapy and pembrolizumab-chemotherapy arms, pembrolizumab (200 mg) was administered once every 3 weeks. Chemotherapy in the pembrolizumab-chemotherapy and EXTREME arms comprised carboplatin (area under the curve 5 mg/mL/min) or cisplatin (100 mg/m2) and 5-fluorouracil (1000 mg/m2 per day for four consecutive days) every 3 weeks for six cycles. Patients in the EXTREME arm also received cetuximab (400-mg/m2 loading dose, then 250 mg/m2 per week). Pembrolizumab was administered until disease progression, intolerable toxicity, physician or participant decision, or completion of 35 cycles, whichever occurred first. After six cycles of EXTREME, patients could continue cetuximab monotherapy until progression, unacceptable toxicity, or withdrawal.
Imaging (computed tomography or magnetic resonance imaging) was performed at baseline, Week 9, and every 6 weeks until Year 1 and then every 9 weeks thereafter. Response was assessed per RECIST 1.1 by blinded independent central review. Survival was assessed every 12 weeks after confirmed disease progression or start of new anticancer therapy. Patients were monitored for adverse events (AEs) throughout treatment and for 30 days after stopping treatment (90 days for serious AEs). AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Baseline PD-L1 expression was assessed in archival or newly obtained tissue samples at a central laboratory using PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Santa Clara, CA, USA). PD-L1 expression was characterized by CPS, defined as the number of PD-L1–staining cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells multiplied by 100.
Outcomes
Primary endpoints were OS and progression-free survival (PFS). OS was defined as the time from randomization to death from any cause. PFS was defined as the time from randomization to radiographically confirmed disease progression or death from any cause, whichever occurred first. Secondary endpoints included objective response rate (ORR), defined as the proportion of patients who have a complete response (CR) or partial response (PR), and safety. Duration of response (DOR), defined as the time from the first documented evidence of CR or PR until disease progression or death, was an exploratory endpoint. Efficacy was evaluated in patients with PD-L1 CPS ≥ 20, patients with PD-L1 CPS ≥ 1, and in the total Japanese population.
Statistical analysis
OS, PFS, and ORR were assessed in all patients allocated to treatment (intention-to-treat population). DOR was assessed in all patients with a confirmed CR or PR. Safety was assessed in all patients who received ≥ 1 dose of study treatment. OS, PFS, and DOR were estimated using the Kaplan–Meier method. HRs and 95% CIs were calculated using a Cox proportional hazards regression model with Efron’s method of tie handling with treatment as a covariate. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). No formal statistical hypothesis testing was performed for this subgroup analysis.
Results
Of 882 patients enrolled in KEYNOTE-048, 67 were enrolled in Japan (pembrolizumab, n = 23; pembrolizumab-chemotherapy, n = 25; EXTREME, n = 19). Patient disposition, reasons for treatment discontinuation, and baseline characteristics (including differences between the treatment groups) have been reported previously [13]. The median follow-up (time from randomization to data cutoff [February 21, 2022]) was 71.0 months (range, 61.2–81.5).
After study drug discontinuation, subsequent anticancer therapy was received by 17 (73.9%), 11 (44.0%), and 14 (73.7%) patients in the pembrolizumab, pembrolizumab-chemotherapy, and EXTREME treatment groups, respectively (Online Resource 1). A subsequent EGFR inhibitor was received by 14 (60.9%), nine (36.0%), and two (10.5%) patients in the pembrolizumab, pembrolizumab-chemotherapy, and EXTREME treatment groups, respectively. Subsequent anti–PD-(L)1 therapy was received by two (8.7%), zero, and nine (47.4%) patients in the pembrolizumab, pembrolizumab-chemotherapy, and EXTREME treatment groups, respectively.
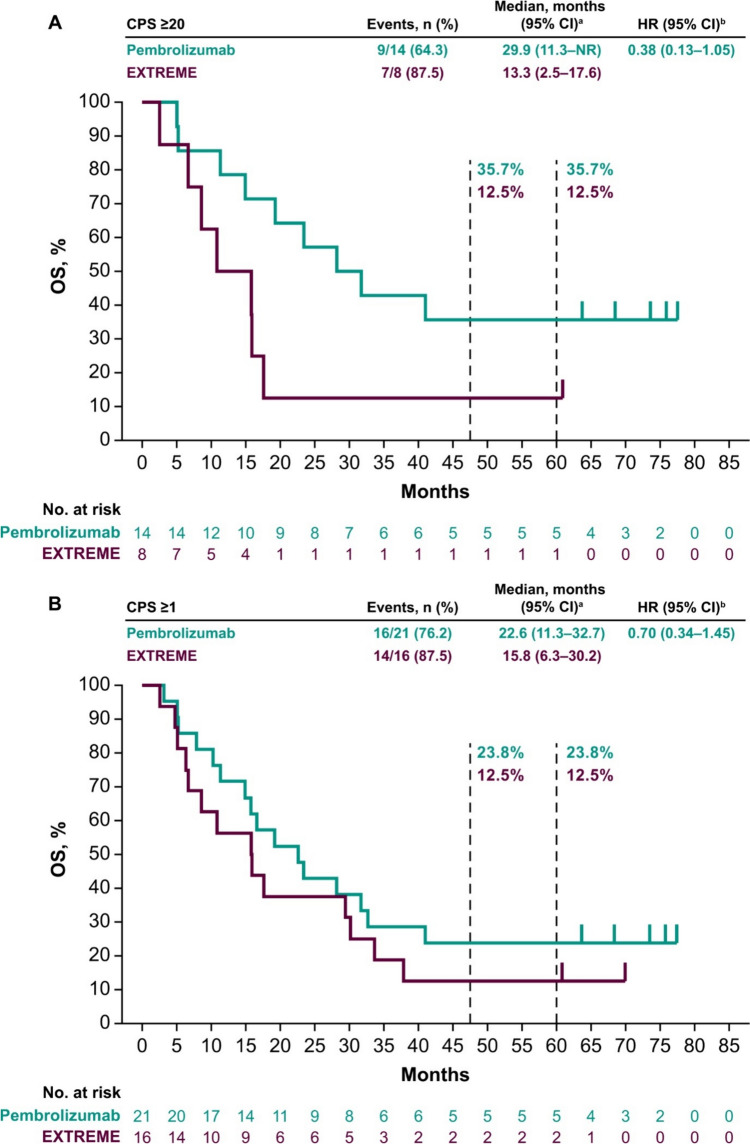
Median OS in the PD-L1 CPS ≥ 20 population was 29.9 months (95% CI 11.3 months–not reached) with pembrolizumab versus 13.3 months (95% CI 2.5–17.6 months) with EXTREME (HR 0.38; 95% CI 0.13–1.05) (Fig. 1A). In the PD-L1 CPS ≥ 1 population, median OS was 22.6 months (95% CI 11.3–32.7 months) with pembrolizumab versus 15.8 months (95% CI 6.3–30.2 months) with EXTREME (HR 0.70; 95% CI 0.34–1.45) (Fig. 1B). In the total Japanese population, median OS was 23.4 months (95% CI 14.9–41.0 months) with pembrolizumab versus 13.6 months (95% CI 6.3–29.4 months) with EXTREME (HR 0.54; 95% CI 0.27–1.07) (Fig. 1C). The 5-year OS rates with pembrolizumab versus EXTREME were 35.7% versus 12.5%, 23.8% versus 12.5%, and 30.4% versus 10.5% in the PD-L1 CPS ≥ 20, PD-L1 CPS ≥ 1, and total Japanese populations, respectively.
