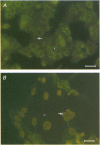
Abstract
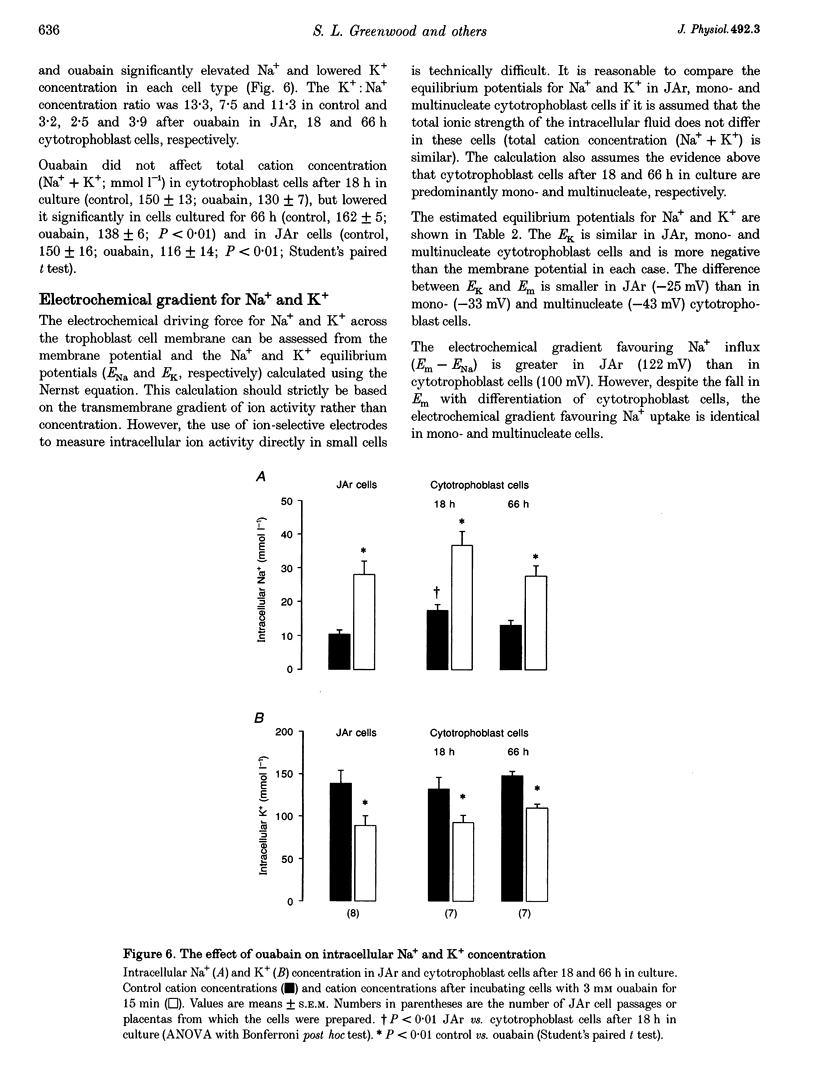
1. The electrochemical gradients for Na+ and K+ were assessed in a cell culture model of trophoblast differentiation. 2. Membrane potential difference (Em), intracellular water and Na+ and K+ contents were measured in choriocarcinoma cells (JAr cell line; 96% of which are undifferentiated trophoblast cells) and in mononucleate and multinucleate (differentiated) cytotrophoblast cells isolated from the human placenta at term. 3. There was a significant fall in Em from -57 mV in JAr cells, to -48 and -40 mV in mono-and multinucleate cytotrophoblast cells, respectively. Treatment with ouabain (1 mM for 15 min) depolarized the JAr cell membrane by 15 mV but did not affect cytotrophoblast cell membrane potential. 4. Intracellular K+ concentration was similar in JAr, mono- and multinucleate cytotrophoblast cells but Na+ concentration was higher in mononucleate cytotrophoblast cells compared with JAr cells. 5. Ouabain treatment (3 mM for 15 min) caused a small increase (4.5%) in cell water in mononucleate cytotrophoblast cells but lowered K+ (approximately 30%) and increased Na+ concentration (approximately 125%) in all the trophoblast cells studied. 6. The K+ equilibrium potential (EK) was more negative than Em in all cells and the difference between EK and Em was smaller in JAr cells (-25 mV) than in mono- and multinucleate cytotrophoblast cells (-33 and -43 mV, respectively). 7. The Na+ equilibrium potential (ENa) was positive in the trophoblast cells and the difference between ENa and Em was 122, 100 and 100 mV in JAr, mono- and multinucleate cytotrophoblast cells, respectively. 8. These results suggest that the electrochemical gradient for K+ is affected by the stage of trophoblast cell differentiation. In contrast, the electrochemical gradient for Na+ is similar in mono- and multinucleate cytotrophoblast cells.
