Abstract
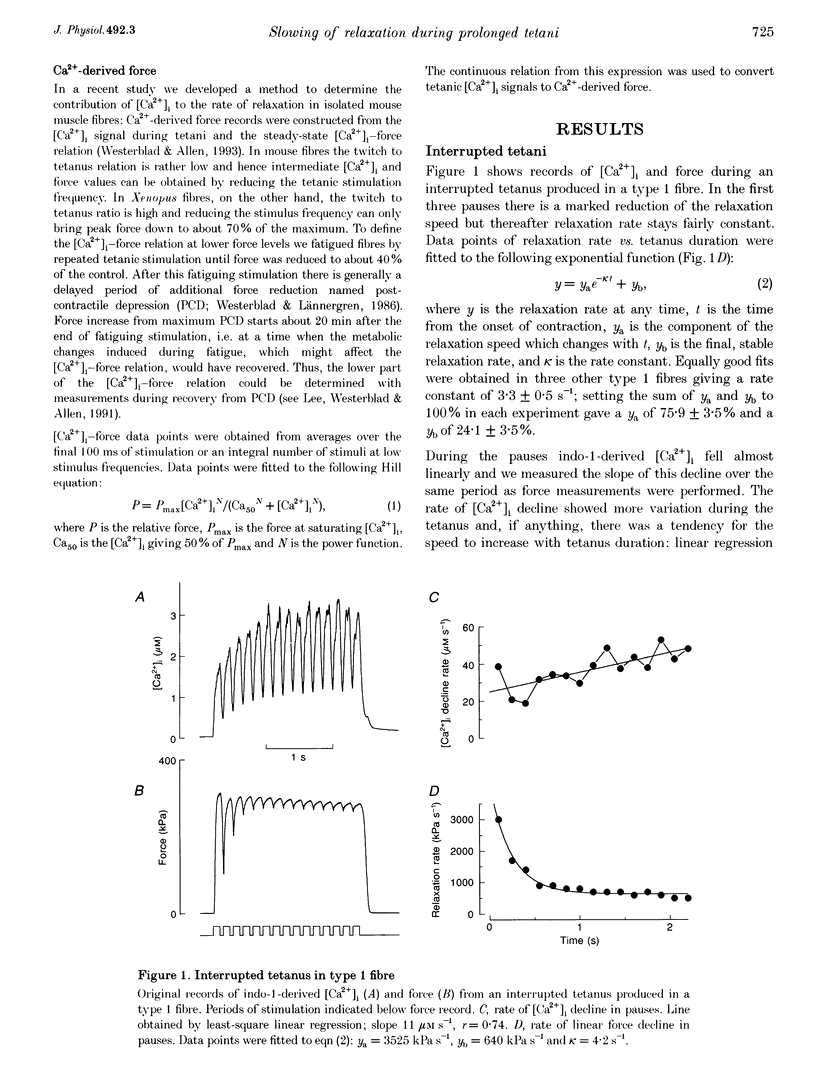
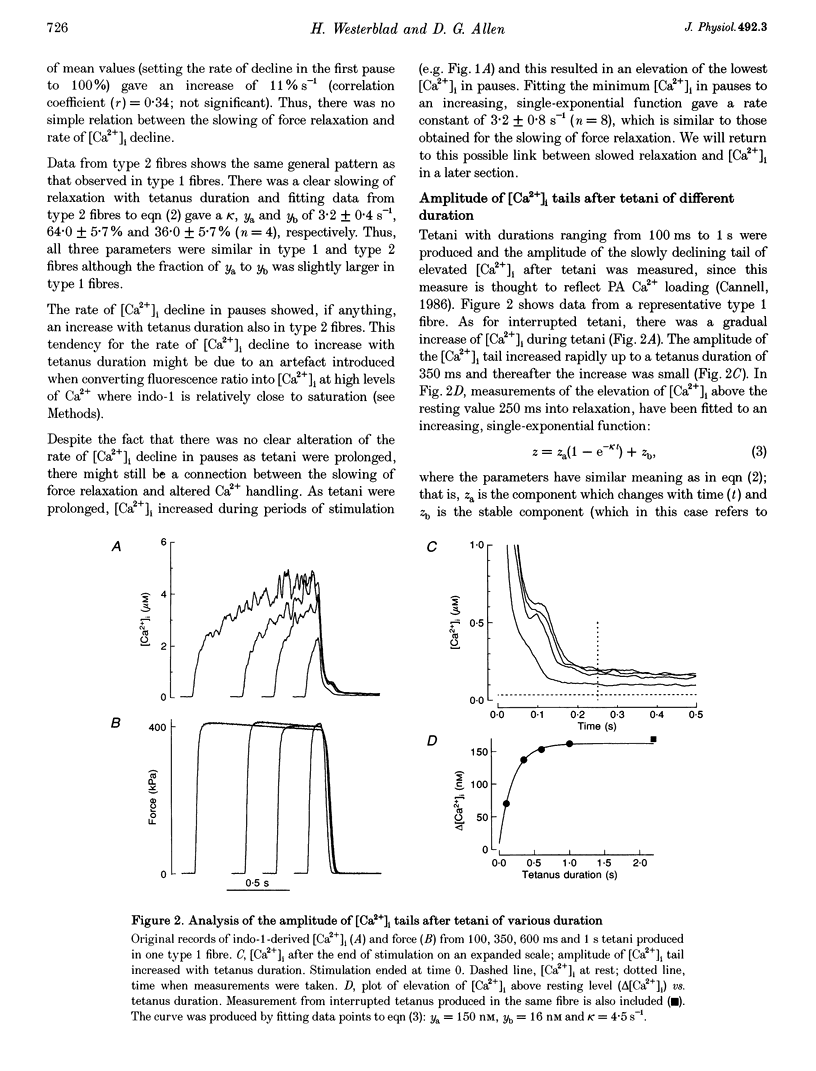
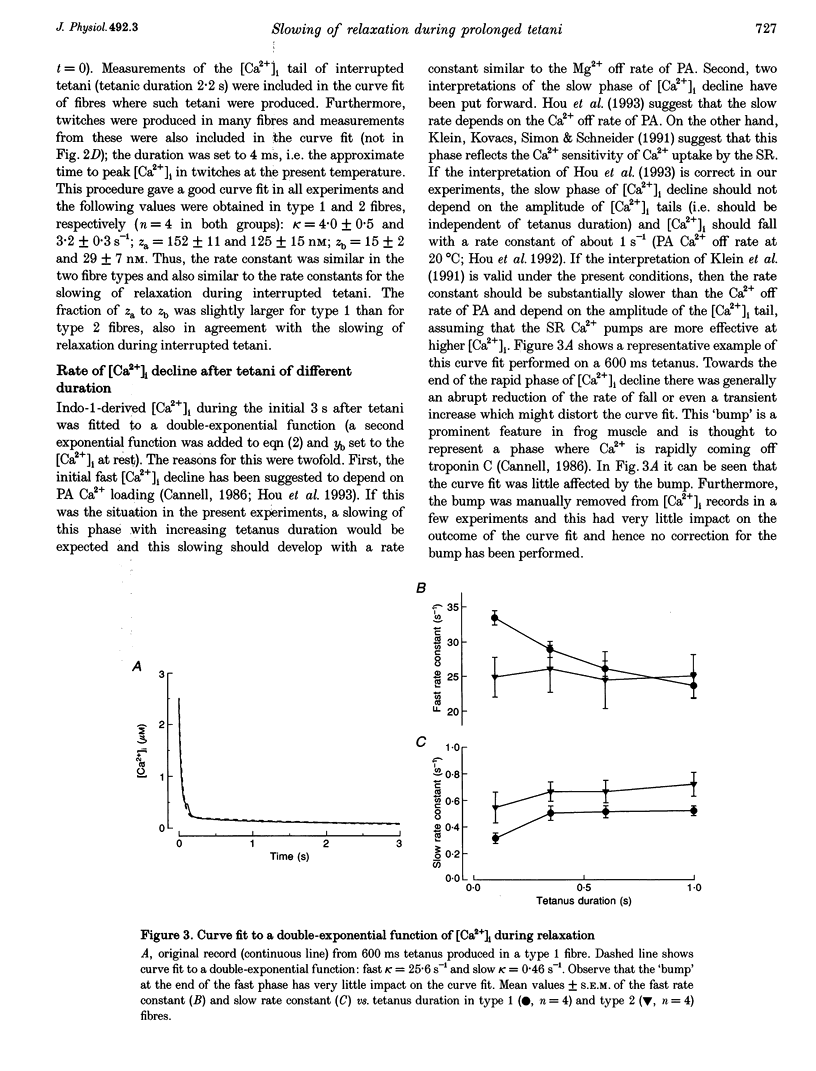
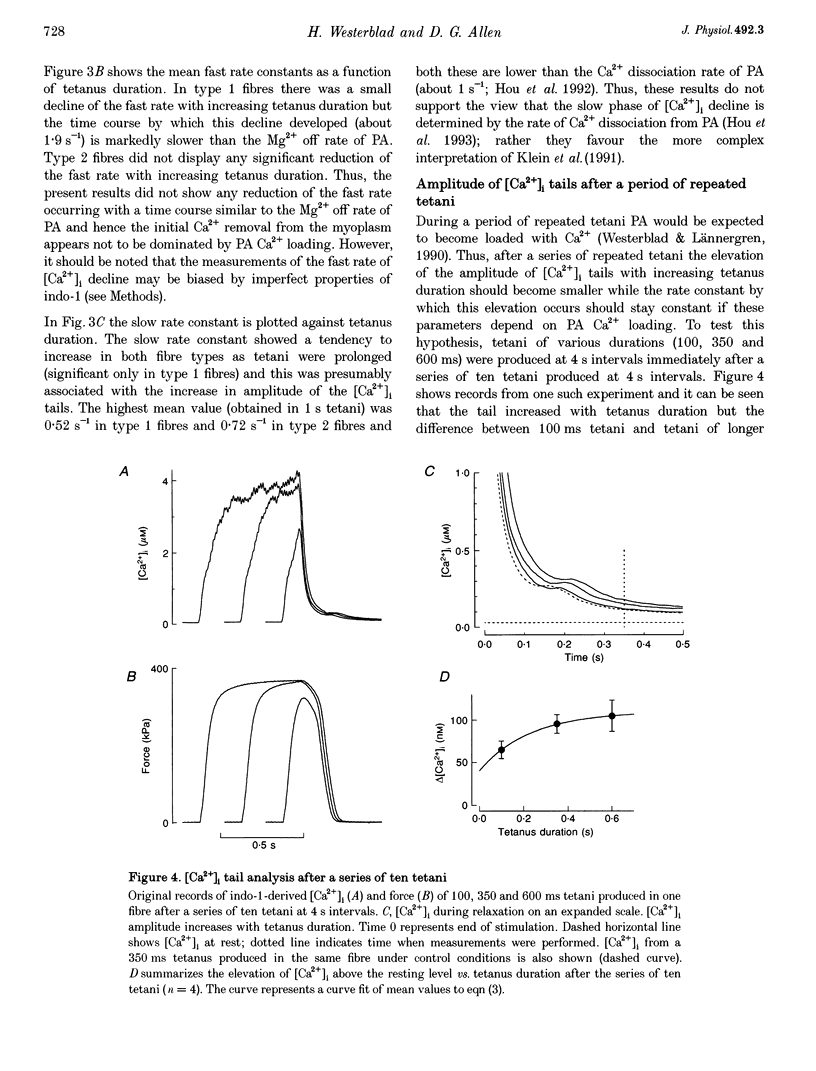
1. Parvalbumin (PA) has been proposed to take up Ca2+ and enhance skeletal muscle relaxation in brief contractions; as the duration of the contraction is increased, PA will become saturated with Ca2+ and no longer contribute to relaxation which therefore will be slowed. The rate of Ca2+ loading of PA is determined by the Mg2+ off rate (about 4 s-1 at 22 degrees C). In the present study we produced prolonged tetani in intact, single fibres of Xenopus frogs while measuring force and the free myoplasmic [Ca2+] ([Ca2+]i) with indo-1. 2. Mean rate constants of slowing of force relaxation with increasing tetanus duration ranged between 3.2 and 4.8 s-1, thus, similar to the Mg2+ off rate of PA. 3. The amplitude of the tail of [Ca2+]i after tetani increased with tetanus duration. This increase developed with a rate constant similar to the Mg2+ off rate of PA 4. Steady-state force-[Ca2+]i curves were produced from tetani of various frequencies and tetani produced when force was depressed after fatiguing stimulation. These curves were used to convert [Ca2+]i records into Ca(2+)-derived force. Relaxation of Ca(2+)-derived force was slowed following a time course similar to that of real force. The lag between Ca(2+)-derived and real force during relaxation was not affected by tetanus duration. 5. Tails of elevated [Ca2+]i after tetani were used to analyse the function of the SR Ca2+ pumps. This analysis showed a marked decline in the rate of Ca2+ uptake with prolonged tetani. 