Abstract
Background
Hemophilia A (HA) treatment strategies aim to manage bleeding episodes and improve patients' quality of life. This study investigates the effectiveness of a preventative approach using intermediate-dose prophylaxis with standard half-life FVIII products in reducing bleeding rates and enhancing the quality of life for patients with severe HA.
Methods
A 4-year prospective longitudinal study followed 35 patients with severe HA (without FVIII inhibitors) who transitioned from a reactive treatment approach to intermediate-dose prophylaxis in Taiwan from 2014 until 2018. The study tracked annual bleeding rates (ABR) and annual joint bleeding rates (AjBR) alongside associated costs and patient-reported quality-of-life measures.
Results
Prophylaxis significantly reduced both ABR and AjBR compared with the previous treatment. After one year, ABR and AjBR decreased by 76.9% and 72.5%, respectively, with further reductions to 91.0% and 90.8% after 4 years (p < 0.001). While the average annual cost of factor VIII concentrate increased by 41.0% in the first year, the incremental cost-effectiveness ratio demonstrated ongoing benefits from ABR avoidance over the 4 years. Additionally, patients reported significant improvements in quality-of-life measures following the switch to prophylaxis (p = 0.036).
Conclusion
Intermediate-dose prophylaxis effectively reduced bleeding rates and improved quality of life in patients with severe HA. Despite initial cost increases, the intervention became cost effective over time. This study provides valuable data for healthcare policymakers, highlighting the long-term benefits of prophylaxis as a preventative approach for managing bleeding and improving overall well-being in patients with severe HA.
Key Points
| Intermediate-dose prophylaxis significantly reduces annual bleeding rates (ABR and AjBR) and enhances patients' quality of life compared with reactive treatment. |
| While initial costs increase, prophylaxis becomes increasingly cost effective over time due to the prevention of bleeding episodes. |
| Pediatric patients may benefit even more from prophylaxis, with potentially lower costs per bleeding event prevented and better long-term outcomes. |
Introduction
Hemophilia affects approximately 1 in 6000–10,000 males globally (World Haemophilia Day organizations, 2024). Hemophilia A (HA) is an inherited X-linked disease caused by a deficiency of coagulation factor VIII (FVIII). The severity of the disease can be classified as mild, moderate, or severe based on the blood FVIII activity level (defined as an FVIII activity level of > 5 IU/dL to < 40 IU/dL, 1 IU/dL to 5 IU/dL, and < 1 IU/dL, respectively) [1, 2]. Patients with severe HA typically present with severe clinical bleeding manifestations, especially in active joints, which can lead to severe chronic arthropathy and may require joint replacement for function recovery or pain relief if hemarthrosis is not adequately treated and prevented. More than 80% of young children with severe HA experience their first bleeding event within the first 2 years of life [3]. In patients with severe hemophilia, hemarthrosis accounts for 80% of all bleeding episodes [1, 4].
FVIII replacement has long been considered an efficacious regimen for both adult and pediatric patients with severe HA. On-demand and prophylactic treatment are two main factor replacement strategies [1]. Prophylactic treatment has been found to be more cost effective than on-demand treatment in numerous studies [5, 6]. The concept of prophylactic hemophilia treatment, which involves the regular infusion of factor concentrates, was first introduced in the 1950s [7, 8]. However, prophylactic FVIII treatment is expensive, especially for patients with severe hemophilia. Studies have shown that prophylactic treatment is efficacious in reducing the incidence of severe bleeding events and the severity of hemophilic arthropathy in patients with severe HA [7, 9]. Primary prophylaxis, defined as regular factor treatment starting before any joint damage caused by repeated bleeding has occurred, has become the standard of care for patients with severe hemophilia because it is associated with a better health-related quality of life (HRQoL) than on-demand treatment [10]. In a 2024 retrospective observational study, HRQoL of 100 people with hemophilia in North Greece was assessed, revealing that disease severity, age, and physical activity significantly impact a patient’s quality of life. Results also showed that younger and more severely affected patients had low HRQoL scores [11].
The Malmö protocol (Swedish high-dose prophylaxis, 25–40 IU/kg, three times a week) and the Utrecht protocol (Dutch intermediate-dose prophylaxis, 15–30 IU/kg, three times a week) are the most widely used FVIII prophylaxis protocols with long-term evidence [1, 12]. According to studies conducted in Sweden and the Netherlands, the incremental benefits of high-dose prophylaxis over intermediate-dose prophylaxis for severe hemophilia appeared to be modest. Although intermediate-dose prophylaxis had slightly inferior clinical outcomes in terms of joint bleeding and joint health, social participation and quality of life were comparable [13, 14]. However, the annual total cost of high-dose prophylaxis was 66% higher than the cost of intermediate-dose prophylaxis. Different countries use different treatment regimens due to differences in healthcare policies and economic considerations.
Launched in 1995, the Taiwan National Health Insurance (NHI) system is a mandatory, single-payer social insurance system that covers almost 100% of the population in Taiwan. Intermediate-dose prophylaxis with standard half-life FVIII products for severe HA has been officially advocated in Taiwan since the regimen was included in our NHI benefit package in 2014, resulting in a rising number of patients receiving prophylactic treatment (73.4% in 2019). However, many patients, especially the elderly, still prefer on-demand episodic treatment. There remains a lack of consensus regarding the cost effectiveness and quality of life of different treatment modalities in Taiwan [15, 16]. Therefore, pharmacoeconomic evaluations of severe hemophilia treatments are needed. This 4-year longitudinal study was a time investigation in Taiwan to evaluate the clinical bleeding outcomes, costs, and cost effectiveness of switching from on-demand treatment to intermediate-dose prophylaxis with standard half-life FVIII products in patients with severe HA without FVIII inhibitors.
Methods
Study Design
The prospective study was designed as an observational study by the Taiwan Society of Thrombosis and Hemostasis (TSTH) Study Group and was conducted at five hemophilia treatment centers in Taiwan. This study protocol was approved by the Institution Review Board of all participating sites in Taiwan [IRB CCH No. 141115, CMUCH104-REC2-024 (TR), KMUHIRB-SV(I)-20210016].
