Abstract
International and national conservation policies almost exclusively focus on conserving species in their historic native ranges, thus excluding species that have been introduced by people and some of those that have extended their ranges on their own accord. Given that many of such migrants are threatened in their native ranges, conservation goals that explicitly exclude these populations may overlook opportunities to prevent extinctions and respond dynamically to rapidly changing environmental and climatic conditions. Focusing on terrestrial mammals, we quantified the number of threatened mammals that have established new populations through assisted migration (i.e., introduction). We devised 4 alternative scenarios for the inclusion of assisted‐migrant populations in mainstream conservation policy with the aim of preventing global species extinctions. We then used spatial prioritization algorithms to simulate how these scenarios could change global spatial conservation priorities. We found that 22% (70 species out of 265) of all identified assisted‐migrant mammals were threatened in their native ranges, mirroring the 25% of all mammals that are threatened. Reassessing global threat statuses by combining native and migrant ranges reduced the threat status of 23 species (∼33% of threatened assisted migrants). Thus, including migrant populations in threat assessments provides a more accurate assessment of actual global extinction risk among species. Spatial prioritization simulations showed that reimagining the role of assisted‐migrant populations in preventing species extinction could increase the importance of overlooked landscapes, particularly in central Australia, Europe, and the southwestern United States. Our results indicated that these various and nonexhaustive ways to consider assisted‐migrant populations, with due consideration of potential conservation conflicts with resident taxa, may provide unprecedented opportunities to prevent species extinctions.
Keywords: climate change, extinction, introduced species, invasion, novel ecosystems, species migration, cambio climático, ecosistema novedoso, especie introducida, extinción, invasión, migración de especies
Abstract
Prevención de la extinción en una época de migración de especies y cambios planetarios
Resumen
Las políticas de conservación nacionales e internacionales casi siempre se enfocan en la conservación de las especies dentro de su distribución histórica y nativa, por lo que se excluyen especies que han sido introducidas por el humano y algunas que se han extendido por cuenta propia más allá de su distribución. Ya que muchas de estas especies migrantes están amenazadas dentro de su distribución nativa, los objetivos de conservación que excluyen explícitamente a estas poblaciones pueden ignorar las oportunidades para prevenir extinciones y responder de forma dinámica a las condiciones ambientales y climáticas que cambian con rapidez. Nos enfocamos en los mamíferos terrestres para cuantificar el número de especies amenazadas que han establecido poblaciones nuevas mediante la migración asistida (introducción). Diseñamos cuatro escenarios alternativos para la inclusión de las poblaciones con migración asistida dentro de las políticas de conservación generales con el objetivo de prevenir extinciones globales de especies. Después usamos algoritmos de priorización espacial para simular cómo estos escenarios podrían cambiar las prioridades de conservación espacial en todo el mundo. Descubrimos que el 22% (70 de 765 especies) de todos los mamíferos con migración asistida están amenazados dentro de su distribución nativa, lo que es similar al 25% de especies amenazadas de todas las especies de mamíferos. La reevaluación de los estados mundiales de amenaza mediante la combinación de la distribución nativa y migrante redujo el estado de amenaza de 23 especies (∼33% de los migrantes asistidos amenazados). Por esto, incluir a las poblaciones migrantes en la evaluación de amenazas proporciona una evaluación más certera del riesgo de extinción que existe entre las especies a nivel mundial. Las simulaciones de priorización espacial mostraron que reinventar el papel que tienen las poblaciones con migración asistida en la prevención de la extinción de especies podría incrementar la importancia de los paisajes ignorados, particularmente en Australia central, Europa y el suroeste de los Estados Unidos. Nuestros resultados indican que estas maneras diversas y no exhaustivas de considerar a las poblaciones con migración asistida, con la debida consideración de los potenciales conflictos de conservación con los taxones residentes, puede proporcionar oportunidades sin precedentes para prevenir la extinción de las especies.
INTRODUCTION
The redistribution of organisms through human introductions has provided opportunities for a number of species outside their historic ranges. Many of these species are threatened or extinct in their native ranges but are thriving in their new ranges, presenting a conservation paradox (Kiacz & Brightsmith, 2021; Lees & Bell, 2008; Lundgren et al., 2018; Marchetti & Engstrom, 2016). This process of biotic redistribution is expected to accelerate, from continuing species introductions and as species migrate in response to ongoing landscape and climatic changes (Twardek et al., 2023). However, migrant species, especially those introduced by humans but also some of those that have dispersed on their own (e.g., rusty crayfish [Orconectes rusticus] and cattle egret [Bubulcus ibis] in North America [Guiaşu & Labib, 2021; Pereyra, 2020]), are widely considered pests, are excluded from biodiversity data sets and threat assessments (Schlaepfer, 2018), and are targeted by conservation eradication and control programs, regardless of whether they are endangered or extinct in their native ranges (Wallach et al., 2020). Although there has been considerable effort placed on understanding and mitigating deleterious effects that introductions can sometimes have, little consideration has been given to investigating how accounting for already established migrant populations may reshape how we might respond to the extinction crisis.
Preventing extinction is one of conservation's primary aims (Soulé, 1985). However, this aim can come into conflict with mainstream preferences for preserving only historically native life (Castelló & Santiago‐Ávila, 2023). These conflicts raise important questions about whether conservation science should respond to widespread ecological and climatic changes by broadening its valuation of organisms beyond those restricted to their historically native ranges. For example, the Javan rusa deer (Rusa timorensis) is threatened by poaching and habitat loss in its native range of Indonesia (IUCN, 2018). However, a population has established in Australia after introduction by humans in the late 1800s (Centre for Invasive Species Solutions, 2009–2023). The primary response of conservation organizations to introduced Javan rusa, and all other deer in Australia (including other threatened species), is eradication (Bengsen et al., 2022; Hampton et al., 2022). What happens if the Javan rusa becomes extinct in its native range while the Australian population lives on? Under existing protocols, would the International Union for Conservation of Nature (IUCN) list the Javan rusa as extinct in the wild or extinct while Australia continued with its eradication plans? An alternative would be to engage in exploratory discussions of how conservation policies could attach value to introduced Javan rusa, either by accommodating their presence in their new range as part of biotic reorganization under planetary change or as a source population for future repatriation to Java.
For the most part, conservation has ignored these paradoxes (but see Bradshaw et al. [2006]) because of claims that introduced organisms have fundamentally different—and unwanted—effects relative to native organisms (Pauchard et al., 2018; Rejmánek & Simberloff, 2017). Although we acknowledge that in specific cases (especially on islands) introduced organisms have contributed to extinctions, multiple meta‐analyses show that it is often impossible to distinguish between native and introduced organisms on the basis of their ecological effects (Boltovskoy et al., 2021; Charlebois & Sargent, 2017; Forgione et al., 2022; Howard et al., 2017; Lundgren et al., 2024; Wallach et al., 2022); some introduced so‐called invasive organisms targeted for eradication turn out to be native endemic species (Weijola et al., 2020); introduced organisms are not a leading cause of biodiversity loss relative to habitat loss and direct exploitation (IPBES, 2019; Jaureguiberry et al., 2022); the temporal baselines of native ranges are defined by arbitrary historic thresholds (e.g., 1500 AD or time of European colonization) and fail to capture long‐term changes in species distributions since prehistory (Monsarrat et al., 2019; Sales et al., 2022); and many introduced organisms sustain ecosystem services and facilitate other species (Bertness & Coverdale, 2013; Lugo, 2004; Lundgren, Ramp, Stromberg, et al., 2021; Mascaro et al., 2012; Schlaepfer et al., 2011). Although there remains considerable debate about these points among conservation scientists, consideration of the contributions migrant species may make to preventing extinctions and replacing lost functional services should be a priority in a time of monumental planetary change.
Earth is undergoing a rapid pace of landscape transformation, primarily from agriculture and development (Powers & Jetz, 2019). Likewise, some climate warming projections set the planet on a course to the Early Eocene (Burke et al., 2018), when northern Greenland was warm temperate forest (Herold et al., 2014; West et al., 2020). Although the concept of a native range is already fraught with ambiguities (Pereyra, 2020), this concern only escalates when organisms are no longer able to live in their historic distributions. Even intentional assisted migrations by conservationists have been met with controversy (McLachlan et al., 2007), despite the fact that these translocations are likely an essential tool to prevent future extinctions (Twardek et al., 2023). Likewise, unassisted species migrations are likely to dovetail with pervasive anthropogenic impacts on habitats, leading to unclear causality (Essl et al., 2019). Conservation's focus on only conserving populations whose distributions are unaffected by human beings may thus be increasingly unrealistic (and potentially always has been, given the extensive prehistoric and historic influences of humans on species distributions [Baker et al., 2024; Faurby & Svenning, 2015]).
For conservation policy to respond proactively and pragmatically to these changes, one must anticipate ways to value biodiversity in a time of species redistribution, including species introduced by humans and species migrating on their own accord (Schlaepfer & Lawler, 2023). Accounting for migrant species in conservation is not to dismiss potential conflicts with resident taxa and broader ecosystem‐level effects but to reveal the novel ecological processes and conservation opportunities of our time.
To provide inspiration for these discussions, we quantified various ways the biotic redistribution of threatened species could be accounted for to prevent species extinctions, focusing on mammals as an example taxonomic group. There are multiple motivations for conserving biodiversity (Chan et al., 2006; Mace, 2014); however, we focused on the common objective of extinction avoidance. Although at least some species that have migrated on their own are considered invasive (Guiaşu & Labib, 2021; Pereyra, 2020), given the scarcity of data on unassisted migrants, we focused on wild populations of assisted migrants whose ranges have expanded through human introductions. We henceforth refer to these organisms as assisted migrants instead of introduced species to align with projected future assisted and unassisted migrations and as a first step, albeit imperfect, toward a value‐neutral terminology free from human–nature ontological dualism (Kopnina & Coghlan, 2022; Sagoff, 2020).
We considered the scale of biotic redistribution of threatened mammals and then devised 4 alternative scenarios for how migrant biodiversity might be valued with the aim of preventing global extinctions. To understand how these differing approaches could influence conservation policy, we tested the relative effect of these various formulations with quantitative spatial prioritization simulations to evaluate future global conservation goals that may be implemented to prevent the extinction of threatened terrestrial mammals.
METHODS
We focused on terrestrial mammals (n = 1225 species) because their threat statuses are well known and their native distributions have been mapped (IUCN 2018, Schipper et al. 2008). We added these ranges to assisted‐migrant ranges (i.e., introduced ranges) digitized from data in the peer‐reviewed literature, government reports, newspaper articles, and a variety of databases (Appendices S1 & S2). Some assisted migrants are described and mapped by the IUCN as native because their introductions were to geographically proximate areas or were intentional conservation translocations (although other intentional conservation assisted migrations are controversial [McLachlan et al., 2007]). These were treated as native following the IUCN to avoid confounding IUCN threat statuses. To describe the overall pattern of biotic redistribution for mammals, we quantified those biogeographic realms (Olson et al., 2001) from which mammals came (donor realms) and realms that received migrant mammals. We then calculated the percentage of threatened species per mammalian family and order that have migrant populations. Biogeographic realms were the Nearctic, Neotropics, Palearctic, Indomalay, Afrotropics, Australasia, Oceania, and Antarctica.
Scenarios
We formulated 4 scenarios based on different formulations of how conservation might value assisted migrants as biodiversity: native only, the conservation of native populations was prioritized; conservative, both native and migrant populations were prioritized; expansive, threat statuses were reassessed based on the full range (migrant plus native); and independent, native populations and migrant populations were valued independently. In the native‐only scenario (i.e., the status quo), we assigned conservation value only to populations of threatened species in their native ranges (near‐threatened species were considered threatened) based on the normative premise that non‐native populations have no conservation value.
In the conservative scenario, the current IUCN Red List threat status of a species was established based only on populations in the native range, but the threat status was then extended to all populations, even those outside their native range and even if the migrant population was larger than the native population. In other words, assisted‐migrant populations were considered akin to bonus populations. We did this under the normative premise that assisted‐migrant populations could one day acquire a value equivalent to native populations and that global stochasticity in human pressures may warrant the most conservative approach to prevent global extinctions. This also acknowledges that redistributed populations have not stood the test of time in their new regions and might therefore be unstable and collapse (Simberloff & Gibbons, 2004). In this scenario, assisted‐migrant populations were assigned a threat status, in contrast with the native‐only scenario, where they were not even considered.
In the expansive scenario, we reevaluated species threat statuses globally based on each species’ full current range (the area of the combined native and migrant ranges). By doing so, some species became downlisted or delisted globally, deprioritizing both their native and migrant ranges. This scenario was based on the normative premise that migrant populations are legitimate components of biodiversity and are thus monitored and protected with equal care across their range. To reassess threat statuses, we used IUCN listing criteria and assumed a linear relationship between range size and population size (following Mogg et al. [2019]). Using the IUCN Red List listing criteria, we considered a 20% change in total range size relative to their native‐only range size as criteria for a one‐step change in threat level (e.g., from critically endangered to endangered).
Finally, in the independent scenario, we maintained current IUCN Red List statuses of species in their native ranges but assigned conservation value to migrant populations independent of native populations. This scenario was based on the normative premise that both native and migrant populations have equal value but should be considered independently due to their unique evolutionary trajectories (Faurby et al., 2022). In other words, assisted‐migrant populations were considered de facto novel species or subspecies. Under the independent scenario, valuing assisted‐migrant populations did not affect the threat status of native populations (unlike in the expansive scenario). The threat status of assisted‐migrant populations was based on the total assisted‐migrant range size relative to the native range size (Table 1).
TABLE 1.
Alternative value scenarios a for how migrant biodiversity (introduced species or assisted migrants) might be valued in conservation policy.
| Scenario | Normative position | Rationale | Change to IUCN Red List threat status b |
|---|---|---|---|
| Native only | Only native populations have value. | Species threat status limited to native populations | Unchanged |
| Conservative | Native and migrant populations have equal value. | Species threat status based solely on native range, but applied throughout the full range because long‐term viability of migrant populations is unknown | Unchanged |
| Expansive | Species threat status based on full range; if species’ combined native and assisted‐migrant range is large enough, entire species is delisted | Changes to include assisted‐migrant population | |
| Independent | Species threat status defined separately for native and assisted‐migrant populations to account for independence of evolutionary trajectories and nonredundant value of native populations | Population in native range unchanged; assisted‐migrant population assigned threat status |
Scenarios applied to conservation prioritization simulations to determine how different values might change how to best prevent species extinctions.
Changes in threat status (in expansive and independent scenarios) affected simulations by removing species (if delisted) and by changing priority weighting (IUCN, International Union for Conservation of Nature).
Spatial prioritization
To gauge how these various scenarios might alter potential conservation action, we conducted spatial prioritization algorithms to identify areas of high conservation importance globally for each scenario. To do so, we rasterized species ranges to produce feature layers for prioritization analysis with the R package exactextractr 0.4.0 (Baston, 2022) with a Mollweide projection at a 30 × 30‐km resolution. Migrant ranges reported at the country scale or within provincial boundaries (n = 12 populations of 8 species) were omitted from spatial prioritization analyses because the large sizes of these political entities would lead to global delisting, even if populations were small. However, to account for these populations (many in central and eastern Asia), 1% of that total area was used in reassessing species’ threat statuses for the expansive value scenario. This cutoff is arbitrary yet conservative for the purposes of this simulation.
We conducted spatial prioritization analyses with the R package prioritizr 5.0.1 (Hanson et al., 2023), which uses integer linear programing techniques to find optimal solutions for spatial conservation planning problems (e.g., by identifying which land units should be conserved to meet conservation objectives). We used a maximum utility objective to find the solution that most cost‐effectively conserved as many species as possible within a specified conservation budget—in this case, the number of land units (e.g., pixels). We iteratively calculated prioritization solutions for each value scenario, increasing the total number of land units in the conservation budget from 1% of Earth's surface to 30%. The resulting solutions were summed to provide a continuous ranking of relative priority per land unit.
Species were assigned weights based on their threat status: 1, near threatened; 3, vulnerable; 5, endangered; 7, critically endangered, extinct in the wild, and extinct. Thus, the prioritization algorithm gave extra importance to protecting the most endangered taxa. In doing so, changes in threat status in the expansive and independent value scenarios altered the importance of those populations in each prioritization simulation.
RESULTS
Biotic redistribution
We identified 265 mammal species with at least one assisted‐migrant (introduced) population. Of these, 70 (22%) are threatened in their native ranges, mirroring the 25% of all terrestrial mammal species that are threatened (IUCN, 2018). Assisted migrants that were introduced to different realms originated from all realms, bar Antarctica and Oceania, and were most commonly donated from the Indomalaya (59 species, 32.1% of redistributed mammals) and the Palearctic (46, 25.0%), followed by the Afrotropics (31, 16.8%), the Nearctic (26, 14.1%), Australasia (11, 6.0%), and the Neotropics (11, 6.0%) (Figure 1a). These species were received primarily by the Palearctic (57, 18.0%), Australasia (54, 17.0%), the Neotropics (51, 16.1%), and the Nearctic (47, 14.8%), followed by Afrotropics (39, 12.3%), Oceania (32, 10.1%), Indomalaya (29, 9.1%), and Antarctica (8, 2.5%) (Figure 1a).
FIGURE 1.

(a) Number of assisted‐migrant mammals from donor biogeographic realms (top) and number received by realms (bottom) (color indicates whether the assisted migrant is threatened in its historic native range); (b) species richness of only native threatened mammals (International Union for Conservation of Nature Red List), of all threatened mammals, including assisted migrants, and of assisted migrants; and (c) percentage of mammal orders that are threatened and have assisted‐migrant populations (see percentage of mammal families in Appendix S2).
Assisted migrants threatened in their native range were donated mostly from Indomalaya (16 species, 32.6%), followed by the Palearctic (14, 28.6%), Afrotropics (13, 26.5%), Nearctic (3, 6.1%), Australasia (2, 4.1%), and Neotropics (1, 2.0%) (Figure 1a). These species were received by the Neotropics (15, 16.1%), Nearctic (15, 16.1%), Australasia (14, 15.1%), and Afrotropics (13, 14.0%), followed by Oceania (12, 12.9%), Palearctic (11, 11.8%), Indomalaya (9, 9.7%), and Antarctica (4, 4.3%).
Biotic redistribution slightly increased threatened mammal species richness in Australia, southwestern Nearctic, the Caribbean, and the Nearctic (Figure 1b). Assistant‐migrant mammals represented threatened species from 9 of 22 terrestrial mammal orders (Figure 1c) and 52 of 115 families, including up to 100% of threatened species in some families (Appendix S2).
Conservative scenario
Only valuing native populations and excluding redistributed migrant populations (native‐only scenario) established tropical parts of South America, Africa, and Asia as the top priority for most effectively protecting the maximum number of threatened mammals per land area (Figure 2a). However, when assisted‐migrant populations of threatened mammals were ascribed equivalent conservation value (conservative scenario), Australia—home to 16 threatened assisted‐migrant mammals—became almost equally important for global conservation of threatened mammals (Figure 2a,b). Likewise, the Caribbean and parts of South America increased in priority, whereas parts of Africa and central Asia become slightly less emphasized (Figure 2b).
FIGURE 2.

Results of conservation prioritization simulations that (a) only take into account native populations of threatened mammals and (b) include assisted‐migrant populations of threatened mammals (i.e., introduced species) based on their threat status in their native range (conservative scenario) and (c) change in conservation priorities between native only and conservative prioritization scenarios (shown are results of conservation prioritization algorithms conducted to determine the optimal way to conserve as many threatened species as possible).
Expansive scenario
Of the 70 threatened assisted‐migrant mammal species, redistribution extended their total range by an average of 781% (between 0.01% and 18,000%). Reassessing the global threat status of these mammals based on their entire range (e.g., the expansive value scenario) reduced the threat status of 23 (∼33%) of these threatened mammal species, of which 20 (∼29%) became least concern (e.g., delisted) (Figure 3a).
FIGURE 3.

(a) Effects on threat status when it is reevaluated based on combined native and assisted‐migrant ranges (expansive scenario) (x‐axis, current threat status; color, change in threat status when assisted‐migrant populations are included) and (b) conservation priorities showed limited changes when species' threat statuses were reevaluated based on combined ranges. However, some areas increased in priority reflecting species that have notfound refuge through assisted migration.
In this scenario, spatial prioritization solutions showed little difference from native‐only solutions, except for the deprioritization of limited areas in Europe and central Asia due to the delisting or reduction in threat of these species. This led to increased prioritization among taxa that did not have migrant populations; thus, conservation resources would flow to those species that did not find refuge through global redistribution (Figure 3b).
Independent scenario
The independent scenario retained the threat status of native populations of species but considered the status of assisted‐migrant populations separately, based on an independent assessment of threat and population resilience. Under this scenario, assisted‐migrant mammals were valued as refuges for species threatened in their native ranges and in their own right and native populations were valued in a nonredundant way to assisted‐migrant populations. Importantly, this scenario reframed the narrative of conservation from one dedicated to recreating historic configurations to a futurist pursuit in which conservation works to promote adaptation while preventing extinction under planetary change.
Under this scenario, 12 of the 70 assisted‐migrant populations had large enough distributions to be treated as Least Concern and could thus be omitted from spatial prioritization (17% of threatened migrant mammals), and 58 assisted‐migrant populations were considered threatened based on their small assisted‐migrant range size, which increased the total number of threatened and (and thus prioritized) mammal populations by 2.7% (Figure 4a). Prioritizing the conservation of these assisted‐migrant populations alongside native populations increased the conservation importance of parts of the Nearctic (Texas and the Caribbean), the Neotropics, southeastern Australia, and Europe (Figure 4b).
FIGURE 4.

(a) The number of assisted‐migrant species by taxonomic family and International Union for Conservation of Nature (IUCN) threat status categorized based on their evolutionary and intrinsic value (independent scenario [assisted‐migrant populations of threatened mammals considered independent of native populations]) and (b) changes in conservation priority when small populations of threatened assisted migrants are prioritized for conservation relative to the native‐only scenario (conservation of native populations only).
Overall, we found that various methods to ascribe value to assisted‐migrant threatened mammal species led to shifts in how one might best prioritize conservation efforts to prevent extinctions. Relisting mammals to include assisted‐migrant ranges (either changing global threat status or adding migrant populations as their own valued entities) led to only slight changes in the total number of threatened mammals overall (Figure 5a), reflective of the broad scale of global mammal endangerment (Harfoot et al., 2021). However, including assisted‐migrant populations did have consequences for the percentage of species’ ranges that were prioritized (Figure 5b), especially for species currently listed as extinct or extinct in the wild. Doing so shifted conservation priorities spatially, increasing the importance of landscapes in Australasia and the Nearctic (Figure 5c).
FIGURE 5.
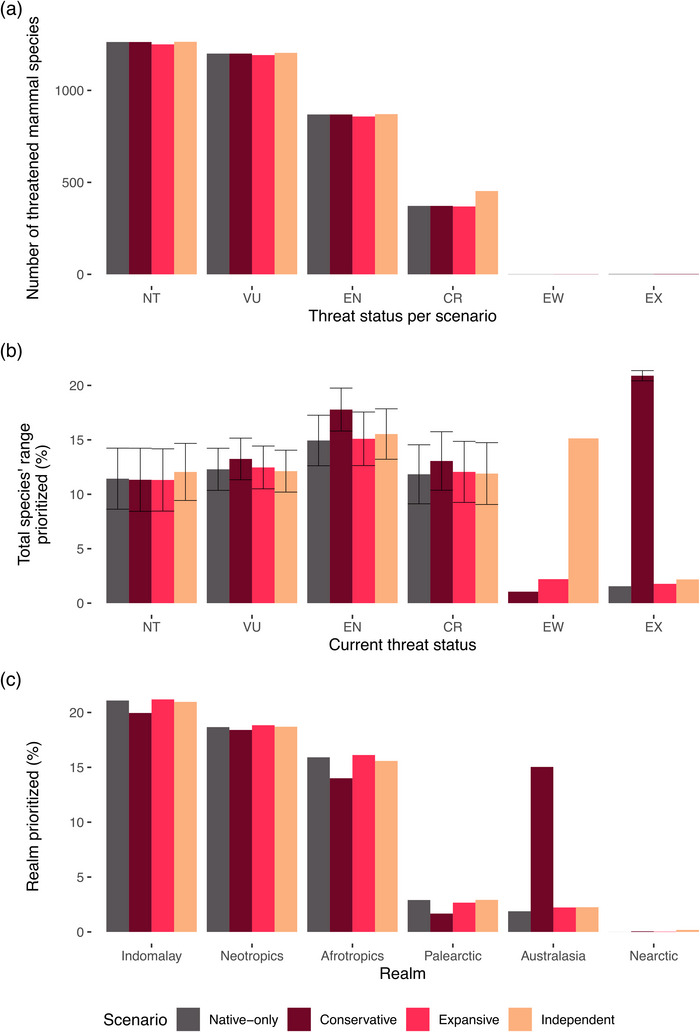
Effect of including assisted‐migrant populations in global prioritization scenarios (native only, only native species prioritized; conservative, assisted migrants prioritized based on their threat status in their native range; expansive, species threat status reassessed based on their combined range size; independent, assisted‐migrant populations prioritized as independent collectives with their own unique threat status) on (a) number of threatened mammal species per scenario, (b) percentage of threatened species’ ranges prioritized for conservation (mean, SD), and (c) percentage of realm prioritized under each scenario.
DISCUSSION
We explored how different conservation policies for assisted migrants could change how conservation effort is allocated. Most current national and international conservation policies, including frameworks such as the IUCN Red List, ignore assisted migrants (Schlaepfer, 2018), rendering populations of 70 of the world's threatened mammal species invisible (Wallach, Lundgren, et al., 2018). Although reconsidering the conservation value of assisted‐migrant populations of threatened mammals did not radically alter the global conservation outlook for the majority of threatened terrestrial mammals—which have not found refuge through migration—some important opportunities for discussion were revealed.
For example, we found that if assisted‐migrant populations were considered as important as native populations, then the rich assisted‐migrant large herbivore (megafauna) community of central Australia could be considered a conservation priority, whose protection could reduce the risk of extinction of 8 of these ecologically important species and their globally endangered functional group (Atwood et al., 2020; Lundgren et al., 2018; Malhi et al., 2016; Ripple et al., 2015; Werner, 2005). Among these Australian assisted‐migrant megafauna are the world's only population of wild dromedary camel (Camelus dromedarius), which went extinct in the wild in its native range 3000–5000 years ago and is therefore not included on the IUCN Red List, as well as feral water buffalo (Bubalus arnee bubalis), critically endangered in its native range, and feral donkeys (Equus africanus asinus), also critically endangered (Figure 6a). Given their unique influences on ecosystem functioning and their global endangerment, such megafauna are key targets for rewilding efforts around the world, which includes the intentional introduction of feral or non‐native megafauna (Svenning et al., 2016). Yet, for the most part, assisted‐migrant mammals have been excluded from this vision (Di Bitetti et al., 2022).
FIGURE 6.

Threatened species whose conservation may benefit, or already has, from inclusivity towards assisted‐migrant populations: (a) African wild ass (Equus africanus) are critically endangered in their native range of Northeast Africa yet have extensive migrant populations in North and South America and Australia due to historic introductions (photo by C. Smeenk, Wikimedia Commons, Creative Commons), (b) fallow deer (Dama dama) are listed as least concern by the International Union for Conservation of Nature but only because of extensive historic introductions across Europe (photo by Michel Langeveld, Wikimedia Commons, Creative Commons), (c) European rabbits (Oryctolagus cuniculus) are endangered in their native European range yet have established at least 187 distinct assisted‐migrant populations around the world (photo by Marie‐Lan Taÿ Pamart, Wikimedia Commons, Creative Commons), and (d) yellow‐crested cockatoo (Cacatua sulphurea) are critically endangered in their native range in Indonesia yet are thriving in novel urban environments in their assisted‐migrant range in Hong Kong (photo by Astrid Andersson).
Many assisted‐migrant populations retain genetic diversity lost from their native conspecific populations (Bradshaw et al., 2006; Marchesini et al., 2021; Todd et al., 2022). Genetic diversity is itself an element of biodiversity, considered by many to have intrinsic value (Crozier, 1997); is important for future evolutionary dynamics; and may be a lifeline to the persistence of these species globally (Booy et al., 2000). Moreover, active conservation efforts in the native ranges of some of these species are difficult, if not impossible (e.g., the remaining 23–200 native African wild asses occur in areas occupied by warring groups [IUCN, 2018]). The success of these and other endangered megafauna in central Australia (and elsewhere in western North America and South America) suggests that these species are in the places with the socioecological conditions most amenable to their survival. Conservation of these migrants would require little direct investment other than policy shifts away from eradication and toward coexistence. Importantly, protecting threatened species in their migrant ranges, which are often in wealthier regions, may help share some of the burden of species conservation with those who can best afford it (Monsarrat & Svenning, 2022).
However, valuing assisted migrant megafauna or any assisted migrant requires discussions that are honest about the limits of empirical evidence and the normative values involved in ascribing harm to the effects of an organism (Sagoff, 2020). For example, dominant views consider Australian assisted‐migrant megafauna to be exclusively harmful by reducing the cover of preferred plants (Box, Nano, et al., 2016), causing trampling and soil compaction (Box, McBurnie, et al., 2016), and reducing small mammal abundance (Legge et al., 2011). However, these effects are common to all megafauna: meta‐analyses and field experiments show that native megafauna also reduce small mammal abundance (Afonso et al., 2024; Daskin & Pringle, 2016; Keesing & Young, 2014; Trepel et al., 2024), reduce the cover of plants (Lundgren et al., 2024; Trepel et al., 2024), and cause trampling and soil compaction (Pringle et al., 2023; Trepel et al., 2024). Describing these impacts as harmful in one location (i.e., where the megafauna is introduced) and beneficial or neutral in another location (i.e., where the megafauna are native) highlights the subjective nature of these designations and the perils of creating science and conservation policy based on normative values of “belonging” (Sagoff, 2020; Wallach, Bekoff, et al., 2018).
In contrast, removals of Australian assisted‐migrant megafauna have led to increased wildfire frequency and intensity (Werner, 2005; Werner et al., 2006) and the extinction of endemic fish populations due to the loss of open water habitat (Kodric‐Brown & Brown, 2007; Kodric‐Brown et al., 2007). As with native organisms, these effects are context dependent (Lundgren et al., 2022; Wallach et al., 2015) and may simultaneously facilitate some species while suppressing others (Macdonald et al., 2007). Treating any organism as an ecological villain or hero simplifies and moralizes ecological science (Lundgren, Ramp, Wu, et al., 2021), excludes people with different values (Cardou & Vellend, 2023; Reo & Ogden, 2018), and can hinder the conservation of these species and their globally endangered functional group (Ripple et al., 2015). Thus, while remaining vigilant of the potential for assisted migrants to contribute to extinctions, we suggest that the safest route to ensure a future with megafauna and their influences (Di Bitetti et al., 2022; Svenning, 2020) would be to consider valuing these assisted‐migrant populations both in terms of their intrinsic value and their contribution to conservation agendas.
Valuing assisted‐migrant populations by including them in global threat assessments (e.g., expansive scenario) delisted 20 species and reduced threat levels for 3 others. Although this is only 1.9% of all threatened mammals, the inclusion of assisted‐migrant populations in global threat assessments reduces threat statuses for more species than the estimated 7–16 mammal extinctions prevented by active conservation interventions between 1993 and 2020 (Bolam et al., 2021). Moreover, assigning threat statuses based on both native and assisted‐migrant populations provides a more accurate description of actual extinction risk. Given the constraints of finite conservation resources, this scenario provides a clear policy pathway for preventing further mammal extinctions by enabling the redirection of resources to those that need it most.
Including assisted‐migrant populations in global threat assessments is not unheard of, particularly when the introduction is old enough and has become culturally adopted as native. For example, despite having a native population of <200 individuals, the fallow deer (Dama dama) is listed as least concern by the IUCN because of ancient introductions across western Europe (Baker et al., 2024; IUCN, 2018) (Figure 6b). This appears to contradict IUCN Red List guidelines. Introduced populations that lack “conservation intent” and that exceed “reasonable” geographic proximity (IUCN Red List 2018 guidelines section 2.1.3) are normally excluded from consideration. However, the case of the fallow deer establishes a useful precedent for engaging in discussions of how assisted‐migrant populations can be considered to reexamine IUCN listings.
The third scenario we examined considered the threat status of assisted‐migrant populations independently from native populations (e.g., independent scenario). This scenario maintained conservation concern for native populations while simultaneously valuing the emerging ecoevolutionary trajectories of assisted‐migrant populations (Faurby et al., 2022). Rapid evolution in assisted‐migrant populations—and interacting native ones—can conceive new taxa and ecological interdependencies (Carroll et al., 2005; Cattau et al., 2018; Herrel et al., 2008; Rozzi & Lomolino, 2017; Schlaepfer et al., 2005; Vellend et al., 2007; Vizentin‐Bugoni et al., 2019). Although biotic redistribution and dispersal are fundamental to evolutionary diversification and potentially to ecosystem resilience (de Queiroz, 2005; Vermeij, 1991), human‐assisted migration of species is generally considered aberrant and harmful. Instead of describing these processes as unnatural, this scenario values the emergence of new ecologies and evolutionary trajectories. For example, the European rabbit (Oryctolagus cuniculus) (Figure 6c) is classified as Endangered (IUCN, 2018) but has established at least 187 distinct populations from the subarctic to the tropics (Appendices S1 & S2). Charles Darwin initially considered one island population of introduced European rabbits to be a new species due to their remarkably divergent morphology (Darwin, 1868). Should consideration be made for the future biodiversity of Oryctolagus, particularly given the threat status of European rabbits in their native range?
These are not straightforward debates, as the example of the European rabbit highlights. Australian farmers have long struggled to reduce rabbits, investing considerable resources to deliver technological solutions aimed at their eradication (Saunders et al., 2010). In the radically altered landscapes of the Anthropocene, emerging ecologies may be unpredictable and may increase conflict. Although this may be true in some circumstances, our results suggest that reimagining current policy frameworks around coexistence may be an effective way to prevent further extinction and biodiversity loss. Ultimately, the exclusion of assisted‐migrant populations does not serve conservation universally well and establishes narratives that mirror political narratives that demonize the redistribution of people (Peretti, 1998). We suggest that the challenge for conservation in an age of biotic shuffling and planetary change is to articulate ways to protect nonhuman organisms and collectives beyond historic valuations of belonging (Schlaepfer & Lawler, 2023).
Doing so may also include broadening conservation concern to include overlooked, modified, and urbanized landscapes—conservation frontiers outside traditional wilderness models—where many threatened migrant populations reside. In addition to mammals, numerous threatened birds have established migrant populations, often in urban environments (Figure 6d). These consist of as many as 17 threatened parrot species (Pruett‐Jones, 2021), including yellow‐crested cockatoos in Hong Kong (Cacatua sulphurea) (critically endangered in their native range, Andersson, 2023), yellow‐headed parrots in Stuttgart, Germany (Amazona oratrix, endangered), and representatives of other families, such as the Javan myna in Singapore (Acridotheres javanicus, vulnerable) (Cardador et al., 2021; Gibson & Yong, 2017; IUCN, 2018; Martens & Woog, 2017). In most cases, the very same process endangering their native populations—the wildlife trade—is the source of these new populations (Gibson & Yong, 2017). Expanding conservation efforts into these anthropogenic environments provides novel opportunities to protect species without land acquisition, finds common ground with efforts to improve urban environments, and may connect the populace with caring for the organisms with whom their lives intersect (Shaffer, 2018).
Global ecosystems, climate, and society are dynamically shifting, giving rise to unpredictable and unprecedented challenges. Excluding assisted‐migrant biodiversity from conservation policy limits our ability to respond creatively and pragmatically. We presented 4 scenarios to investigate how valuing assisted‐migrant species in different ways might influence global conservation priorities and frameworks such as the IUCN Red List. Indeed, it may be possible to conceive of how policy frameworks might utilize input from all these scenarios (and others we have not considered) to arrive at context‐dependent decision‐making that best protects ecosystems and prevents extinctions. Together, our results suggest that stewarding migrant populations of threatened species, with due consideration for potential conservation conflicts with resident taxa, may make important contributions to preventing global extinctions.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank M. Jung for advice regarding spatial prioritization methods and M. Davis for feedback on earlier drafts of this manuscript. E.J.L. was funded by the International Postgraduate Research Scholarship from the University of Technology Sydney. This work was funded by an Australian Research Council Future Fellow to A.D.W. (FT210100243). J.C.S. considers this work a contribution to the Center for Ecological Dynamics in a Novel Biosphere (ECONOVO), funded by Danish National Research Foundation (grant DNRF173), and his VILLUM Investigator project “Biodiversity Dynamics in a Changing World,” funded by VILLUM FONDEN (grant 16549).
Lundgren, E. J. , Wallach, A. D. , Svenning, J.‐C. , Schlaepfer, M. A. , Andersson, A. L. A. , & Ramp, D. (2024). Preventing extinction in an age of species migration and planetary change. Conservation Biology, 38, e14270. 10.1111/cobi.14270
Article impact statement: Accounting for introduced and migrant populations can provide new opportunities to prevent extinctions and ensure a biodiverse future.
REFERENCES
- Afonso, B. C. , Rosalino, L. M. , Henriques, J. , Tinoco Torres, R. , Wauters, J. , & Carvalho, J. (2024). The effects of wild ungulates on small mammals: A systematic review and meta‐analysis. Mammal Review, 54(2), 121–132. 10.1111/mam.12331 [DOI] [Google Scholar]
- Andersson, A. L. A. (2023). Ongoing and emergent threats to yellow‐crested cockatoos (Cacatua sulphurea): A critically endangered species surviving in a city (Thesis). Hong Kong University. [Google Scholar]
- Atwood, T. B. , Valentine, S. A. , Hammill, E. , McCauley, D. J. , Madin, E. M. P. , Beard, K. H. , & Pearse, W. D. (2020). Herbivores at the highest risk of extinction among mammals, birds, and reptiles. Science Advances, 6, eabb8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, K. H. , Miller, H. , Doherty, S. , Gray, H. W. I. , Daujat, J. , Çakırlar, C. , Spassov, N. , Trantalidou, K. , Madgwick, R. , Lamb, A. L. , Ameen, C. , Atici, L. , Baker, P. , Beglane, F. , Benkert, H. , Bendrey, R. , Binois‐Roman, A. , Carden, R. F. , Curci, A. , … Sykes, N. (2024). The 10,000‐year biocultural history of fallow deer and its implications for conservation policy. Proceedings of the National Academy of Sciences of the United States of America, 121, e2310051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baston, D. (2022). exactextractr: Fast extraction from raster datasets using polygons . https://CRAN.R‐project.org/package=exactextractr
- Bengsen, A. J. , Forsyth, D. M. , Pople, A. , Brennan, M. , Amos, M. , Leeson, M. , Cox, T. E. , Gray, B. , Orgill, O. , Hampton, J. O. , Crittle, T. , & Haebich, K. (2022). Effectiveness and costs of helicopter‐based shooting of deer. Wildlife Research, 50, 617–631. [Google Scholar]
- Bertness, M. D. , & Coverdale, T. C. (2013). An invasive species facilitates the recovery of salt marsh ecosystems on Cape Cod. Ecology, 94, 1937–1943. [DOI] [PubMed] [Google Scholar]
- Bolam, F. C. , Mair, L. , Angelico, M. , Brooks, T. M. , Burgman, M. , Hermes, C. , Hoffmann, M. , Martin, R. W. , McGowan, P. J. K. , Rodrigues, A. S. L. , Rondinini, C. , Westrip, J. R. S. , Wheatley, H. , Bedolla‐Guzmán, Y. , Calzada, J. , Child, M. F. , Cranswick, P. A. , Dickman, C. R. , Fessl, B. , … Butchart, S. H. M. (2021). How many bird and mammal extinctions has recent conservation action prevented? Conservation Letters, 14, e12762. [Google Scholar]
- Boltovskoy, D. , Correa, N. M. , Burlakova, L. E. , Karatayev, A. Y. , Thuesen, E. V. , Sylvester, F. , & Paolucci, E. M. (2021). Traits and impacts of introduced species: A quantitative review of meta‐analyses. Hydrobiologia, 848, 2225–2258. [Google Scholar]
- Booy, G. , Hendriks, R. J. J. , Smulders, M. J. M. , Van Groenendael, J. M. , & Vosman, B. (2000). Genetic diversity and the survival of populations. Plant Biology, 2, 379–395. [Google Scholar]
- Box, J. B. , McBurnie, G. , Strehlow, K. , Guest, T. , Campbell, M. , Bubb, A. , McConnell, K. , Willy, S. , Uluru, R. , Kulitja, R. , Bell, B. , Burke, S. , James, R. , Kunoth, R. , & Stockman, B. (2016). The impact of feral camels (Camelus dromedarius) on remote waterholes in central Australia. Rangeland Journal, 38, 191–200. [Google Scholar]
- Box, J. B. , Nano, C. E. M. , McBurnie, G. , Waller, D. M. , McConnell, K. , Brock, C. , Paltridge, R. , McGilvray, A. , Bubb, A. , & Edwards, G. P. (2016). The impact of feral camels (Camelus dromedarius) on woody vegetation in arid Australia. Rangeland Journal, 38, 181–190. [Google Scholar]
- Bradshaw, C. J. A. , Isagi, Y. , Kaneko, S. , Bowman, D. M. J. S. , & Brook, B. W. (2006). Conservation value of non‐native banteng in northern Australia. Conservation Biology, 20, 1306–1311. [DOI] [PubMed] [Google Scholar]
- Burke, K. D. , Williams, J. W. , Chandler, M. A. , Haywood, A. M. , Lunt, D. J. , & Otto‐Bliesner, B. L. (2018). Pliocene and Eocene provide best analogs for near‐future climates. Proceedings of the National Academy of Sciences of the United States of America, 115, 13288–13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardador, L. , Abellán, P. , Anadón, J. D. , Carrete, M. , & Tella, J. L. (2021). The world parrot trade. In Pruett‐Jones S. (Eds.), Naturalized parrots of the world: Distribution, ecology, and impacts of the world's most colorful colonizers (pp. 13–21). Princeton University Press. [Google Scholar]
- Cardou, F. , & Vellend, M. (2023). Stealth advocacy in ecology and conservation biology. Biological Conservation, 280, 109968. [Google Scholar]
- Carroll, S. P. , Loye, J. E. , Dingle, H. , Mathieson, M. , Famula, T. R. , & Zalucki, M. P. (2005). And the beak shall inherit—Evolution in response to invasion. Ecology Letters, 8, 944–951. [DOI] [PubMed] [Google Scholar]
- Castelló, P. P. , & Santiago‐Ávila, F. J. (2023). Conservation after biodiversity: An analysis of Michael E. Soulé’s “What is Conservation Biology?” Biological Conservation, 287, 110313. [Google Scholar]
- Cattau, C. E. , Fletcher Jr, R. J. , Kimball, R. T. , Miller, C. W. , & Kitchens, W. M. (2018). Rapid morphological change of a top predator with the invasion of a novel prey. Nature Ecology & Evolution, 2, 108–115. [DOI] [PubMed] [Google Scholar]
- Centre for Invasive Species Solutions . (2009–2023). FeralScan . https://www.feralscan.org.au/
- Chan, K. M. A. , Shaw, M. R. , Cameron, D. R. , Underwood, E. C. , & Daily, G. C. (2006). Conservation planning for ecosystem services. PLoS Biology, 4, e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlebois, J. A. , & Sargent, R. D. (2017). No consistent pollinator‐mediated impacts of alien plants on natives. Ecology Letters, 20, 1479–1490. [DOI] [PubMed] [Google Scholar]
- Crozier, R. H. (1997). Preserving the information content of species: Genetic diversity, phylogeny, and conservation worth. Annual Review of Ecology and Systematics, 28, 243–268. [Google Scholar]
- Darwin, C. (1868). The variation of animals and plants under domestication. John Murray. [PMC free article] [PubMed] [Google Scholar]
- Daskin, J. H. , & Pringle, R. M. (2016). Does primary productivity modulate the indirect effects of large herbivores? A global meta‐analysis. The Journal of Animal Ecology, 85, 857–868. [DOI] [PubMed] [Google Scholar]
- de Queiroz, A. (2005). The resurrection of oceanic dispersal in historical biogeography. Trends in Ecology & Evolution, 20, 68–73. [DOI] [PubMed] [Google Scholar]
- Di Bitetti, M. S. , Mata, J. , & Svenning, J.‐C. (2022). Exotic mammals and rewilding in the Neotropics. Mastozoologia Neotropical, 29, 1–15. [Google Scholar]
- Essl, F. , Dullinger, S. , Genovesi, P. , Hulme, P. E. , Jeschke, J. M. , Katsanevakis, S. , Kühn, I. , Lenzner, B. , Pauchard, A. , Pyšek, P. , Rabitsch, W. , Richardson, D. M. , Seebens, H. , Van Kleunen, M. , Van Der Putten, W. H. , Vilà, M. , & Bacher, S. (2019). A conceptual framework for range‐expanding species that track human‐induced environmental change. Bioscience, 69, 908–919. [Google Scholar]
- Faurby, S. , Pedersen, R. Ø. , Svenning, J.‐C. , & Antonelli, A. (2022). The counteracting effects of anthropogenic speciation and extinction on mammal species richness and phylogenetic diversity. Global Ecology and Biogeography, 31, 1810–1823. [Google Scholar]
- Faurby, S. , & Svenning, J.‐C. (2015). Historic and prehistoric human‐driven extinctions have reshaped global mammal diversity patterns. Diversity & Distributions, 21, 1155–1166. [Google Scholar]
- Forgione, L. , Bacher, S. , & Vimercati, G. (2022). Are species more harmful in their native, neonative or alien range? Insights from a global analysis of bark beetles. Diversity and Distributions, 28, 1832–1849. [Google Scholar]
- Gibson, L. , & Yong, D. L. (2017). Saving two birds with one stone: Solving the quandary of introduced, threatened species. Frontiers in Ecology and the Environment, 15, 35–41. [Google Scholar]
- Guiaşu, R. C. , & Labib, M. (2021). The unreliable concept of native range as applied to the distribution of the rusty crayfish (Faxonius rusticus) in North America. Hydrobiologia, 848, 1177–1205. [Google Scholar]
- Hampton, J. O. , Mackenzie, D. I. , & Forsyth, D. M. (2022). Animal welfare outcomes of professional vehicle‐based shooting of peri‐urban rusa deer in Australia. Wildlife Research, 50, 603–616. [Google Scholar]
- Hanson, J. O. , Schuster, R. , Morrell, N. , Strimas‐Mackey, M. , Edwards, B. P. M. , Watts, M. E. , Arcese, P. , Bennett, J. , & Possingham, H. P. (2023). prioritizr: Systematic conservation prioritization in R . https://CRAN.R‐project.org/package=prioritizr [DOI] [PMC free article] [PubMed]
- Harfoot, M. B. J. , Johnston, A. , Balmford, A. , Burgess, N. D. , Butchart, S. H. M. , Dias, M. P. , Hazin, C. , Hilton‐Taylor, C. , Hoffmann, M. , Isaac, N. J. B. , Iversen, L. L. , Outhwaite, C. L. , