Abstract
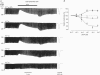
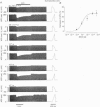
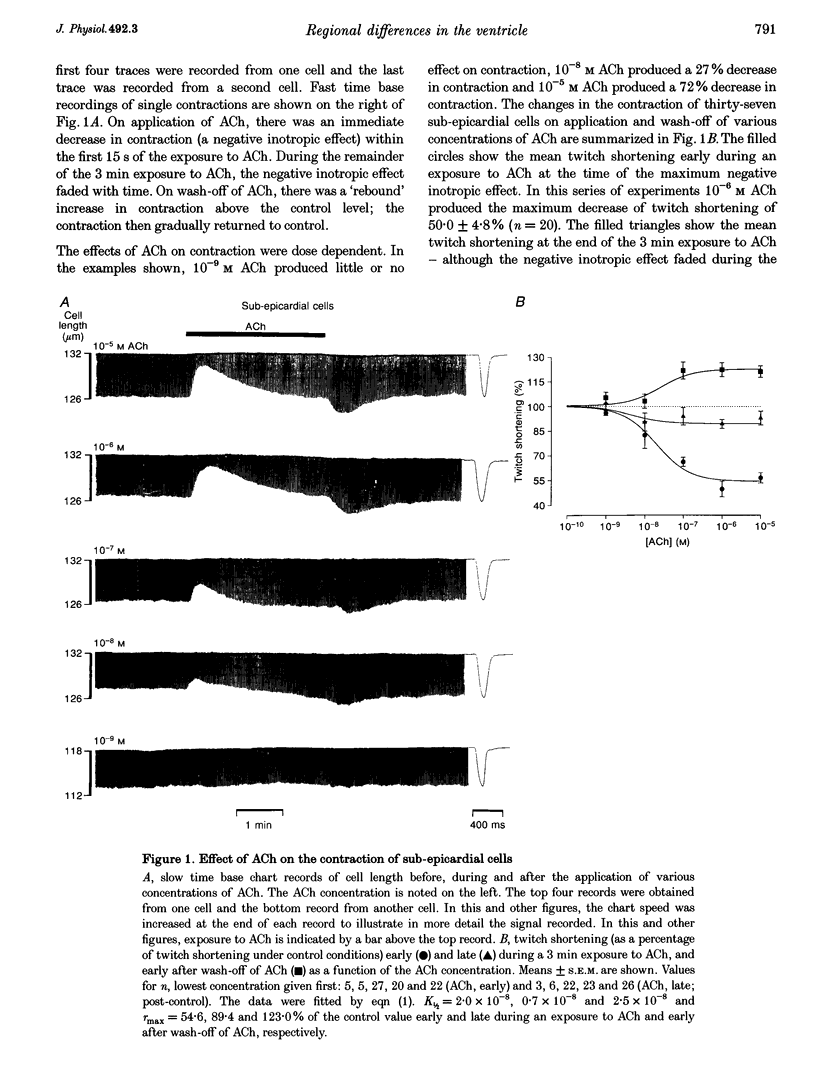
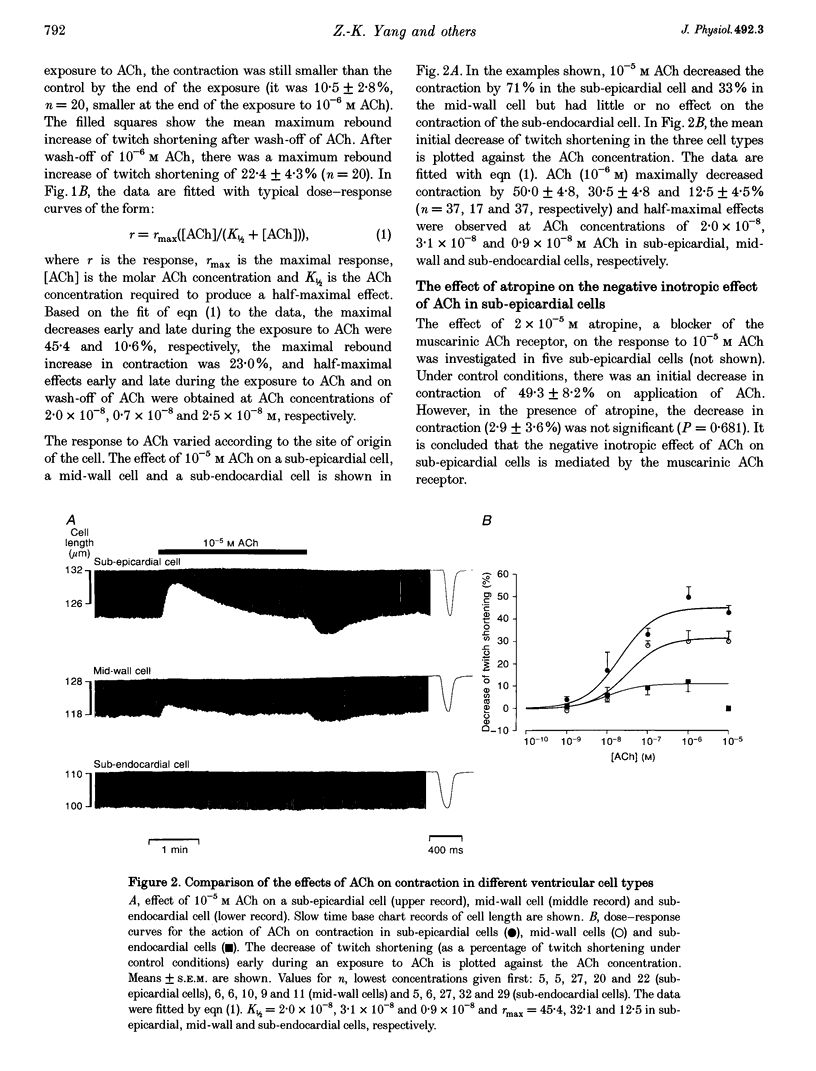
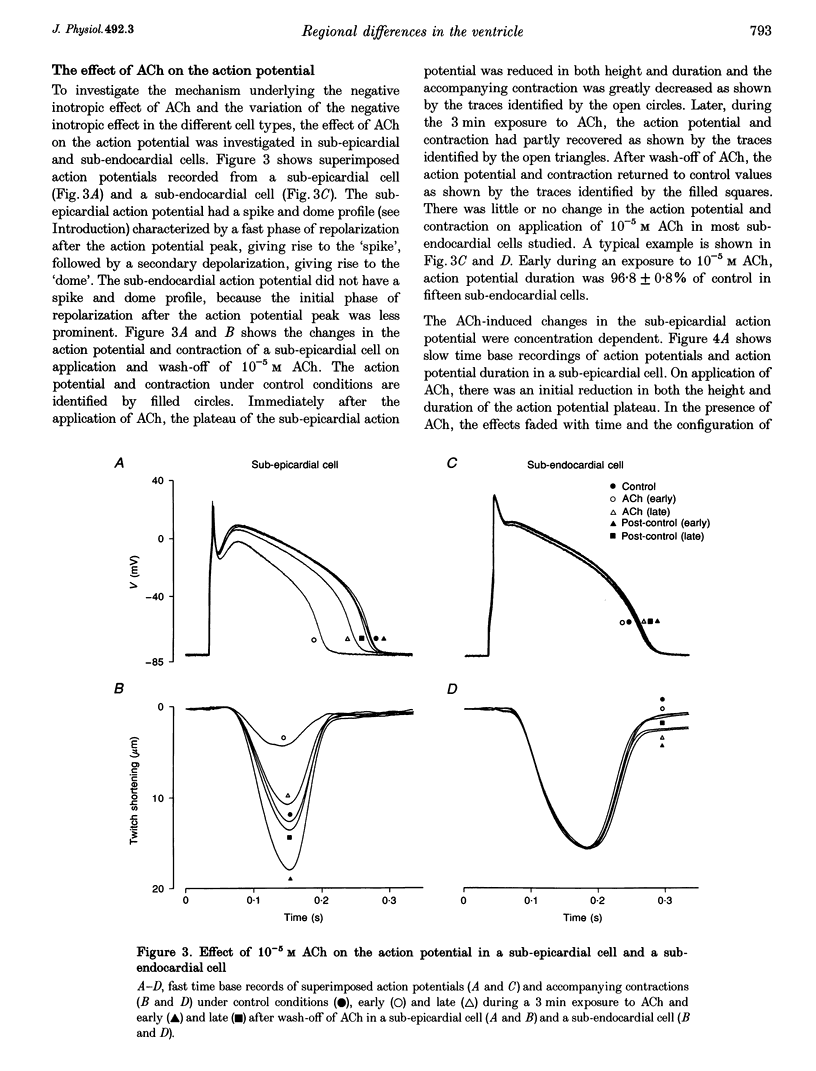
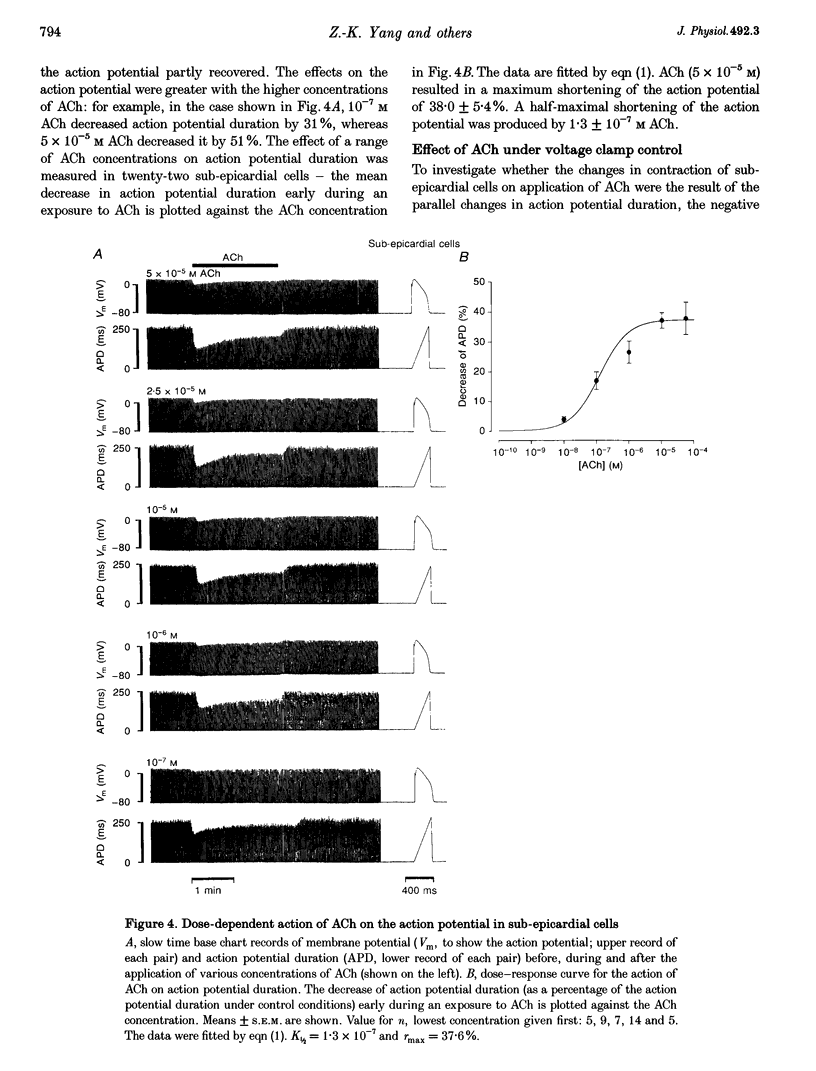
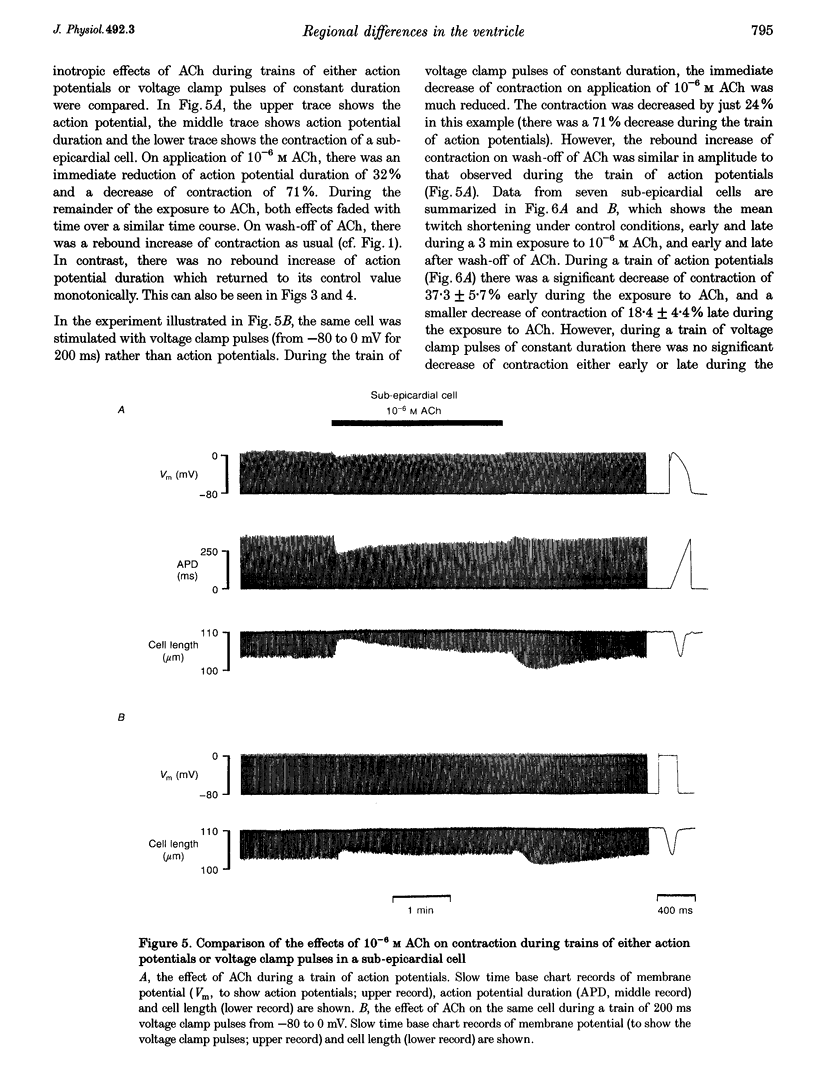
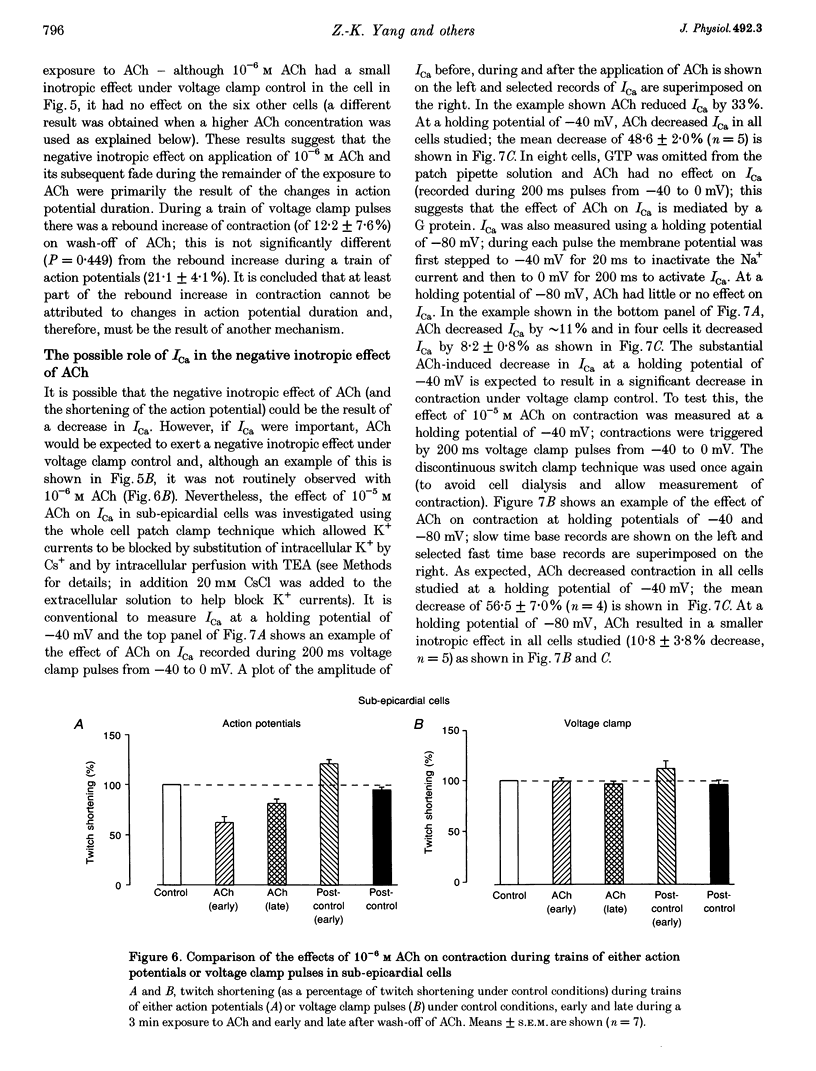
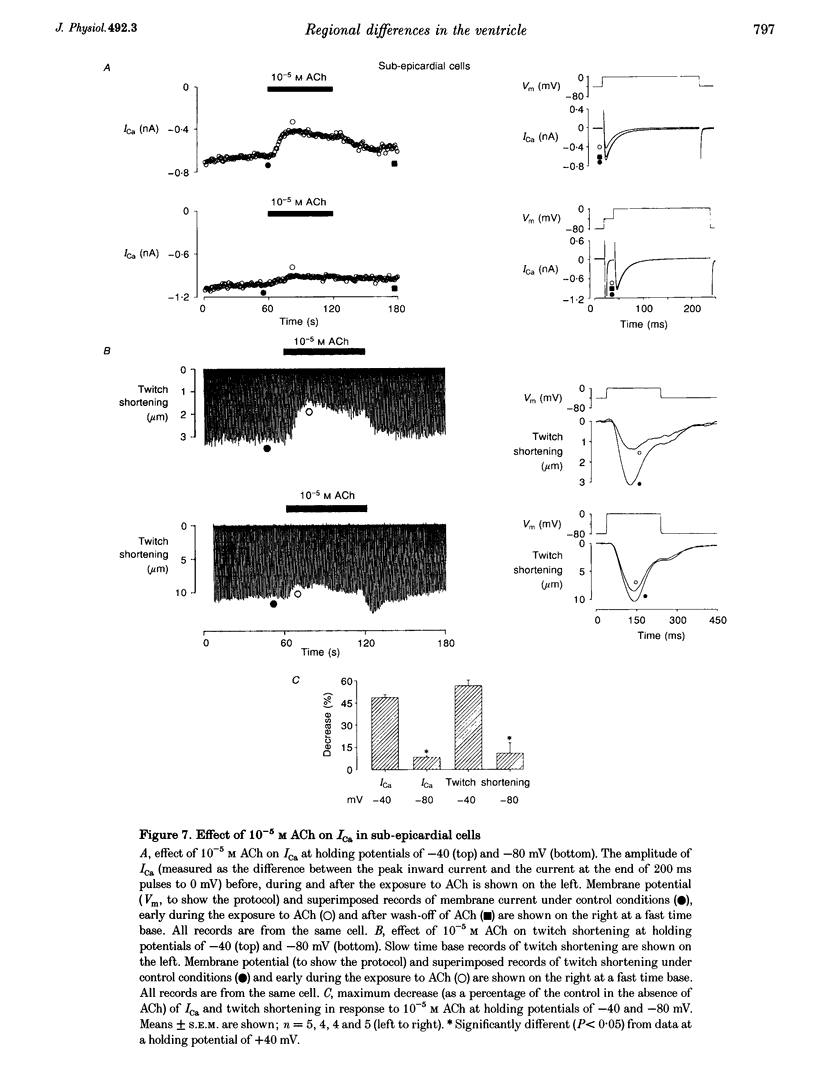
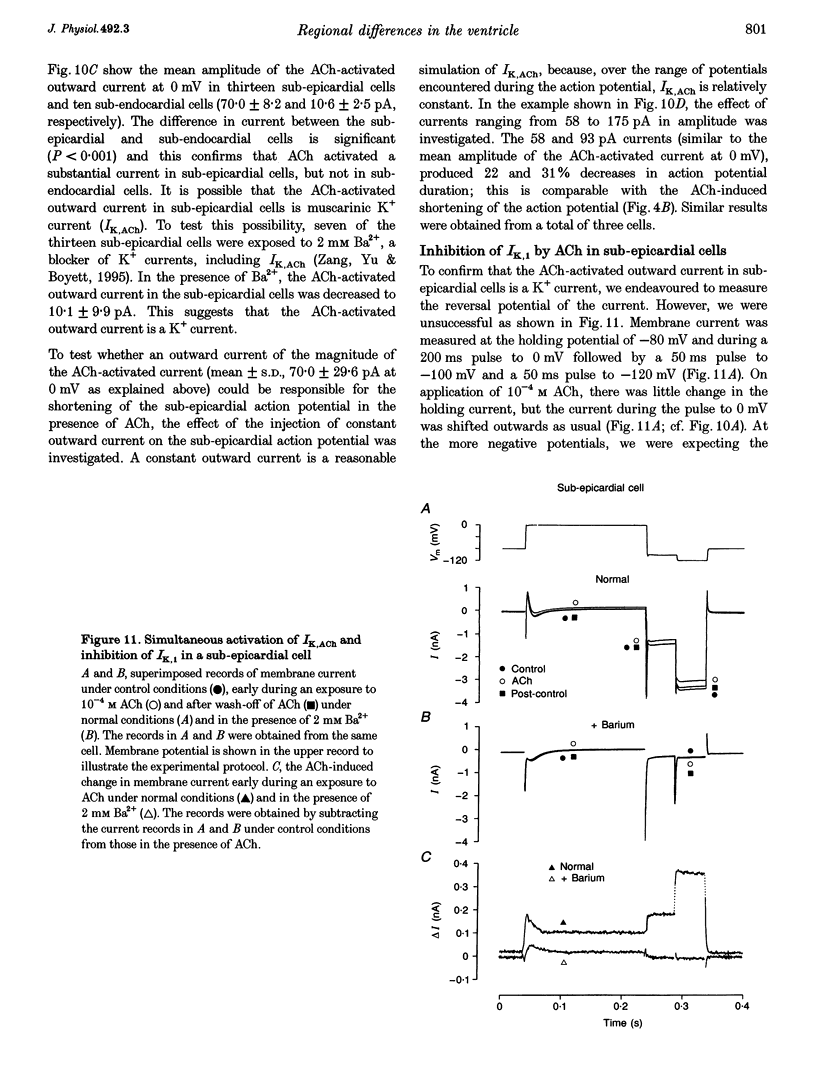
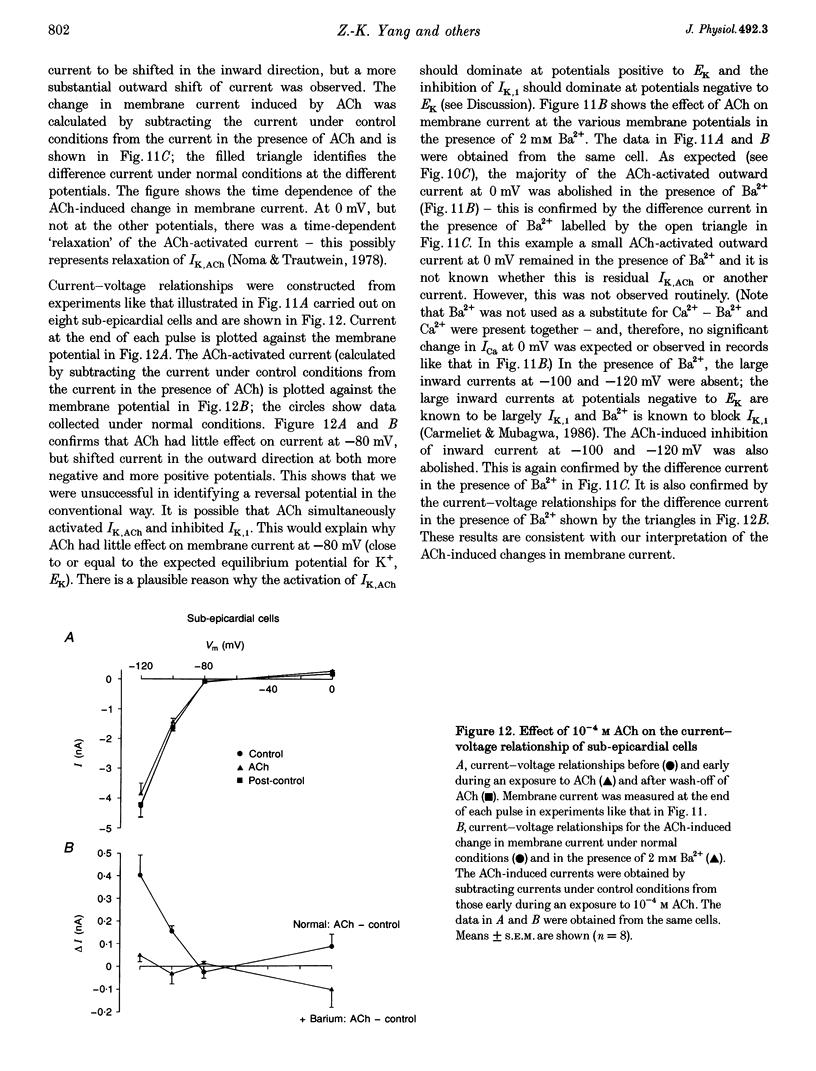