Patient Population and Demographic Characteristics
A cohort of 35 patients with severe hemophilia A without FVIII inhibitors (inhibitor activity <0.6 Bethesda units) who switched from the reactive treatment to intermediate-dose prophylaxis (standard half-life FVIII products 15–25 IU/kg, 1–3 times a week) were enrolled for analysis between 2014 and 2018. A medical record detailing the bleeding episodes from at least 1 year before enrollment was required. The assessment includes the patient’s basic information, clinical examination data, and hemophilia-related questionnaires. Prophylactic treatments were classified according to the World Federation of Hemophilia guidelines [1]. Thirty-four patients received tertiary prophylaxis after the onset of documented joint disease, and one received secondary prophylaxis after two or more joint bleeds.
Measurements
In our study, compliance of hemophilia patients was defined by FVIII inhibitor serology tests at least every 3 months, intermediate-dose prophylaxis 15–30 IU/kg 3 times a week for at least 45 of a total 52 weeks, annual bleeding rate (ABR), and annual joint bleeding rate (AjBR). The definition of a target joint is three or more spontaneous bleeds into the same single joint within a consecutive 6-month period. If there are two or fewer bleeds into the joint within a consecutive 12-month period, the joint is no longer considered a target joint [17].
Hemophilia Activity List (HAL), Hemophilia Joint Health Score (HJHS), and Hemophilia-Specific Quality of Life Index (Haemo-QoL and Haem-A-QoL questionnaires) of children and adults were used to assess clinical outcomes [18, 19]. The HAL is a hemophilia-specific questionnaire designed to evaluate self-perceived functioning in patients with hemophilia [20]. The HAL assessment includes daily living functions such as lying, sitting, kneeling, standing, leg function, use of transportation, self-care, sports, and others [20]. The HJHS is the current global measure of joint health and a higher score indicates worse joint health [21]. The HJHS includes an assessment of specific characteristics of joints as well as an assessment of overall joint swelling and duration of swelling, muscle and joint pain, strength, etcetera [21].
Cost analysis was conducted from the perspective of the Taiwan national health system, and only direct drug costs were considered. The drug cost was based on the Taiwan National Health Insurance formulary price index. The costs of the FVIII were converted from New Taiwan Dollars (NTD) to United States Dollars (USD) using a conversion factor of 0.03619 on January 3, 2022, as obtained from the Central Bank of Taiwan's website. The dose and schedule of prophylactic treatment, bleeding episodes, clinical symptoms, and patients’ self-reported data were collected and reviewed by physicians during patients’ clinical visits to hemophilia treatment centers.
Statistical Analysis
Descriptive statistics, the Wilcoxon signed-rank test, and multiple linear regressions were used to analyze the data. The number and percentage of patients in each demographic characteristic were presented. Bar charts were used to present the mean ABR, AjBR, and annual FVIII cost for each year before and after the treatment modality switch. Box plots were used to present the median ABR and AjBR for each year before and after the treatment modality switch, and a Wilcoxon signed-rank test was used to determine whether there was a significant decrease in ABR or AjBR over time. A multiple linear regression model was used to examine the relationship between the clinical outcomes and explanatory variables and to determine the significance of each variable. A p-value of <0.050 is considered statistically significant. All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA)
