Abstract
Introduction
Psychotropic drugs have been reported to cause urinary retention (UR) via anticholinergic and other mechanisms. However, UR has not received much attention because of its non-fatal symptoms. We investigated the occurrence of UR associated with psychotropic drugs using the Japanese Adverse Drug Event Report (JADER) database.
Methods
Using the JADER database, we calculated reporting odds ratios for UR for 74 psychotropic drugs. Multivariate logistic regression analysis was used to adjust for the effects of sex, underlying disease, and age on UR. Variable selection included forced entry for sex, age, benign prostatic hyperplasia (BPH), depression, and backward-forward stepwise selection for each drug.
Results
A total of 887,704 cases were reported, of which 4653 (0.52%) had UR. In terms of sex, 0.79% (3401/429,372 cases) and 0.43% (1797/415,358 cases) of male and female patients had UR. In terms of age, 0.31% (892/288,676 cases) and 0.68% (3463/506,907 cases) of patients aged < 60 years and 60 years or older had UR. Among the underlying diseases, 8.22% (930/11,316 cases) and 0.43% (3723/876,388 cases) of patients with BPH and without BPH had UR, respectively. Further, 1.99% (337/16,959 cases) and 0.50% (4316/870,745 cases) of patients with depression and without depression had UR, respectively. Overall, 38 psychotropic drugs met the criteria for signal detection. In logistic regression, a total of 783,083 patients of discernible age and sex were included. The selected variables were sex, age, BPH, depression, and 23 drugs, including quetiapine [adjusted reporting odds ratio (ROR) 95% confidence interval (CI): 1.46–2.81], chlorpromazine (adjusted ROR 95%CI: 1.29–3.13), etizolam (adjusted ROR 95%CI: 1.47–3.09), maprotiline (adjusted ROR 95%CI: 1.99–8.34), mirtazapine (adjusted ROR 95%CI: 1.37–2.88), and duloxetine (adjusted ROR 95%CI: 2.15–4.21).
Conclusions
Many psychotropic drugs induce UR, which may be owing to their pharmacological effects. Appropriate monitoring is needed, especially in patients with other risk factors for UR.
Key Points
| Multivariate analysis using the JADER database showed a significant increase in ROR for 23 psychotropic drugs, even after adjustment for sex, age, and medical history of BPH and depression. When considering the risk of drug-induced urinary retention, various pharmacologic factors may come into play. In addition to anticholinergic effects, we need to look at sympathomimetic pharmacologic effects and actively check with the patient regarding the presence or absence of urinary retention. |
Introduction
Urinary retention (UR) generally presents as the inability to pass urine and is usually accompanied by lower abdominal and/or suprapubic discomfort, which causes considerable distress to patients [1]. Various factors contribute to the development of UR, including drug therapy [2]. The most common drugs that cause UR are anticholinergic and sympathomimetic agents. Anticholinergic drugs reduce urinary bladder smooth muscle contraction [3], whereas sympathetic stimulation causes contraction of the urethral sphincter and relaxation of the erector sphincter, thereby increasing the risk of urinary retention [4]. These pharmacological effects have led to UR being reported as a typical side effect of psychotropic agents, such as benzodiazepines (BZs), antipsychotics, and antidepressants [2].
In recent years, several studies have compared the effects of psychotropic drugs on detrusor or urethra smooth muscles by either anticholinergic or sympathomimetic stimulation [5–9]. However, only a few studies have comprehensively investigated the association between antipsychotic agents and UR. Nonetheless, medical “big data” have become available and many studies involving analyses of information from databases have been published. In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) collects spontaneous reports on drug-induced adverse events (AEs). In addition, the PMDA has created a database of spontaneous reports on its website, the Japanese Adverse Drug Event Report (JADER), where data have been freely available for download since April 2012 [10]. However, no JADER-based analyses of UR and related drugs have been reported to date. The JADER database collects spontaneous AE reports in Japan and is the best big data on adverse events for identifying and analyzing UR cases. In addition, the data are publicly available and can be revalidated, as anyone can re-evaluate the data after it has been updated. Therefore, we decided to use this database for this study.
In this study, we examined the association between psychotropic agents and UR in Japan, using spontaneous AE reports registered in the JADER database.
Materials and Methods
Database
The JADER database was used to collect data from April 2004 to March 2024 to investigate drug-induced UR. The JADER database was accessed from the PMDA website [5]. The database consists of four data tables: patient demographic information (demo.csv), drug information (drug.csv), AE (reac.csv), and primary disease (hist.csv). The contents of each file were as follows: the “demo.csv” file contained basic patient information such as identification number, sex, age, weight, height, and reporting year; the “drug.csv” file contained information about the drug administered, including generic name, brand name, route of administration, start and end dates of administration, reason for administering the drug, and identification number; the “reac.csv” file contained information about adverse events, outcomes, and onset dates and identification number; the “hist.csv” file contained information about the patient’s primary disease and identification number. These data were provided as files in CSV format which were read using R version 4.2.3. Because of repeated data in the JADER data tables, the identification number in the “demo” file was used to remove these duplicates.
Selection of Drugs and Identification of Adverse Events
Nine classes of psychotropic agents, comprising 74 drugs, were included in this study (Table 1). Adverse drug events are coded according to the preferred terminology of the Medical Dictionary for Regulatory Activities®/Japanese (MedDRA®/J) (https://www.jmo.pmrj.jp). The Standardized MedDRA® Queries (SMQ) index consists of groupings of MedDRA® terms, ordinarily at the preferred term level, which are related to a defined medical condition or area of interest. We used SMQ version 27.0 to extract case reports related to UR (preferred terms: 10046555; “Urinary retention”). This search was performed using “reac.csv,” and the data was tabulated as binary data according to the presence or absence of condition matches.
