Abstract
Background
Monkeypox (MPOX) caused a public health emergency of international concern (PHEIC) outbreak between 2022 and 2023, with a recent rise in cases that prompted the World Health Organization (WHO) to declare the disease a PHEIC once again. There is little information on its long‐term scarring sequelae.
Objectives
The objective of this study was to assess the risk and characteristics of scarring in patients with MPOX in a tertiary hospital.
Methods
This is a prospective cohort study including patients diagnosed using polymerase chain reaction (PCR) tests. Clinical data were collected and followed up at 12–15 months to assess scarring and its impact on quality of life.
Results
Of the 40 patients, 19 (47.5%) developed scars, which were more common in those with initial cutaneous manifestations. Scars significantly affected the quality of life, especially in the genital and mucosal areas. The limited sample and loss to follow‐up may affect the validity of the results.
Conclusion
Scarring is a frequent and disfiguring sequela of MPOX, particularly in patients with early skin symptoms. Prevention and close follow‐up are crucial in mitigating these complications.
Keywords: monkeypox, mpox, scar, STI, epidemiology, sexually transmitted infection
Introduction
MPOX, formerly known as monkeypox, is a viral infection caused by its namesake poxvirus that caused a public health emergency of international concern (PHEIC) between 2022 and 2023, with cases still being reported daily in 2024. On August 14, 2024, the World Health Organization (WHO) declared the disease a PHEIC for the second time due to a worrying increase in cases in non‐endemic areas of Africa and in Europe. 1 , 2 , 3 This is particularly interesting to dermatologists, who are generally involved in the diagnosis, given the variety of mucocutaneous manifestations of the disease that usually constitute the most distinctive clinical presentation.
Clinical presentation occurs after a prodromal period of 2–4 days, including cutaneous and systemic manifestations that may or may not be concurrent. Patients develop whitish papules on the skin on an erythematous base, also called pseudopustules, which evolve in 1–2 weeks to central umbilication with subsequent centrifugally progressing necrosis. The most frequent systemic manifestations include fever, malaise, lymphadenopathy, headache, and arthromyalgia. 4 , 5
Cicatricial sequelae, including hypertrophic, keloid, atrophic scars, or pigmentary changes, have been reported anecdotally, both in the current outbreak 6 , 7 , 8 and in endemic cases in Africa. 9 , 10 However, the risk of scarring after lesions is unknown, and little objective information has been published due to the lack of long‐term follow‐up. 11 , 12 , 13 Some authors have estimated this risk at 13%–20%. 14 , 15 This is the first study, to the best of our knowledge, to examine the risk of scarring in these patients. This work aims to analyze the factors associated with the presence of scarring and the characteristics of scarring in patients with MPOX in the sexually transmitted infection (STI) unit of a tertiary hospital.
Materials and methods
Data collection and analysis
This study included cases with a confirmed diagnosis of MPOX in a tertiary referral hospital in Valencia, Spain. Confirmation was performed by polymerase chain reaction (PCR) testing of skin, oropharyngeal, urethral, and/or anal samples using the VIASURE Monkeypox Virus reverse transcription (RT)‐PCR kit. Epidemiological, clinical, and microbiological data were collected from electronic medical records, patient history, and physical examinations at the initial and follow‐up visits. At 12–15 months after the initial diagnosis, a follow‐up consultation was performed to collect information on the presence of scarring and, if applicable, the clinical characteristics of the lesions. Patients without a follow‐up consultation were excluded from the study. Patients with scars were asked to evaluate the impact on their quality of life using a scale from 0 to 10, where 0 represented no impact, and 7 to 10 had a substantial impact.
Ethical aspects
The research was conducted in compliance with the ethical principles of the Declaration of Helsinki, maintaining integrity, transparency, and respect for the human dignity and privacy of all patients. Informed consent was obtained for the use of clinical images.
Statistical analysis
A preliminary analysis of the numerical variables was performed with the Shapiro–Wilk test, with the student's t‐test chosen for variables with normal distribution and the Mann–Whitney U test for those without normal distribution. Pearson's chi‐squared and Fisher's exact tests were used for categorical variables to determine the differences between patients with and without scars. A P‐value of less than 0.05 was considered significant. The analyses were performed with SPSS version 28 (IBM, Armonk, NY, USA).