Results
Patient Characteristics
This prospective study included 35 male patients who had severe HA without FVIII inhibitors. The demographic data of the patients are shown in Table 1, including their age, education, blood type, body mass index (BMI), occupation, and previous history of FVIII inhibitors. At the end of the study, all participants remained on prophylactic treatment. The mean follow-up time was 3.6 years (range: 1–4 years).
Table 1.
Demographic characteristics of the study cohort
| Patient number (n = 35) | |
|---|---|
| Age group, n [mean ± SD] | |
| > 18 years | 29 [37.2 ± 9.3] |
| ≤ 18 years | 6 [13.5 ± 6.1] |
| All patients | 35 [33.2 ± 12.6] |
| Education, n [%] | |
| College/university | 13 [37.1] |
| High school | 21 [60.0] |
| Preschool | 1 [2.9] |
| Blood type, n [%] | |
| Non-O type | 21 [60.0] |
| O type | 14 [40.0] |
| Body mass index, n [%] | |
| < 25 kg/m2 | 21 [60.0] |
| ≥ 25 kg/m2 | 14 [40.0] |
| Occupation, n [%] | |
| Employed | 24 [68.6] |
| Unemployed | 3 [8.6] |
| Student | 8 [22.8] |
| Previous history of FVIII inhibitor, n [%] | |
| ≤ 0.6 BU | 18 [51.4] |
| > 0.6 BU | 17 [48.6] |
BU Bethesda unit, FVIII factor VIII, SD standard deviation
Assessments of Bleeding Episodes, Clinical and Joint Function, and Quality of Life
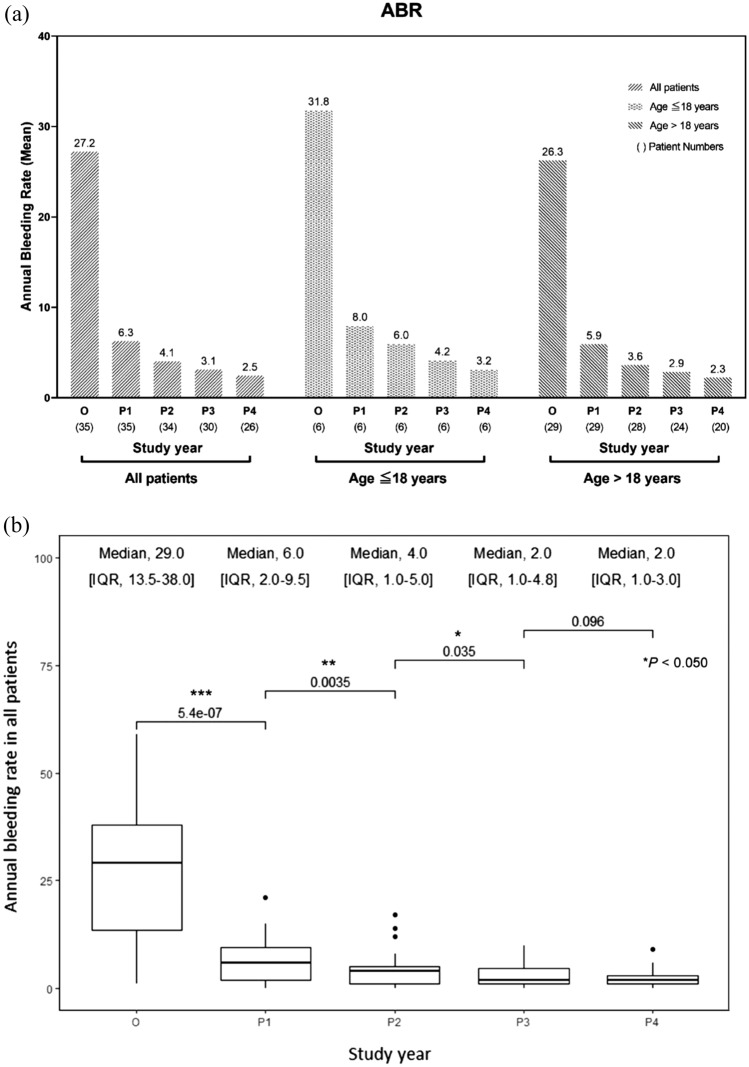
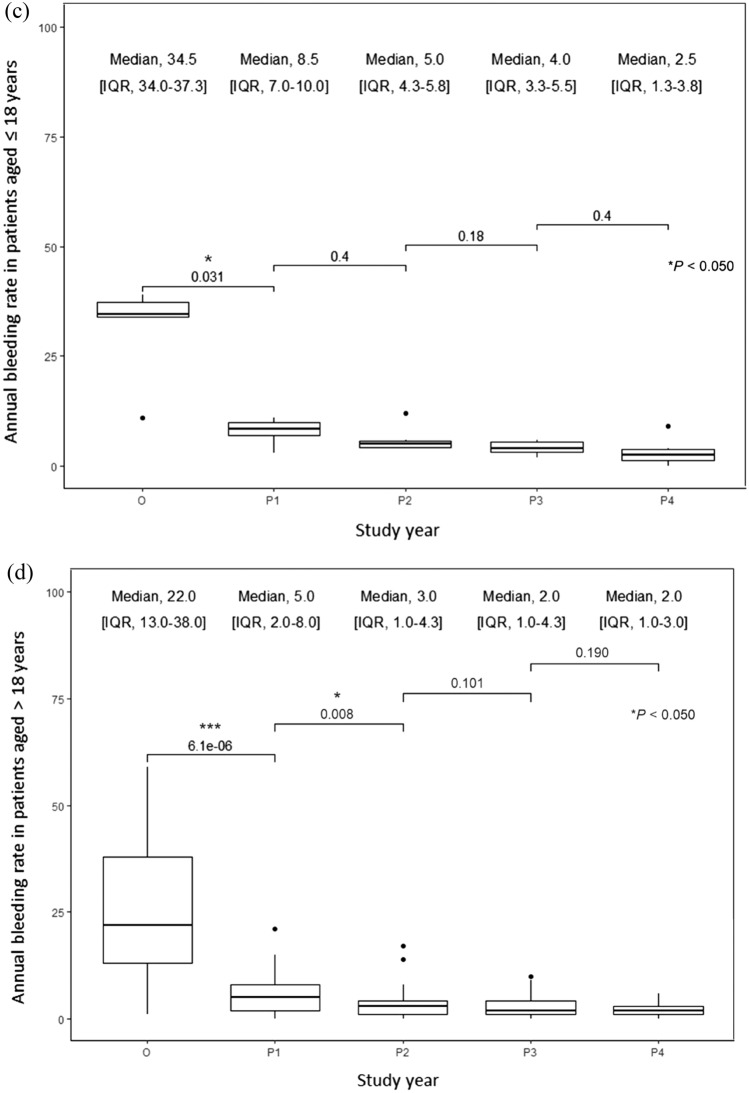
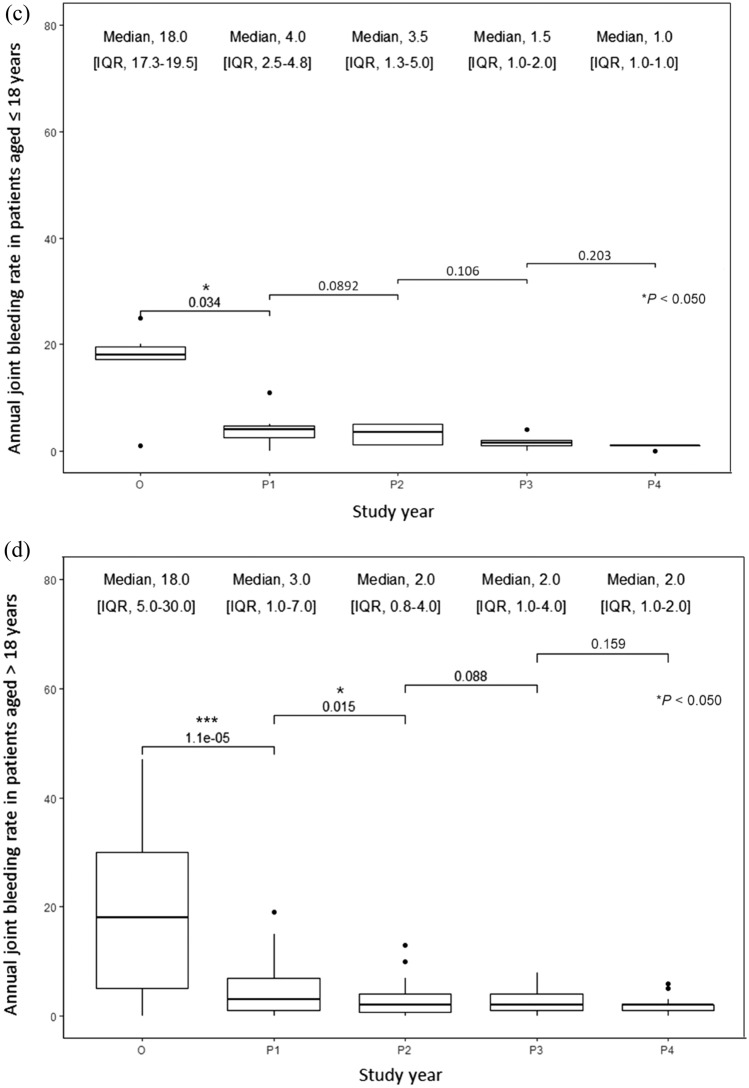
The mean and median ABRs decreased significantly by 76.9% and 79.3%, respectively, after 1 year of switching from the reactive to prophylactic treatment and continued to decrease in the following years in all patients (Fig. 1a–d). After 4 years of the switch, the mean and median ABRs decreased by 91.0% and 93.1%, respectively. The mean and median AjBRs decreased significantly by 72.5% and 77.8%, respectively, after 1 year of switching from the reactive to prophylactic treatment and continued to decrease in the following years in all patients (Fig. 2a–d). After 4 years of the switch, the mean and median AjBRs decreased by 90.8% and 94.4%, respectively. Patients with a previous history of FVIII inhibitors had higher ABR and AjBR compared with those without a history. Multiple linear regression analyses showed that age, education, blood type, BMI, and occupation were not significant predictors of bleeding manifestations (Table 2). To assess for multicollinearity, variance inflation factors (VIF) were calculated for the ABR and AjBR regression models. The resulting VIF values of 1.754 and 1.613, respectively, indicate a very low level of collinearity among the independent variables in both models, suggesting that multicollinearity is not a significant issue in this analysis. The ABR was higher in pediatric patients than in adult patients in the first 3 years of intermediate-dose prophylaxis (p = 0.039). Results of the Wilcoxon Signed Rank test indicate HAL scores did not show significant improvement in adult patients after prophylaxis, however significant improvements were shown in pediatric patients (p = 0.021). HJHS did not show significant improvement in adult and pediatric patients. Quality of life after prophylactic treatment showed a significant improvement in a single institution study (p = 0.036) (Table 3).