Table 1.
Analyzed drugs
| Drug category | Drug name |
|---|---|
| SGA (11 types) | Clozapine, risperidone, paliperidone, perospirone, asenapine, olanzapine, quetiapine, aripiprazole, blonanserin, brexpiprazole, and lurasidone |
| FGA (20 types) | Chlorpromazine, fluphenazine, prochlorperazine, propericiazine, perphenazine, levomepromazine, spiperone, timiperone, haloperidol, pimozide, bromperidol, pipamperone, sultopride, sulpiride, tiapride, nemonapride, clocapramine, mosapramine, oxypertine, and zotepine |
| BZs (22 types) | Eszopiclone, zopiclone, zolpidem, triazolam, brotizolam, rilmazafone, lormetazepam, estazolam, nitrazepam, flunitrazepam, quazepam, haloxazolam, flurazepam, etizolam, clotiazepam, flutazolam, lorazepam, alprazolam, bromazepam, diazepam, cloxazolam, and fludiazepam |
| Antidepressant (21 types) | |
| SSRI (4 types) | Escitalopram, sertraline, and paroxetine, fluvoxamine |
| SNRI (3 types) | Duloxetine, venlafaxine, and milnacipran |
| S-RIM (1 type) | Vortioxetine |
| NaSSA (1 type) | Mirtazapine |
| TCA (8 types) | Amitriptyline, amoxapine, imipramine, clomipramine, dosulepin, trimipramine, nortriptyline, and lofepramine |
| TeCA (4 types) | Setiptiline, maprotiline, mianserin, and trazodone |
SGA, second-generation antipsychotics; FGA, first-generation antipsychotics; BZs, benzodiazepines; SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin noradrenaline reuptake inhibitors; S-RIM, serotonin reuptake inhibitor and modulator; NaSSA, noradrenergic and specific serotonergic antidepressant; TCA, tricyclic antidepressants; TeCA, tetracyclic antidepressants
Statistical Analysis
The outcome was the inclusion of UR as an AE in the database. In this study, we used the reporting odds ratio (ROR) to evaluate the relationship between the investigated drugs and AEs. The ROR is the odds ratio of reports of an AE of interest to reports of other events with the same drug compared with the odds of reporting the AE of interest with all other drugs in the database. The patient data from the database were divided into two groups: “Case” for patients with UR and “No Case” for patients with other AEs. If benign prostatic hyperplasia (BPH) or depression is included as the primary disease using “hist.csv” or the reason for administering the drug using “drug.csv,” the patient is determined to have BPH or depression. Sex (male or female) and age (60 years or older) were also determined using “demo.csv.” The use of the target drug was determined using the generic name using “drug.csv.” All of these were tabulated as binary data, and a two-by-two contingency table was created for each to calculate the ROR. Univariate analysis was used to calculate the ROR and a 95% confidence interval (CI) was set for each drug and class. The UR signal was considered detected if the lower limit of the ROR was above 1. For the RORs, we performed a chi-square test. Multivariate logistic regression analysis was used to adjust for the effects of sex, underlying disease, and age on UR. Variable selection included forced entry for sex (male or female), age (≥ 60 years old or not), BPH, depression, and backward-forward stepwise selection for each drug. Multicollinearity was evaluated based on the generalized variance inflation factor (GVIF) with a cut-off of 10. The inclusion and exclusion thresholds for the variables were set at p < 0.05. The dataset for the logistic regression was limited to those cases in which sex or age was specified in the “demo” file. The “drug” file was divided into three categories, “suspected drug,” “interacting drug,” and “concomitant drug,” on the basis of the involvement of the drug in the occurrence of UR. Herein, “suspected drugs,” which were considered to be the most involved, were analyzed. All analyses were performed using R version 4.2.3.
Results
Univariate Analysis of UR
Figure 1 shows UR reporting ratios according to patient demographics. In terms of sex, 0.79% (3401/429,372 cases) and 0.43% (1797/415,358 cases) of male and female patients, respectively, had UR. UR was significantly more common among males (p = 7.05×10–99). In terms of age, 0.31% (892/288,676 cases) and 0.68% (3463/506,907 cases) of patients aged < 60 years and 60 years or older had UR, with the condition being more significantly common among patients aged 60 years or older (p = 3.66×10−182). Patients in whom sex and age were unknown were excluded. Among the underlying diseases, 8.22% (930/11,316 cases) and 0.43% (3723/876,388 cases) of patients with BPH and without BPH had UR, respectively. Further, 1.99% (337/16,959 cases) and 0.50% (4316/870,745 cases) of patients with depression and without depression had UR. UR was significantly more common among patients with BPH (p = 1.40×10−2825) and depression (p = 9.73×10−156).
Fig. 1.
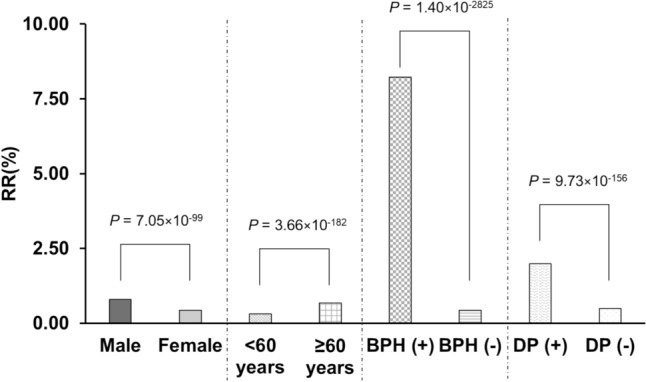
UR reporting ratio according to patient demographics. RR, risk ratio
Table 2 summarizes the second generation antipsychotics (SGA)-induced UR reports in the JADER database from April 2004 to March 2024. SGA are also known as atypical antipsychotics. In general, SGA carry a lower risk of extrapyramidal symptoms and tardive dyskinesia than first-generation antipsychotics (FGA). A total of 887,704 cases were reported, of which 4653 (0.52%) had UR. For SGA, eight drugs met the signal detection criteria.
