Abstract
Background
Pediatric pemphigus is a rare bullous disease that represents a diagnostic and therapeutic challenge; evidence on patients' response to various treatments and long‐term surveillance data are lacking. We aimed to investigate pediatric pemphigus patients' characteristics, diagnosis, therapeutics, response, and long‐term follow‐up.
Methods
This is a retrospective study of all pemphigus patients aged <18 years, diagnosed between 2000 and 2023, from three tertiary medical centers in Israel. The diagnosis was confirmed by positive immunofluorescence.
Results
Twelve pediatric pemphigus patients were included (mean age 10.7 ± 4.3 years, male:female ratio 1:1). Mean diagnostic delay was 11.1 ± 12.6 months (range 1.8–36 months). Most patients had pemphigus vulgaris with mucosal involvement (58.3%). First‐line treatment for all patients included systemic corticosteroids (sCS), with a treatment duration (including tapering down) of 28 ± 18.4 months. Hospitalization did not yield better outcomes. Only three patients achieved sustained complete response with sCS treatment (25.0%), and the rest required additional therapeutics, most commonly rituximab. Rituximab showed a good safety profile and therapeutic response. Follow‐up was recorded up to 18.1 years after diagnosis (mean: 5.6 years). Three of five patients with information available more than 5 years after the pemphigus diagnosis still exhibited disease symptoms.
Conclusions
Pediatric pemphigus is associated with a significant diagnostic delay. While sCS can induce remission in most patients as a first‐line treatment, long‐term disease control requires additional immunomodulators. Long‐term follow‐up reveals a chronic yet mostly benign disease course in this population and advocates for the use of rituximab in pediatric pemphigus patients.
Keywords: corticosteroids, immunomodulators, pemphigus foliaceus, pemphigus vulgaris, pediatric dermatology, rituximab
Introduction
Pemphigus is a rare, autoimmune blistering disease affecting the skin and mucous membranes. It is characterized by the presence of circulating autoantibodies directed against desmogleins (in most cases), which are the adhesion molecules responsible for maintaining the integrity of intercellular junctions in the epidermis and mucosal epithelia. The resulting loss of cell‐to‐cell adhesion leads to the formation of flaccid blisters and erosions that are prone to secondary infection and may cause significant morbidity and mortality.
Although pemphigus is most commonly seen in adults, it can also affect children, with an estimated incidence of eight cases per million per year. 1 , 2 With only 1.4%–2.9% of all pemphigus vulgaris (PV) cases consisting of patients younger than 12 years, pediatric pemphigus is a diagnostic and therapeutic challenge. 3 Furthermore, it often presents with atypical clinical and histological features. 4 Thus, the diagnosis of pediatric pemphigus is often substantially delayed. 5 , 6 Given the infrequency of pediatric pemphigus, with most of the evidence on the disease provided from case reports and small case series, response rates to various treatments are not well characterized. Moreover, long‐term surveillance of patients for disease recurrence is lacking.
Usually, the first line of treatment in pemphigus is high‐dose systemic corticosteroid (sCS). 7 Recent advances in our understanding of the pathogenesis of pemphigus have led to the use of novel targeted therapies, such as rituximab, a monoclonal antibody directed against CD20‐positive B lymphocytes, which has been shown to induce remission in refractory cases of pemphigus in both adults and children. 8 , 9 However, the optimal management of pediatric pemphigus remains uncertain, and further studies are needed to elucidate the natural history, common diagnostic pitfalls, prognostic factors, and treatment outcomes of this disease in the pediatric population. A better understanding of the long‐term trajectory of pediatric pemphigus may enhance disease management and optimize therapeutic interventions while also allowing improved patient education.
In this retrospective study, we aim to provide a detailed report of the presentation, diagnosis, and treatment of a cohort encompassing 12 pediatric pemphigus patients from three outpatient pediatric dermatology clinics with up to 18 years of follow‐up after the initial symptoms.