Fig. 1.
a Mean annual bleeding rate (ABR) in all patients, patients aged ≤ 18 years and patients aged > 18 years; b–d median ABR in all patients, patients aged ≤ 18 years, and patients aged > 18 years, respectively, in severe hemophilia A patients before and after the treatment modality switch. Wilcoxon signed-rank test: * p < 0.05; ** p < 0.005; *** p < 0.0005. IQR interquartile range, O on-demand treatment year (one year before prophylaxis), P1 1st year of prophylactic treatment, P2 2nd year of prophylactic treatment, P3 3rd year of prophylactic treatment, P4 4th year of prophylactic treatment
Fig. 2.
a Mean annual joint bleeding rate (AjBR) in all patients, patients aged ≤ 18 years, and patients aged > 18 years; b–d median AjBR in all patients, patients aged ≤ 18 years, and patients aged > 18 years in severe hemophilia A patients, respectively, before and after the treatment modality switch. Wilcoxon signed-rank test: * p < 0.05; ** p < 0.005; *** p < 0.0005. IQR interquartile range, O on-demand treatment year (1 year before prophylaxis), P1 1st year of prophylactic treatment, P2 2nd year of prophylactic treatment, P3 3rd year of prophylactic treatment, P4 4th year of prophylactic treatment
Table 2.
Multiple linear regression analyses of the (a) annual bleeding rate (ABR) and (b) annual joint bleeding rate (AjBR)
| Estimate | SE | p-Value | |
|---|---|---|---|
| (a) Annual bleeding rate | |||
| Age (years) | 0.05 | 0.07 | 0.528 |
| Education (0 = high school or preschool, 1 = college or higher) | − 0.15 | 1.68 | 0.927 |
| Blood type (0 = Non-O type, 1 = O type) | 1.47 | 1.75 | 0.401 |
| Body mass index (kg/m2) | 0.68 | 1.69 | 0.688 |
| Occupation (0 = employed or under-employed, 1 = student) | − 3.24 | 2.01 | 0.110 |
| History of FVIII inhibitor (0 = FVIII inhibitor ≤ 0.6 BU, 1 = FVIII inhibitor > 0.6 BU) | 4.76 | 1.57 | 0.003** |
| Follow-up year (1 = 1st follow-up year, 2, 3, 4 = 2nd, 3rd, 4th follow-up year) | − 5.63 | 0.56 | < 0.001** |
| R2 | 0.43 | ||
| 1st year of prophylactic treatment | − 20.94 | 2.55 | < 0.001** |
| 2nd year of prophylactic treatment | − 23.29 | 2.60 | < 0.001** |
| 3rd year of prophylactic treatment | − 24.47 | 2.67 | < 0.001** |
| 4th year of prophylactic treatment | − 25.41 | 2.87 | < 0.001** |
| (b) Annual joint bleeding rate | |||
| Age (years) | 0.10 | 0.05 | 0.076 |
| Education (0 = high school or preschool, 1 = college or higher) | 0.36 | 1.25 | 0.776 |
| Blood type (0 = Non-O type 1 = O type) | 1.25 | 1.30 | 0.339 |
| Body mass index (kg/m2) | 0.22 | 1.25 | 0.864 |
| Occupation (0 = employed or under-employed, 1 = student) | − 1.79 | 1.50 | 0.235 |
| History of FVIII inhibitor (0 = FVIII inhibitor ≤ 0.6 BU, 1 = FVIII inhibitor > 0.6 BU) | 3.53 | 1.17 | 0.003** |
| Follow-up year (1 = 1st follow-up year, 2, 3 ,4 = 2nd, 3rd, 4th follow-up year) | − 3.71 | 0.42 | < 0.001** |
| R2 | 0.38 | ||
| 1st year of prophylactic treatment | − 12.97 | 2.14 | < 0.001** |
| 2nd year of prophylactic treatment | − 15.01 | 2.10 | < 0.001** |
| 3rd year of prophylactic treatment | − 15.67 | 2.19 | < 0.001** |
| 4th year of prophylactic treatment | − 16.31 | 2.34 | < 0.001** |
BU Bethesda unit, FVIII factor VIII, SE standard error
**p < 0.005
Table 3.