Results
Seventy‐two individuals with a confirmed monkeypox (MPOX) diagnosis by PCR tests were included in the analysis. Thirty‐two patients were excluded due to the lack of at least one follow‐up visit between 12 and 15 months after initial diagnosis. Of the 40 patients selected, 19 (47.5%) developed scarring as a complication, whereas 21 (52.5%) were free of this sequela. Table 1 summarizes the clinical and demographic data of the patients stratified according to the presence or absence of scarring.
Table 1.
Epidemiological and clinical characteristics of patients with and without scars and associated statistical significance
| Patient information | Patients without scars | Patients with scars | Test | Statistical significance |
|---|---|---|---|---|
| Age (range) | 42.5 (22–69) | 36.2 (23–49) | Student's t‐test | 0.042 |
| Male | 20 (95) | 19 (100) | Chi‐square | 1 |
| Female | 1 (5) | 0 (0) | ||
| Sexual orientation | ||||
| Homosexual | 14 (66.7) | 17 (89.5) | Chi‐square | 0.202 |
| Bisexual | 5 (23.8) | 1 (5.2) | ||
| Heterosexual | 2 (9.5) | 1 (5.2) | ||
| Vaccination | 8 (38.1) | 2 (10.5) | Chi‐square | 0.100 |
| Non‐vaccination | 13 (61.9) | 17 (89.5) | ||
| HIV positive | 6 (28.6) | 4 (21.1) | Chi‐square | 0.855 |
| HIV negative | 15 (71.4) | 15 (78.9) | ||
| Nationality | ||||
| Spain | 16 (76.2) | 11 (57.9) | Chi‐square | 0.176 |
| South America | 5 (23.8) | 4 (21.1) | ||
| Europe | 0 (0) | 3 (15.8) | ||
| Asia | 0 (0) | 1 (5.3) | ||
| Total number of lesions (range) | 8.5 (1–22) | 33.4 (1–458) | Mann–Whitney U test | 0.654 |
| 9.8 (1–28)* | ||||
| Systemic symptoms | ||||
| Yes | 14 (66.7) | 14 (74) | Chi‐square | 0.648 |
| No | 7 (33.3) | 5 (26) | ||
| Fever | ||||
| Yes | 8 (38) | 12 (63) | Chi‐square | 0.205 |
| No | 13 (62) | 7 (37) | ||
| Arthromyalgia | ||||
| Yes | 8 (38) | 8 (42) | Chi‐square | 1 |
| No | 13 (62) | 11 (58) | ||
| Asthenia | ||||
| Yes | 9 (43) | 11 (58) | Chi‐square | 0.527 |
| No | 12 (57) | 8 (42) | ||
| Headache | ||||
| Yes | 6 (29) | 6 (32) | Chi‐square | 1 |
| No | 15 (71) | 13 (68) | ||
| Adenopathies | ||||
| Yes | 12 (57) | 13 (68) | Chi‐square | 0.683 |
| No | 9 (43) | 6 (32) | ||
| Pharyngitis | ||||
| Yes | 6 (29) | 4 (21) | Chi‐square | 0.855 |
| No | 15 (71) | 15 (79) | ||
| Urethritis | ||||
| Yes | 4 (19) | 2 (11) | Chi‐square | 0.756 |
| No | 17 (81) | 17 (89) | ||
| Proctitis | ||||
| Yes | 5 (24) | 3 (16) | Chi‐square | 0.812 |
| No | 16 (76) | 16 (84) | ||
| Clinical debut: | ||||
| Systemic | 12 (57) | 5 (26) | Chi‐square |
Chi‐square: 0.022 Fischer's test: 0.018 |
| Cutaneous | 2 (10) | 9 (47) | ||
| Cutaneous only | 7 (33) | 5 (26) | ||
Excluding the subject with 458 lesions.
The mean age of the patients was 42.5 years among patients without scars and 36.2 years among those with scars, showing statistically significant differences between the two groups. All 19 patients with scars were male, whereas 20 of the 21 (95%) patients without scars were female. Six patients in the group without scars had HIV infection (28.6%), whereas four in the scarred group were seropositive (21.1%). Regarding the smallpox vaccination status of the patients, 8 (38.1%) were vaccinated among those without scarring, and 2 (10.5%) were vaccinated among those who developed this complication with no statistically significant differences (P = 0.1).
Systemic symptoms accompanying the cutaneous manifestations were observed in 14 patients (67%) in the group without scars and 14 (74%) in the group with scars, with no statistically significant differences between groups. Details on the manifestations of these systemic symptoms can be found in Table 1. There were statistically significant differences between groups regarding the initial presentation of the infection: systemic or cutaneous. Twelve patients (57%) without scars initially showed systemic symptoms, whereas 2 (10%) had cutaneous manifestations initially. In patients with scars, 5 (26%) initially experienced systemic symptoms, and 9 (47%) had skin lesions as first signs.
All 19 patients had between 1 and 300 scars. Ten (52.6%) had 1 scar lesion, whereas one patient developed 300 scars. The mean number of scars per patient was 19.7 and 4.1 if the one with 300 was excluded. The median was one lesion.
Table S1 shows the distribution of active lesions and the distribution of scars in patients with these lesions. The areas presenting lesions most frequently during the active phase of the disease were the upper limbs (28) and lower limbs (21), genital area (21), trunk (18), and perianal area (13). The most frequently scarred area was the genital area (9). The areas with active lesions where the highest percentage of patients developed scarring were the nose (50%) and genital area (43%). Five (26.3%) patients had mucosal scarring. Two were on the genital mucosa, two on the perianal mucosa, and one on the tongue.
Bacterial superinfection was present in 3 (15.7%) of the 19 patients with scarring. Severe pain in the acute phase of the disease occurred in 4 (21%), whereas perilesional edema was documented in 8 (42.1%) of these patients. No such complications were recorded in patients without scarring.
Thirteen of the 19 patients (68.4%) had lesions with a depressed appearance. In terms of color, 8 (42%) had lesions with hypopigmentation, many of them pearly white in appearance; 6 (31.5%) had some degree of associated erythema, 3 (15.8%) had associated hyperpigmentation, and 5 (26.3%) had skin‐colored scars. Fourteen (73.7%) of the 19 patients had oval or round‐looking scars, three (15.8%) of them had stellate‐looking lesions, two on the nose and one on the penis, and 3 (15.8%) had fusiform or linear lesions. Table S2 summarizes the clinical appearance of the scars.
The range in scar diameter was 2–31 mm for all lesions. The mean minimum diameter of the smallest scar among all patients was 4.1 mm, and the maximum diameter was 10.6 mm. Eight of the 10 patients with scars in hairy areas (beard and genital area most frequently) had scarring alopecia as a sequela of their lesions.
The quality of life impairment of patients with scars showed a mean of 5.2 and a median of 5 out of 10 on the visual numerical scale. The mode was 3 of 10 points. Seven patients (36.8%) had a score with substantial impairment of quality of life. Three had scars in the nasal and perioral areas, and four in the genital area.
Discussion
Scarring in almost half of our cohort's patients contrasts with previous estimates that placed the risk as low as 20%. This may be because it is a complication that can often be overlooked or because many of the studies performed did not include long‐term follow‐up.
The age difference between groups may be due to younger patients' tendency to develop more unaesthetic or hypertrophic scars. 16 Younger patients may be a population group to monitor more closely during the course of the disease and in whom scar treatments such as fractional CO2 laser could even be considered early. 17
Vaccination against smallpox has been shown to provide cross‐protection against MPOX even in the long term. 18 Some studies show a lower clinical severity of disease among smallpox‐vaccinated patients who become infected with MPOX. 19 In our cohort, there is a lower proportion of vaccinated patients among those who developed scarring, although we did not find statistically significant differences. Vaccines developed against MPOX have been shown to reduce the risk of infection, 20 , 21 as well as the severity of the disease. 22 It seems reasonable to assume that vaccination will reduce the risk of scarring because it is associated with a less severe clinical picture and probably less extensive and destructive lesions, although further studies are needed to support this hypothesis.