Materials and methods
This is a retrospective cohort study including all patients aged <18 years who were diagnosed with PV or foliaceous (PF) in outpatient clinics of three tertiary medical centers in Israel between 2000 and 2023: Schneider Children's Medical Center, Sheba Medical Center, and Emek Medical Center. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of all participating medical centers. PV and PF diagnosis was established clinically and supported by pathology and immunofluorescence studies. The following data were retrieved from patients' files after Institutional Review Board approval: age, gender, ethnicity, clinical manifestations and distribution, comorbidities, delay since first signs of disease and pemphigus diagnosis, treatments (including drug name and dosing regimen), adverse events, response, and last follow‐up visit.
Data were transferred to Microsoft Office Excel and subjected to statistical analysis by the StatPlus statistical package software (StatPlus Prov.7, AnalystSoft Inc., Walnut, CA, USA). Categorical variables were compared using the chi‐square test, with P < 0.05 considered significant. A two‐sided t‐test was used for numerical data.
Results
Patient characteristics
Twelve pediatric pemphigus patients from three different pediatric dermatology outpatient hospital clinics were included in this cohort (Table 1). Patients included in the cohort were diagnosed with pemphigus between 2005 and 2021. The mean age was 10.7 ± 4.3 years at the time of diagnosis, and the male to female ratio was 1:1. Two patients were of Arab origin, and the rest were Jewish. Only one patient had a positive family history of pemphigus—a paternal cousin with PV. Two patients had attention deficit and hyperactivity disorder (ADHD), one patient had a history of asthma with no recent activity of the disease, and one patient had a history of macrohematuria but negative workup for a membranous kidney disease prior to pemphigus symptom initiation.
Table 1.
Patient demographics
| Pt number | Gender | Diagnosis/type | Age at diagnosis | Diagnosis delay (months) | Differential diagnosis | sCS Tx duration (months) | #2 line treatment | #3 line treatment | F/U from diagnosis (months) | Disease activity b on last F/U |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | PF | 9.3 | 4.8 | NA | 45 | IVIG | AZA | 126.4 | No |
| 2 | F | PF | 9.1 | 1.8 | NA | 36 | 71.0 | Yes | ||
| 3 | M | PV/MC | 4.2 | 3.6 | Impetigo | 12 | 23.1 | Yes | ||
| 4 | F | PV/MC | 11.9 | 26.0 | Aphtous stomatitis | 42 | RTX | 191.7 | No | |
| 5 | M | PV/C | 6.6 | 2.0 | Impetigo | 6 | 11.1 | No | ||
| 6 | M | PV/M | 13.5 | 24.4 | Behcet disease | Ongoing | RTX | 97.6 | Yes | |
| 7 | F | PF a | 5.9 | 2.0 | Linear IgA disease | 18 | Dapsone | RTX | 23.8 | Yes |
| 8 | M | PF | 17.0 | 5.0 | SLE | Ongoing | AZA | 30.6 | Yes | |
| 9 | M | PV/M | 12.0 | 36.0 | Aphtosis, Crohn's disease | 20 | Dapsone | 19.3 | No | |
| 10 | F | PV/MC | 15.0 | 2.0 | FMF | 60 | RTX | 68.6 | No | |
| 11 | M | PV/MC | 16.6 | 2.0 | NA | NA | Dapsone | Mycophenolate mofetil | 4.0 | No |
| 12 | F | PV/MC | 7.0 | 24.0 | MMP, EBA | 13 | Dapsone | RTX | 23.4 | No |
Abbreviations: AZA, azathioprine; C, cutaneous; EBA, epidermolysis bullos acquisita; FMF, familial Mediterranean fever; F/U, follow‐up; M, mucosal; MC, mucocutaneous; MMP, mucous membrane pemphigoid; NA, not available; PF, pemphigus foliaceus; PV, pemphigus vulgaris; RTX, rituximab; sCS, systemic corticosteroids; SLE, systemic lupus erythematosus; Tx, treatment.
Herpetiformis phenotype.
Continued active treatment.