1. Regional differences in the effects of ACh on sub-epicardial, mid-wall and sub-endocardial cells of the dog left ventricle have been studied. 2. ACh produced a dose-dependent, atropine-sensitive negative inotropic effect that was greatest in sub-epicardial cells and small or absent in sub-endocardial cells. 3. In sub-epicardial (but not sub-endocardial) cells, ACh also resulted in a dose-dependent decrease in action potential duration. The inotropic effect of ACh on sub-epicardial cells was primarily the result of the decrease of action potential duration, because during trains of voltage clamp pulses the inotropic effect of ACh was reduced or abolished. At a holding potential of -80 mV, 10(-5)M ACh decreased L-type Ca2+ current by approximately 8% and this is thought to be responsible for the small inotropic effect during trains of pulses. 4. Although 4-AP, a blocker of the transient outward current (I(to)), abolished the "spike and dome' morphology of the sub-epicardial action potential, it had little or no effect on the actions of ACh on sub-epicardial cells. ACh had no effect on I(to) in sub-epicardial cells in voltage clamp experiments. 5. ACh activated a Ba(2+)-sensitive outward current (IK,ACh) in sub-epicardial cells, but little or no such current in sub-endocardial cells. In sub-epicardial cells, ACh also inhibited the inward rectifier current, IK,1. 6. It is concluded that in left ventricular sub-epicardial cells, ACh activates IK,ACh. This results in a shortening of the action potential and, therefore, a negative inotropic effect. In subendocardial cells, ACh activates little or no IK,ACh and, therefore, it has little or no negative inotropic effect. This may result from a regional variation in the expression of the muscarinic K+ channel.