HIV infection has been associated with much more profound and necrotic ulcers with a very high associated mortality rate. 23 , 24 , 25 Some authors have even proposed considering ulceronecrotic MPOX as a diagnostic criterion for acquired immunodeficiency syndrome (AIDS) due to its characteristic clinical presentation. 26 Large lesions with greater associated necrosis are likely to have a higher risk of scarring as a sequela. 27 Nevertheless, in our patients, we have not found HIV to be a risk factor for this complication. This is probably because HIV‐positive patients in our cohort had an undetectable or very close to undetectable viral load, and the disease did not manifest itself in them as it does in the majority of immunocompromised patients in whom ulceronecrotic MPOX is described. One of the HIV‐positive patients had atypical MPOX with many lesions of widespread distribution, affecting the entire integument. When the lesions healed, they left biopsy‐confirmed anetoderma lesions. We noted the presence of highly necrotic lesions with large, deep ulcers leading to extensive scarring in the genital area in a patient with iatrogenic immunosuppression who was being treated with infliximab for Crohn's disease (Figure 1, further images depicting the case are found in Appendix S1).
Figure 1.
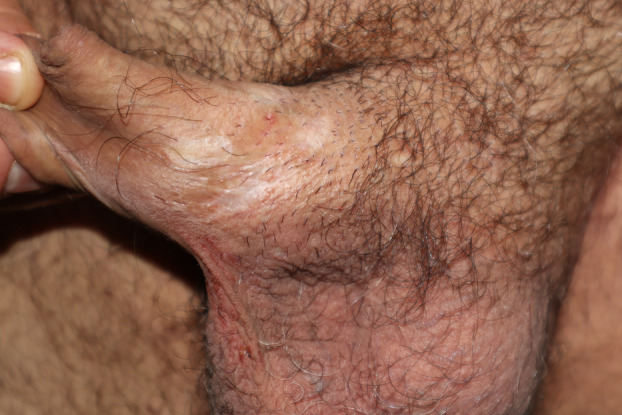
Extensive and multiple pearly white cicatricial plaques on the dorsum of the penis
During the current outbreak in 2024, two distinct routes of transmission have been described in patients with MPOX. The respiratory route is the first and most frequent in endemic cases in Africa. It is associated with higher viremia and generalized skin lesions. The second route of infection, which was the predominant one during the PHEIC, was the cutaneous route. In these cases, the skin lesions appear first and they are more localized in contact areas with the infected patient. The perioral, perianal, and genital areas have been frequently affected. Locoregional inflammatory lymphadenopathies are very often associated, and viremia has been delayed in time and at lower titers. This may explain the lower infectivity of these patients through the respiratory tract and the lower number of generalized lesions. 28 , 29 In patients with occupational disease transmission, more inflammatory and scarring lesions have been observed at the inoculation site. 30 , 31 In a 2003 outbreak in the USA involving 47 patients, Reynolds et al. 32 described the differences depending on how the infection was transmitted. They noted that patients infected through more invasive exposure (bitten or scratched by infected animals) had more pronounced systemic symptoms and risk of hospitalization. They also found that these patients had a shorter incubation period and often had an earlier onset of skin symptoms than fever. These similarities to the typical picture during the current outbreak may be due to a superimposable pathogenesis.
In our patients, the presence or absence of systemic manifestations does not seem to confer a greater or lesser risk of scarring. On the other hand, the clinical onset of MPOX by cutaneous manifestations seems to be associated with a higher risk of subsequent scarring. This may be due to more extensive initial lesions at the site of inoculation in the subgroup of patients infected by cutaneous inoculation. Lesions secondary to viremia, which are more disseminated but generally smaller, less necrotic, and less inflammatory, usually heal without scarring.
The number of scars is usually small, but in many cases, it is limited to a single lesion. Usually, it is in relation to the larger, more inflammatory lesion(s) during the active phase. An exception was one HIV‐positive patient with numerous lesions in the active phase and 300 anetoderma lesions as sequelae.
The distribution of lesions in our patients was similar to that reported in the literature, with lesions predominating in the genital and perianal area associated with several secondary lesions that were not very numerous and widespread, with greater involvement of the trunk and limbs. 33 On the other hand, scars were predominantly distributed in the genital area as well as the nasal and perioral areas. The perioral and nasal areas were high‐risk locations as few patients had lesions in the active phase, but those who did often experienced scarring. These findings are consistent with isolated reports describing intensely inflammatory lesions in these regions with significant scarring sequelae. 8 , 27 , 34 The trunk and limbs appear to be low‐risk areas as lesions are very often seen to heal without scarring. The mucous membranes presented scarring lesions, including one case of lingual depapillation and two perianal polypoid lesions. Early vaccination as post‐exposure prophylaxis could be particularly important in patients with lesions in at‐risk areas.