Full text
PDF



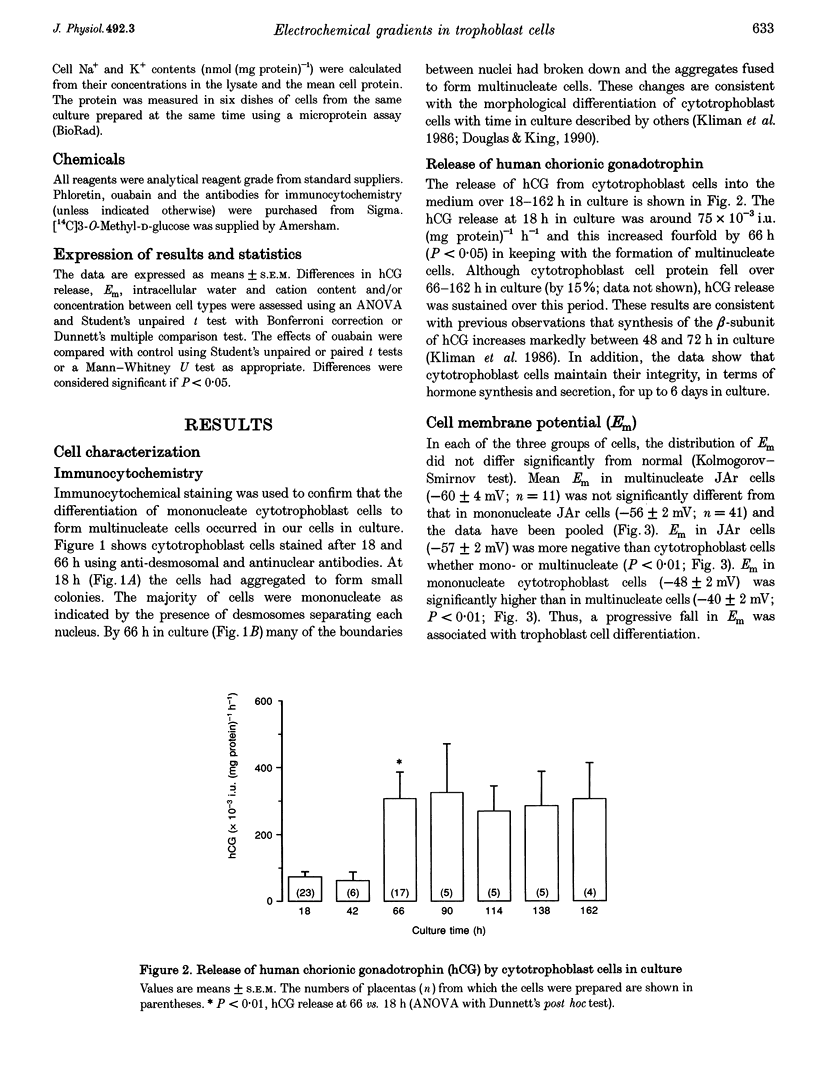
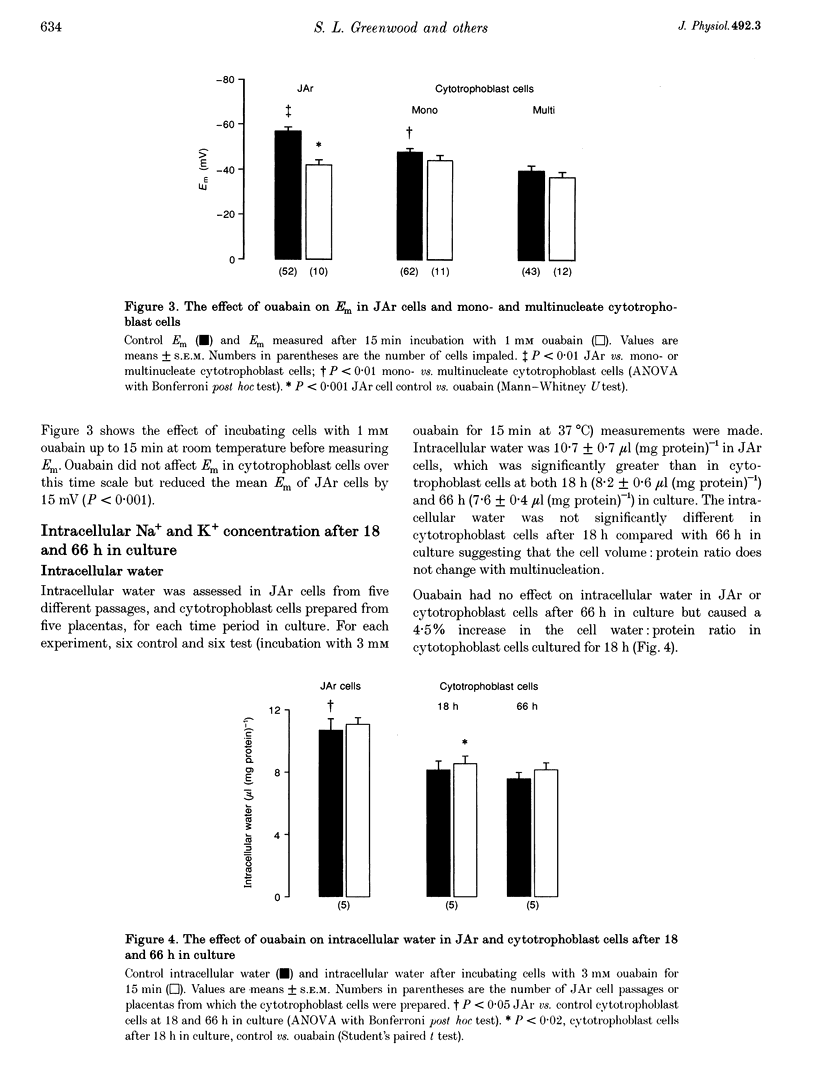
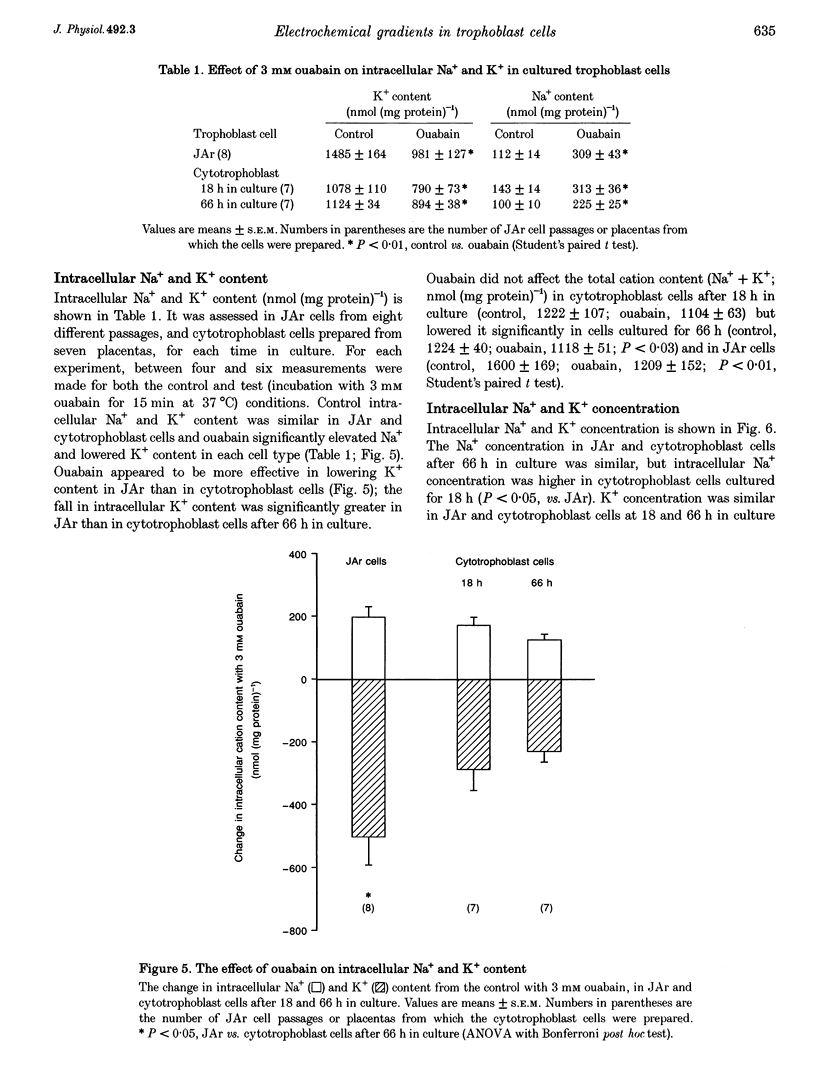




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Pasternak C. A. Plasma membrane potential of Lettré cells does not depend on cation gradients but on pumps. J Membr Biol. 1984;79(3):275–284. doi: 10.1007/BF01871066. [DOI] [PubMed] [Google Scholar]
- Beck F., Bauer R., Bauer U., Mason J., Dörge A., Rick R., Thurau K. Electron microprobe analysis of intracellular elements in the rat kidney. Kidney Int. 1980 Jun;17(6):756–763. doi: 10.1038/ki.1980.88. [DOI] [PubMed] [Google Scholar]
- Hochberg A., Sibley C., Pixley M., Sadovsky Y., Strauss B., Boime I. Choriocarcinoma cells increase the number of differentiating human cytotrophoblasts through an in vitro interaction. J Biol Chem. 1991 May 5;266(13):8517–8522. [PubMed] [Google Scholar]
- Hoshina M., Boothby M., Hussa R., Pattillo R., Camel H. M., Boime I. Linkage of human chorionic gonadotrophin and placental lactogen biosynthesis to trophoblast differentiation and tumorigenesis. Placenta. 1985 Mar-Apr;6(2):163–172. doi: 10.1016/s0143-4004(85)80066-7. [DOI] [PubMed] [Google Scholar]