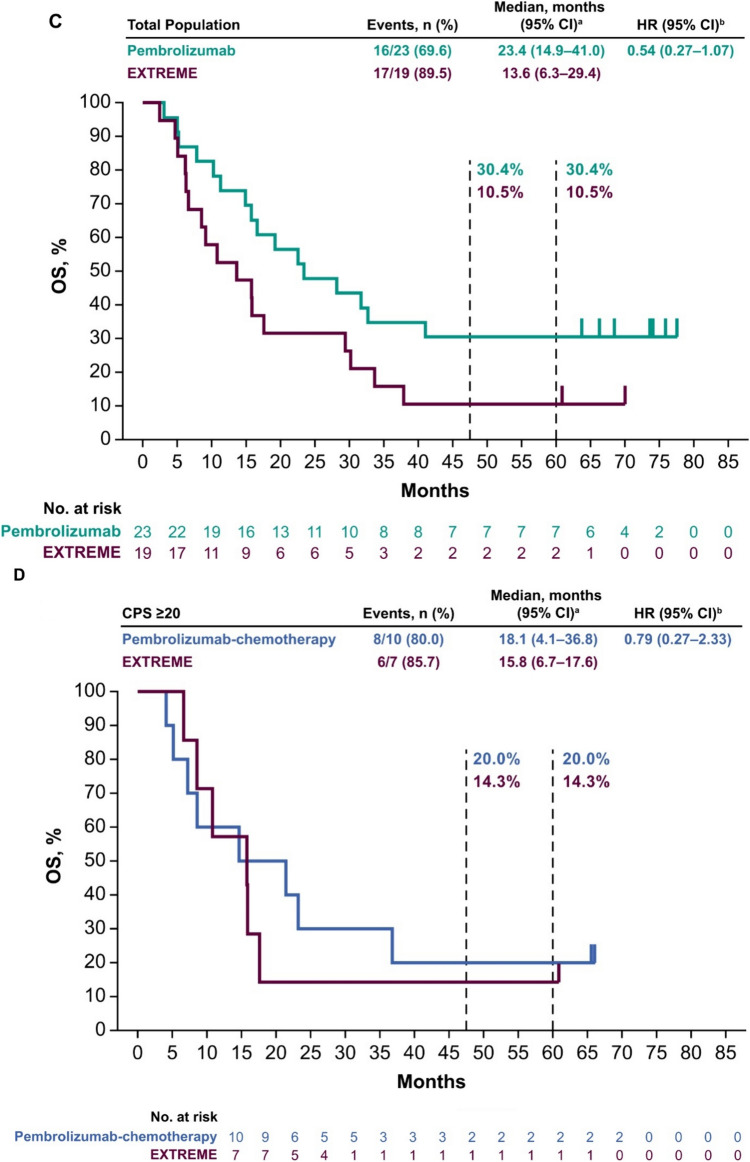
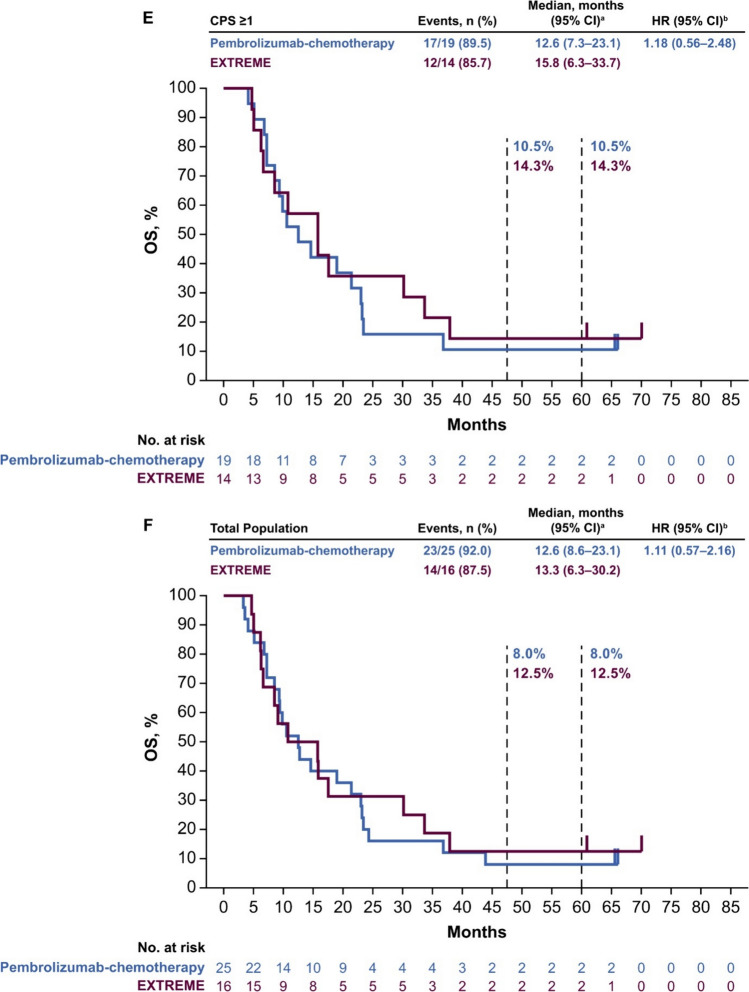
Fig. 1.