Bacterial superinfection and edema affected a similar percentage of patients to those described previously. 35 They have been associated with longer disease recovery time. Patients in whom they were present had nasal, perioral, and genital lesions, increasing the degree of inflammation and possibly contributing to a larger scar after disease resolution (Figure 2; further images depicting these cases are found in Appendix S1). Active surveillance of these patients, especially those with lesions in high‐risk areas, may be key to early treatment of superinfection and reduction of the aesthetic sequelae. Some authors have proposed the use of topical or intralesional cidofovir in these areas when there is a significant inflammatory component. 36 , 37
Figure 2.
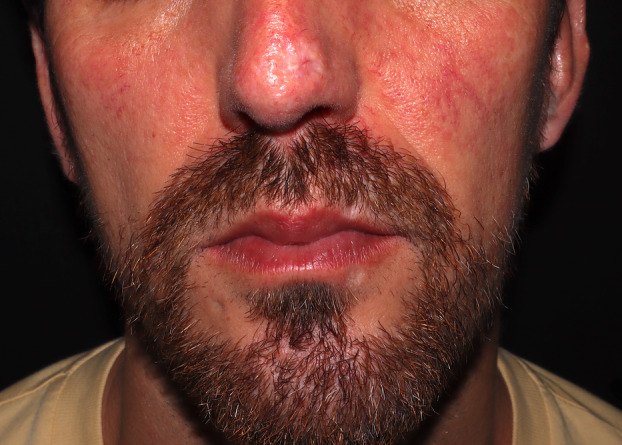
Facial scar lesions distributed in the nasal and perioral areas
The depressed appearance of the lesions, similar to smallpox scars, was frequent and has already been reported. 38 , 39 Hypopigmentation was the most common alteration in the color of the scars, followed by erythema and skin‐colored scars. Hyperpigmentation was less frequent than in African patients; 40 it was more common in dark phototypes and hypopigmented lesions in light phototypes. Erythema appears to be an intermediate stage that disappears with time. 41 The size of scars and lesions varied, with larger lesions causing larger scars.
MPOX scars have received little attention during the current PHEIC but can be highly disfiguring. This study shows that some lesions significantly impact quality of life, leading to problems with self‐esteem, anxiety, and depression. A total of 36.8% of patients had quality of life impairment scores of 7–10, with large scars in the central facial or genital areas. Therefore, patients with nasal, perioral, or genital lesions with a significant associated inflammatory component deserve close follow‐up and even adjuvant psychological treatment.
Limitations
The most important limitation of this work is the sample size, which limits the external validity of the findings. A significant percentage of patients have not been included due to the need for a follow‐up visit after 12–15 months.
Conclusions
We present a prospective study with the greatest long‐term follow‐up, to the best of our knowledge, in MPOX patients, focusing on the risk of scarring after infection. Scarring as a sequela of MPOX is a more frequent complication than previously estimated that can be highly disfiguring and have a major impact on patients' quality of life and psychological wellbeing. Young patients with risk factors for this infection are a group at high risk of significant sequelae, where primary prevention through vaccination is of particular importance. Central facial and genital lesions, especially if they are the initial manifestation of the disease, require close follow‐up and early treatment of complications to avoid these sequelae.
Patient consent
Patients provided informed consent to participate in the study. All procedures, examinations, and data handling were performed considering patients' privacy and following ethical standards for medical research.
Supporting information
Figure S1. Deep facial ulcers in the perioral and nasal area with large perilesional inflammation after the crust has been removed.
Figure S2. Scars of depressed and atrophic appearance on the nasal tip.
Figure S3. (A) Inflammatory ulcer with a fibrinous background on the dorsum of the tongue. (B) After healing of the lesion, lingual depapillation can be seen in the area of the stellate scar.
Figure S4. (A) Multiple perianal ulcers with radial distribution and endoanal involvement. (B) Nacreous perianal scarring in the areas of previous ulceration and polypoid lesion at the anal margin.
Figure S5. (A) Clustered ulcers of linear arrangement at the root of the penis. (B) Linear spindle‐shaped scar as sequelae of the lesions.
Figure S6. (A) Pseudopustule with central necrosis in the proximal foreskin. (B) The resulting scar has a linear and hypopigmented appearance.
Figure S7. (A) Oval scar with a varioliform appearance in the dorsum of the penis. (B) Smaller fusiform scar.