- Illsley N. P., Sellers M. C. Ion conductances in the microvillous and basal membrane vesicles isolated from human placental syncytiotrophoblast. Placenta. 1992 Jan-Feb;13(1):25–34. doi: 10.1016/0143-4004(92)90004-d. [DOI] [PubMed] [Google Scholar]
- Karl P. I., Alpy K. L., Fisher S. E. Amino acid transport by the cultured human placental trophoblast: effect of insulin on AIB transport. Am J Physiol. 1992 Apr;262(4 Pt 1):C834–C839. doi: 10.1152/ajpcell.1992.262.4.C834. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986 Apr;118(4):1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Leichtweiss H. P., Carstensen M., Schröder H., Rachor D. Some physiological properties of the isolated human placenta. Contrib Gynecol Obstet. 1985;13:70–76. [PubMed] [Google Scholar]
- Mitchell A. M., Yap A. S., Payne E. J., Manley S. W., Mortimer R. H. Characterization of cell polarity and epithelial junctions in the choriocarcinoma cell line, JAR. Placenta. 1995 Jan;16(1):31–39. doi: 10.1016/0143-4004(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Pressley T. A., Haber R. S., Loeb J. N., Edelman I. S., Ismail-Beigi F. Stimulation of Na,K-activated adenosine triphosphatase and active transport by low external K+ in a rat liver cell line. J Gen Physiol. 1986 Apr;87(4):591–606. doi: 10.1085/jgp.87.