Pemphigus diagnosis
The mean duration of diagnosis delay from first symptoms to pemphigus diagnosis was almost a year (11.1 ± 12.6 months, range—1.8–36 months; Table 1). Most patients had PV (eight patients/66.7%) with mucosal involvement (seven patients/58.3%) (Figure 1), of which only two patients had exclusively mucosal lesions (oral and anal). The oral mucosa was most commonly involved (in six patients/50.0%). Ocular or anal lesions were recorded in one patient each. Four patients (33.3%) were diagnosed with PF. Alternative diagnoses until a definite diagnosis of pemphigus was established included impetigo or impetiginized atopic dermatitis (two patients), cutaneous manifestation of FMF, Behcet disease, linear IgA disease, cutaneous manifestation of suspected systemic lupus erythematosus, Crohn's disease or aphthous stomatitis, mucous membrane pemphigoid (MMP), and epidermolysis bullosa acquisita (EBA) (one patient for each of these diagnoses). The diagnosis was supported by a characteristic skin biopsy showing suprabasilar intraepidermal acantholysis in most patients and by positive immunofluorescence in all patients. The pathology of the PF patient with herpetiformis morphology presented no acantholysis with an intradermal blister coupled with positive direct and indirect immunofluorescence testing, as previously reported for this pediatric pemphigus subtype. 10
Figure 1.
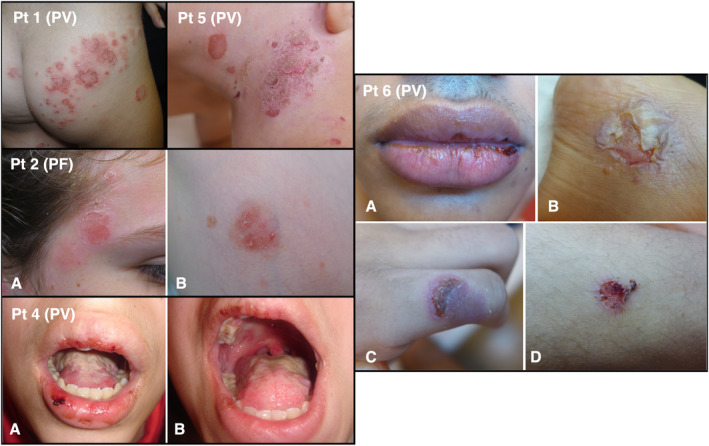
Clinical manifestations of pediatric pemphigus in Patients (Pt) 1, 2, 4, 5, and 6 (based on Table 1). PF, pemphigus foliaceus; PV, pemphigus vulgaris
Treatment
The first line of treatment for all patients included sCS, with dosing ranging from 1 mg/kg/day (10/12 of patients) to 2 mg/kg/day (equivalent to prednisone). Half of the patients were hospitalized for the initiation of high‐dose sCS therapy for remission induction and close monitoring. The patients treated with the higher 2 mg/kg/day dosing (two patients with PV) were among those hospitalized, while 6/10 patients treated with 1 mg/kg/day were closely monitored in an outpatient clinic setting. Adverse events related to this treatment included weight gain and acne (3/25.0% of patients for each), striae, moon face, and sleep/mood disturbances (2/16.7% of patients for each), one patient with short stature (suspected as a related adverse event), and one patient with telogen effluvium (8.3% for each). The overall duration of sCS treatment (any dose, including tapering down) was 2818.4 months, with two patients still treated with an ongoing low‐dose sCS regimen. The mean duration of treatment was shorter in PF patients (25.5 ± 21.0 months) as compared with PV patients (33.0 ± 13.7 months), but the difference was not significant (P = 0.59). Treatment duration was not associated with age or delay in diagnosis. There was also no association between hospitalization and sCS treatment duration and no difference in sCS‐related adverse events across hospitalized patients and those treated in outpatient clinics. Overall, only three patients achieved complete and sustained response on exclusive sCS treatment (25.0%), and the rest of the patients were treated with additional therapeutics to achieve disease remission.