Pembrolizumab versus EXTREME in the A PD-L1 CPS ≥ 20, B PD-L1 CPS ≥ 1, and C total Japanese populations at long-term follow-up and pembrolizumab-chemotherapy versus EXTREME in the D PD-L1 CPS ≥ 20, E PD-L1 CPS ≥ 1, and F total Japanese populations. aFrom the product-limit (Kaplan–Meier) method for censored data. bOn the basis of a Cox proportional hazards regression model with the Efron method of tie handling with treatment as a covariate. CI confidence interval, CPS combined positive score, HR hazard ratio, OS overall survival, PD-L1 programmed cell death ligand 1
Median OS in the PD-L1 CPS ≥ 20 population was 18.1 months (95% CI 4.1–36.8 months) with pembrolizumab-chemotherapy versus 15.8 months (95% CI 6.7–17.6 months) with EXTREME (HR 0.79; 95% CI 0.27–2.33) (Fig. 1D). In the PD-L1 CPS ≥ 1 population, median OS was 12.6 months (95% CI 7.3–23.1 months) with pembrolizumab-chemotherapy versus 15.8 months (95% CI 6.3–33.7 months) with EXTREME (HR 1.18; 95% CI 0.56–2.48) (Fig. 1E). In the total Japanese population, median OS was 12.6 months (95% CI 8.6–23.1 months) with pembrolizumab-chemotherapy versus 13.3 months (95% CI 6.3–30.2 months) with EXTREME (HR 1.11; 95% CI 0.57–2.16) (Fig. 1F). The 5-year OS rates with pembrolizumab-chemotherapy versus EXTREME were 20.0% versus 14.3%, 10.5% versus 14.3%, and 8.0% versus 12.5% in the PD-L1 CPS ≥ 20, PD-L1 CPS ≥ 1, and total Japanese populations, respectively.
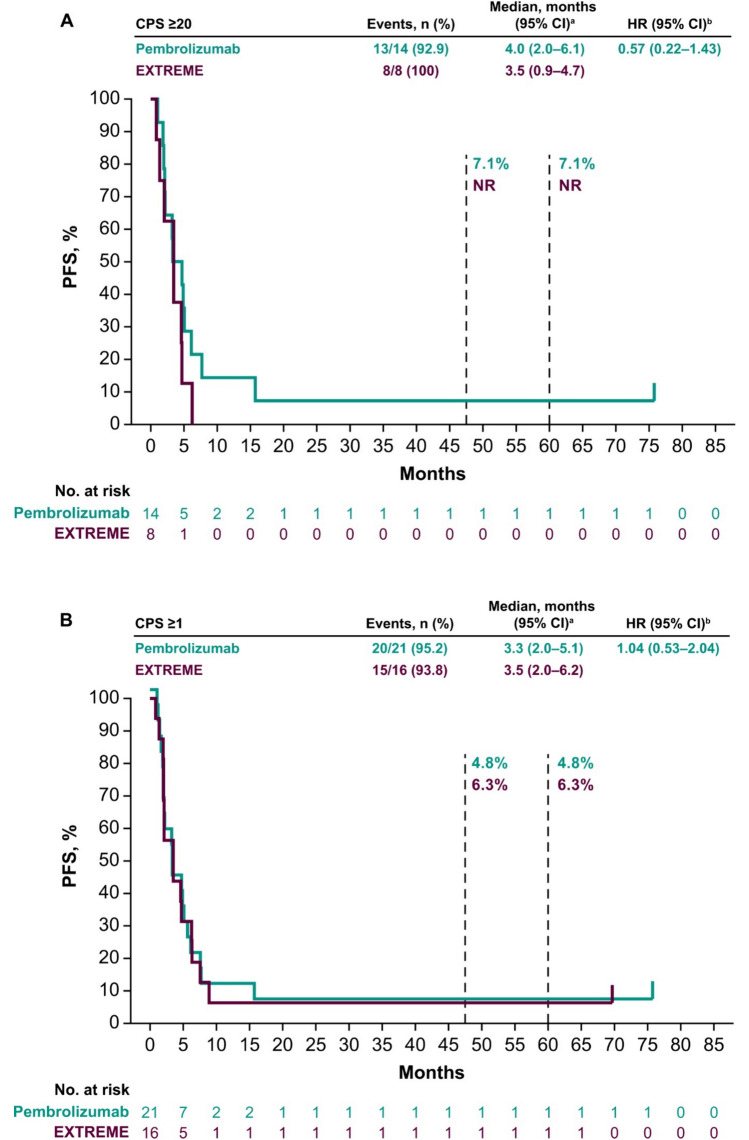
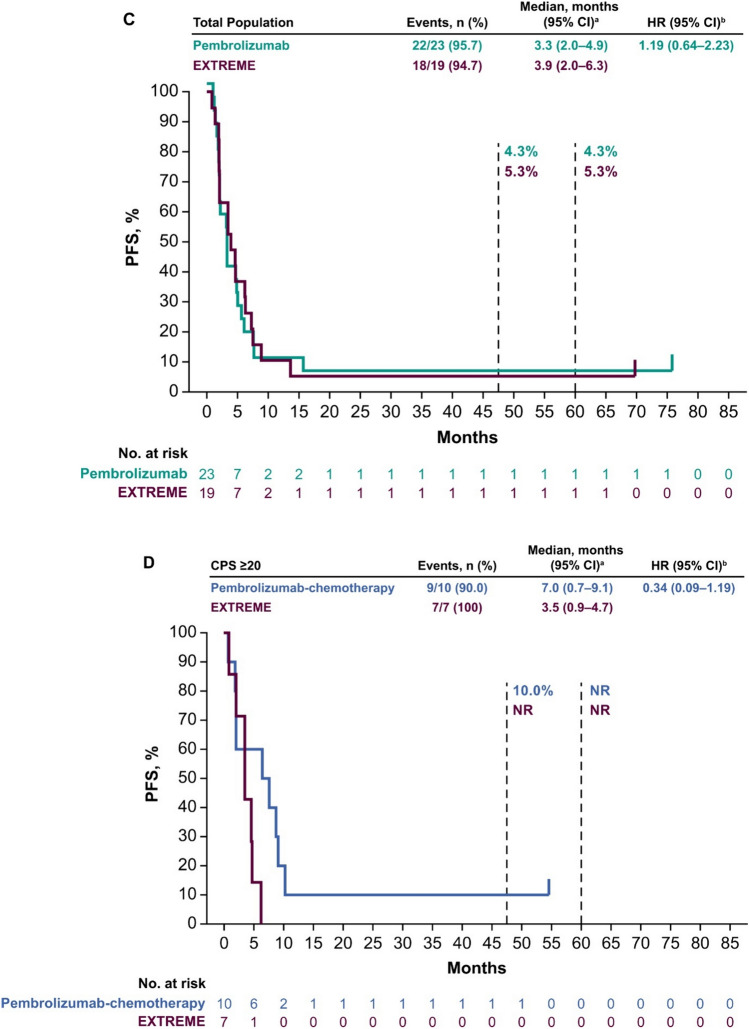
Median PFS in the PD-L1 CPS ≥ 20 population was 4.0 months (95% CI 2.0–6.1 months) with pembrolizumab versus 3.5 months (95% CI 0.9–4.7 months) with EXTREME (HR 0.57; 95% CI 0.22–1.43) (Fig. 2A). In the PD-L1 CPS ≥ 1 population, median PFS was 3.3 months (95% CI 2.0–5.1 months) with pembrolizumab versus 3.5 months (95% CI 2.0–6.2 months) with EXTREME (HR 1.04; 95% CI 0.53–2.04 months) (Fig. 2B). In the total Japanese population, median PFS was 3.3 months (95% CI 2.0–4.9 months) with pembrolizumab versus 3.9 months (95% CI 2.0–6.3 months) with EXTREME (HR 1.19; 95% CI 0.64–2.23) (Fig. 2C).
Fig. 2.
Pembrolizumab versus EXTREME in the A PD-L1 CPS ≥ 20, B PD-L1 CPS ≥ 1, and C total Japanese populations at long-term follow-up and pembrolizumab-chemotherapy versus EXTREME in the D PD-L1 CPS ≥ 20, E PD-L1 CPS ≥ 1, and F total Japanese populations. aFrom the product-limit (Kaplan–Meier) method for censored data. bOn the basis of a Cox proportional hazards regression model with the Efron’s method of tie handling with treatment as a covariate. CI confidence interval, CPS combined positive score, HR hazard ratio, PD-L1 programmed cell death ligand 1, PFS progression-free survival
Median PFS in the PD-L1 CPS ≥ 20 population was 7.0 months (95% CI 0.7–9.1 months) with pembrolizumab-chemotherapy versus 3.5 months (95% CI 0.9–4.7 months) with EXTREME (HR 0.34; 95% CI 0.09–1.19) (Fig. 2D). In the PD-L1 CPS ≥ 1 population, median PFS was 6.4 months (95% CI 2.0–8.8 months) with pembrolizumab-chemotherapy versus 3.5 months (95% CI 2.0–6.2 months) with EXTREME (HR 0.66; 95% CI 0.31–1.37) (Fig. 2E). In the total Japanese population, median PFS was 6.2 months (95% CI 2.1–7.6 months) with pembrolizumab-chemotherapy versus 3.7 months (95% CI 2.0–6.2 months) with EXTREME (HR 0.72; 95% CI 0.37–1.39) (Fig. 2F).
The ORR in the PD-L1 CPS ≥ 20 population was 28.6% (one CR, three PRs) with pembrolizumab versus 12.5% (one PR) with EXTREME, and median DOR was 8.4 versus 2.6 months (Table 1). In the PD-L1 CPS ≥ 1 population, the ORR was 19.0% (one CR, three PRs) with pembrolizumab versus 25.0% (one CR, three PRs) with EXTREME, and median DOR was 8.4 versus 5.5 months. In the total Japanese population, the ORR was 17.4% (one CR, three PRs) with pembrolizumab versus 36.8% (one CR, six PRs) with EXTREME, and median DOR was 8.4 versus 4.1 months.
Table 1.
Summary of confirmed objective response and duration of response (per RECIST 1.1 by BICR) by PD-L1 CPS population with pembrolizumab versus EXTREME in the Japanese population
| PD-L1 CPS ≥ 20 | PD-L1 CPS ≥ 1 | Total population | ||||
|---|---|---|---|---|---|---|
| Pembrolizumab (n = 14) | EXTREME (n = 8) | Pembrolizumab (n = 21) | EXTREME (n = 16) | Pembrolizumab (n = 23) | EXTREME (n = 19) | |
| ORR | 4 (28.6; 8.4–58.1) | 1 (12.5; 0.3–52.7) | 4 (19.0; 5.4–41.9) | 4 (25.0; 7.3–52.4) | 4 (17.4; 5.0–38.8) | 7 (36.8; 16.3–61.6) |
| Best overall response | ||||||
| CR | 1 (7.1) | 0 | 1 (4.8) | 1 (6.3) | 1 (4.3) | 1 (5.3) |
| PR | 3 (21.4) | 1 (12.5) | 3 (14.3) | 3 (18.8) | 3 (13.0) | 6 (31.6) |
| SD | 5 (35.7) | 5 (62.5) | 8 (38.1) | 6 (37.5) | 9 (39.1) | 6 (31.6) |
| PD | 5 (35.7) | 2 (25.0) | 9 (42.9) | 6 (37.5) | 10 (43.5) | 6 (31.6) |
| Median (range) duration of response, months | 8.4 (3.2–73.8 +) | 2.6 | 8.4 (3.2–73.8 +) | 5.5 (2.6–66.5 +) | 8.4 (3.2–73.8 +) | 4.1 (2.0–66.5 +) |
Data are n (%; 95% CI) or n (%). ORR was defined as a best overall response of CR or PR. Duration of response was defined as the time from the first documented evidence of CR or PR until disease progression or death (whichever occurred first)
BICR blinded independent central review, CI confidence interval, CPS combined positive score, CR complete response; ORR objective response rate, PD progressive disease, PD-L1 programmed cell death ligand 1, PR partial response, RECIST Response Evaluation Criteria in Solid Tumors, SD stable disease
aFrom product-limit (Kaplan–Meier) method for censored data; “ + ” indicates that there was no progressive disease by the time of last disease assessment
The ORR in the PD-L1 CPS ≥ 20 population was 50.0% (one CR, four PRs) with pembrolizumab-chemotherapy versus 14.3% (one PR) with EXTREME, and median DOR was 6.9 versus 2.6 months (Table 2). In the PD-L1 CPS ≥ 1 population, the ORR was 31.6% (one CR, five PRs) with pembrolizumab-chemotherapy versus 21.4% (one CR, two PRs) with EXTREME, and median DOR was 7.5 versus 4.1 months. In the total Japanese population, the ORR was 32.0% (one CR, seven PRs) with pembrolizumab-chemotherapy versus 31.3% (one CR, four PRs) with EXTREME, and median DOR was 7.5 versus 4.1 months.