Table 2.
SGA induced urinary retention
| Case (n) | No case (n) | Total | % | ROR | 95%CI | |
|---|---|---|---|---|---|---|
| All | 4653 | 883,051 | 887,704 | 0.52 | ||
| Clozapine | 8 | 3605 | 3613 | 0.22 | 0.42 | 0.210–0.841 |
| Risperidone | 57 | 4404 | 4461 | 1.28 | 2.47 | 1.902–3.218 |
| Paliperidone | 12 | 1721 | 1733 | 0.69 | 1.32 | 0.750–2.338 |
| Perospirone | 4 | 589 | 593 | 0.67 | 1.29 | 0.482–3.448 |
| Asenapine | 7 | 544 | 551 | 1.27 | 2.44 | 1.159–5.154 |
| Olanzapine | 43 | 3185 | 3228 | 1.33 | 2.58 | 1.905–3.486 |
| Quetiapine | 52 | 3392 | 3444 | 1.51 | 2.93 | 2.225–3.860 |
| Aripiprazole | 43 | 3983 | 4026 | 1.07 | 2.06 | 1.522–2.784 |
| Blonanserin | 18 | 1130 | 1148 | 1.57 | 3.03 | 1.901–4.833 |
| Brexpiprazole | 12 | 575 | 587 | 2.04 | 3.97 | 2.239–7.034 |
| Lurasidone | 21 | 577 | 598 | 3.51 | 6.93 | 4.482–10.727 |
SGA, second-generation antipsychotics; ROR, reporting odds ratio; 95%CI, 95% confidence interval
Table 3 summarizes the first generation antipsychotics (FGA)-induced UR reports. FGA are also known as typical antipsychotics. FGA are known to have a significant potential to cause extrapyramidal symptoms and tardive dyskinesia. For FGA, five drugs met the signal detection criteria.
Table 3.
FGA induced UR
| Case (n) | No case (n) | Total | % | ROR | 95%CI | |
|---|---|---|---|---|---|---|
| All | 4653 | 883,051 | 887,704 | 0.52 | ||
| Chlorpromazine | 24 | 1632 | 1656 | 1.45 | 2.80 | 1.869–4194 |
| Fluphenazine | 0 | 133 | 133 | 0.00 | 0.00 | NA |
| Prochlorperazine | 6 | 230 | 236 | 2.54 | 4.96 | 2.202–11.152 |
| Propericiazine | 1 | 131 | 132 | 0.76 | 1.45 | 0.203–10.365 |
| Perphenazine | 1 | 98 | 99 | 1.01 | 1.94 | 0.270–13.890 |
| Levomepromazine | 10 | 1256 | 1266 | 0.79 | 1.51 | 0.811–2.819 |
| Spiperone | 0 | 2 | 2 | 0.00 | 0.00 | NA |
| Timiperone | 1 | 40 | 41 | 2.44 | 4.75 | 0.652–34.526 |
| Haloperidol | 22 | 2077 | 2099 | 1.05 | 2.01 | 1.323–3.070 |
| Pimozide | 1 | 60 | 61 | 1.64 | 3.16 | 0.438–22.832 |
| Bromperidol | 3 | 170 | 173 | 1.73 | 3.35 | 1.070–10.497 |
| Pipamperone | 0 | 21 | 21 | 0.00 | 0.00 | NA |
| Sultopride | 1 | 85 | 86 | 1.16 | 2.23 | 0.311–16.039 |
| Sulpiride | 12 | 1883 | 1895 | 0.63 | 1.21 | 0.685–2.136 |
| Tiapride | 7 | 478 | 485 | 1.44 | 2.78 | 1.318–5.870 |
| Nemonapride | 0 | 16 | 16 | 0.00 | 0.00 | NA |
| Clocapramine | 0 | 25 | 25 | 0.00 | 0.00 | NA |
| Mosapramine | 0 | 26 | 26 | 0.00 | 0.00 | NA |
| Oxypertine | 0 | 18 | 18 | 0.00 | 0.00 | NA |
| Zotepine | 5 | 480 | 485 | 1.03 | 1.98 | 0.819–4.776 |
FGA, first-generation antipsychotics; ROR, reporting odds ratio; 95%CI, 95% confidence interval; NA, not available
Table 4 summarizes reports of BZ-induced UR. For BZs, seven drugs met the signal detection criteria.
Table 4.
BZs induced seizure related AEs
| Case (n) | No case (n) | Total | % | ROR | 95%CI | |
|---|---|---|---|---|---|---|
| All | 4653 | 883,051 | 887,704 | 0.52 | ||
| Eszopiclone | 4 | 273 | 277 | 1.44 | 2.78 | 1.036–7.469 |
| Zopiclone | 8 | 907 | 915 | 0.87 | 1.68 | 0.835–3.362 |
| Zolpidem | 21 | 2725 | 2746 | 0.76 | 1.46 | 0.952–2.252 |
| Triazolam | 11 | 847 | 858 | 1.28 | 2.47 | 1.361–4.477 |
| Brotizolam | 24 | 1623 | 1647 | 1.46 | 2.82 | 1.880–4.218 |
| Rilmazafone | 0 | 121 | 121 | 0.00 | 0.00 | |
| Lormetazepam | 2 | 155 | 157 | 1.27 | 2.45 | 0.607–9.885 |
| Estazolam | 1 | 360 | 361 | 0.28 | 0.53 | 0.074–3.753 |
| Nitrazepam | 19 | 2479 | 2498 | 0.76 | 1.46 | 0.927–2.289 |
| Flunitrazepam | 13 | 2012 | 2025 | 0.64 | 1.23 | 0.711–2.118 |
| Quazepam | 3 | 240 | 243 | 1.23 | 2.37 | 0.760–7.413 |
| Haloxazolam | 0 | 3 | 3 | 0.00 | 0.00 | |
| Flurazepam | 0 | 22 | 22 | 0.00 | 0.00 | |
| Etizolam | 40 | 2253 | 2293 | 1.74 | 3.39 | 2.476–4.640 |
| Clotiazepam | 19 | 358 | 377 | 5.04 | 10.11 | 6.367–16.051 |
| Flutazolam | 0 | 11 | 11 | 0.00 | 0.00 | |
| Alprazolam | 8 | 635 | 643 | 1.24 | 2.39 | 1.191–4.810 |
| Bromazepam | 10 | 1088 | 1098 | 0.91 | 1.75 | 0.936–3.256 |
| Lorazepam | 4 | 626 | 630 | 0.63 | 1.21 | 0.454–3.243 |
| Diazepam | 13 | 1107 | 1120 | 1.16 | 2.23 | 1.291–3.859 |
| Cloxazolam | 1 | 105 | 106 | 0.94 | 1.81 | 0.252–12.956 |
| Fludiazepam | 4 | 13 | 17 | 23.53 | 58.44 | 19.049–179.308 |
BZs, benzodiazepines; ROR, reporting odds ratio; 95%CI, 95% confidence interval; NA, not available
Table 5 summarizes the antidepressant-induced UR reports. For antidepressants, 18 drugs met the signal detection criteria.
Table 5.
Antidepressant induced UR
| Case (n) | No case (n) | Total | % | ROR | 95%CI | |
|---|---|---|---|---|---|---|
| All | 4653 | 883,051 | 887,704 | 0.52 | 3.372–7.881 | |
| Escitalopram | 22 | 813 | 835 | 2.63 | 5.16 | 2.130–4.706 |
| Sertraline | 25 | 1504 | 1529 | 1.64 | 3.17 | 1.697–3.149 |
| Paroxetine | 41 | 3383 | 3424 | 1.20 | 2.31 | 1.602–4.178 |
| Fluvoxamine | 17 | 1250 | 1267 | 1.34 | 2.59 | 3.445–6.056 |
| Duloxetine | 50 | 2095 | 2145 | 2.33 | 4.57 | 24.485–34.483 |
| Venlafaxine | 155 | 1046 | 1201 | 12.91 | 29.06 | 3.033–8.784 |
| Milnacipran | 14 | 516 | 530 | 2.64 | 5.16 | 1.915–7.774 |
| Vortioxetine | 8 | 394 | 402 | 1.99 | 3.86 | 4.677–8.287 |
| Mirtazapine | 49 | 1507 | 1556 | 3.15 | 6.23 | 4.045–10.980 |
| Amitriptyline | 16 | 457 | 473 | 3.38 | 6.66 | 1.774–6.202 |
| Amoxapine | 10 | 573 | 583 | 1.72 | 3.32 | 2.437–9.932 |
| Imipramine | 8 | 309 | 317 | 2.52 | 4.92 | 3.133–9.890 |
| Clomipramine | 12 | 410 | 422 | 2.84 | 5.57 | 1.486–83.922 |
| Dosulepin | 1 | 17 | 18 | 5.56 | 11.17 | NA |
| Trimipramine | 0 | 4 | 4 | 0.00 | 0.00 | 1.330–22.126 |
| Nortriptyline | 2 | 70 | 72 | 2.78 | 5.42 | NA |
| Lofepramine | 0 | 8 | 8 | 0.00 | 0.00 | NA |
| Setiptiline | 0 | 30 | 30 | 0.00 | 0.00 | NA |
| Maprotiline | 10 | 306 | 316 | 3.16 | 6.21 | 3.307–11.672 |
| Mianserin | 7 | 389 | 396 | 1.77 | 3.42 | 1.618–7.223 |
| Trazodone | 10 | 705 | 715 | 1.40 | 2.70 | 1.443–5.035 |
ROR, reporting odds ratio; 95%CI, 95% confidence interval; NA, not available
Multivariate Analysis of UR
Table 6 summarizes the results of the final logistic regression model. For this analysis, 783,083 patients of discernible age and sex were entered. All the variables included in the final model were significant. For patient demographic variables, the adjusted ROR of being male, over 60 years old, having BPH, and depression were 1.35 (95% CI: 1.25‒1.46), 2.32 (95% CI: 2.12‒2.54), 13.96 (95% CI: 12.74‒15.30), and 1.36 (95% CI: 1.13‒1.63), respectively. For drugs, 6 SGA, including quetiapine (adjusted ROR 95%CI: 1.46‒2.81); 2 FGA, including chlorpromazine (adjusted ROR 95%CI: 1.29‒3.13); 3 BZs, including etizolam (adjusted ROR 95%CI: 1.47‒3.09); and 12 antidepressants, including maprotiline (adjusted ROR 95%CI: 1.99‒8.34), mirtazapine (adjusted ROR 95%CI: 1.37‒2.88), and duloxetine (adjusted ROR 95%CI: 2.15‒4.21) were selected by stepwise variable selection and incorporated into the final model. Because all GVIFs were ≤ 10, we considered that there was no multicollinearity between these variables.
