Abstract
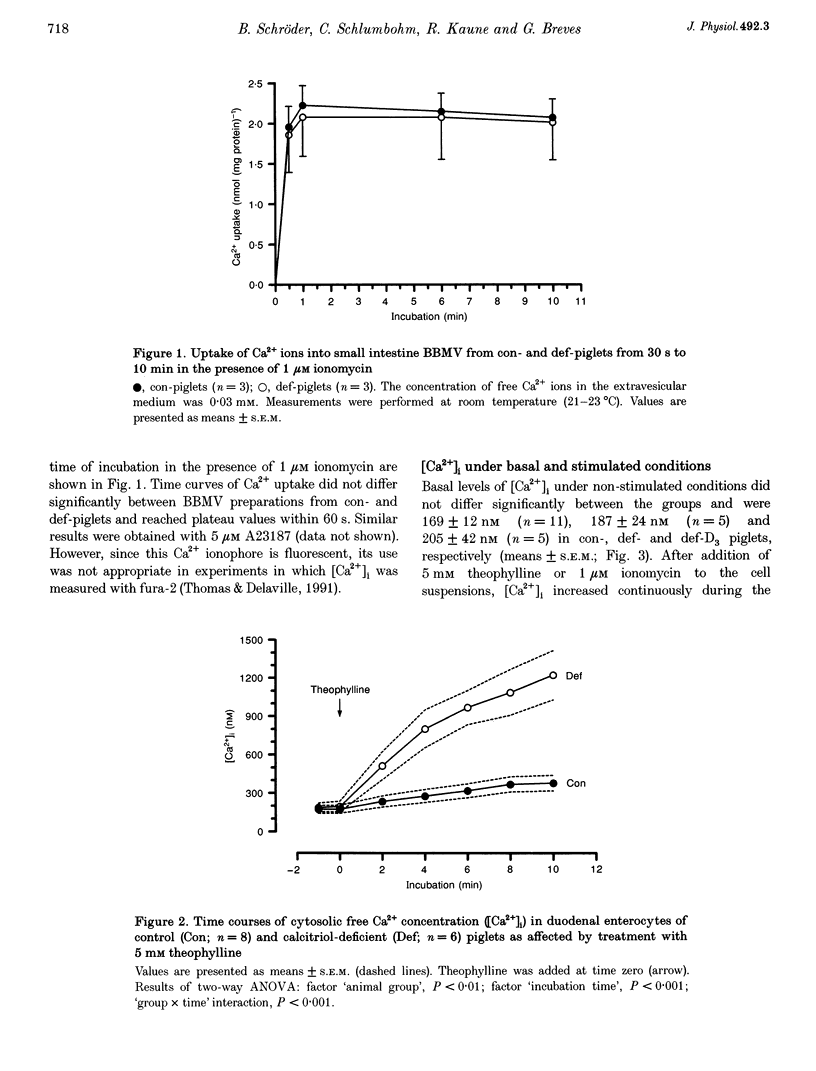
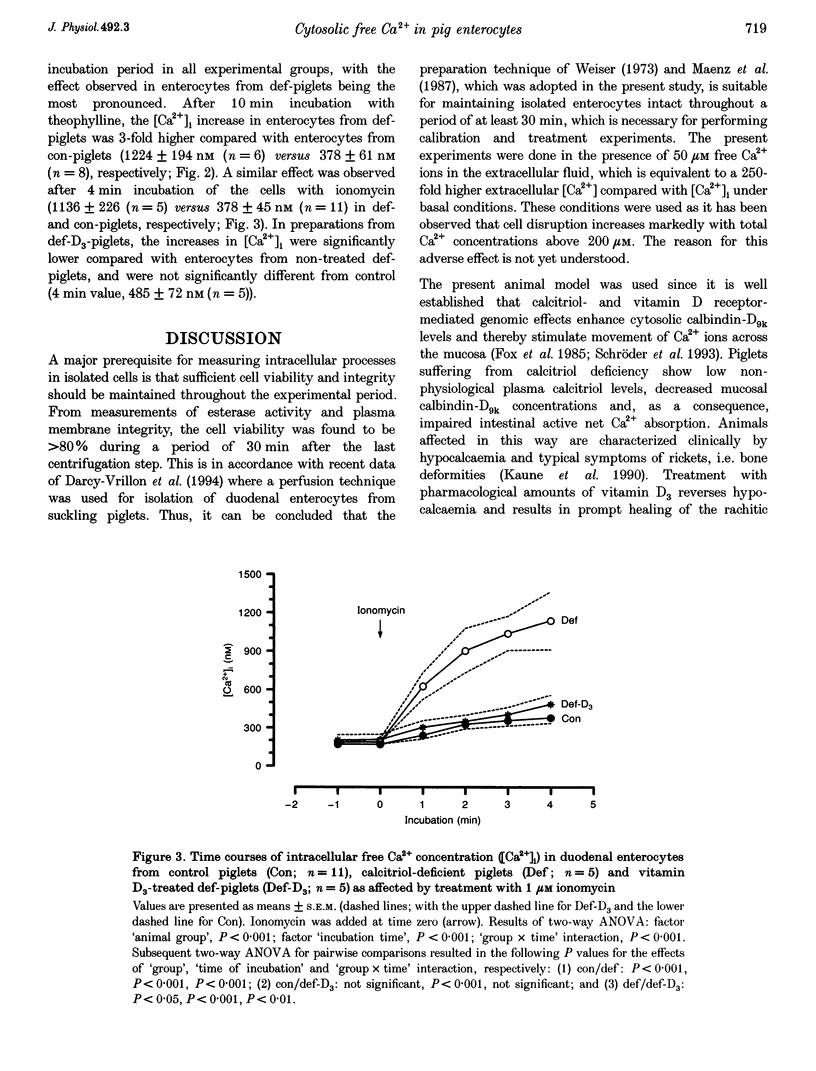
1. The aim of the present study was to test whether the vitamin D-dependent Ca(2+)-binding protein calbindin-D9k could function as an important cytosolic Ca2+ buffer in duodenal enterocytes while facilitating transepithelial active transport of Ca2+ ions. For the investigations we used dual-wavelength, fluorescence ratio imaging, with fura-2 as the Ca(2+)-sensitive dye, to measure changes in cytosolic concentrations of free Ca2+ ions ([Ca2+]i) in isolated pig duodenal enterocytes affected by different cytosolic calbindin-D9k concentrations. 2. Epithelial cells were obtained from weaned piglets with normal calbindin-D9k concentrations (con-piglets), from piglets with low calbindin-D9k levels due to inherited calcitriol deficiency caused by defective renal 25-hydroxycholecalciferol D3-1 alpha-hydroxylase activity (def-piglets), and from piglets with reconstituted calbindin-D9k concentrations, i.e. def-animals treated with high doses of vitamin D3 which elevated plasma calcitriol levels by extrarenal production (def-D3-piglets). Basal levels of [Ca2+]i ranged between 170 and 205 nM and did not differ significantly between the groups. 3. After addition of 5 mM theophylline, the [Ca2+]i in enterocytes from con-piglets doubled during the 10 min incubation. This effect, however, was three times higher in enterocytes from def-piglets compared with those from con-piglets. Similar results were obtained after 4 min incubation of enterocytes from con- and def-piglets in the presence of 1 microM ionomycin. In preparations from def-D3-piglets, ionomycin-induced increases in [Ca2+]i were significantly lower compared with enterocytes from def-piglets and were not different from the control values. 4. From the results, substantial support is given for the hypothesis that one of the major functions of mucosal calbindin-D9k is the effective buffering of Ca2+ ions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amoah J., Williams C., Long R. G. Calmodulin content and activity in normal and coeliac duodenum. Gut. 1992 Mar;33(3):303–306. doi: 10.1136/gut.33.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenzio R. A., Liacos J., Berschneider H. M., Whipp S. C., Robertson D. C. Effect of heat-stable enterotoxin of Escherichia coli and theophylline on ion transport in porcine small intestine. Can J Comp Med. 1984 Jan;48(1):14–22. [PMC free article] [PubMed] [Google Scholar]