Clinical significance of the Hemophilia Activities List (HAL) and Hemophilia Joint Health Score (HJHS) in patients with severe hemophilia A before and after prophylaxis, and quality of life (QoL) in patients with severe hemophilia A before and after prophylaxis, from a single institution
| Before prophylaxis | After prophylaxis | p-value | |
|---|---|---|---|
| Hemophilia Activities List (HAL), mean (SE) | |||
| Age > 18 years (n = 27) | 78.06 (4.39) | 79.59 (3.93) | 0.735 |
| Age ≤ 18 years (n = 6) | 79.82 (4.75) | 98.57 (0.55) | 0.021* |
| Hemophilia Joint Health Score (HJHS) | |||
| Age > 18 years (n = 27) | 37.15 (4.12) | 33.27 (4.13) | 0.681 |
| Age ≤ 18 years (n = 6) | 5.40 (1.99) | 5.00 (0.11) | 0.410 |
| Quality of life (QoL) | |||
| A single institution (n = 18) | 38.88 (3.64) | 23.24 (2.45) | 0.036* |
* p < 0.05
Incremental Cost per Unit of Clinical Outcome (ABR, AjBR)
The average annual FVIII cost increased by 41.0% in the first year of switching from reactive to prophylactic treatment and held steady in the following years (Table 4). The incremental cost-effectiveness ratios (ICERs) of all 35 patients were USD2304 per ABR avoided and USD3721 per AjBR avoided in the first year of the switch. After the fourth year of prophylactic treatment, the ICERs dropped to USD1780 per ABR avoided and USD2716 per AjBR avoided, respectively, in 26 patients followed up for 4 years. The ICERs were lower in pediatric patients than in adult patients, indicating that the additional cost of avoiding one bleeding episode was lower in pediatric patients (Table 4). In the fourth year of prophylactic treatment, intermediate-dose prophylaxis prevented 28.6 and 24.0 bleeding episodes per year on average in pediatric and adult patients, respectively, compared with when the patients were receiving reactive therapy. The ABR and AjBR in the fourth year of prophylactic treatment were 2.5 and 1.7, respectively, in all patients. When compared with the reactive treatment, prophylactic treatment was more expensive but more effective in preventing bleeding episodes (Table 4). Prophylactic treatment improved quality of life and was a cost-effective option.
Table 4.
Incremental cost-effectiveness ratio (ICER) in severe hemophilia A patients after the treatment modality switch
| Study year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | P1 | P2 | P3 | P4 | ∆P1 – O | ∆P2 – O | ∆P3 – O | ∆P4 – O | |
| All patients | |||||||||
| Patient number | 35 | 35 | 34 | 30 | 26 | ||||
| Average annual FVIII consumption (IU/year) | 155,394 | 219,036 | 210,182 | 212,989 | 213,536 | 63,641 | 54,788 | 57,594 | 58,142 |
| Average cost of FVIII (USD) | 117,840 | 166,100 | 159,387 | 161,515 | 161,930 | 48,261 | 41,547 | 43,675 | 44,090 |
| Average ABR (bleeds/year) | 27.2 | 6.3 | 4.1 | 3.1 | 2.5 | 20.9 | 23.1 | 24.1 | 24.7 |
| Average AjBR (bleeds/year) | 17.9 | 4.9 | 3.0 | 2.3 | 1.7 | 13.0 | 14.9 | 15.6 | 16.2 |
| USD per ABR avoided | 2304 | 1793 | 1813 | 1780 | |||||
| USD per AjBR avoided | 3721 | 2786 | 2796 | 2716 | |||||
| ABR, median (IQR) | 29.0 (13.5–38.0) | 6.0 (2.0–9.5) | 4.0 (1.0–5.0) | 2.0 (1.0–4.8) | 2.0 (1.0–3.0) | ||||
| AjBR, median (IQR) | 18.0 (6.0–26.0) | 4.0 (1.0–7.0) | 2.0 (1.0–4.0) | 2.0 (1.0–3.8) | 1.0 (1.0–2.0) | ||||
| Age ≤ 18 years | |||||||||
| Patient number | 6 | 6 | 6 | 6 | 6 | ||||
| Average annual FVIII consumption (IU/year) | 118,292 | 131,500 | 132,667 | 125,792 | 130,903 | 13,208 | 14,375 | 7500 | 12,611 |
| Average cost of FVIII (USD) | 89,704 | 99,720 | 100,605 | 95,391 | 99,267 | 10,016 | 10,901 | 5687 | 9563 |
| Average ABR (bleeds/year) | 31.8 | 8.0 | 6.0 | 4.2 | 3.2 | 23.8 | 25.8 | 27.6 | 28.6 |
| Average AjBR (bleeds/year) | 16.5 | 4.3 | 3.2 | 1.7 | 0.8 | 12.2 | 13.3 | 14.8 | 15.7 |
| USD per ABR avoided | 420 | 422 | 206 | 334 | |||||
| USD per AjBR avoided | 823 | 818 | 383 | 610 | |||||
| ABR, median (IQR) | 34.5 (34.0–37.3) | 8.5 (7.0–10.0) | 5.0 (4.3–5.8) | 4.0 (3.3–5.5) | 2.5 (1.3–3.8) | ||||
| AjBR, median (IQR) | 18.0 (17.3–19.5) | 4.0 (2.5–4.8) | 3.5 (1.3–5.0) | 1.5 (1.0–2.0) | 1.0 (1.0–1.0) | ||||
| Age > 18 years | |||||||||
| Patient number | 29 | 29 | 28 | 24 | 20 | ||||
| Average annual FVIII consumption (IU/year) | 163,071 | 237,147 | 226,793 | 234,788 | 238,326 | 74,076 | 63,722 | 71,717 | 75,255 |
| Average cost of FVIII (USD) | 123,661 | 179,834 | 171,983 | 178,046 | 180,729 | 56,174 | 48,322 | 54,385 | 57,068 |
| Average ABR (bleeds/year) | 26.3 | 5.9 | 3.6 | 2.9 | 2.3 | 20.4 | 22.7 | 23.4 | 24.0 |
| Average AjBR (bleeds/year) | 18.2 | 5.0 | 2.9 | 2.4 | 1.9 | 13.2 | 15.3 | 15.8 | 16.3 |
| USD per ABR avoided | 2761 | 2135 | 2324 | 2375 | |||||
| USD per AjBR avoided | 4276 | 3170 | 3452 | 3507 | |||||
| ABR, median (IQR) | 22.0 (13.0–38.0) | 5.0 (2.0–8.0) | 3.0 (1.0–4.3) | 2.0 (1.0–4.3) | 2.0 (1.0–3.0) | ||||
| AjBR, median (IQR) | 18.0 (5.0–30.0) | 3.0 (1.0–7.0) | 2.0 (0.8–4.0) | 2.0 (1.0–4.0) | 2.0 (1.0–2.0) | ||||
ABR annual bleeding rate, AjBR annual joint bleeding rate, FVIII factor VIII, IQR interquartile range, IU international unit, O on-demand treatment year (1 year before prophylaxis), P1 1st year of prophylactic treatment, P2 2nd year of prophylactic treatment, P3 3rd year of prophylactic treatment, P4 4th year of prophylactic treatment, USD United States dollars
Discussion
FVIII prophylaxis is considered the main treatment modality for severe HA patients [1, 6, 18, 19, 21]. In this 4-year longitudinal study, the ABR and AjBR decreased rapidly in the first year of switching from the reactive treatment to intermediate-dose prophylaxis and continued to decrease in the following years, suggesting that longer prophylactic treatment is associated with better clinical outcomes. Our study also found that after prophylactic treatment, patients’ quality of life improved, indicating that their life functions had improved. After switching to intermediate-dose prophylaxis, pediatric patients had higher ABRs than adult patients, which could be attributed to insufficient FVIII levels, a shorter FVIII half-life, and higher activity levels. Higher and more frequent FVIII dosing, as well as individualized pharmacokinetic-driven prophylaxis, which has been found to be more cost effective than standard prophylaxis in treating children with hemophilia, may be required for better bleeding control in young children [22, 23]. A 10-year study showed that intermediate-dose prophylaxis helped adolescent patients with severe hemophilia A maintain joint function below the critical level for most of their lifetime [19]. HAL score and HJHS showed no significant change in these follow-up years after switching to prophylaxis treatment. Yet, pediatric patients exhibited better HAL scores after switching, which may be due to fewer chronic joint conditions in these patients. QoL showed a significant improvement after switching to prophylaxis treatment (Table 3).