Table 6.
Multiple logistic regression analysis
| Variable selection | Factor | Adjusted ROR | 95% CI | p | GVIF | |
|---|---|---|---|---|---|---|
| Forced | Patient demographics | Male (ref: female) | 1.35 | 1.25–1.46 | 3.07 × 10−15 | 1.15 |
| Over 60 years old (ref: under 60 years old) | 2.32 | 2.12–2.54 | 2.55 × 10−75 | 1.10 | ||
| BPH | 13.96 | 12.74–15.30 | 1.02 × 10−690 | 1.18 | ||
| Depression | 1.36 | 1.13–1.63 | 1.11 × 10−3 | 1.86 | ||
| Forward-backward stepwise selection | SGA | Risperidone | 2.30 | 1.67–3.15 | 2.59 × 10−7 | 1.11 |
| Quetiapine | 2.02 | 1.46–2.81 | 2.40 × 10−5 | 1.10 | ||
| Aripiprazole | 1.56 | 1.09–2.22 | 0.015 | 1.09 | ||
| Blonanserin | 2.19 | 1.29–3.73 | 3.65 × 10−3 | 1.09 | ||
| Brexpiprazole | 2.64 | 1.31–5.31 | 6.52 × 10−3 | 1.03 | ||
| Lurasidone | 6.31 | 3.59–11.10 | 1.98 × 10−10 | 1.06 | ||
| FGA | Chlorpromazine | 2.01 | 1.29–3.13. | 2.00 × 10−3 | 1.09 | |
| Prochlorperazine | 5.16 | 2.25–11.87 | 1.11 × 10−4 | 1.00 | ||
| BZ | Etizolam | 2.13 | 1.47–3.09 | 6.55 × 10−5 | 1.14 | |
| Clotiazepam | 8.36 | 4.91–14.21 | 4.53 × 10−15 | 1.12 | ||
| Fludiazepam | 81.56 | 22.33–297.89 | 2.75 × 10−11 | 1.07 | ||
| Antidepressant | Escitalopram | 3.12 | 1.92–5.06 | 4.21 × 10−6 | 1.05 | |
| Sertraline | 2.84 | 1.82–4.42 | 4.03 × 10−6 | 1.09 | ||
| Paroxetine | 1.71 | 1.17–2.49 | 5.47 × 10−3 | 1.12 | ||
| Fluvoxamine | 1.77 | 1.05–3.00 | 0.033 | 1.10 | ||
| Duloxetine | 3.01 | 2.15–4.21 | 1.16 × 10−10 | 1.05 | ||
| Venlafaxine | 30.88 | 24.24–39.32 | 4.00 × 10−170 | 1.43 | ||
| Milnacipran | 2.92 | 1.62–5.28 | 3.82 × 10−4 | 1.12 | ||
| Vortioxetine | 3.64 | 1.67–7.92 | 1.16 × 10−3 | 1.03 | ||
| Mirtazapine | 1.99 | 1.37–2.88 | 2.82 × 10−4 | 1.12 | ||
| Amitriptyline | 3.93 | 2.26–6.84 | 1.22 × 10−6 | 1.06 | ||
| Imipramine | 3.23 | 1.38–7.55 | 6.34 × 10−3 | 1.03 | ||
| Maprotiline | 4.08 | 1.99–8.34 | 1.17 × 10−4 | 1.08 |
BPH, benign prostatic hyperplasia; BZs, benzodiazepines; FGA, first-generation antipsychotics; GVIF: generalized variance inflation factor, SGA, second-generation antipsychotics; ROR, reporting odds ratio; 95%CI, 95% confidence interval
Discussion
To the best of our knowledge, this is the first study to use the JADER database to estimate the association between UR and psychotropic agents. With over 700,000 case reports included in this database, we think we were able to handle a sufficient number of cases for analysis. It was also possible to comprehensively examine 74 psychotropic drugs.
In this study, more spontaneous reports of UR were seen in males (chi-square test: p = 7.05×10−99, 95% CI of adjusted ROR: 1.25‒1.46). UR has been estimated to occur in approximately 5 cases per 1000 person-years in the male population [11]. In contrast, the occurrence of UR has been estimated to be approximately 7 cases per 100,000 persons in the female population, with a male-to-female ratio of 13:1 [12]. Herein, there was no extreme sex difference, as previously reported, but there was a tendency for males to more likely develop UR than females.
More spontaneous reports of UR were seen in patients with BPH than in those without (chi-square test: p = 1.40×10−2825, 95% CI of adjusted ROR: 12.74‒15.30). The mechanisms of UR are complex, with multiple mechanisms involved in its development. Urinary outflow tract obstruction is the most common cause; in males, BPH is considered a cause of outflow tract obstruction owing to the presence of the prostate gland, and the risk of UR is reportedly three times higher in patients with the disease [13]. In females, obstruction of the urinary outflow tract is based on anatomical obstruction, such as a gynecological mass, thus differing from the cause in males because women do not have a prostate gland [14]. Herein, the ROR for BPH was very large, which may be owing to the fact that the control for the BPH group was “no BPH,” a group that included both males and females. In other words, the ROR includes the effects of sex differences.