- Argenzio R. A., Whipp S. C. Effect of Escherichia coli heat-stable enterotoxin, cholera toxin and theophylline on ion transport in porcine colon. J Physiol. 1981 Nov;320:469–487. doi: 10.1113/jphysiol.1981.sp013962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasitus T. A., Dudeja P. K., Eby B., Lau K. Correction by 1-25-dihydroxycholecalciferol of the abnormal fluidity and lipid composition of enterocyte brush border membranes in vitamin D-deprived rats. J Biol Chem. 1986 Dec 15;261(35):16404–16409. [PubMed] [Google Scholar]
- Bryant D. T., Andrews P. A simple procedure for purifying mammalian duodenal Ca2+-binding proteins on a 100 mg scale and an investigation of the stoichiometry of their high-affinity binding of Ca2+ ions. Biochem J. 1983 Jun 1;211(3):709–716. doi: 10.1042/bj2110709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Darcy-Vrillon B., Posho L., Morel M. T., Bernard F., Blachier F., Meslin J. C., Duée P. H. Glucose, galactose, and glutamine metabolism in pig isolated enterocytes during development. Pediatr Res. 1994 Aug;36(2):175–181. doi: 10.1203/00006450-199408000-00007. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Welsh M. J. Ca2+ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol. 1986;48:135–150. doi: 10.1146/annurev.ph.48.030186.001031. [DOI] [PubMed] [Google Scholar]
- Fox J., Maunder E. M., Randall V. A., Care A. D. Vitamin D-dependent rickets type I in pigs. Clin Sci (Lond) 1985 Nov;69(5):541–548. doi: 10.1042/cs0690541. [DOI] [PubMed] [Google Scholar]
- Gleason W. A., Jr, Lankford G. L. Rat intestinal calcium-binding protein: rapid purification with AG MP-1 ion-exchange chromatography. Anal Biochem. 1981 Sep 15;116(2):256–263. doi: 10.1016/0003-2697(81)90353-5. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kaune R., Kassianoff I., Schröder B., Harmeyer J. The effects of 1,25-dihydroxyvitamin D-3 deficiency on Ca(2+)-transport and Ca(2+)-uptake into brush-border membrane vesicles from pig small intestine. Biochim Biophys Acta. 1992 Aug 24;1109(2):187–194. doi: 10.1016/0005-2736(92)90082-w. [DOI] [PubMed] [Google Scholar]