Given the above, our study demonstrated that intermediate-dose FVIII prophylaxis is a highly effective treatment for severe hemophilia A in Taiwan, although our patients are mostly adults with advanced arthropathy. The proportion of patients experiencing zero joint bleeding events increased from 0 to 17.6% after switching to prophylactic treatment. To achieve zero bleeding events and improve clinical outcomes even further, developing an individualized treatment plan based on the patient’s age, bleeding phenotype, physical activity, specific clinical scenario, and pharmacokinetic profile is essential [1, 19, 21, 24]. FVIII prophylaxis in severe hemophilia should be started as early as possible, especially in young patients. During the study period, adult patients only showed minor and insignificant improvement in HAL and HJHS after switching to prophylactic treatment. However, if the patients are followed for a longer period, the findings may show more improvement due to better joint stability and the utilization of rehabilitation therapies, as our patients showed good adherence to the treatment protocol in this study [19].
Many countries, particularly developing countries, are concerned about the economic burden of long-term FVIII prophylactic treatment when developing national health policies. Numerous pharmacoeconomic studies comparing on-demand versus prophylactic treatment have been done to aid in the development of national health policy [15, 25, 26]. Although there are studies showing a trend for decreasing ABR with the use of extended half-life (EHL) FVIII products for prophylactic treatment in severe HA, it has not resulted in an obvious improvement in HJHS or quality of life. Both products may require long-term observational records [27]. The use of a short half-life (SHL) FVIII products instead of EHL would amount to cost savings for the National Health System in some countries [28–31]. However, the majority of pharmacoeconomic studies were conducted in Western countries, and they focused on prophylactic treatment initiated at a young age with short follow-up periods [12, 23, 26, 32, 33]. Additionally, on-demand and prophylactic treatments are rarely compared in the same cohort of patients, which might need more individual adjustment statistically. In this longitudinal study, we prospectively analyzed the cost effectiveness of on-demand treatment versus late/tertiary prophylaxis in the same cohort of patients for 4 years. Patients who initiate prophylactic treatment after developing varying degrees of chronic joint diseases may incur higher costs due to increased FVIII consumption. However, less bleeding and improved joint health will result in a reduction in long-term medical costs associated with complications and comorbidities. Improved quality of life and activity levels may also have a beneficial effect on cost effectiveness, thus justifying investment in prophylactic treatment.
Our results demonstrate that the ICERs decreased to USD1780 for each ABR averted and USD2716 for each AjBR avoided throughout the fourth year of preventative treatment. These values were in line with or less than those reported in other studies (USD2538 and USD4355) [12, 15, 34]. Gringeri et al. reported an ICER of USD8537 per bleeding event avoided following a median follow-up of 4 years when comparing primary prophylaxis with reactive treatment. Our study showed that prophylactic treatment was more cost effective in pediatric patients than in adult patients, owing to the reduced cost of FVIII. Prophylactic treatment initiated at a young age can have a larger beneficial effect and help maintain joint health as children grow into adulthood.
This study also has limitations. It does not fully cover all expenses, such as medical expenses, consumable pharmaceuticals, etc. Although the reactive treatment is associated with a lower FVIII cost, other direct and indirect economic impacts on patients and their families should not be overlooked. In this study, we focused exclusively on FVIII consumption expenses and omitted other direct medical costs associated with clinic visits, laboratory and imaging assessments, hospitalization, port insertion, medications, long-term rehabilitation, dental treatment, and joint replacement [33]. In terms of these indirect costs, they may include economic loss due to patients and family members being unable to work, transportation for treatment care, psychosocial support, handicap services, early medical retirement, labor productivity loss, and poor quality of life [18, 19, 33, 35, 38, 39].
Prophylactic treatment should be tailored to each patient, and many patients may do well on lower doses of FVIII [35]. Previous studies have demonstrated that short-term low-dose prophylaxis (10 IU/kg, twice a week) may also be a feasible and a beneficial option in resource-limited countries [36, 37].
This study compared the cost effectiveness of intermediate-dose prophylaxis treatment and reactive treatment with comparable outcomes shown with other studies, which can assist health policymakers in determining which regimen to pay for when working with a limited budget [13]. Our clinical outcome results can be adopted for cost-effectiveness and pharmacoeconomic evaluation by government healthcare policymakers. However, a limitation of this method is that it doesn't account for the impact on patients' quality of life. A reduction in bleeding events might significantly improve quality of life, which isn't captured by this approach. Further pharmacoeconomic research may evaluate the impact of rare but costly hemophilia treatment-related complications, such as bleeding in the central nervous system, gastrointestinal tract, or renal system. Other pharmacoeconomic studies, such as cost-utility analyses that evaluate the costs and health effects (measured in quality-adjusted life-years [QALYs], a core measure of how well different treatment options improve the quantity and quality of life) of alternative interventions, may give more extensive and comprehensive information, however, the results of these studies varied. Standardization of the analytic method is required. In addition, most studies were funded by pharmaceutical companies, which increases the possibility of sponsor bias [16, 20].