Figure S8. Multiple hyperpigmented papules with a parchment‐like appearance with histology compatible with anetoderma.
Table S1. Number of active lesions and scars in patients according to location.
Table S2. Characteristics of the scars in the patients in our sample.
Conflict of interest: None.
Funding source: None.
Contributor Information
Andrés Grau‐Echevarría, Email: ageche@outlook.es.
Daniel Blaya‐Imbernón, Email: daniblayaimbernon@gmail.com.
Data availability statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.
References
- 1. Kozlov M. Growing Mpox outbreak prompts WHO to declare global health emergency. Nature. 2024;632:718–719. 10.1038/d41586-024-02607-y [DOI] [PubMed] [Google Scholar]
- 2. Eurosurveillance Editorial Team . Note from the editors: WHO declares Mpox outbreak a public health emergency of international concern. Euro Surveill. 2024;29(33):pii=240815v. 10.2807/1560-7917.ES.2024.29.33.240815v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Disease outbreak news; Mpox (monkeypox) in South Africa. 2024. https://www.who.int/emergencies/disease‐outbreak‐news/item/2024‐DON525
- 4. Long B, Koyfman A, Gottlieb M, Liang SY, Carius BM, Chavez S, et al. Monkeypox: a focused narrative review for emergency medicine clinicians. Am J Emerg Med. 2022;61:34–43. 10.1016/j.ajem.2022.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maronese CA, Avallone G, Aromolo IF, Spigariolo CB, Quattri E, Ramoni S, et al. Mpox: an updated review of dermatological manifestations in the current outbreak. Br J Dermatol. 2023;189(3):260–270. 10.1093/bjd/ljad151 [DOI] [PubMed] [Google Scholar]
- 6. Urban N, Valencak J, Bauer WM, Thalhammer F, Handisurya A. Diary of human monkeypox: Illustrations of the clinical course. J Eur Acad Dermatol Venereol. 2023;37(5):e672–e674. [DOI] [PubMed] [Google Scholar]
- 7. Ciccarese G, Di Biagio A, Drago F, Mastrolonardo M, Pipoli A, Lo Caputo S, et al. Monkeypox virus infection mimicking primary syphilis. Infez Med. 2023;31(1):113–115. 10.53854/liim-3101-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bertin C, Tarhini H, Rahi M, Deconinck L, Perrineau S, Merlant M, et al. Complicated scarring following mpox infection. Br J Dermatol. 2023;189(2):225–226. 10.1093/bjd/ljad126 [DOI] [PubMed] [Google Scholar]
- 9. Besombes C, Mbrenga F, Malaka C, Gonofio E, Schaeffer L, Konamna X, et al. Investigation of a mpox outbreak in Central African Republic, 2021‐2022. One Health. 2023;16:100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13(10):e0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Català A, Clavo‐Escribano P, Riera‐Monroig J, Martín‐Ezquerra G, Fernandez‐Gonzalez P, Revelles‐Peñas L, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross‐sectional study of 185 cases. Br J Dermatol. 2022;187(5):765–772. 10.1111/bjd.21790 [DOI] [PubMed] [Google Scholar]
- 12. Thornhill JP, Antinori A, Orkin CM. Monkeypox virus infection across 16 countries—April‐June 2022. Reply N Engl J Med. 2022;387(25):e69. 10.1056/NEJMc2213969 [DOI] [PubMed] [Google Scholar]
- 13. Mailhe M, Beaumont AL, Thy M, Le Pluart D, Perrineau S, Houhou‐Fidouh N, et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: an observational cohort study. Clin Microbiol Infect. 2023;29(2):233–239. 10.1016/j.cmi.2022.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prasad S, Galvan Casas C, Strahan AG, Fuller LC, Peebles K, Carugno A, et al. A dermatologic assessment of 101 mpox (monkeypox) cases from 13 countries during the 2022 outbreak: Skin lesion morphology, clinical course, and scarring. J Am Acad Dermatol. 2023;88(5):1066–1073. 10.1016/j.jaad.2022.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ciccarese G, Di Biagio A, Bruzzone B, Guadagno A, Taramasso L, Oddenino G, et al. Monkeypox outbreak in Genoa, Italy: clinical, laboratory, histopathologic features, management, and outcome of the infected patients. J Med Virol. 2023;95(2):e28560. 10.1002/jmv.28560 [DOI] [PubMed] [Google Scholar]
