Abstract
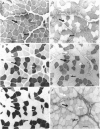
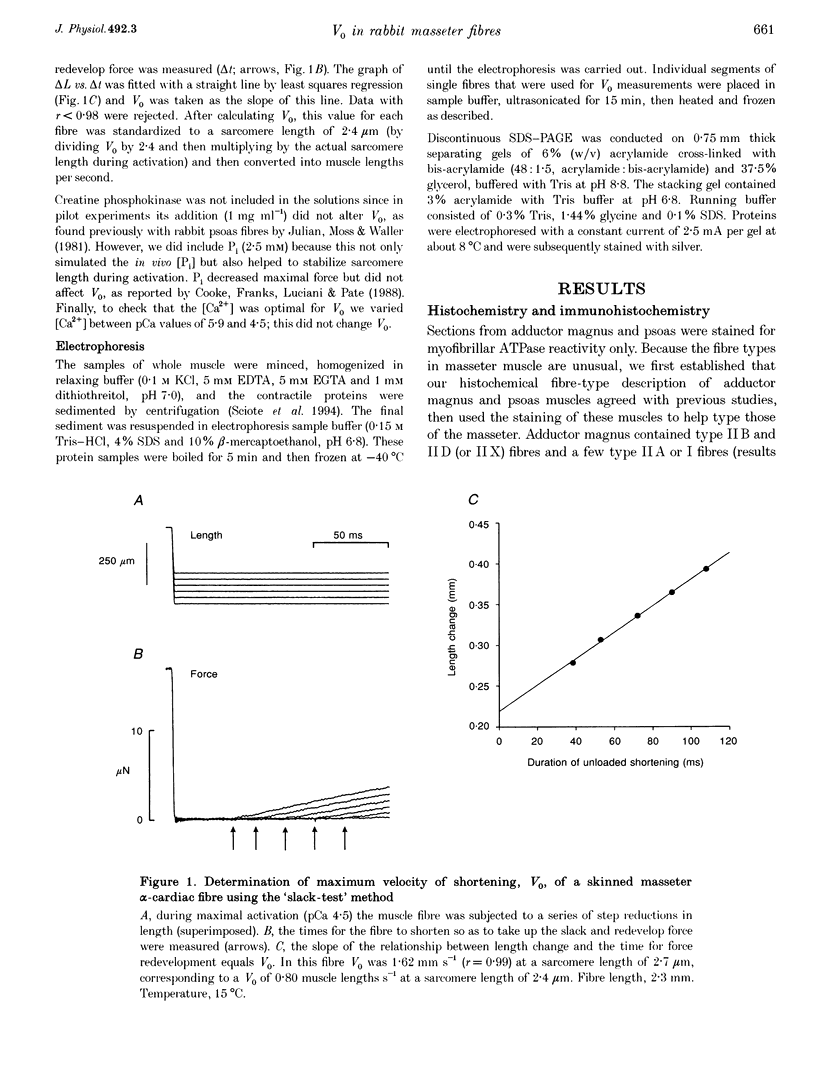
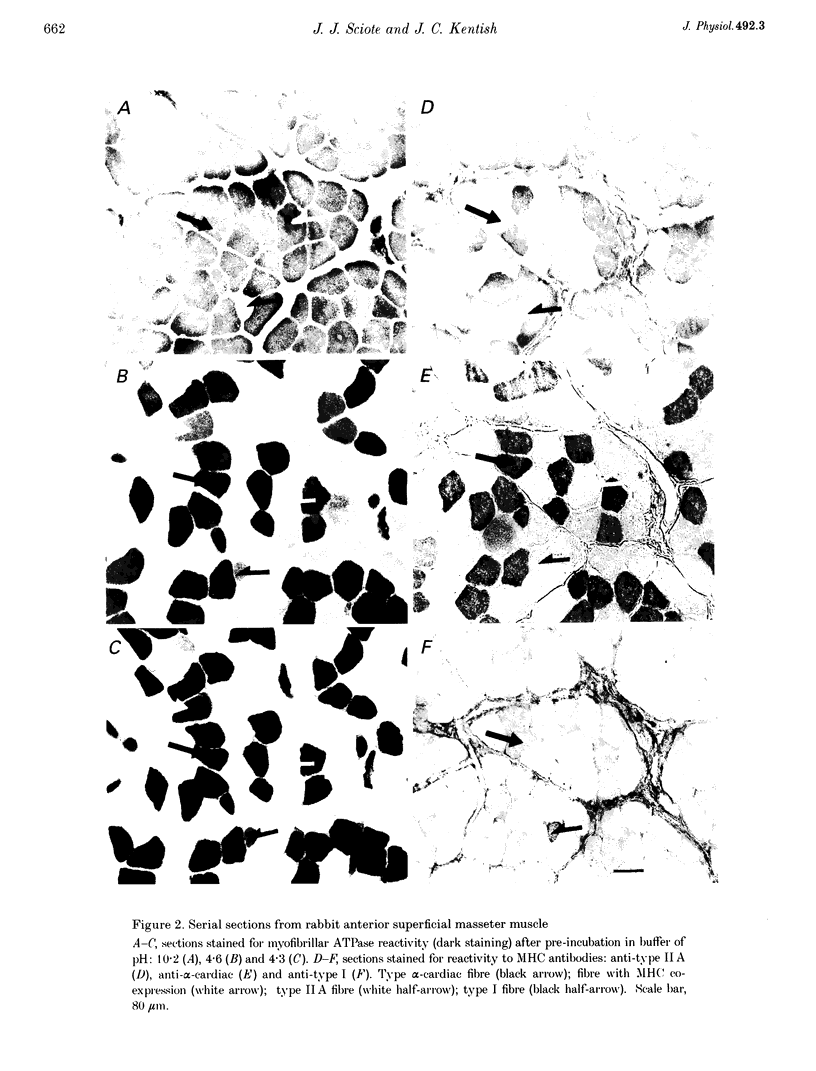
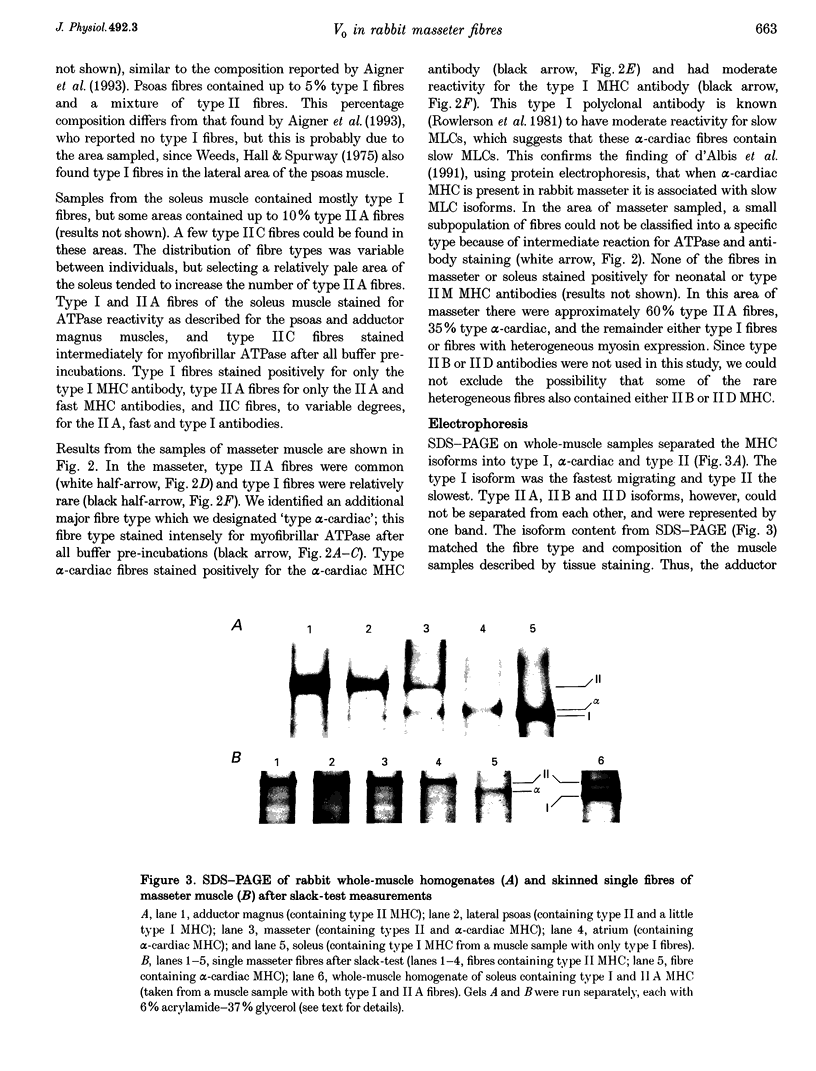
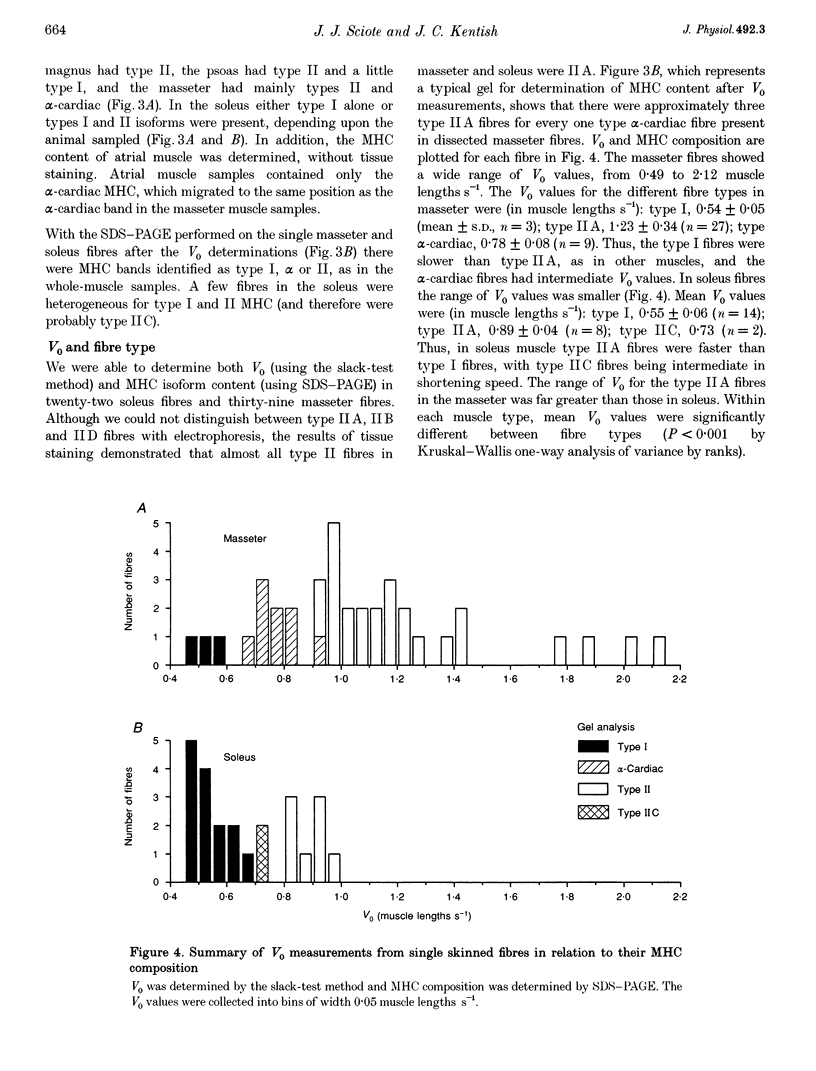
1. Some rabbit masseter fibres express the alpha-cardiac myosin heavy chain (MHC). To compare the biochemical and physiological properties of these fibres with other skeletal fibre types, we examined the histochemical and immunohistochemical staining characteristics, maximum velocity of shortening (V(zero)) and MHC isoform content of fibres from rabbit masseter and soleus muscles. 2. The fibre-type composition of muscle sections was determined with MHC antibodies and myofibrillar ATPase histochemistry. Fibres we designated 'type alpha-cardiac' were different from type I and type II fibres in that they stained positively with the alpha-cardiac MHC antibody and they maintained. ATPase reactivity after acid and alkali pre-incubations. Samples of superficial masseter contained a few type I fibres, with the majority of fibres classified as either type IIA or type alpha-cardiac. Soleus samples contained type I, IIA and IIC fibres. 3. The V(zero) of chemically skinned fibres was determined by the slack-test method. Each fibre was subsequently characterized as type I, IIA, IIC or alpha-cardiac from MHC identification using gel electrophoresis (SDS-PAGE). In masseter fibres the V(zero) values were (in muscle lengths s-1): type I, 0.54 +/- 0.05 (mean +/- S.D., n = 3); type IIA, 1.23 +/- 0.34 (n = 27); type alpha-cardiac, 0.78 +/- 0.08 (n = 9). In soleus fibres V(zero) values were: type I, 0.55 +/- 0.06 (n = 14); type IIA, 0.89 +/- 0.04 (n = 8); type IIC, 0.73 (n = 2). 