More spontaneous reports of UR were seen in those aged 60 years or older (chi-square test: p = 3.66×10–182, 95% CI of adjusted ROR: 2.12‒2.54). Age has been reported to be an obvious risk factor for UR, with the risk being directly proportional [11, 13, 15, 16]. In males, the prevalence of BPH increases with age, in turn leading to an increased risk of UR [15]. In addition, it has been reported that the contractile capacity of the muscles involved in urinary drainage decreases with age [17].
More spontaneous reports of UR were seen in patients with depression than in those without (chi-squared test: p = 9.73×10–156, 95% CI of adjusted ROR: 1.13‒1.63). A high prevalence of depression in patients with UR has been reported [18, 19]. As UR is heavily influenced by age, sex, and medical history, the present study included these factors using the forced introduction method in a multiple logistic regression analysis.
The drugs were selected as stepwise variables after forced entry of previously reported factors associated with UR, including sex, age, BPH, and depression.
For the SGA, risperidone, asenapine, olanzapine, quetiapine, aripiprazole, blonanserin, brexpiprazole, and lurasidone met the signal detection criteria. Risperidone, quetiapine, aripiprazole, blonanserin, brexpiprazole, and lurasidone were identified as significant factors. SGA have been reported to be a risk factor for UR in logistic regression [20]. Several studies have suggested an association between UR and risperidone, olanzapine, quetiapine, aripiprazole, and lurasidone [4, 21–25]. However, no study has reported an association between UR and asenapine, blonanserin, and brexpiprazole. For the FGA, chlorpromazine, prochlorperazine, haloperidol, bromperidol, and tiapride met the signal detection criteria. Chlorpromazine and prochlorperazine were selected as significant factors. An association between UR and chlorpromazine, prochlorperazine, and haloperidol has also been suggested [26, 27]. Nonetheless, no study has reported an association between bromperidole, tiapride, and UR. In pharmacological experiments, chlorpromazine, levomepromazine, zotepine, olanzapine, quetiapine, and clozapine have shown anticholinergic effects at clinically therapeutic blood levels [5]; this study did not investigate lurasidone. Among the drugs with reported anticholinergic effects at clinically therapeutic blood concentrations, chlorpromazine, olanzapine, and quetiapine showed UR signals. The detection of UR signals for these three drugs may be explained by their high blood concentrations. A signal detection was not evident for levomepromazine, zotepine, and clozapine; however, a small number of UR cases were reported (levomepromazine, ten cases; zotepine, five cases; clozapine, eight cases; Tables 2 and 3). It has been reported that risperidone, aripiprazole, blonanserin, and prochlorperazine, the compounds with signal detection in this study, do not exhibit anticholinergic effects at clinically therapeutic concentrations. Antipsychotic-induced UR is thought to be the result of not only anticholinergic effects but also central serotonergic mechanisms combined with blockade of central dopamine 2 receptors and stimulation of peripheral α1-adrenergic receptors in the urinary tract [28, 29]. Serotonin stimulation enhances the sympathetic reflex pathway and inhibits the parasympathetic urinary drainage pathway, promoting urine retention. In addition, dopamine 1 receptors play a role in the inhibition of bladder activity, whereas dopamine 2 receptors promote urination [2]. Therefore, the effects of antipsychotic-induced UR may be complicated by the drug’s strong or weak affinity for muscarinic, dopamine, serotonin, and α1-adrenergic receptors. Antipsychotic drug metabolites may also be associated with the development of UR. For example, N desmethyl clozapine, a metabolite of clozapine reported to have strong anticholinergic effects, is a partial agonist of choline receptors [30, 31]. Therefore, it is necessary to evaluate the metabolites in vivo as well as conducting more detailed studies in actual patients. These abovementioned mechanisms are thought to function in complex ways to cause UR; therefore, we believe that real-world case studies are important for assessing the risk of UR.
For BZs, triazolam, etizolam, brotizolam, clotiazepam, alprazolam, diazepam, and fludiazepam met the signal detection criteria. Etizolam, clotiazepam, and fludiazepam were selected as the significant factors. An association between diazepam and UR has been suggested [32]; however, that of the other abovementioned compounds with detected signals has not been reported. In pharmacological experiments, clotiazepam and diazepam significantly inhibited acetylcholine-induced contractions at clinically therapeutic concentrations [7, 9]; these studies did not investigate fludiazepam. However, it has been reported that triazolam, etizolam, and alprazolam, compounds that showed a signal detection in this study, did not exhibit anticholinergic effects at clinically therapeutic concentrations.