Table 2.
Summary of confirmed objective response and duration of response (per RECIST 1.1 by BICR) by PD-L1 CPS population with pembrolizumab-chemotherapy versus EXTREME in the Japanese population
| PD-L1 CPS ≥ 20 | PD-L1 CPS ≥ 1 | Total population | ||||
|---|---|---|---|---|---|---|
| Pembrolizumab-chemotherapy (n = 10) | EXTREME (n = 7) | Pembrolizumab-chemotherapy (n = 19) | EXTREME (n = 14) | Pembrolizumab-chemotherapy (n = 25) | EXTREME (n = 16) | |
| ORR | 5 (50.0; 18.7–81.3) | 1 (14.3; 0.4–57.9) | 6 (31.6; 12.6–56.6) | 3 (21.4; 4.7–50.8) | 8 (32.0; 14.9–53.5) | 5 (31.3; 11.0–58.7) |
| Best overall response | ||||||
| CR | 1 (10.0) | 0 | 1 (5.3) | 1 (7.1) | 1 (4.0) | 1 (6.3) |
| PR | 4 (40.0) | 1 (14.3) | 5 (26.3) | 2 (14.3) | 7 (28.0) | 4 (25.0) |
| SD | 1 (10.0) | 5 (71.4) | 5 (26.3) | 6 (42.9) | 7 (28.0) | 6 (37.5) |
| PD | 4 (40.0) | 1 (14.3) | 7 (36.8) | 5 (35.7) | 8 (32.0) | 5 (31.3) |
| Non-CR/non-PD | 0 | 0 | 0 | 0 | 1 (4.0) | 0 |
| Not evaluable | 0 | 0 | 1 (5.3) | 0 | 1 (4.0) | 0 |
| Median (range) duration of response, months | 6.9 (5.7–52.5 +) | 2.6 (2.6–2.6) | 7.5 (5.7–52.5 +) | 4.1 (2.6–66.5 +) | 7.5 (4.1–52.5 +) | 4.1 (2.0–66.5 +) |
Data are n (%; 95% CI) or n (%). Objective response rate was defined as a best overall response of CR or PR. Duration of response was defined as the time from the first documented evidence of CR or PR until disease progression or death (whichever occurred first)
BICR blinded independent central review, CI confidence interval, CPS combined positive score, CR complete response, ORR objective response rate, PD progressive disease, PD-L1 programmed cell death ligand 1, PR partial response, RECIST Response Evaluation Criteria in Solid Tumors, SD stable disease
aFrom product-limit (Kaplan–Meier) method for censored data; “ + ” indicates that there was no progressive disease by the time of last disease assessment
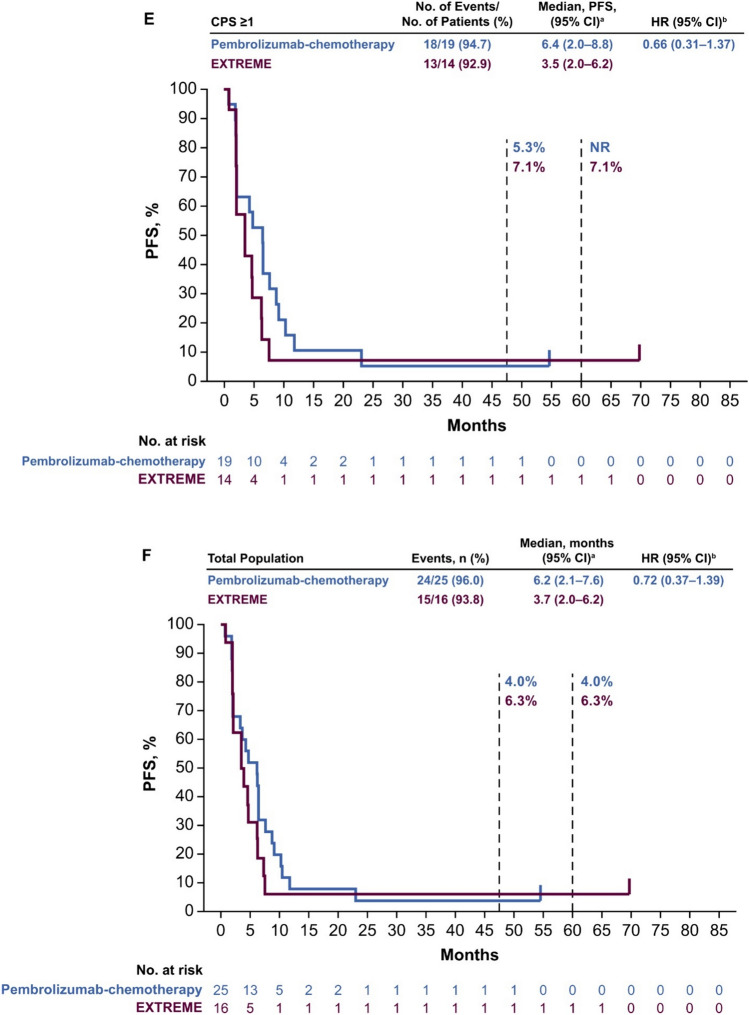
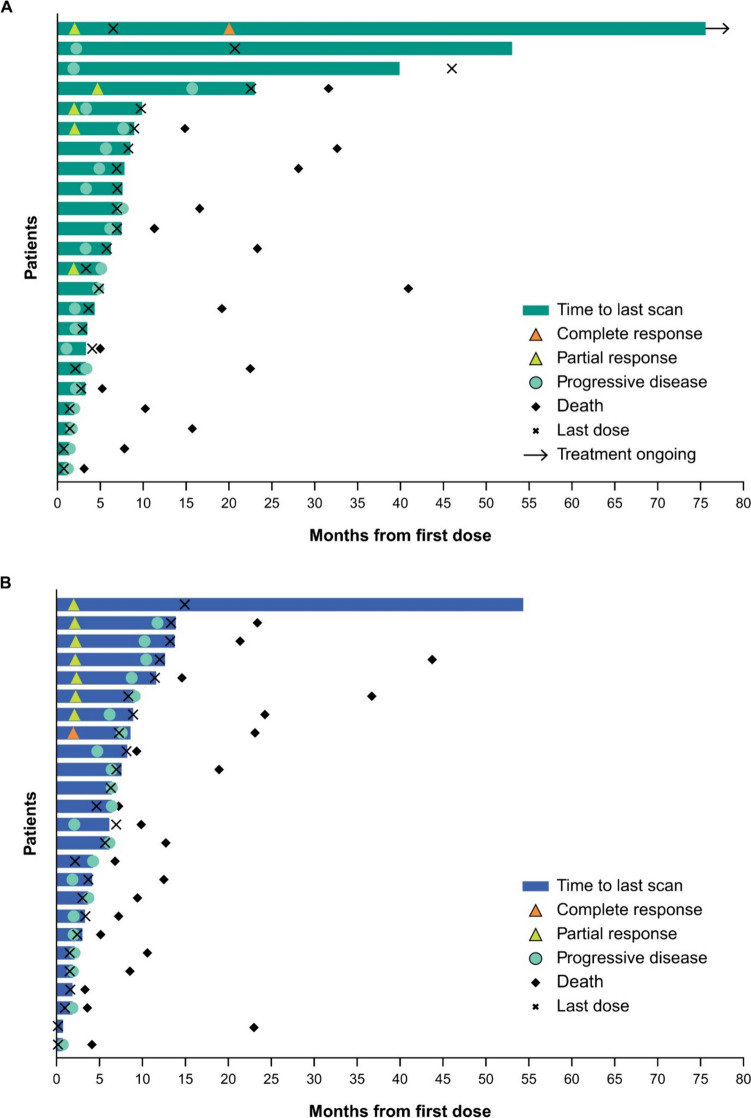
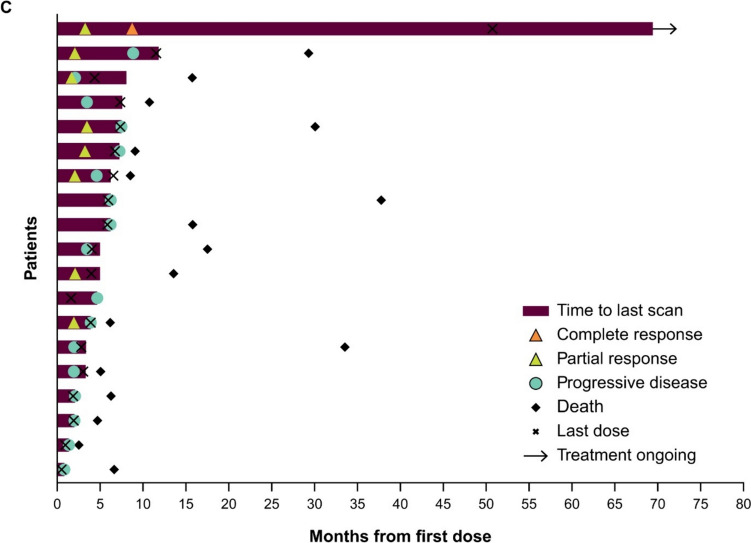
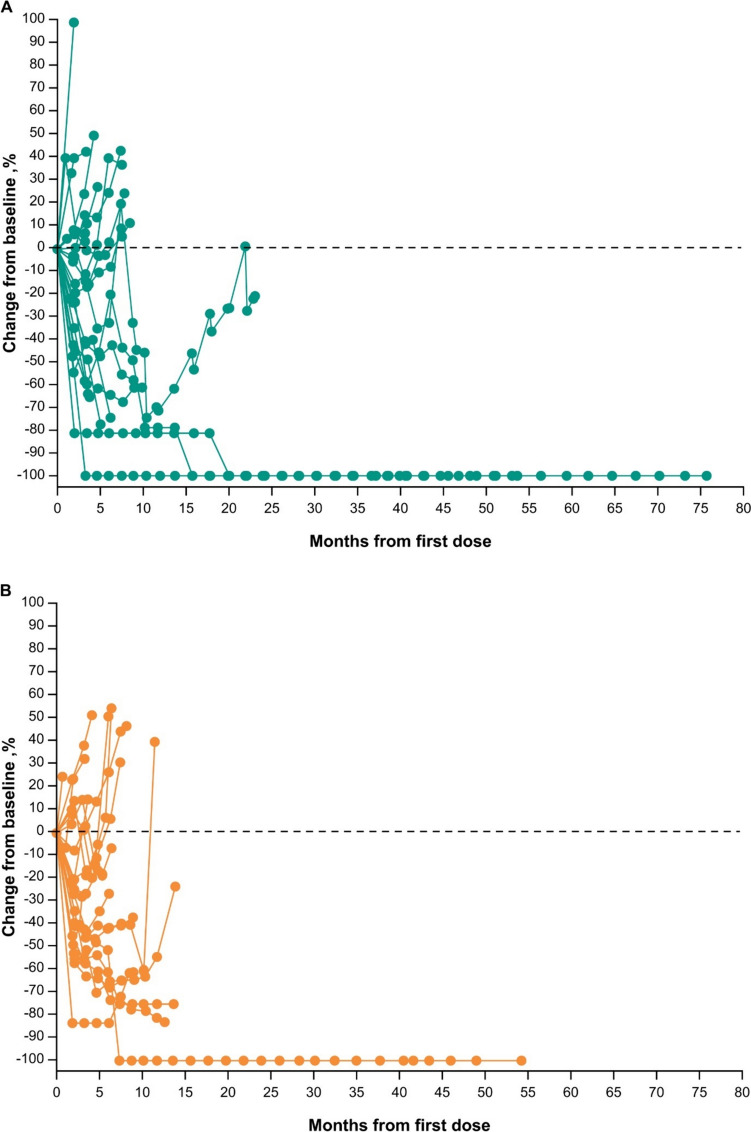
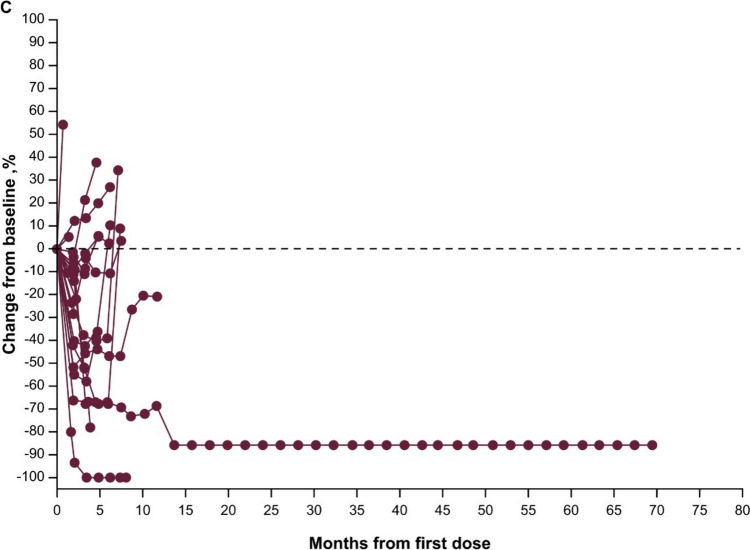
Responses were durable with pembrolizumab (Fig. 3A), and response was ongoing in two patients with CR (one with pembrolizumab, one with EXTREME). Reductions in target lesion size from baseline were generally durable with all three treatments over time (Fig. 4A–C).