4. We conclude that the rabbit masseter muscle contains an 'alpha-cardiac' fibre type that is distinct from other skeletal fibres. This fibre type expresses only the alpha-cardiac MHC, has unusual myofibrillar ATPase reactivity and has a V(zero) intermediate between type I and type II fibres.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aigner S., Gohlsch B., Hämäläinen N., Staron R. S., Uber A., Wehrle U., Pette D. Fast myosin heavy chain diversity in skeletal muscles of the rabbit: heavy chain IId, not IIb predominates. Eur J Biochem. 1993 Jan 15;211(1-2):367–372. doi: 10.1111/j.1432-1033.1993.tb19906.x. [DOI] [PubMed] [Google Scholar]
- Bottinelli R., Betto R., Schiaffino S., Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994 Jul 15;478(Pt 2):341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredman J. J., Wessels A., Weijs W. A., Korfage J. A., Soffers C. A., Moorman A. F. Demonstration of 'cardiac-specific' myosin heavy chain in masticatory muscles of human and rabbit. Histochem J. 1991 Apr;23(4):160–170. doi: 10.1007/BF01046587. [DOI] [PubMed] [Google Scholar]
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983 Jan;41(1):99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelGaudio J. M., Sciote J. J., Carroll W. R., Escalmado R. M. Atypical myosin heavy chain in rat laryngeal muscle. Ann Otol Rhinol Laryngol. 1995 Mar;104(3):237–245. doi: 10.1177/000348949510400310. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L., Waller G. S. Mechanical properties and myosin light chain composition of skinned muscle fibres from adult and new-born rabbits. J Physiol. 1981 Feb;311:201–218. doi: 10.1113/jphysiol.1981.sp013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L., Moss R. L. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993 Dec;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S., Kentish J. C. The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibres. J Physiol. 1994 Oct 1;480(Pt 1):45–60. doi: 10.1113/jphysiol.1994.sp020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope B., Hoh J. F., Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Lett. 1980 Sep 8;118(2):205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Kasper C. E., Greaser M. L., Moss R. L. Functional significance of myosin transitions in single fibers of developing soleus muscle. Am J Physiol. 1988 May;254(5 Pt 1):C605–C613. doi: 10.1152/ajpcell.1988.254.5.C605. [DOI] [PubMed] [Google Scholar]
- Sartore S., Mascarello F., Rowlerson A., Gorza L., Ausoni S., Vianello M., Schiaffino S. Fibre types in extraocular muscles: a new myosin isoform in the fast fibres. J Muscle Res Cell Motil. 1987 Apr;8(2):161–172. doi: 10.1007/BF01753992. [DOI] [PubMed] [Google Scholar]