6. In conclusion, in Xenopus fibres the slowing of relaxation with increasing tetanus duration can be explained by altered Ca2+ handling due to PA Ca2+ loading and impaired SR Ca2+ uptake. This contrasts to our previous results in mouse fibres and the difference can be explained by a markedly lower rate of SR Ca2+ uptake resulting in higher tetanic [Ca2+]i in Xenopus fibres.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C. The heat production associated with the maintenance of a prolonged contraction and the extra heat produced during large shortening. J Physiol. 1951 Feb;112(3-4):438–445. doi: 10.1113/jphysiol.1951.sp004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Westerblad H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. J Physiol. 1995 Sep 1;487(Pt 2):331–342. doi: 10.1113/jphysiol.1995.sp020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., Stéfani E. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol. 1988 Apr;398:441–473. doi: 10.1113/jphysiol.1988.sp017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer M. W., Neering I. R. Actions of caffeine on fast- and slow-twitch muscles of the rat. J Physiol. 1989 Sep;416:435–454. doi: 10.1113/jphysiol.1989.sp017770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J. M. Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochim Biophys Acta. 1985 Jun 3;811(2):97–145. doi: 10.1016/0304-4173(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Haiech J., Derancourt J., Pechère J. F., Demaille J. G. Magnesium and calcium binding to parvalbumins: evidence for differences between parvalbumins and an explanation of their relaxing function. Biochemistry. 