Five patients were treated with rituximab (41.7%) as the second (in three patients) or third line (in two patients, after sCS + dapsone) of treatment (Table 2). Almost all patients treated with rituximab had PV with mucosal involvement, except one with PF with substantial skin involvement and a herpetiformis morphology. This patient was also the youngest patient treated with rituximab in our cohort, starting the treatment at the age of 6.5 years. There was no statistically significant difference in mean age between those treated with rituximab and those treated with complete remission on other immunomodulators/steroid‐sparing drugs. All patients treated with rituximab started this treatment after being treated with sCS for at least 1 year. Three of five patients achieved complete remission after two to three doses of rituximab, and two patients needed continued concomitant therapies due to partial response. Of note, one of the patients presenting partial response was treated with rituximab as an adult (age 28.3 years) after a prolonged disease course from childhood (started rituximab treatment 14 years after disease diagnosis). All patients were treated with two consecutive doses on Days 1 and 15, but the amount of rituximab per dose varied across patients (Table 2). No rituximab‐related adverse events were recorded.
Table 2.
Rituximab‐treated patients
| Pt number a | Gender | Age at rituximab initiation (years) | Duration of disease before rituximab (months) | # of rituximab doses (mg per dose) b | Rituximab response |
|---|---|---|---|---|---|
| F | 28.3 | 168.3 | 2 (1000 mg) | PR | |
| 6 | M | 15.8 | 1.2 | 2 (500 mg) | PR |
| 7 | F | 6.5 | 9.7 | 2 (345 mg) | CR |
| 10 | F | 15.9 | 9.7 | 3 (1000 mg) | CR |
| 12 | F | 7.3 | 6.1 | 2 (500 mg) | CR |
Abbreviations: CR, complete response; PR, partial response.
Based on Table 1.
Two doses were given on Days 1 and 15. A third dose was given to Patient 10, 3.5 years after previous doses due to disease relapse.
Additional second‐line treatments included dapsone (four patients, induced complete response in one of them), azathioprine (two patients, induced complete response as a monotherapy in one patient, and in the other patient, concomitant low‐dose sCS was continued), IVIG (one patient with PV, overall four infusions—with no sufficient response), and mycophenolate mofetil (one patient, induced partial response with continued low‐dose sCS treatment; Table 1). Of these treatments, only one patient discontinued the drug due to adverse events (cyanosis and dyspnea due to dapsone treatment), and the rest discontinued treatment due to complete or insufficient response.
Long‐term follow‐up and recurrence
The maximum duration of follow‐up from symptom initiation was 18.1 years (mean: 5.6 ± 5.2 years), and the maximum duration from pemphigus diagnosis was 16 years (mean: 4.8 ± 4.7 years). Of the five patients who were followed up for more than 5 years from disease initiation, three patients still had symptoms indicative of active disease (Patients 2, 3, and 6).
During the follow‐up period, only one patient initiated a second course of high‐dose sCS treatment (Patient 3), which was relatively short (2 months), a year after remission induction by a first course of sCS (with no additional treatments). The rest of the patients were maintained on various therapeutics, including low‐dose sCS, rituximab, and azathioprine.
Until the end of the follow‐up period, no new comorbidities were detected in our cohort. One patient had a significant learning disability and severe ADHD that were recorded as a potential long‐term consequence of her PV, as reported in their medical records. The rest of the patients had no new reported pemphigus treatment‐related adverse events necessitating additional therapeutics or designated medical care during the long‐term follow‐up period.
Discussion
Pemphigus, a rare autoimmune blistering disease, is often difficult to diagnose and treat, with even more significant therapeutic challenges in the pediatric population. Moreover, only a limited number of studies provide long‐term evidence on pediatric pemphigus course, 7 while 41.6% of the patients in our cohort had a follow‐up of at least 5 years after disease initiation.