Apart from the added cost, treatment adherence may add another layer of challenge to prophylactic treatment. The main reason for the premature termination of prophylactic treatment is frequent infusions [22, 23]. The use of extended half-life factor replacement therapy will result in fewer infusions, a lower treatment burden, and improved adherence, all of which will contribute to achieving the goal of zero bleeding events.
Conclusion
This 4-year study provided compelling evidence for the long-term benefits of switching to intermediate-dose prophylaxis in severe HA. Not only did annual bleeding rates (ABR and AjBR) and patient-reported quality of life improve significantly, but the cost effectiveness of prophylaxis also increased over time. While the initial cost of FVIII rises with prophylaxis, our findings demonstrate a continuous decrease in the incremental cost per bleeding event prevented. This suggests that prophylaxis becomes progressively more cost effective the longer patients receive it. Furthermore, the study suggests that pediatric patients might benefit even more from this approach. Early initiation and potentially incorporating pharmacokinetic-guided dosing adjustments could further optimize cost effectiveness, particularly in this age group. Overall, this study strengthens the case for prophylaxis as a valuable approach for managing HA, but future research can provide even more robust data on its long-term cost-effectiveness profile.
Acknowledgments
The authors express sincere gratitude to Fu-Wen Liang and Yu-Chuan Liu of Kaohsiung Medical University for their invaluable contributions to the statistical analysis of this study. We are deeply indebted to the clinicians and staff of the Taiwan Society of Thrombosis and Hemostasis (TSTH) Study Group for their dedicated efforts in patient recruitment and data collection.
Declarations
Funding
No funding was provided for this research.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Ethics Approval
This study protocol was approved by the Institution Review Board of all participating sites in Taiwan (IRB CCH No. 141115, CMUCH104-REC2-024 (TR), KMUHIRB-SV(I)-20210016).
Consent to Participate
Informed consent was obtained from all participants prior to their involvement in the study.
Consent for Publication
Not applicable.
Availability of Data and Material
The data supporting the results reported in this article are available upon reasonable request. Please contact the corresponding author for further information.
Code Availability
The code used for the statistical analyses is available upon request. Please contact the corresponding author for more details.
Authors’ Contributions
SS Chiou substantially contributed to the conception and design of the study, drafted the manuscript, and interpreted the data. MC Shen contributed to the conception and interpretation of the study. JD Wang contributed to the design of the study and analyzed the data. CY Lin, TF Weng, CT Peng, PC Lin, and YM Liao acquired and collected data and participated in patient recruitment. SC Chou collected data, contributed to interpretation, and participated in the conception of the study. L Lai conducted the statistical analysis, interpreted the results, and critically reviewed and revised the manuscript. All authors have read and approved the final submitted manuscript and agree to be accountable for all aspects of the work, ensuring that any questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Contributor Information
Leanne Lai, Email: LL33317@gmail.com.
Ming-Ching Shen, Email: 111710@cch.org.tw.
Taiwan Society of Thrombosis, Hemostasis (TSTH) Study Group:
Shyh-Shin Chiou, Ching-Yeh Lin, Te-Fu Weng, Jiaan-Der Wang, Sheng-Chieh Chou, Ching-Tien Peng, and Ming-Ching Shen
References
- 1.Srivastava A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47. [DOI] [PubMed] [Google Scholar]
- 2.Blanchette V, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–9. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni R, et al. Complications of haemophilia in babies (first two years of life): a report from the Centers for Disease Control and Prevention Universal Data Collection System. Haemophilia. 2017;23:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gringeri A, Ewenstein B, Reininger AJH. The burden of bleeding in haemophilia: is one bleed too many? Haemophilia. 2014;20:459–63. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z-Y, et al. Burden of illness: direct and indirect costs among persons with hemophilia A in the United States. J Med Econ. 2015;18:457–65. [DOI] [PubMed] [Google Scholar]
- 6.Colombo GL, Di Matteo S, Mancuso ME, Santagostino E, CEOR OR. Cost–utility analysis of prophylaxis versus treatment on demand in severe hemophilia A. ClinicoEcon Outcomes Res. 2011;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson I, Berntorp E, Löfqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. [DOI] [PubMed] [Google Scholar]
- 8.Ahlberg Å. Haemophilia in Sweden VII. Incidence, treatment and prophylaxis of arthropathy and other musculo-skeletal manifestations of haemophilia A and B. Acta Orthop Scand Suppl. 1965;36:3–132. [DOI] [PubMed] [Google Scholar]
- 9.Manco-Johnson M, et al. Randomized, controlled, parallel-group trial of routine prophylaxis vs. on-demand treatment with sucrose-formulated recombinant factor VIII in adults with severe hemophilia A (SPINART). J Thromb Haemost. 2013;11:1119–27. [DOI] [PubMed] [Google Scholar]
- 10.Royal S, et al. Quality-of-life differences between prophylactic and on-demand factor replacement therapy in European haemophilia patients. Haemophilia. 2002;8:44–50. [DOI] [PubMed] [Google Scholar]
- 11.Moka E, Ntova Z, Gavrilaki E, et al. A retrospective observational study of quality of life in a northern greece population of people with hemophilia. Life. 2024;14(6):697:1-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szucs T, Öffner A, Schramm WH. Socioeconomic impact of haemophilia care: results of a pilot study. Haemophilia. 1996;2(4):211–7. [DOI] [PubMed] [Google Scholar]