Full text
PDF

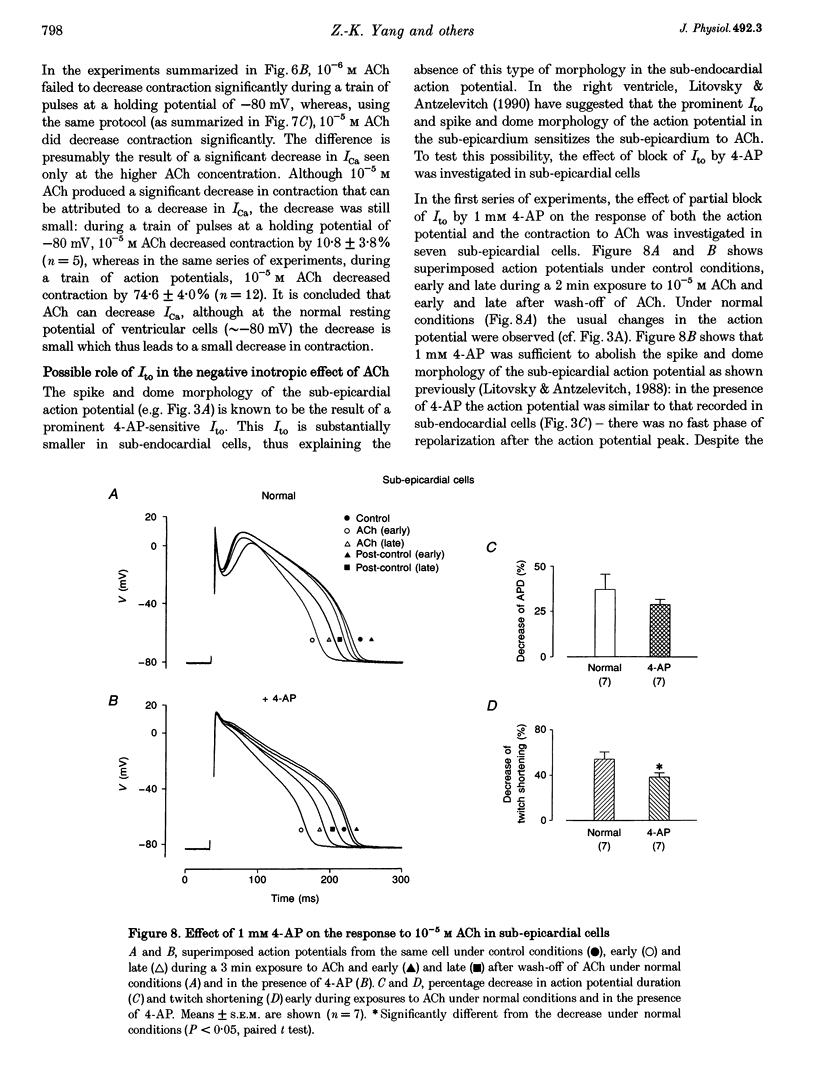
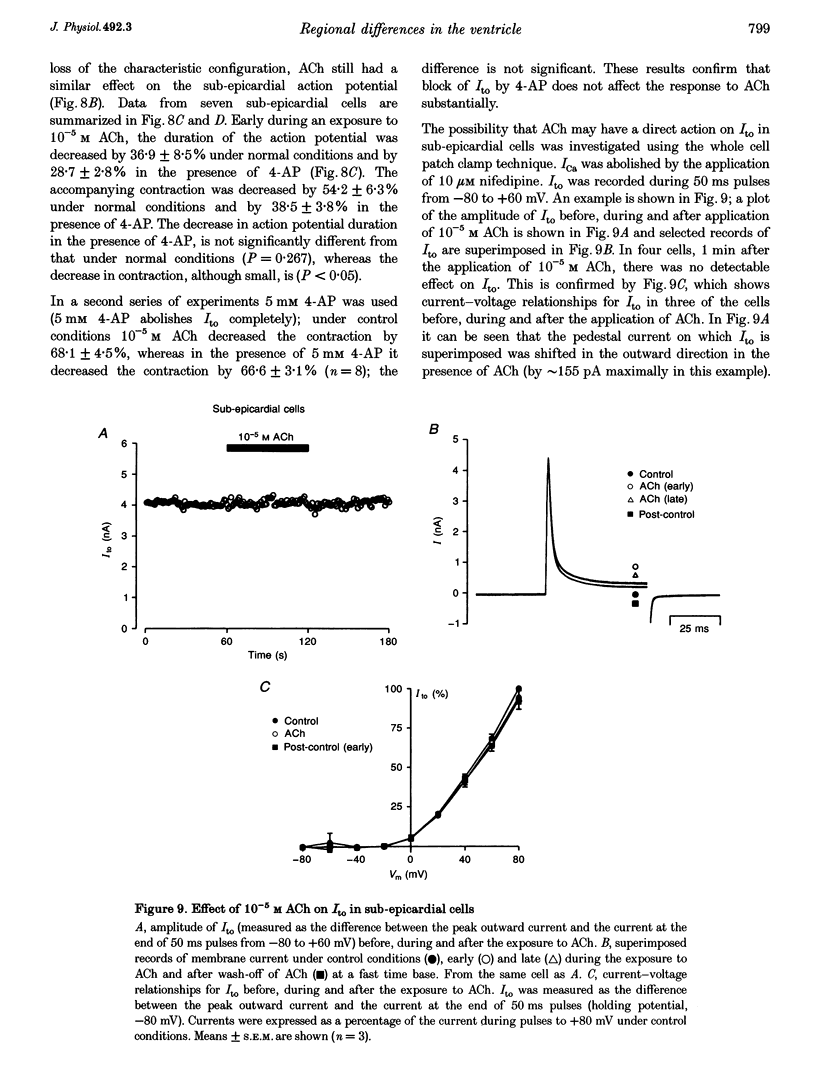




Images in this article
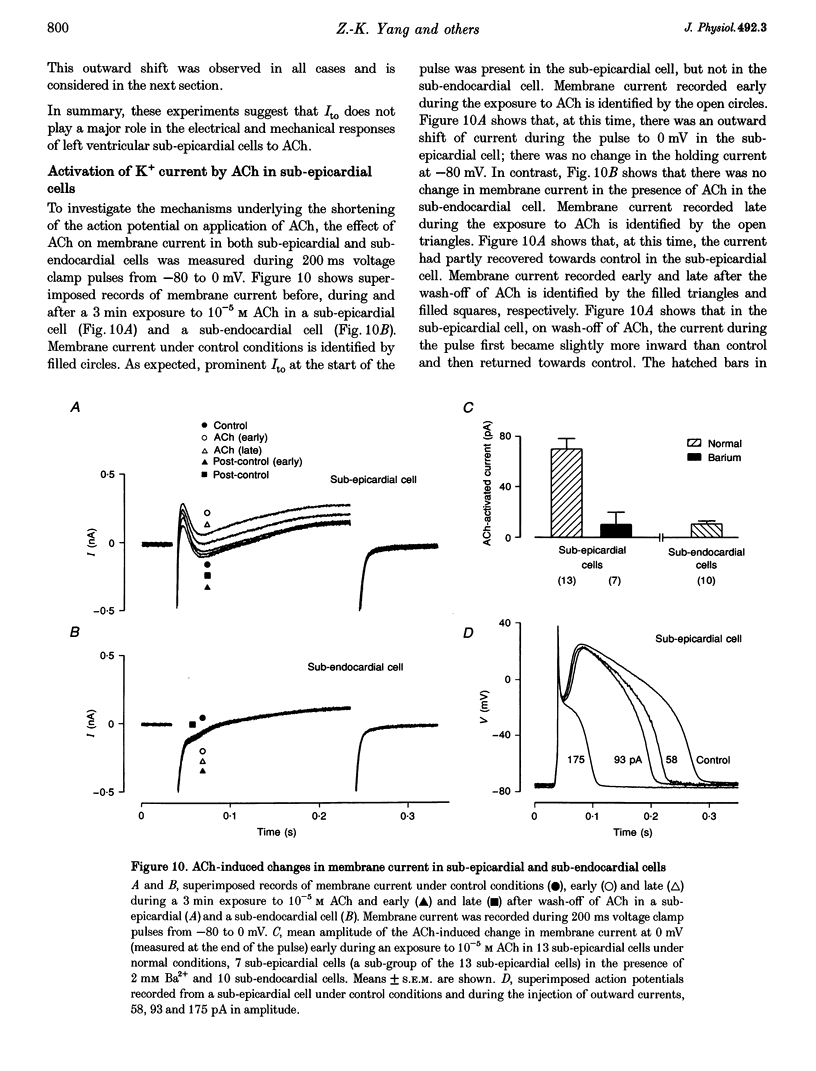
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antzelevitch C., Sicouri S., Litovsky S. H., Lukas A., Krishnan S. C., Di Diego J. M., Gintant G. A., Liu D. W. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991 Dec;69(6):1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Kirby M. S., Orchard C. H., Roberts A. The negative inotropic effect of acetylcholine on ferret ventricular myocardium. J Physiol. 1988 Oct;404:613–635. doi: 10.1113/jphysiol.1988.sp017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Levi A. J. A simple electronic circuit for determining the twitch force and resting force of small heart muscle preparations. Pflugers Arch. 1987 Oct;410(3):340–341. doi: 10.1007/BF00580287. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Moore M., Jewell B. R., Montgomery R. A., Kirby M. S., Orchard C. H. An improved apparatus for the optical recording of contraction of single heart cells. Pflugers Arch. 1988 Dec;413(2):197–205. doi: 10.1007/BF00582531. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Lederer W. J. A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch. 1986 May;406(5):536–539. doi: 10.1007/BF00583378. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Characterization of the acetylcholine-induced potassium current in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:219–237. doi: 10.1113/jphysiol.1986.sp015970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamunorwa J. P., O'Neill S. C. Regional differences in rest decay and recoveries of contraction and the calcium transient in rabbit ventricular muscle. Pflugers Arch. 1995 Jun;430(2):195–204. doi: 10.1007/BF00374650. [DOI] [PubMed] [Google Scholar]
- Dixon J. E., McKinnon D. Quantitative analysis of potassium channel mRNA expression in atrial and ventricular muscle of rats. Circ Res. 1994 Aug;75(2):252–260. doi: 10.1161/01.res.75.2.252. [DOI] [PubMed] [Google Scholar]
- Figueredo V. M., Brandes R., Weiner M. W., Massie B. M., Camacho S. A. Endocardial versus epicardial differences of intracellular free calcium under normal and ischemic conditions in perfused rat hearts. Circ Res. 1993 May;72(5):1082–1090. doi: 10.1161/01.res.72.5.1082. [DOI] [PubMed] [Google Scholar]
- Honjo H., Kodama I., Zang W. J., Boyett M. R. Desensitization to acetylcholine in single sinoatrial node cells isolated from rabbit hearts. Am J Physiol. 1992 Dec;263(6 Pt 2):H1779–H1789. doi: 10.1152/ajpheart.1992.263.6.H1779. [DOI] [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C., Boyett M. R. A simple electronic circuit for monitoring changes in the duration of the action potential. Pflugers Arch. 1983 Aug;398(3):233–235. doi: 10.1007/BF00657157. [DOI] [PubMed] [Google Scholar]
- Korth M., Kühlkamp V. Muscarinic receptor-mediated increase of intracellular Na+-ion activity and force of contraction. Pflugers Arch. 1985 Mar;403(3):266–272. doi: 10.1007/BF00583598. [DOI] [PubMed] [Google Scholar]
- Korth M., Sharma V. K., Sheu S. S. Stimulation of muscarinic receptors raises free intracellular Ca2+ concentration in rat ventricular myocytes. Circ Res. 1988 Jun;62(6):1080–1087. doi: 10.1161/01.res.62.6.1080. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990 Sep;67(3):615–627. doi: 10.1161/01.res.67.3.615. [DOI] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988 Jan;62(1):116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- Liu D. W., Gintant G. A., Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res. 1993 Mar;72(3):671–687. doi: 10.1161/01.res.72.3.671. [DOI] [PubMed] [Google Scholar]