1979 Jun 26;18(13):2752–2758. doi: 10.1021/bi00580a010. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W., Berchtold M. W., Rowlerson A. M. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Effect of temperature on relaxation rate and Ca2+, Mg2+ dissociation rates from parvalbumin of frog muscle fibres. J Physiol. 1992 Apr;449:399–410. doi: 10.1113/jphysiol.1992.sp019092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Rapid 'give' and the tension 'shoulder' in the relaxation of frog muscle fibres. J Physiol. 1970 Sep;210(1):32P–33P. [PubMed] [Google Scholar]
- Jackson A. P., Timmerman M. P., Bagshaw C. R., Ashley C. C. The kinetics of calcium binding to fura-2 and indo-1. FEBS Lett. 1987 May 25;216(1):35–39. doi: 10.1016/0014-5793(87)80752-4. [DOI] [PubMed] [Google Scholar]
- Klein M. G., Kovacs L., Simon B. J., Schneider M. F. Decline of myoplasmic Ca2+, recovery of calcium release and sarcoplasmic Ca2+ pump properties in frog skeletal muscle. J Physiol. 1991 Sep;441:639–671. doi: 10.1113/jphysiol.1991.sp018771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. G., Simon B. J., Szucs G., Schneider M. F. Simultaneous recording of calcium transients in skeletal muscle using high- and low-affinity calcium indicators. Biophys J. 1988 Jun;53(6):971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N., Harkins A. B., Baylor S. M. Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibers. Biophys J. 1993 Jun;64(6):1934–1960. doi: 10.1016/S0006-3495(93)81564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Westerblad H., Allen D. G. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol. 1991 Feb;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Elzinga G., Stienen G. J. Force relaxation, labile heat and parvalbumin content of skeletal muscle fibres of Xenopus laevis. J Physiol. 1993 Apr;463:123–140. doi: 10.1113/jphysiol.1993.sp019587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Force and membrane potential during and after fatiguing, continuous high-frequency stimulation of single Xenopus muscle fibres. Acta Physiol Scand. 1986 Nov;128(3):359–368. doi: 10.1111/j.1748-1716.1986.tb07989.x. [DOI] [PubMed] [Google Scholar]
- Pagala M. K. Effect of length and caffeine on isometric tetanus relaxation of frog sartorius muscles. Biochim Biophys Acta. 1980 Jun 10;591(1):177–186. doi: 10.1016/0005-2728(80)90231-5. [DOI] [PubMed] [Google Scholar]
- Peckham M., Woledge R. C. Labile heat and changes in rate of relaxation of frog muscles. J Physiol. 1986 May;374:123–135. doi: 10.1113/jphysiol.1986.sp016070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonides W. S., van Hardeveld C. Identification and quantification in single muscle fibers of four isoforms of parvalbumin in the iliofibularis muscle of Xenopus laevis. Biochim Biophys Acta. 1989 Oct 5;998(2):137–144. doi: 10.1016/0167-4838(89)90265-3. [DOI] [PubMed] [Google Scholar]
- Stienen G. J., van Graas I. A., Elzinga G. Uptake and caffeine-induced release of calcium in fast muscle fibers of Xenopus laevis: effects of MgATP and P(i). Am J Physiol. 1993 Sep;265(3 Pt 1):C650–C657. doi: 10.1152/ajpcell.1993.265.3.C650. [DOI] [PubMed] [Google Scholar]