Consistent with prior findings, PV was more prevalent than PF, 1 , 2 , 9 the mean age was 10.7 years, and the male to female ratio was 1:1. 11 In contrast to a previous report primarily including adult pemphigus patients from Israel, the distribution of Jewish to Arab patients in our pediatric cohort was similar to the ratio of these major ethnic groups in the general Israeli population. 12
Common differential diagnoses (or misdiagnoses) included other bullous/erosive dermatoses, such as impetigo, linear IgA, Behcet disease, EBA, and MMP among others, resulting in an average delay in diagnosis of almost 1 year from symptom initiation (11.1 ± 12.6 months). This delay is slightly longer than those previously reported, ranging from 6 to 9 months (in oral autoimmune vesiculobullous diseases 13 , 14 and pediatric pemphigus 5 ). Nevertheless, in our cohort, in line with a previous report by Metry et al., 5 delays in disease diagnosis did not correlate with an extended treatment duration or any other outcomes.
While osteoarthritis, renal failure, hypothyroidism, and weight loss were previously reported as comorbidities in solid association with pediatric pemphigus, 2 comorbidities in our cohort included ADHD and learning disability, asthma, and asymptomatic macrohematuria. The variety of reported comorbidities across cohorts could be attributed to the heterogeneity of the disease in pediatric patients, as could be appreciated by the relatively wide age range and clinical manifestations.
For all patients, the first line of treatment included sCS with a dosing regimen of 1–2 mg/kg/day, yet complete response was induced in only three patients (25.0%) using sCS monotherapy. As previously reported, 9 PF patients responded faster than PV patients, but the difference was insignificant. Half of our patients started high‐dose sCS treatment while hospitalized for close monitoring of adverse events and therapeutic response, but most of the patients in the cohort were treated with a dose equivalent to 1 mg/kg/day prednisone at outpatient clinics with similar overall treatment duration, outcomes, and adverse events as hospitalized patients. These findings raise the question of whether hospitalization of pediatric pemphigus patients in all cases is clinically beneficial.
Dapsone was the most prevalent steroid‐sparing agent used as the second line of treatment. Nevertheless, only one patient responded thoroughly to dapsone and did not require further therapeutic interventions. This response rate is considerably lower than previously reported in adult PV and PF patients (ranging 83.6%–55.6%), 15 , 16 potentially implying lower efficacy for dapsone in pediatric pemphigus patients.
On the other hand, most patients (3/5) achieved complete response with rituximab after the first two doses. These results are aligned with the outcomes reported by Kianfar et al. and Vinay et al., 17 , 18 and are slightly lower than response rates found in a recent literature review. 19 These findings suggest that the use of the two‐dose rituximab protocol regimen (as given in our cohort, on Days 1 and 15, also known as the rheumatoid arthritis protocol) may be appropriate for pediatric pemphigus cohorts, although some reported administration of the lymphoma 4‐week protocol in this population. 20 , 21 Of note, while a 375 mg/m2 dose is generally recommended in the pediatric literature, rituximab dosing widely varied in our study, and due to the small cohort, no clear trends between regimens and response were detected. Additionally, a patient with PF and a pemphigus herpetiformis morphology, which is more common in pediatric patients 5 and often responds well to sCS and dapsone, 22 , 23 failed to achieve remission on these treatments and was eventually successfully treated with rituximab. While infusion reactions, angioedema, reduction in peripheral B‐cell counts, and the onset of sepsis are the most commonly reported adverse events in pediatric pemphigus patients treated with rituximab, such adverse events were not recorded in our cohort. 19
Long‐term follow‐up demonstrated that most pediatric patients will present persistent yet manageable disease without needing a second high‐dose sCS course to induce remission. In contrast to data from adult patients, 24 most of the patients with follow‐up information available more than 5 years after diagnosis continued to exhibit signs of disease activity (including two patients treated with rituximab), presenting an overall chronic course in pediatric patients. Given the potentially severe adverse events with long‐term sCS usage in children, including delayed puberty, reduced bone density, susceptibility to severe infections, increased body weight, insulin resistance, growth suppression and short stature, and more, 25 our results may suggest that rituximab should be a first‐line treatment for pediatric pemphigus. Of note, long‐term rituximab usage in the pediatric population had shown an overall acceptable safety profile for other indications, with no increase in adverse events with more treatment courses nor a higher cumulative dose. 26
In conclusion, pediatric pemphigus is a rare disease that represents a diagnostic challenge and, thus, a significant delay in diagnosis. While high‐dose sCS was the first line of treatment in all patients, most patients advanced to steroid‐sparing therapeutics, and hospitalization did not yield better outcomes for patients treated with a 1 mg/kg/day dosing. Our extended follow‐up data portray pediatric pemphigus as a chronic disease that could be well controlled (after remission induction) with low‐dose sCS and steroid‐sparing therapeutics and advocate for the use of rituximab in pediatric pemphigus patients. These findings underscore the importance of long‐term planning for pediatric pemphigus patients to consider the potential implications of prolonged immunomodulation on patients' health and growth.