Fig. 3.
Time to response and response duration at long-term follow-up in the total Japanese population receiving A pembrolizumab, B pembrolizumab-chemotherapy, and C EXTREME
Fig. 4.
Change from baseline in target lesion size at long-term follow-up in the total Japanese population receiving A pembrolizumab monotherapy, B pembrolizumab-chemotherapy, and C EXTREME
The median duration of treatment with pembrolizumab was 5.8 months (range, 0.7–24.0), and the median number of cycles administered was nine (range, 2–33). The median duration of treatment with pembrolizumab-chemotherapy was 5.7 months (range, 0.2–15.0), and the median number of cycles administered was seven (range, 1–22). The median duration of treatment with EXTREME was approximately 4.2 months (range, 0.5–51.0), and the median number of cycles administered was six (range, 1–52).
Detailed safety data for the Japanese population have been published previously [13]. Any-grade treatment-related AEs occurred in 17 (73.9%) patients in the pembrolizumab alone group, 25 (100%) in the pembrolizumab-chemotherapy group, and 19 (100%) in the EXTREME group (Table 3). Grade ≥ 3 treatment-related AEs were reported in five (21.7%), 19 (76.0%), and 17 (89.5%) patients, respectively. As reported previously, one Japanese patient receiving pembrolizumab-chemotherapy died because of treatment-related pneumonitis [13].
Table 3.
Summary of treatment-related AEs in the Japanese population
| Pembrolizumab (n = 23) | Pembrolizumab-chemotherapy (n = 25) | EXTREME (n = 19) |
|
|---|---|---|---|
| Any treatment-related AEsa | 17 (73.9) | 25 (100) | 19 (100) |
| Grade 3–5 | 5 (21.7) | 19 (76.0) | 17 (89.5) |
| Serious | 3 (13.0) | 7 (28.0) | 5 (26.3) |
| Death | 0 | 1 (4.0) | 0 |
| Discontinuation due to treatment-related AEs | 2 (8.7) | 1 (4.0) | 4 (21.1) |
| Discontinuation due to serious treatment-related AEs | 2 (8.7) | 1 (4.0) | 2 (10.5) |
Data are n (%). Non-serious AEs within 30 days of the last dose and serious AEs within 90 days of the last dose are included. aDetermined by the investigator to be related to study treatment
AE adverse event
Discussion
With an extended follow-up of 5 years, pembrolizumab monotherapy and pembrolizumab-chemotherapy conferred long-term clinical benefit as first-line treatment for Japanese patients with R/M HNSCC. While the number of patients enrolled in Japan was low and the populations were small, the results from the Japanese population are generally consistent with those from the global population in KEYNOTE-048 [14]. In the current analysis, pembrolizumab monotherapy improved OS compared with those treated with EXTREME in the total Japanese population. In Japanese patients with PD-L1 CPS ≥ 20, OS was numerically longer in patients treated with pembrolizumab-chemotherapy than in those treated with EXTREME. In patients in the PD-L1 CPS ≥ 1 population and in the total population, there was no apparent OS benefit in patients treated with pembrolizumab-chemotherapy compared with those treated with EXTREME. As reported previously [13], differences in baseline characteristics, such as ECOG PS, between treatment arms may have influenced the results of the current analysis, in addition to the higher number of patients in the EXTREME arm and the lower number of patients in the pembrolizumab-chemotherapy arm who received subsequent anti–PD-(L)1 therapy. Furthermore, the small sample size and wide confidence intervals limit interpretation of these results.
Survival rates observed in Japanese clinical practice are consistent with the 1-year rates we previously reported in the total Japanese population of KEYNOTE-048 [13] and in the global KEYNOTE-048 population [12]. The 1-year OS rates were 74% and 52% with pembrolizumab monotherapy and pembrolizumab-chemotherapy, respectively, in the total Japanese population [13] and 49% and 53%, respectively, in the total global population [12]. It is worth noting that real-world studies included patients with uncommon primary lesions, such as those in the paranasal sinuses and external auditory canal, who were excluded from KEYNOTE-048; outcomes in patients with HNSCC of the oropharynx, hypopharynx, oral cavity, and larynx in this real-world study were similar to those in KEYNOTE-048 [2, 15].
In conclusion, after 5 years of follow-up, first-line pembrolizumab monotherapy and pembrolizumab-chemotherapy showed long-term clinical benefit in Japanese patients with R/M HNSCC. Results from this study further support pembrolizumab and pembrolizumab-chemotherapy as first-line treatment options for Japanese patients with R/M HNSCC.
Author contributors
Conceptualization: NN, BG. Data curation: YS, TYok, RY, NN, BG. Investigation: NO, ST, KT, KM, TYok, MT, NH, HY, HH, TYos, NM, TF, NN, BG, NL. Methodology: KT, NN, BG, NL. Project administration: ST, YF, KM, NH, HH. Formal analysis: KT, NN, BG. Writing—original draft preparation: NN, BG. Writing—review and editing: NO, ST, KT, YS, YF, KM, TYok, TYa, TU, NH, HH, NM, TF, KT, NN, BG, NL, MT. Funding acquisition: None. Resources: TU, KS, KM. Supervision: NO, BG, NL. Validation: KT, BG.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and their families and caregivers for participating in this trial, and all investigators and site personnel. We also thank Yasuhiko Murata from MSD K.K., Tokyo, Japan, for communication support with external authors. Medical writing or editorial assistance was provided by Matthew Grzywacz, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Data availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and elsewhere, or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses. For the MSD data sharing website, see https://engagezone.msd.com/ds_documentation.php.