- Kaune R., Schroeder B., Harmeyer J. Binding properties of plasma vitamin D-binding protein and intestinal 1,25-dihydroxyvitamin D3 receptor in piglets with pseudo-vitamin D-deficiency rickets, type I: treatment effects with pharmacological doses of vitamin D3. Arch Biochem Biophys. 1990 Nov 1;282(2):326–332. doi: 10.1016/0003-9861(90)90124-h. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maenz D. D., Gabriel S. E., Forsyth G. W. Calcium transport affinity, ion competition and cholera toxin effects on cytosolic Ca concentration. J Membr Biol. 1987;96(3):243–249. doi: 10.1007/BF01869306. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D3 on phospholipid metabolism in chick duodenal mucosal cell. Relationship to its mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3354–3360. [PubMed] [Google Scholar]
- Schoenmakers T. J., Visser G. J., Flik G., Theuvenet A. P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992 Jun;12(6):870-4, 876-9. [PubMed] [Google Scholar]
- Schröder B., Kaune R., Harmeyer J. Effects of calcitriol on stimulation of ion transport in pig jejunal mucosa. J Physiol. 1991 Feb;433:451–465. doi: 10.1113/jphysiol.1991.sp018437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder B., Kaune R., Schlumbohm C., Breves G., Harmeyer J. Evidence for vitamin D-independent active calcium absorption in newborn piglets. Calcif Tissue Int. 1993 Apr;52(4):305–309. doi: 10.1007/BF00296656. [DOI] [PubMed] [Google Scholar]
- Semrad C. E., Chang E. B. Calcium-mediated cyclic AMP inhibition of Na-H exchange in small intestine. Am J Physiol. 1987 Mar;252(3 Pt 1):C315–C322. doi: 10.1152/ajpcell.1987.252.3.C315. [DOI] [PubMed] [Google Scholar]
- Timmermans J. A., Kaune R., Bindels R. J., van Os C. H. Quantification of Ca(2+)-ATPases in porcine duodenum. Effects of 1,25(OH)2D3 deficiency. Biochim Biophys Acta. 1991 Jun 18;1065(2):177–184. doi: 10.1016/0005-2736(91)90228-z. [DOI] [PubMed] [Google Scholar]