Conflict of interest: None.
Funding source: None.
References
- 1. Hubner F, Konig IR, Holtsche MM, Zillikens D, Linder R, Schmidt E. Prevalence and age distribution of pemphigus and pemphigoid diseases among paediatric patients in Germany. J Eur Acad Dermatol Venereol. 2020;34(11):2600–2605. [DOI] [PubMed] [Google Scholar]
- 2. Ren Z, Hsu DY, Silverberg NB, Silverberg JI. The inpatient burden of autoimmune blistering disease in US children: analysis of nationwide inpatient sample data. Am J Clin Dermatol. 2017;18(2):287–297. [DOI] [PubMed] [Google Scholar]
- 3. Mabrouk D, Ahmed AR. Analysis of current therapy and clinical outcome in childhood pemphigus vulgaris. Pediatr Dermatol. 2011;28(5):485–493. [DOI] [PubMed] [Google Scholar]
- 4. Schultz B, Hook K. Bullous diseases in children: a review of clinical features and treatment options. Paediatr Drugs. 2019;21(5):345–356. [DOI] [PubMed] [Google Scholar]
- 5. Metry DW, Hebert AA, Jordon RE. Nonendemic pemphigus foliaceus in children. J Am Acad Dermatol. 2002;46(3):419–422. [DOI] [PubMed] [Google Scholar]
- 6. Weston WL, Morelli JG, Huff JC. Misdiagnosis, treatments, and outcomes in the immunobullous diseases in children. Pediatr Dermatol. 1997;14(4):264–272. [DOI] [PubMed] [Google Scholar]
- 7. Fuertes I, Guilabert A, Mascaro JM Jr, Iranzo P. Rituximab in childhood pemphigus vulgaris: a long‐term follow‐up case and review of the literature. Dermatology. 2010;221(1):13–16. [DOI] [PubMed] [Google Scholar]
- 8. Bilgic‐Temel A, Ozgen Z, Harman M, Kapicioglu Y, Uzun S. Rituximab therapy in pediatric pemphigus patients: a retrospective analysis of five Turkish patients and review of the literature. Pediatr Dermatol. 2019;36(5):646–650. [DOI] [PubMed] [Google Scholar]
- 9. Mahajan R, Handa S, Kumar S, Chatterji D, Saikia UN, De D. Pediatric autoimmune blistering disorders ‐ a five‐year demographic profile and therapy experience. Int J Dermatol. 2022;61(12):1511–1518. [DOI] [PubMed] [Google Scholar]
- 10. Evans MS, Culton DA, Diaz LA, Googe PB, Morrell DS. Childhood pemphigus foliaceus presenting as a polycyclic eruption: case report and review of the literature. Pediatr Dermatol. 2019;36(2):236–241. [DOI] [PubMed] [Google Scholar]
- 11. Lins GT, Barbosa NLS, de Abreu EMV, da Costa KVT, Meneses KCB, Silva RN, et al. Childhood pemphigus vulgaris is a challenging diagnosis. Autops Case Rep. 2021;11:e2021267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kridin K, Zelber‐Sagi S, Khamaisi M, Cohen AD, Bergman R. Remarkable differences in the epidemiology of pemphigus among two ethnic populations in the same geographic region. J Am Acad Dermatol. 2016;75(5):925–930. [DOI] [PubMed] [Google Scholar]
- 13. Petruzzi M, Della Vella F, Squicciarini N, Lilli D, Campus G, Piazzolla G, et al. Diagnostic delay in autoimmune oral diseases. Oral Dis. 2023;29(7):2614–2623. [DOI] [PubMed] [Google Scholar]
