Abstract
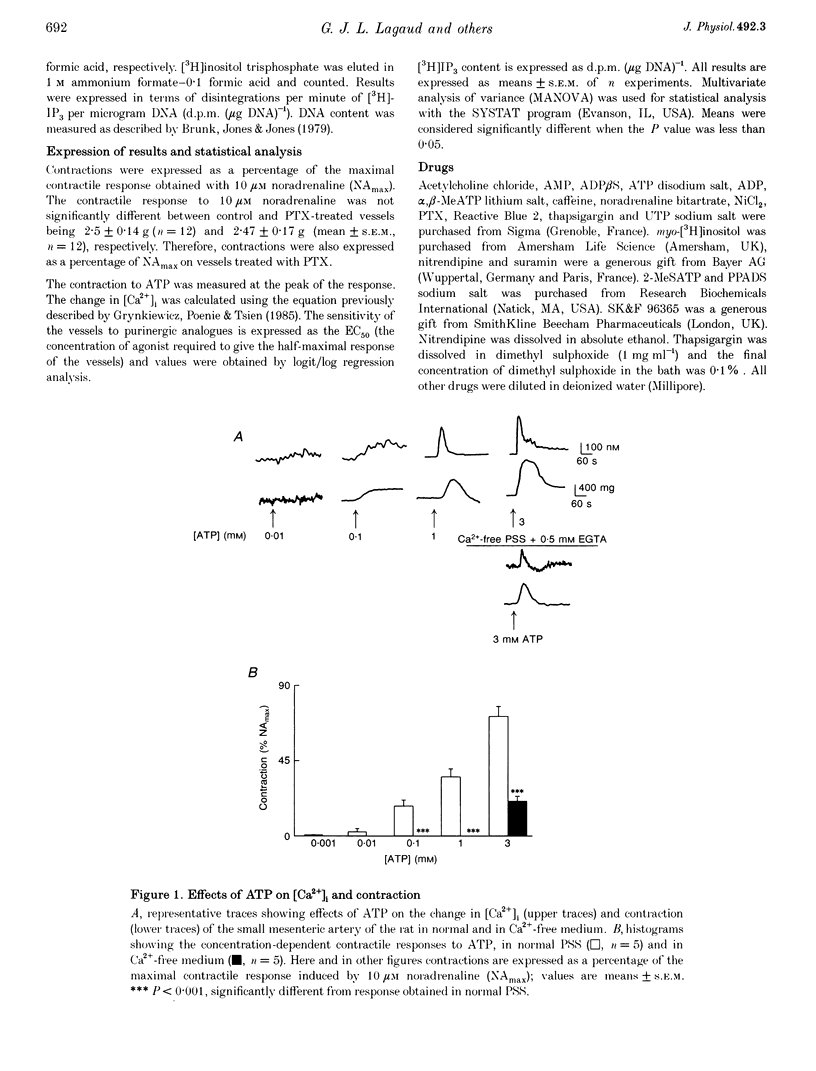
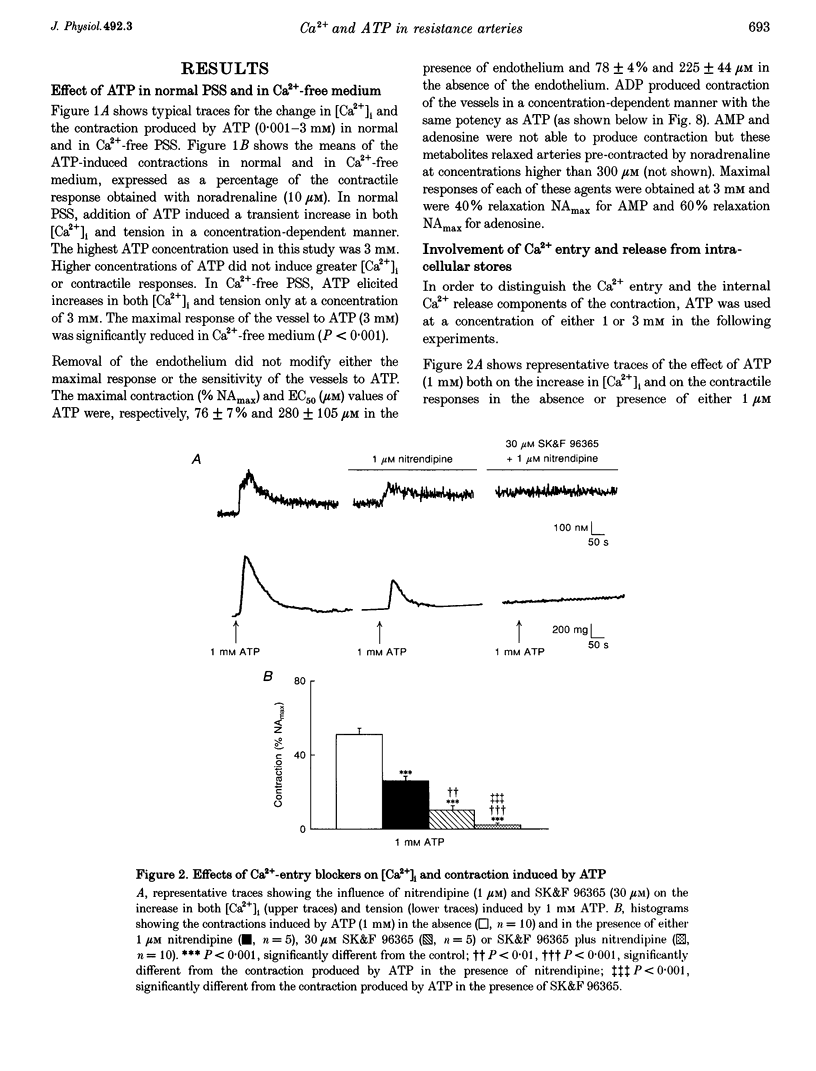
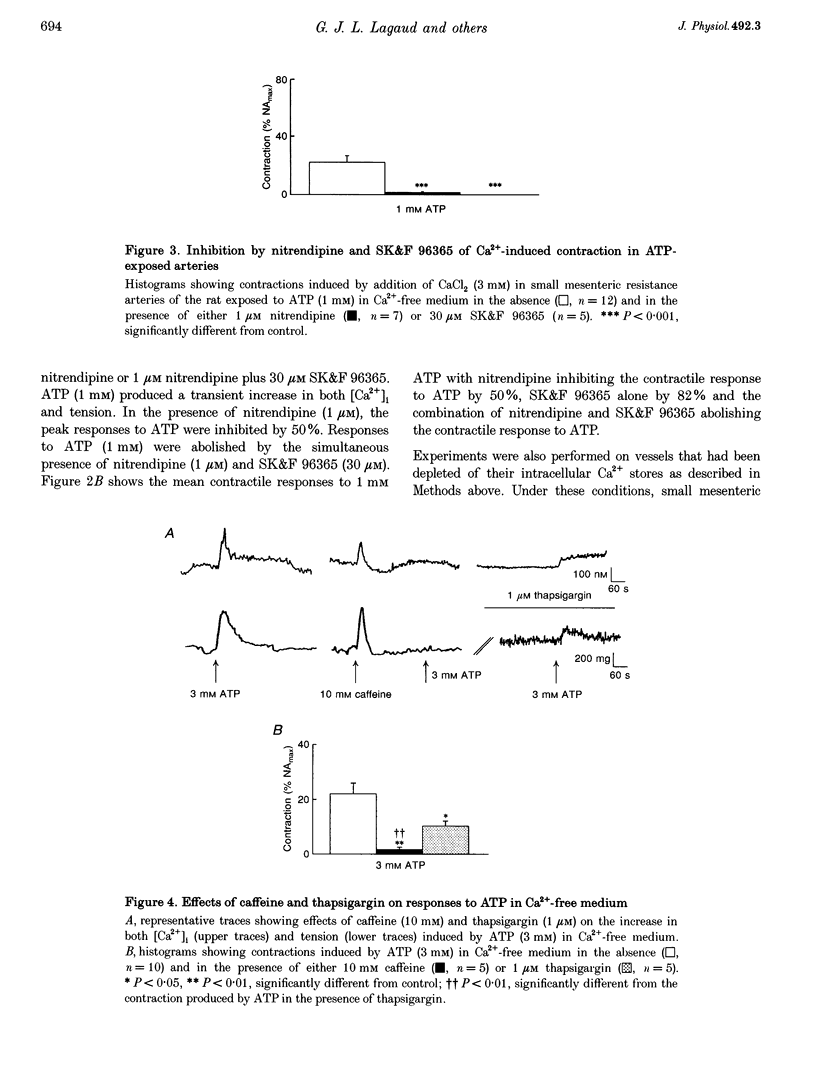
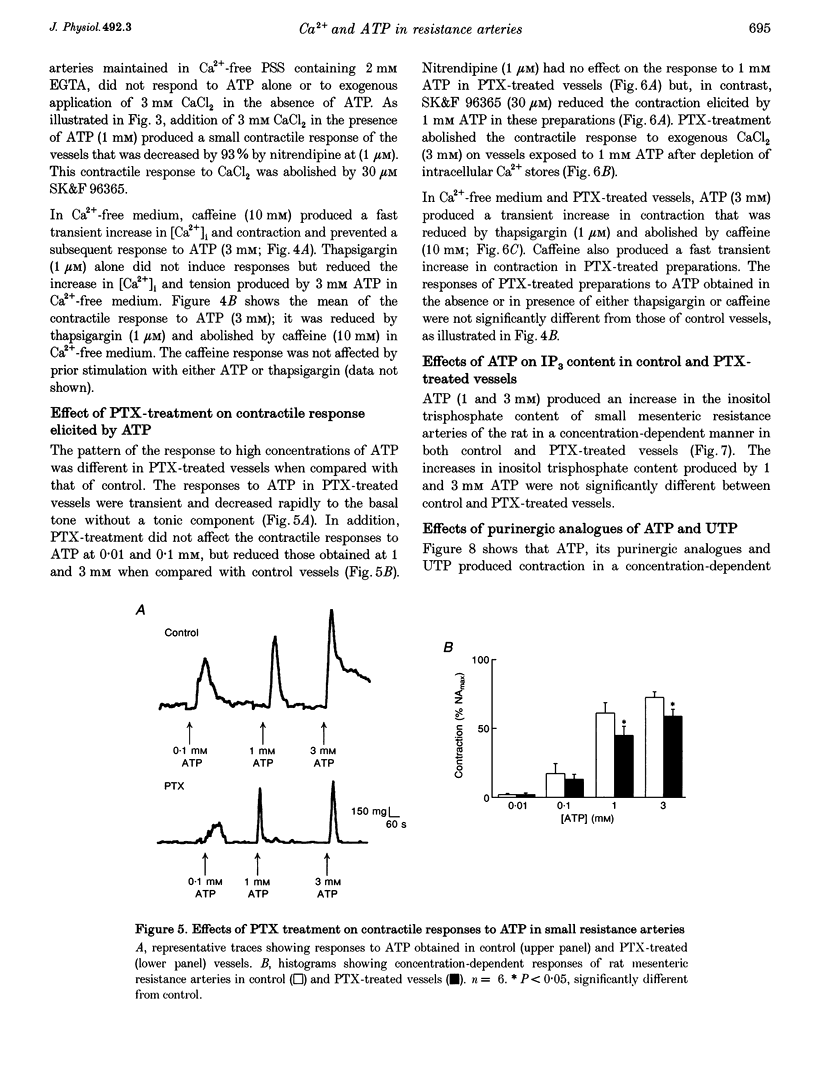
1. The relationship between the stimulation of ATP receptors, the increase in intracellular free calcium concentration ([Ca2+]i; measured using the fluorescent indicator fura-2), contraction and the subtypes of purinoceptors involved were investigated in the small mesenteric artery of the rat. 2. In normal physiological solution, ATP (0.001-3 mM) caused concentration-dependent increases in both [Ca2+]i and contraction. Both responses produced by ATP (1 mM) were inhibited by 50% in the presence of nitrendipine (1 microM) and were abolished in the presence of nitrendipine plus SK&F 96365 (30 microM). 3. In Ca(2+)-free medium, ATP (3 mM) elicited a transient increase in both [Ca2+]i and tension which were abolished by caffeine and decreased by 65% by thapsigargin (1 microM). Moreover, ATP (1 and 3 mM) produced increases in the [3H]D-myo-inositol 1,4,5-trisphosphate ([3H]IP3) content of vessels in a concentration-dependent manner. 