- Sciote J. J., Rowlerson A. M., Hopper C., Hunt N. P. Fibre type classification and myosin isoforms in the human masseter muscle. J Neurol Sci. 1994 Oct;126(1):15–24. doi: 10.1016/0022-510x(94)90089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron R. S., Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry. 1986;86(1):19–23. doi: 10.1007/BF00492341. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Termin A., Pette D. Myosin heavy-chain-based isomyosins in developing, adult fast-twitch and slow-twitch muscles. Eur J Biochem. 1991 Jan 30;195(2):577–584. doi: 10.1111/j.1432-1033.1991.tb15740.x. [DOI] [PubMed] [Google Scholar]
- Wada M., Hämäläinen N., Pette D. Isomyosin patterns of single type IIB, IID and IIA fibres from rabbit skeletal muscle. J Muscle Res Cell Motil. 1995 Jun;16(3):237–242. doi: 10.1007/BF00121132. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Hall R., Spurway N. C. Characterization of myosin light chains from histochemically identified fibres of rabbit psoas muscle. FEBS Lett. 1975 Jan 1;49(3):320–324. doi: 10.1016/0014-5793(75)80776-9. [DOI] [PubMed] [Google Scholar]
- Weijs W. A., Jüch P. J., Kwa S. H., Korfage J. A. Motor unit territories and fiber types in rabbit masseter muscle. J Dent Res. 1993 Nov;72(11):1491–1498. doi: 10.1177/00220345930720110601. [DOI] [PubMed] [Google Scholar]
- d'Albis A., Anger M., Lompré A. M. Rabbit masseter expresses the cardiac alpha myosin heavy chain gene. Evidence from mRNA sequence analysis. FEBS Lett. 1993 Jun 14;324(2):178–180. doi: 10.1016/0014-5793(93)81388-g. [DOI] [PubMed] [Google Scholar]