- Stienen G. J., van der Laarse W. J., Diegenbach P. C., Elzinga G. Relation between force and calcium ion concentration in different fibre types of the iliofibularis muscle of Xenopus laevis. Pflugers Arch. 1987 Jan;408(1):63–67. doi: 10.1007/BF00581841. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Methods for calibration of fluorescent calcium indicators in skeletal muscle fibers. Biophys J. 1994 Mar;66(3 Pt 1):926–928. doi: 10.1016/s0006-3495(94)80870-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Relaxation, [Ca2+]i and [Mg2+]i during prolonged tetanic stimulation of intact, single fibres from mouse skeletal muscle. J Physiol. 1994 Oct 1;480(Pt 1):31–43. doi: 10.1113/jphysiol.1994.sp020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. J Physiol. 1994 Jan 15;474(2):291–301. doi: 10.1113/jphysiol.1994.sp020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Decreased Ca2-buffering contributes to slowing of relaxation in fatigued Xenopus muscle fibres. Acta Physiol Scand. 1990 May;139(1):243–244. doi: 10.1111/j.1748-1716.1990.tb08918.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Force and membrane potential during and after fatiguing, intermittent tetanic stimulation of single Xenopus muscle fibres. Acta Physiol Scand. 1986 Nov;128(3):369–378. doi: 10.1111/j.1748-1716.1986.tb07990.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Slowing of relaxation during fatigue in single mouse muscle fibres. J Physiol. 1991 Mar;434:323–336. doi: 10.1113/jphysiol.1991.sp018472. [DOI] [PMC free article] [PubMed] [Google Scholar]