Declarations
Conflict of interest
Nobuhiko Oridate has received honoraria from MSD, Ono Pharmaceutical, Bristol Myers Squibb Japan, Merck Biopharm Japan, and Taiho Pharmaceutical. Shunji Takahashi has received honoraria from MSD, Eisai, and Ono Pharmaceutical and grants from Taiho Pharmaceutical, Daiichi Sankyo, Novartis, Ono Pharmaceutical, Eisai, BMS, Bayer, and Astra Zeneca. Kaoru Tanaka has received personal fees from Astra Zeneca, Merck Biopharma, Eisai, Bristol Myers Squibb, Ono Pharmaceutical, MSD, Kyowa Kirin, Chugai Pharmaceutical, Takeda Pharmaceutical, Taiho Pharmaceutical, and Novartis Pharma, outside the submitted work. Yasushi Shimizu has received research funding from MSD. Koji Matsumoto has received honoraria from MSD, Kyowa Kirin, Daiichi Sankyo, Eli Lilly, and Chugai Pharmaceutical Co. and received trial fee from Daiichi Sankyo, MSD, Eli Lilly, Gildea Sciences, and Eisai. Tomoya Yokota has received research funding from MSD. Masanobu Takahashi has received lecture fees from Daiichi Sankyo, Ono Pharmaceuticals, Bristol Myers Squibb, Taiho Pharmaceutical Co. and research funding from Ono Pharmaceutical, Chugai Pharmaceutical, and MSD, outside the submitted work. Tsutomu Ueda has received research funding from Lilly and personal fees from Rakuten Medical, Merck Biopharma, Mitsubishi Tanabe Pharma, MSD, and Bristol Myers Squibb. Nobuhiro Hanai has received honoraria from Rakuten Medical K.K. and research funding from MSD K.K. and Adlai Nortye USA Inc. Hiroki Hara has received honoraria from Bristol Myers Squibb, Chugai, Daiichi Sankyo, Lilly, Merck Biopharma, Miyarisan, MSD, Ono Pharmaceutical, Taiho Pharmaceutical, Takeda, and Yakult Honsha and research funding from ALX oncology, Amgen, Astellas, Astra Zeneca, Bayer, BioGene, Chugai, Daiichi Sankyo, Janssen, Jazz Pharma, MSD, Ono Pharmaceutical, and Taiho Pharmaceutical. Kenichi Takahashi is an employee of MSD K.K., Tokyo, Japan and owns stock/stock options in Merck & Co., Inc., Rahway, NJ, USA. Nijiro Nohata is an employee of MSD K.K., Tokyo, Japan and owns stock/stock options in Merck & Co., Inc., Rahway, NJ, USA. Burak Gumuscu is an employee of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and owns stock/stock options in Merck & Co., Inc., Rahway, NJ, USA. Nati Lerman is an employee of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and owns stock/stock options in Merck & Co., Inc., Rahway, NJ, USA. Makoto Tahara has received honoraria from Eisai, MSD, Merck Biopharma, Lilly and research funding from Bayer, Astra Zeneca, Ono Pharmaceutical, and Pfizer. Yasushi Fujimoto, Tomoko Yamazaki, Hironori Yamaguchi, Tomokazu Yoshizaki Ryuji Yasumatsu, Masahiro Nakayama, Kiyoto Shiga, Takashi Fujii, and Kenji Mitsugi declare they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gupta B, Johnson NW, Kumar N (2016) Global epidemiology of head and neck cancers: a continuing challenge. Oncology 91(1):13–23. 10.1159/000446117 [DOI] [PubMed] [Google Scholar]
- 2.Sano D, Tokuhisa M, Takahashi H et al (2022) Real-world therapeutic outcomes of the pembrolizumab regimen as first-line therapy for recurrent/metastatic squamous cell carcinoma of the head and neck: a single-center retrospective cohort study in Japan. Anticancer Res 42(9):4477–4484. 10.21873/anticanres.15948 [DOI] [PubMed] [Google Scholar]
- 3.Matsuda T, Saika K (2018) Cancer burden in Japan based on the latest cancer statistics: need for evidence-based cancer control programs. Ann Cancer Epidemiol 2:2 [Google Scholar]
- 4.International Agency for Research on Cancer (2021) GLOBOCAN 2020–2021. International Agency for Research on Cancer. https://gco.iarc.fr/today/home. Accessed 12 Sep 2024
- 5.Nibu KI, Hayashi R, Asakage T et al (2017) Japanese clinical practice guideline for head and neck cancer. Auris Nasus Larynx 44(4):375–380. 10.1016/j.anl.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Fukuda N, Yunokawa M, Fujiwara Y et al (2021) Comparison of the efficacy and safety of the EXTREME regimen for treating recurrent or metastatic head and neck squamous cell carcinoma in older and younger adult patients. Cancer Rep (Hoboken) 4(2):e1322. 10.1002/cnr2.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keam B, Machiels JP, Kim HR et al (2021) Pan-Asian adaptation of the EHNS-ESMO-ESTRO clinical practice guidelines for the diagnosis, treatment and follow-up of patients with squamous cell carcinoma of the head and neck. ESMO Open 6(6):100309. 10.1016/j.esmoop.2021.100309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machiels JP, René Leemans C, Golusinski W et al (2020) Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31(11):1462–1475. 10.1016/j.annonc.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz E, Ismaila N, Bauman JE et al (2023) Immunotherapy and biomarker testing in recurrent and metastatic head and neck cancers: ASCO Guideline. J Clin Oncol 41(5):1132–1146. 10.1200/jco.22.02328 [DOI] [PubMed] [Google Scholar]
- 10.Ferris RL, Blumenschein G Jr, Fayette J et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375(19):1856–1867. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen EEW, Soulieres D, Le Tourneau C et al (2019) Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 393(10167):156–167. 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 12.Burtness B, Harrington KJ, Greil R et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394(10212):1915–1928. 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S, Oridate N, Tanaka K et al (2022) First-line pembrolizumab ± chemotherapy for recurrent/metastatic head and neck cancer: Japanese subgroup of KEYNOTE-048. Int J Clin Oncol 27(12):1805–1817. 10.1007/s10147-022-02233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahara M, Greil R, Rischin D et al (2022) Pembrolizumab with or without chemotherapy for first-line treatment of recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): 5-year results from KEYNOTE-048. Ann Oncol 33:844 [Google Scholar]
- 15.Nakano T, Yasumatsu R, Hashimoto K et al (2022) Real-world experience with pembrolizumab for advanced-stage head and neck cancer patients: a retrospective, multicenter study. Anticancer Res 42(7):3653–3664. 10.21873/anticanres.15854 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and elsewhere, or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses. For the MSD data sharing website, see https://engagezone.msd.com/ds_documentation.php.