4. Treatment of the vessels with Bordetella pertussis toxin (PTX) inhibited contractions to ATP linked to the influx of calcium through nitrendipine-sensitive mechanisms, but not those linked to the release of Ca2+ from intracellular stores nor the capacity of ATP in increasing IP3 content of the vessels. 5. The order of potency of ATP and its analogues in eliciting contraction was alpha, beta-methylene-ATP (alpha, beta-MeATP) > 2-methylthio-ATP (2-MeSATP) > ATP = ADP. The response to ATP was inhibited by suramin. Reactive Blue 2 (up to 100 microM) did not affect the contractile response to ATP. Pyridoxal-phosphate-6-azophenyl-2',4'-disulphonic acid 4-sodium (PPADS) and alpha, beta-MeATP abolished the response to low concentrations of ATP and reduced contractions elicited by high concentrations of ATP. 6. After blockade of P2X-purinoceptors with PPADS, the order of potency of ATP and its analogues was 2-MeSATP > ATP = ADP. UTP produced concentration-dependent contractions which were not affected by suramin, Reactive Blue 2, PPADS or alpha, beta-MeATP, suggesting the presence of P2U-purinoceptors. 7. The results suggest that low concentrations of ATP activate P2X-purinoceptors and produce an influx of calcium through both voltage-dependent calcium channels sensitive to nitrendipine and through receptor-operated calcium channels sensitive to SK&F 96365. High concentrations of ATP activate P2Y-purinoceptors which promote firstly a nitrendipine-sensitive calcium influx via a PTX-sensitive G protein and secondly a release of Ca2+ from an internal source via the production of IP3.
Full text
PDF


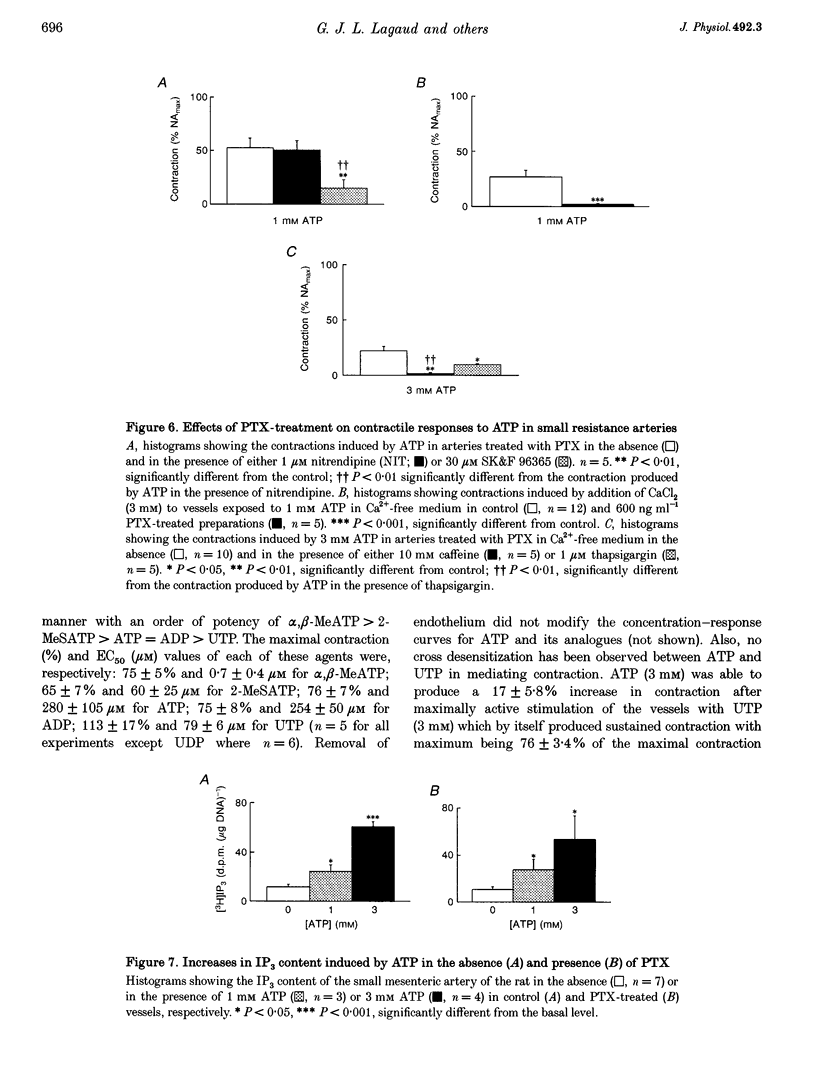
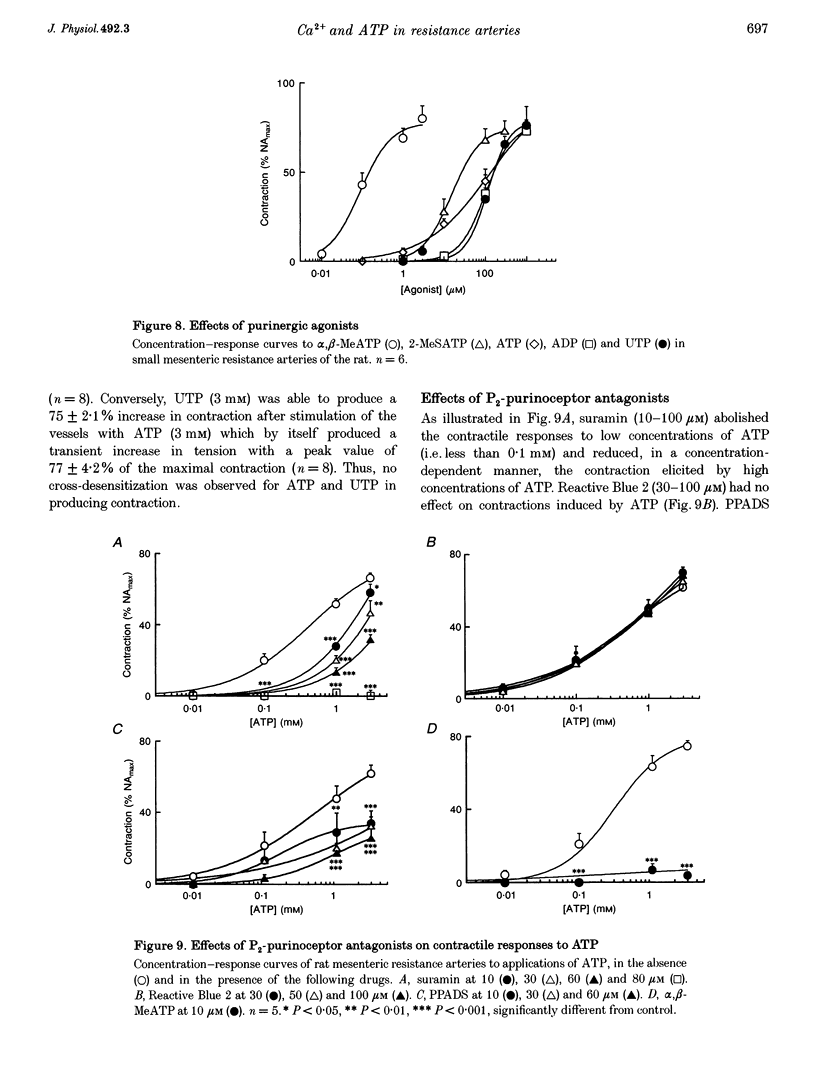
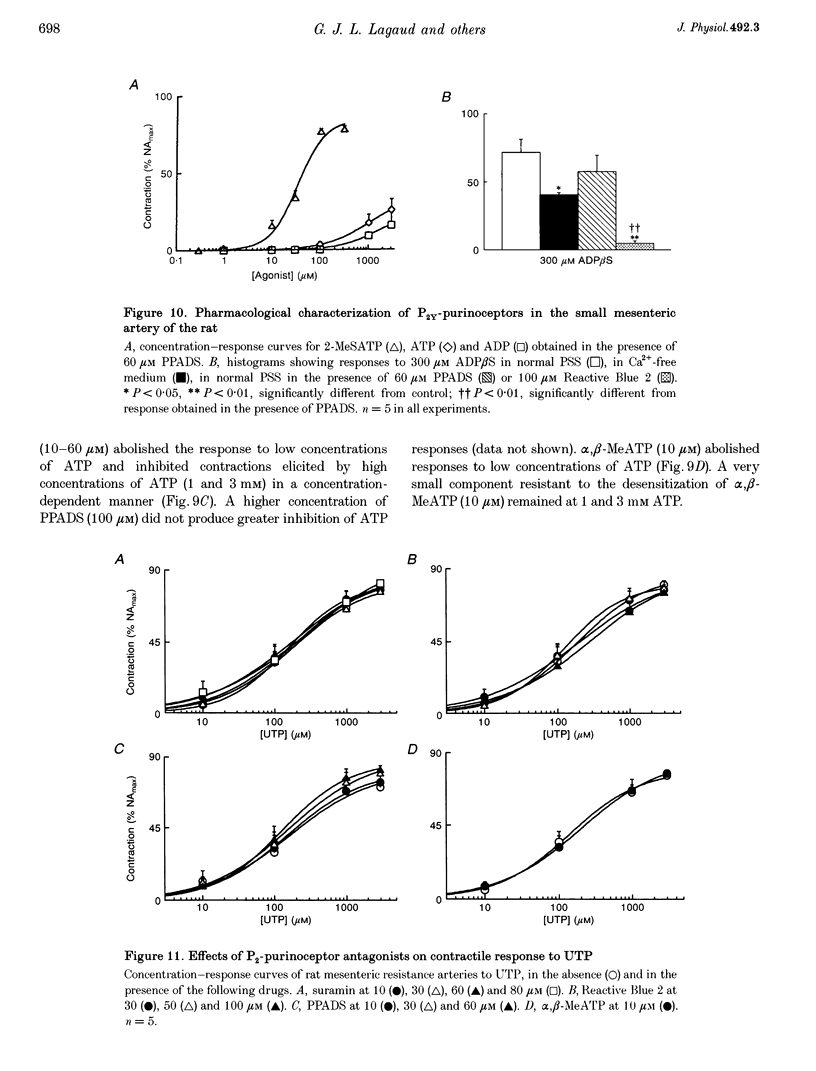





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriantsitohaina R., Andre P., Stoclet J. C. Pertussis toxin abolishes the effect of neuropeptide Y on rat resistance arteriole contraction. Am J Physiol. 1990 Nov;259(5 Pt 2):H1427–H1432. doi: 10.1152/ajpheart.1990.259.5.H1427. [DOI] [PubMed] [Google Scholar]
- Andriantsitohaina R., Lagaud G. J., Andre A., Muller B., Stoclet J. C. Effects of cGMP on calcium handling in ATP-stimulated rat resistance arteries. Am J Physiol. 1995 Mar;268(3 Pt 2):H1223–H1231. doi: 10.1152/ajpheart.1995.268.3.H1223. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Jones K. C., James T. W. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979 Jan 15;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriu C., André P., Schott C., Michel M., Stoclet J. C. ANG II receptor expression and function during phenotypic modulation of rat aortic smooth muscle cells. Am J Physiol. 1994 Feb;266(2 Pt 2):H631–H636. doi: 10.1152/ajpheart.1994.266.2.H631. [DOI] [PubMed] [Google Scholar]
- Cuthbert N. J., Gardiner P. J., Nash K., Poll C. T. Roles of Ca2+ influx and intracellular Ca2+ release in agonist-induced contractions in guinea pig trachea. Am J Physiol. 1994 Jun;266(6 Pt 1):L620–L627. doi: 10.1152/ajplung.1994.266.6.L620. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Cowen D. S., Meuller L. M. Activation of inositol phospholipid breakdown in HL60 cells by P2-purinergic receptors for extracellular ATP. Evidence for mediation by both pertussis toxin-sensitive and pertussis toxin-insensitive mechanisms. J Biol Chem. 1988 Dec 5;263(34):18108–18117. [PubMed] [Google Scholar]
- Fasolato C., Pizzo P., Pozzan T. Receptor-mediated calcium influx in PC12 cells. ATP and bradykinin activate two independent pathways. J Biol Chem. 1990 Nov 25;265(33):20351–20355. [PubMed] [Google Scholar]
- Juul B., Lüscher M. E., Aalkjaer C., Plesner L. Nucleotide hydrolytic activity of isolated intact rat mesenteric small arteries. Biochim Biophys Acta. 1991 Aug 26;1067(2):201–207. doi: 10.1016/0005-2736(91)90044-9. [DOI] [PubMed] [Google Scholar]
- Juul B., Plesner L., Aalkjaer C. Effects of ATP and UTP on [Ca2+]i, membrane potential and force in isolated rat small arteries. J Vasc Res. 1992 Sep-Oct;29(5):385–395. doi: 10.1159/000158955. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Leff P. How should P2X purinoceptors be classified pharmacologically? Trends Pharmacol Sci. 1995 May;16(5):168–174. doi: 10.1016/s0165-6147(00)89010-0. [DOI] [PubMed] [Google Scholar]
- Kitajima S., Ozaki H., Karaki H. The effects of ATP and alpha,beta-methylene-ATP on cytosolic Ca2+ level and force in rat isolated aorta. Br J Pharmacol. 