- 16. Butzelaar L, Ulrich MM, Mink van der Molen AB, Niessen FB, Beelen RH. Currently known risk factors for hypertrophic skin scarring: a review. J Plast Reconstr Aesthet Surg. 2016;69(2):163–169. [DOI] [PubMed] [Google Scholar]
- 17. Pimentel B, Palmeiro A, Catorze G. Combined laser therapy in a Mpox scar. Act Dermo‐sifiliograficas. 2023. Epub ahead of print. 10.1016/j.ad.2023.06.019 [DOI] [PubMed] [Google Scholar]
- 18. Akter F, Hasan TB, Alam F, Das A, Afrin S, Maisha S, et al. Effect of prior immunisation with smallpox vaccine for protection against human Mpox: a systematic review. Rev Med Virol. 2023;33(4):e2444. [DOI] [PubMed] [Google Scholar]
- 19. Karem KL, Reynolds M, Hughes C, Braden Z, Nigam P, Crotty S, et al. Monkeypox‐induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vaccine Immunol. 2007;14(10):1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Payne AB, Ray LC, Kugeler KJ, Fothergill A, White EB, Canning M, et al. Incidence of Monkeypox among unvaccinated persons compared with persons receiving ≥1 JYNNEOS vaccine dose—32 U.S. jurisdictions. Morb Mortal Wkly Rep. 2022;71(40):1278–1282. 10.15585/mmwr.mm7140e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Payne AB, Ray LC, Cole MM, Canning M, Houck K, Shah HJ, et al. Reduced risk for Mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons—43 U.S. Jurisdictions. Morb Mortal Wkly Rep. 2022;71(49):1560–1564. 10.15585/mmwr.mm7149a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allard R, Leclerc P, Bergeron G, Cadieux G. Breakthrough cases of mpox: one‐dose vaccination is associated with milder clinical manifestations. J Infect Public Health. 2024;17(4):676–680. 10.1016/j.jiph.2024.02.015 [DOI] [PubMed] [Google Scholar]
- 23. Shin H, Shin H, Rahmati M, Koyanagi A, Jacob L, Smith L, et al. Comparison of clinical manifestations in mpox patients living with HIV versus without HIV: a systematic review and meta‐analysis. J Med Virol. 2023;95(4):e28713. [DOI] [PubMed] [Google Scholar]
- 24. Crosato V, Degli Antoni M, Izzo I, Cerini C, Pennati F, Gulletta M, et al. Atypical monkeypox presentation in a previously vaccinated MSM HIV‐positive adult. Infection. 2023;51(3):783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boesecke C, Monin MB, van Bremen K, Schlabe S, Hoffmann C. Severe monkeypox‐virus infection in undiagnosed advanced HIV infection. Infection. 2022;50(6):1633–1634. 10.1007/s15010-022-01901-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitjà O, Alemany A, Marks M, Lezama Mora JI, Rodríguez‐Aldama JC, Torres Silva MS, et al. Mpox in people with advanced HIV infection: a global case series. Lancet. 2023;401(10380):939–949. 10.1016/S0140-6736(23)00273-8 [DOI] [PubMed] [Google Scholar]
- 27. González‐Torres J, Méndez‐Flores S, García‐Hidalgo L, Domínguez‐Cherit J, Quiles Martínez B. Atypical presentation of monkeypox with verrucous lesions on the face and genitalia in a patient with human immunodeficiency virus (HIV). Int J Dermatol. 2023;62(11):1397–1399. 10.1111/ijd.16755 [DOI] [PubMed] [Google Scholar]
- 28. Mitjà O, Ogoina D, Titanji BK, Galvan C, Muyembe JJ, Marks M, et al. Monkeypox. Lancet (London, England). 2023;401(10370):60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giacomelli A, Moschese D, Pozza G, Casalini G, Cossu MV, Rizzardini G, et al. Route of monkeypox viral inoculum as a determinant of atypical clinical presentation. J Med Virol. 2023;95(1):e28112. [DOI] [PubMed] [Google Scholar]
- 30. Alarcón J, Kim M, Balanji N, Davis A, Mata F, Karan A, et al. Occupational Monkeypox virus transmission to healthcare worker, California, USA, 2022. Emerg Infect Dis. 2023;29(2):435–437. 10.3201/eid2902.221750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Castaño‐Fernández JL, Grau‐Pérez M. Microblading‐transmitted Monkeypox (mpox) infection: fomites matter. Br J Dermatol. 2023;188(6):e40. 10.1093/bjd/ljad063 [DOI] [PubMed] [Google Scholar]