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick R., Beck F. X., Dörge A., Thurau K. Intracellular ion concentrations in the frog cornea epithelium during stimulation and inhibition of Cl secretion. J Membr Biol. 1987;95(3):229–240. doi: 10.1007/BF01869485. [DOI] [PubMed] [Google Scholar]
- Shalmi M., Kibble J. D., Day J. P., Christensen P., Atherton J. C. Improved analysis of picomole quantities of lithium, sodium, and potassium in biological fluids. Am J Physiol. 1994 Oct;267(4 Pt 2):F695–F701. doi: 10.1152/ajprenal.1994.267.4.F695. [DOI] [PubMed] [Google Scholar]
- Sibley C. P., Boyd R. D. Control of transfer across the mature placenta. Oxf Rev Reprod Biol. 1988;10:382–435. [PubMed] [Google Scholar]
- Sibley C. P., Hochberg A., Boime I. Bromo-adenosine stimulates choriogonadotropin production in JAr and cytotrophoblast cells: evidence for effects on two stages of differentiation. Mol Endocrinol. 1991 Apr;5(4):582–586. doi: 10.1210/mend-5-4-582. [DOI] [PubMed] [Google Scholar]
- Sjodin R. A. Contributions of electrogenic pumps to resting membrane potentials: the theory of electrogenic potentials. Soc Gen Physiol Ser. 1984;38:105–127. [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Serum stimulates the Na+,K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5560–5564. doi: 10.1073/pnas.75.11.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]