1993 Sep;110(1):263–268. doi: 10.1111/j.1476-5381.1993.tb13803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Cantley L. C., Talamo B. R. Extracellular ATP elevates intracellular free calcium in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):523–530. doi: 10.1016/0006-291x(87)90399-8. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Grégoire G., Loirand G. Release of Ca2+ from intracellular store in smooth muscle cells of rat portal vein by ATP-induced Ca2+ entry. Br J Pharmacol. 1994 Oct;113(2):457–462. doi: 10.1111/j.1476-5381.1994.tb17011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp B., Enyedi A., Kovács T., Sarkadi B., Wuytack F., Thastrup O., Gárdos G., Bredoux R., Levy-Toledano S., Enouf J. Demonstration of two forms of calcium pumps by thapsigargin inhibition and radioimmunoblotting in platelet membrane vesicles. J Biol Chem. 1991 Aug 5;266(22):14593–14596. [PubMed] [Google Scholar]
- Suzuki H. Electrical responses of smooth muscle cells of the rabbit ear artery to adenosine triphosphate. J Physiol. 1985 Feb;359:401–415. doi: 10.1113/jphysiol.1985.sp015592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawada Y., Furukawa K., Shigekawa M. ATP-induced calcium transient in cultured rat aortic smooth muscle cells. J Biochem. 1987 Dec;102(6):1499–1509. doi: 10.1093/oxfordjournals.jbchem.a122197. [DOI] [PubMed] [Google Scholar]
- Tribe R. M., Borin M. L., Blaustein M. P. Functionally and spatially distinct Ca2+ stores are revealed in cultured vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5908–5912. doi: 10.1073/pnas.91.13.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994 Oct 6;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Ziganshin A. U., Hoyle C. H., Lambrecht G., Mutschler E., Bümert H. G., Burnstock G. Selective antagonism by PPADS at P2X-purinoceptors in rabbit isolated blood vessels. Br J Pharmacol. 1994 Mar;111(3):923–929. doi: 10.1111/j.1476-5381.1994.tb14827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol Sci. 1991 Sep;12(9):319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]
- von der Weid P. Y., Serebryakov V. N., Orallo F., Bergmann C., Snetkov V. A., Takeda K. Effects of ATP on cultured smooth muscle cells from rat aorta. Br J Pharmacol. 1993 Mar;108(3):638–645. doi: 10.1111/j.1476-5381.1993.tb12854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