- Löffelholz K., Pappano A. J. The parasympathetic neuroeffector junction of the heart. Pharmacol Rev. 1985 Mar;37(1):1–24. [PubMed] [Google Scholar]
- Matsumoto K., Pappano A. J. Sodium-dependent membrane current induced by carbachol in single guinea-pig ventricular myocytes. J Physiol. 1989 Aug;415:487–502. doi: 10.1113/jphysiol.1989.sp017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Hoffman B. F. Two components of transient outward current in canine ventricular myocytes. Circ Res. 1989 Apr;64(4):633–647. doi: 10.1161/01.res.64.4.633. [DOI] [PubMed] [Google Scholar]
- Wang D., Belardinelli L. Mechanism of the negative inotropic effect of adenosine in guinea pig atrial myocytes. Am J Physiol. 1994 Dec;267(6 Pt 2):H2420–H2429. doi: 10.1152/ajpheart.1994.267.6.H2420. [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Korth M. Effects of doxorubicin on excitation-contraction coupling in guinea pig ventricular myocardium. Circ Res. 1995 Apr;76(4):645–653. doi: 10.1161/01.res.76.4.645. [DOI] [PubMed] [Google Scholar]
- Yin F. C. Ventricular wall stress. Circ Res. 1981 Oct;49(4):829–842. doi: 10.1161/01.res.49.4.829. [DOI] [PubMed] [Google Scholar]
- Zang W. J., Yu X. J., Boyett M. R. Barium block of the muscarinic potassium current in guinea-pig atrial cells. Pflugers Arch. 1995 Jul;430(3):348–357. doi: 10.1007/BF00373909. [DOI] [PubMed] [Google Scholar]
- Zang W. J., Yu X. J., Honjo H., Kirby M. S., Boyett M. R. On the role of G protein activation and phosphorylation in desensitization to acetylcholine in guinea-pig atrial cells. J Physiol. 1993 May;464:649–679. doi: 10.1113/jphysiol.1993.sp019656. [DOI] [PMC free article] [PubMed] [Google Scholar]