The antidepressants escitalopram, sertraline, paroxetine, fluvoxamine, duloxetine, venlafaxine, milnacipran, vortioxetine, mirtazapine, amitriptyline, amoxapine, imipramine, clomipramine, dosulepin, nortriptyline, maprotiline, mianserin, and trazodone met the criteria for signal detection. In other words, all antidepressants met the criteria except trimipramine, lofepramine, and setiptiline, which were not reported in a single case. Escitalopram, sertraline, paroxetine, fluvoxamine, duloxetine, milnacipran, vortioxetine, mirtazapine, amitriptyline, imipramine, and maprotiline were selected as significant factors. Pharmacological studies have shown that imipramine, amitriptyline, trimipramine, clomipramine, nortriptyline, amoxapine, maprotiline, and mirtazapine exert anticholinergic effects at clinically therapeutic concentrations [8]. As in antipsychotics, antidepressant-induced UR is thought to be the result of not only anticholinergic effects but also central serotonergic mechanisms combined with blockade of central dopamine 2 receptors and stimulation of peripheral α1-adrenergic receptors in the urinary tract [28, 29]. In another pharmacological experiment, desipramine (an active metabolite of imipramine), nortriptyline, amoxapine, maprotiline, paroxetine, milnacipran, and duloxetine showed noradrenaline reuptake inhibitory effects at clinically therapeutic concentrations [6]; this study did not investigate venlafaxine, vortioxetine, dosulepin, lofepramine, and setiptiline. These results suggest that caution should be exercised regarding UR when using antidepressants. Further caution should be exercised in patients with depression, as background factors for depression were also noticeable independent factors during the multivariate analysis in the present study. The present study has several limitations. First, because we used the JADER database, the target patients were mostly Japanese. Therefore, it is unclear whether these findings can be applicable to other ethnicities. Second, because the JADER database is based on spontaneous reports, basic information, such as sex, weight, age, and drug dosage, is often missing, making the database prone to reporting bias. Owing to this, the reported results may have been distorted by reporting bias. Third, there may be limitations in the interpretation of RORs, given that each drug is expected to have a different profile of reported side effects other than urinary retention, and each disease has few patients who are not treated with the drug. Lastly, studies using the JADER database are subject to over-reporting, under-reporting, missing data, exclusion of healthy individuals, missing denominators, and the presence of various confounding factors. Therefore, it is necessary to conduct prospective research in future for verification prior to translation of these findings into real-world clinical practice.
Conclusions
When using psychotropic medications, clinicians should consider the risk of UR. In particular, a history of depression or BPH, being male, and elderly are factors associated independently of drug use. The same caution should be exercised with anticholinergic drugs, as well as with drugs that act on the sympathetic nervous system. Some of these pharmacologic effects can be explained by results in animal studies, while others cannot. More detailed studies on human subjects will be needed in the future. To detect UR in practice, it is necessary to question the patient more aggressively before the condition becomes serious. This may lead to safer psychotropic drug treatment.
Acknowledgements
We thank Editage (www.editage.jp) for English language editing.
Declarations
Statement of Ethics
This study analyzed a publicly available database; therefore, it did not require ethical approval.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There are no funding sources for this study.
Author Contributions
K.K., T.Y., and K.M. conceived and designed the study; S.U., K.M., Y.H., and Y.O. analyzed the data; S.U. and K.M. wrote the manuscript. All authors read and approved the final version.
Data Availability Statement
The data that support the findings of this study are not publicly available because the dataset is too large but are available from S.U. (shuusuke.uekusa@phar.toho-u.ac.jp) upon reasonable request.
References
- 1.Thomas K, Chow K, Kirby RS. Acute urinary retention: a review of the aetiology and management. Prostate Cancer Prostatic Dis. 2004;7:32–7. 10.1038/sj.pcan.4500700. [DOI] [PubMed] [Google Scholar]
- 2.Verhamme KM, Sturkenboom MC, Stricker BH, Bosch R. Drug-induced urinary retention: incidence, management and prevention. Drug Saf. 2008;31:373–88. 10.2165/00002018-200831050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Obara K, Chino D, Tanaka Y. The recovery effects of distigmine on guinea pig detrusor underactivity induced by anticholinergic drugs. Ōyō Yakuri Pharmacometr. 2016;91:25–39. [Google Scholar]
- 4.Trinchieri M, Perletti G, Magri V, Stamatiou K, Montanari E, Trinchieri A. Urinary side effects of psychotropic drugs: a systematic review and metanalysis. Neurourol Urodyn. 2021;40:1333–48. 10.1002/nau.24695. [DOI] [PubMed] [Google Scholar]
- 5.Obara K, Matsuoka Y, Iwata N, Abe Y, Ikegami Y, Shioda N, et al. Inhibitory effects of antipsychotics on the contractile response to acetylcholine in rat urinary bladder smooth muscles. Biol Pharm Bull. 2021;44:1140–50. 10.1248/bpb.b21-00363. [DOI] [PubMed] [Google Scholar]
- 6.Obara K, Imanaka S, Fukuhara H, Yamaki F, Matsuo K, Yoshio T, et al. Evaluation of the potentiating effects of antidepressants on the contractile response to noradrenaline in guinea pig urethra smooth muscles. Clin Exp Pharmacol Physiol. 2019;46:444–55. 10.1111/1440-1681.13072. [DOI] [PubMed] [Google Scholar]
- 7.Obara K, Ao L, Ogawa T, Ikarashi T, Yamaki F, Matsuo K, et al. Assessment of inhibitory effects of hypnotics on acetylcholine-induced contractions in isolated rat urinary bladder smooth muscle. Biol Pharm Bull. 2019;42:280–8. 10.1248/bpb.b18-00829. [DOI] [PubMed] [Google Scholar]
- 8.Uno J, Obara K, Suzuki H, Miyatani S, Chino D, Yoshio T, et al. Inhibitory effects of antidepressants on acetylcholine-induced contractions in isolated guinea pig urinary bladder smooth muscle. Pharmacology. 2017;99:89–98. 10.1159/000452221. [DOI] [PubMed] [Google Scholar]
- 9.Obara K, Ao L, Shimada T, Horiguchi S, Ikarashi T, Ogawa T, et al. Pharmacological characteristics of anxiolytics on acetylcholine-induced contractions in rat detrusor smooth muscle. Pharmacology. 2020;105:369–76. 10.1159/000503885. [DOI] [PubMed] [Google Scholar]
- 10.Pharmaceuticals and Medical Devices Agency. [Japanese adverse drug event report database]. Accessed 2021 Dec 1. https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0004.html (in Japanese)
- 11.Meigs JB, Barry MJ, Giovannucci E, Rimm EB, Stampfer MJ, Kawachi I. Incidence rates and risk factors for acute urinary retention: the health professionals followup study. J Urol. 1999;162:376–82. 10.1016/S0022-5347(05)68563-1. [PubMed] [Google Scholar]
- 12.Klarskov P, Andersen JT, Asmussen CF, Brenøe J, Jensen SK, Jensen IL, et al. Acute urinary retention in women: a prospective study of 18 consecutive cases. Scand J Urol Nephrol. 1987;21:29–31. 10.3109/00365598709180286. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–7. 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey S, Palmer M. The management of female urinary retention. Int Urol Nephrol. 2006;38:533–5. 10.1007/s11255-005-5790-9. [DOI] [PubMed] [Google Scholar]
- 15.Verhamme KM, Dieleman JP, van Wijk MA, Bosch JL, Stricker BH, Sturkenboom MC. Low incidence of acute urinary retention in the general male population: the triumph project. Eur Urol. 2005;47:494–8. 10.1016/j.eururo.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Cathcart P, van der Meulen J, Armitage J, Emberton M. Incidence of primary and recurrent acute urinary retention between 1998 and 2003 in England. J Urol. 2006;176:200–4. 10.1016/S0022-5347(06)00509-X. [DOI] [PubMed] [Google Scholar]
- 17.Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology. 2007;69:436–40. 10.1016/j.urology.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 18.De Ridder D, Ost D, Bruyninckx F. The presence of Fowler’s syndrome predicts successful long-term outcome of sacral nerve stimulation in women with urinary retention. Eur Urol. 2007;51:229–33. 10.1016/j.eururo.2006.06.031. (discussion 233–4). [DOI] [PubMed] [Google Scholar]
- 19.Hoeritzauer I, Stone J, Fowler C, Elneil-Coker S, Carson A, Panicker J. Fowler’s syndrome of urinary retention: a retrospective study of co-morbidity. Neurourol Urodyn. 2016;35:601–3. 10.1002/nau.22758. [DOI] [PubMed] [Google Scholar]
- 20.Hwang YJ, Dixon SN, Reiss JP, Wald R, Parikh CR, Gandhi S, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med. 2014;161:242–8. 10.7326/M13-2796. [DOI] [PubMed] [Google Scholar]
- 21.Ou H, Chang HA, Kao YC, Tzeng NS. Risperidone-induced acute urinary retention in a patient with major depressive disorder with psychotic features: a case report. Am J Ther. 2021;28:e585–7. 10.1097/MJT.0000000000001414. [DOI] [PubMed] [Google Scholar]
- 22.Cohen R, Wilkins KM, Ostroff R, Tampi RR. Olanzapine and acute urinary retention in two geriatric patients. Am J Geriatr Pharmacother. 2007;5:241–6. 10.1016/j.amjopharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, McCombs JS, Park SH. A retrospective cohort study of acute kidney injury risk associated with antipsychotics. CNS Drugs. 2017;31:319–26. 10.1007/s40263-017-0421-4. [DOI] [PubMed] [Google Scholar]
- 24.Hsu WY, Chang TG, Chiu NY. Aripiprazole associated urine retention in a male schizophrenia patient. Gen Hosp Psychiatry. 2013;35(680):e11–2. 10.1016/j.genhosppsych.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Kahn B, Boazak M, Ragazino J, Sineath RC, Kapral T. An additive mix? Acute urinary retention in a patient with benign prostatic hyperplasia treated with Suboxone, lurasidone, and trazodone. Focus (Am Psychiatr Publ). 2018;16:292–8. 10.1176/appi.focus.20180007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill DC, Markland C. Vesical dysfunction induced by the major tranquilizers. J Urol. 1972;107:769–71. 10.1016/s0022-5347(17)61136-4. [DOI] [PubMed] [Google Scholar]
- 27.Ulmar G, Schunck H, Kober C. Urinary retention in the course of neuroleptic therapy with haloperidol. Pharmacopsychiatry. 1988;21:208–9. 10.1055/s-2007-1014677. [DOI] [PubMed] [Google Scholar]
- 28.Xomalis D, Bozikas VP, Garyfallos G, Nikolaidis N, Giouzepas J, Fokas K. Urinary hesitancy and retention caused by ziprasidone. Int Clin Psychopharmacol. 2006;21:71–2. 10.1097/01.yic.0000182117.49887.e7. [DOI] [PubMed] [Google Scholar]
- 29.Bozikas V, Petrikis P, Karavatos A. Urinary retention caused after fluoxetine-risperidone combination. J Psychopharmacol. 2001;15:142–3. 10.1177/026988110101500201. [DOI] [PubMed] [Google Scholar]
- 30.Davies MA, Compton-Toth BA, Hufeisen SJ, Meltzer HY, Roth BL. The highly efficacious actions of N-desmethylclozapine at muscarinic receptors are unique and not a common property of either typical or atypical antipsychotic drugs: is M1 agonism a pre-requisite for mimicking clozapine’s actions? Psychopharmacol (Berl). 2005;178:451–60. 10.1007/s00213-004-2017-1. [DOI] [PubMed] [Google Scholar]
- 31.Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, et al. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate receptor activity. Proc Natl Acad Sci U S A. 2003;100:13674–9. 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maany I, Greenfield H, Dhopesh V, Woody G. Urinary retention as a possible complication of long-term diazepam abuse. Am J Psychiatry. 1991;148:685. 10.1176/ajp.148.5.685b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available because the dataset is too large but are available from S.U. (shuusuke.uekusa@phar.toho-u.ac.jp) upon reasonable request.