- 32. Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773–780. [DOI] [PubMed] [Google Scholar]
- 33. Mazzotta V, Hazra A, Silva M, Montenegro‐Idrogo JJ, Gebo K, Ghosn J, et al. Mpox in people with advanced HIV infection: a global case series. Lancet (London, England). 2023;401(10380):939–949. [DOI] [PubMed] [Google Scholar]
- 34. Labate L, Brucci G, Ciccarese G, Bruzzone B, Ricucci V, Stefanelli F, et al. Nasal monkeypox virus infection successfully treated with cidofovir in a patient newly diagnosed with HIV. Int J STD AIDS. 2023;34(3):208–210. 10.1177/09564624221141152 [DOI] [PubMed] [Google Scholar]
- 35. Moody S, Lamb T, Jackson E, Beech A, Malik N, Johnson L, et al. Assessment and management of secondary bacterial infections complicating Mpox (Monkeypox) using a telemedicine service. A prospective cohort study. Int J STD AIDS. 2023;34(7):434–438. 10.1177/09564624231162760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Escudero‐Tornero R, Sobral‐Costas TG, De Moraes‐Souza R. Monkeypox lesions affecting the nose: a therapeutical challenge. J Eur Acad Dermatol Venereol. 2023;37(3):e361–e362. 10.1111/jdv.18540 [DOI] [PubMed] [Google Scholar]
- 37. Buechler CR, Anderson ZJ, Kullberg SA, Miller DD, Ahiskali A, Schut R, et al. Successful treatment of recalcitrant Mpox lesions with intralesional cidofovir in a patient with HIV/AIDS. JAMA Dermatol. 2024;160(2):235–236. 10.1001/jamadermatol.2023.4727 [DOI] [PubMed] [Google Scholar]
- 38. Pisano L, Lagi F, Turco M, Gaggioli S, Bartoloni A, Pimpinelli N. Monkeypox: a novel pitfall in clinical dermatology. Travel Med Infect Dis. 2022;50:102480. 10.1016/j.tmaid.2022.102480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scotti B, Piraccini BM, Gaspari V. Hypertrophic verrucous lesions after monkeypox virus infection. Lancet Infect Dis. 2023;23(2):259. 10.1016/S1473-3099(22)00695-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ogoina D, Iroezindu M, James HI, Oladokun R, Yinka‐Ogunleye A, Wakama P, et al. Clinical course and outcome of human Monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210–e214. 10.1093/cid/ciaa143 [DOI] [PubMed] [Google Scholar]
- 41. Aromolo IF, Maronese CA, Avallone G, Beretta A, Boggio FL, Murgia G, et al. Clinical spectrum of human monkeypox: An Italian single‐centre case series. J Eur Acad Dermatol Venereol. 2023;37(3):e368–e371. 10.1111/jdv.18612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Deep facial ulcers in the perioral and nasal area with large perilesional inflammation after the crust has been removed.
Figure S2. Scars of depressed and atrophic appearance on the nasal tip.
Figure S3. (A) Inflammatory ulcer with a fibrinous background on the dorsum of the tongue. (B) After healing of the lesion, lingual depapillation can be seen in the area of the stellate scar.
Figure S4. (A) Multiple perianal ulcers with radial distribution and endoanal involvement. (B) Nacreous perianal scarring in the areas of previous ulceration and polypoid lesion at the anal margin.
Figure S5. (A) Clustered ulcers of linear arrangement at the root of the penis. (B) Linear spindle‐shaped scar as sequelae of the lesions.
Figure S6. (A) Pseudopustule with central necrosis in the proximal foreskin. (B) The resulting scar has a linear and hypopigmented appearance.
Figure S7. (A) Oval scar with a varioliform appearance in the dorsum of the penis. (B) Smaller fusiform scar.
Figure S8. Multiple hyperpigmented papules with a parchment‐like appearance with histology compatible with anetoderma.
Table S1. Number of active lesions and scars in patients according to location.
Table S2. Characteristics of the scars in the patients in our sample.
Data Availability Statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.