- 14. Daltaban O, Ozcentik A, Akman Karakas A, Ustun K, Hatipoglu M, Uzun S. Clinical presentation and diagnostic delay in pemphigus vulgaris: a prospective study from Turkey. J Oral Pathol Med. 2020;49(7):681–686. [DOI] [PubMed] [Google Scholar]
- 15. Gurcan HM, Ahmed AR. Efficacy of dapsone in the treatment of pemphigus and pemphigoid: analysis of current data. Am J Clin Dermatol. 2009;10(6):383–396. [DOI] [PubMed] [Google Scholar]
- 16. Werth VP, Fivenson D, Pandya AG, Chen D, Rico MJ, Albrecht J, et al. Multicenter randomized, double‐blind, placebo‐controlled, clinical trial of dapsone as a glucocorticoid‐sparing agent in maintenance‐phase pemphigus vulgaris. Arch Dermatol. 2008;144(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vinay K, Kanwar AJ, Sawatkar GU, Dogra S, Ishii N, Hashimoto T. Successful use of rituximab in the treatment of childhood and juvenile pemphigus. J Am Acad Dermatol. 2014;71(4):669–675. [DOI] [PubMed] [Google Scholar]
- 18. Kianfar N, Dasdar S, Mahmoudi H, Tavakolpour S, Balighi K, Daneshpazhooh M. Rituximab in childhood and juvenile autoimmune bullous diseases as first‐line and second‐line treatment: a case series of 13 patients. J Dermatolog Treat. 2022;33(2):869–874. [DOI] [PubMed] [Google Scholar]
- 19. Patel MH, Brumfiel CM, Bohrer N, Marsch AF. Efficacy of rituximab in pediatric pemphigus: a literature review. JAAD Int. 2022;6:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kincaid L, Weinstein M. Rituximab therapy for childhood pemphigus vulgaris. Pediatr Dermatol. 2016;33(2):e61–e64. [DOI] [PubMed] [Google Scholar]
- 21. Carver C, Kalesinskas M, Dheden N, Ahmed AR. Treatment of pediatric pemphigus foliaceuss. Cureus. 2023;15(9):e45373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peterman CM, Vadeboncoeur S, Schmidt BA, Gellis SE. Pediatric pemphigus herpetiformis: case report and review of the literature. Pediatr Dermatol. 2017;34(3):342–346. [DOI] [PubMed] [Google Scholar]
- 23. Galambrun C, Cambazard F, Clavel C, Versini P, Stephan JL. Pemphigus foliaceus. Arch Dis Child. 1997;77(3):255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shimanovich I, Baumann T, Schmidt E, Zillikens D, Hammers CM. Long‐term outcomes of rituximab therapy in pemphigus. J Eur Acad Dermatol Venereol. 2020;34(12):2884–2889. [DOI] [PubMed] [Google Scholar]
- 25. Bell JM, Shields MD, Watters J, Hamilton A, Beringer T, Elliott M, et al. Interventions to prevent and treat corticosteroid‐induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2017;1(1):CD010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan EY, Yu ELM, Angeletti A, Arslan Z, Basu B, Boyer O, et al. Long‐term efficacy and safety of repeated rituximab to maintain remission in idiopathic childhood nephrotic syndrome: an international study. J Am Soc Nephrol. 2022;33(6):1193–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]