- 13.Fischer K, Steen Carlsson K, Petrini P, Holmström M, Ljung R, Van Den Berg HM, et al. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. J Am Soc Hematol. 2013;122(7):1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer K, van der Bom JG, Mauser-Bunschoten EP, Roosendaal G, Prejs R, Grobbee DE, et al. Prophylactic treatment for severe haemophilia: comparison of an intermediate-dose to a high-dose regimen. Haemophilia. 2002;8(6):753–60. [DOI] [PubMed] [Google Scholar]
- 15.Cortesi PA, Castaman G, Mantovani LG, Ceresa IF, Linari S, et al. Modern treatments of haemophilia: review of cost-effectiveness analyses and future directions. Pharmacoeconomics. 2018;36(3):263–84. [DOI] [PubMed] [Google Scholar]
- 16.Thorat T, Neumann PJ, Chambers JD. Hemophilia burden of disease: a systematic review of the cost-utility literature for hemophilia. J Manag Care Spec Pharm. 2018;24(6):632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St-Louis J, Cloutier S, Derzko C, Grigoropoulos K, Laliberté S, Roshdy H, et al. The hemophilia joint health score version 2.1 validation in adult patients study: a multicenter international study. Res Pract Thromb Haemost. 2022;6(1): e12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton DR. Hemophilia in the managed care setting. Am J Manag Care. 2015;21(6 Suppl):S123–30. [PubMed] [Google Scholar]
- 19.Kim JY, Lee DJ, Chun TJ, You CW. Impact of intermediate-dose prophylaxis on progression of haemarthropathy in patients with severe haemophilia A: A 10-year, single-centre experience in Korea. Haemophilia. 2018;24(5):e402–9. [DOI] [PubMed] [Google Scholar]
- 20.van Genderen F, Westers P, Heijnen L, van der Net J, Schrijvers A, Helders PJ. Measuring patients’ perceptions on their functional abilities: validation of the Haemophilia Activities List. Haemophilia. 2006;12(1):36–46. [DOI] [PubMed] [Google Scholar]
- 21.Fischer K, Konkle B, Broderick C, Kessler CJH. Prophylaxis in real life scenarios. Haemophilia. 2014;20:106–13. [DOI] [PubMed] [Google Scholar]
- 22.Iannazzo S, et al. Cost-effectiveness analysis of pharmacokinetic-driven prophylaxis vs. standard prophylaxis in patients with severe haemophilia A. Blood Coagul Fibrinolysis. 2017;28(5):425–30. [DOI] [PubMed] [Google Scholar]
- 23.Pasca S, Milan M, Sarolo L, Zanon E. PK-driven prophylaxis versus standard prophylaxis: When a tailored treatment may be a real and achievable cost-saving approach in children with severe hemophilia A. Thromb Res. 2017;157:58–63. [DOI] [PubMed] [Google Scholar]
- 24.Oldenburg JJ. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. J Am Soc Hematol. 2015;125(13):2038–44. [DOI] [PubMed] [Google Scholar]
- 25.Zahedi Z, Karimi M, Keshavarz K, Haghpanah S, Ravangard RJ. A cost-effectiveness analysis of the prophylaxis versus on-demand regimens in severe hemophilia A patients under 12 years old in southern Iran. Hematology. 2021;26(1):240–8. [DOI] [PubMed] [Google Scholar]
- 26.Coppola A, et al. Cost-effectiveness analysis of late prophylaxis vs. on-demand treatment for severe haemophilia A in Italy. Haemophilia. 2017;23(4):422–9. [DOI] [PubMed] [Google Scholar]
- 27.Malec LM, Witmer CM, Jaffray J, Kouides PA, Haley KM, Sidonio RF Jr, Johnson K, Recht M, White G, Croteau SE, Ragni MV. The impact of extended half-life factor concentrates on prophylaxis for severe hemophilia in the United States. Am J Hematol. 2020;95(8):960–5. [DOI] [PubMed] [Google Scholar]
- 28.Cafuir L, Chen E, Hinds D, Prince P, Thorburn J, Mead H, Kempton CL. Early real-world experience with emicizumab and concomitant factor VIII replacement products in adult males with Hemophilia A without inhibitors. J Med Econ. 2022;25:984–992. [DOI] [PubMed]
- 29.Kim HK, Rubio-Rodríguez D, Rubio-Terrés C. Cost of patients with hemophilia A treated with standard half-life or extended half-life FVIII in Spain. Expert Rev Pharmacoecon Outcomes Res. 2021;21(6):839–46. [DOI] [PubMed] [Google Scholar]
- 30.Chhabra A, Tortella BJ, Spurden D, Alvir J, McDonald M, Hodge J, Pleil AM. Real-world analysis of dispensed international units of coagulation factor VIII and resultant expenditures for hemophilia a patients: a comparison between standard half-life and extended half-life products. Manag Care. 2018;27(6):39–46. [PubMed] [Google Scholar]
- 31.Yu JK, Keepanasseril AW, Iorio A, Edginton AN. Cost-utility analysis of emicizumab for the treatment of severe hemophilia A patients in Canada. Haemophilia. 2023;29(5):488–97. [DOI] [PubMed] [Google Scholar]
- 32.Daliri AAK, Haghparast H, Mamikhani J. Cost-effectiveness of prophylaxis against on-demand treatment in boys with severe hemophilia A in Iran. Int J Technol Assess Health Care. 2009;25(4):584–7. [DOI] [PubMed] [Google Scholar]
- 33.Risebrough N, et al. Cost-utility analysis of Canadian tailored prophylaxis, primary prophylaxis and on-demand therapy in young children with severe haemophilia A. Haemophilia. 2008;14(6):743–52. [DOI] [PubMed] [Google Scholar]
- 34.Gringeri A, et al. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9(4):700–10. [DOI] [PubMed] [Google Scholar]
- 35.Chen SL. Economic costs of hemophilia and the impact of prophylactic treatment on patient management. Am J Manag Care. 2016;22(5 Suppl):s126–33. [PubMed] [Google Scholar]
- 36.Verma S, et al. A randomized study of very low-dose factor VIII prophylaxis in severe haemophilia–a success story from a resource limited country. Haemophilia. 2016;22(3):342–8. [DOI] [PubMed] [Google Scholar]
- 37.Tang L, et al. Short-term low-dose secondary prophylaxis for severe/moderate haemophilia A children is beneficial to reduce bleed and improve daily activity, but there are obstacle in its execution: a multi-centre pilot study in China. Haemophilia. 2013;19(1):27–34. [DOI] [PubMed] [Google Scholar]
- 38.Carlsson KS, et al. Costs of on-demand and prophylactic treatment for severe haemophilia in Norway and Sweden. Haemophilia. 2004;10(5):515–26. [DOI] [PubMed] [Google Scholar]
- 39.Petrova G, Tachkov K, Georgieva S, Dimitrova MJ. Humanistic and economic aspects of haemophilia treatment in Bulgaria. Comparison between two therapeutic approaches: prophylactic vs. on-demand treatment. Biotechnol Biotechnol Equip. 2014;28(6):576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]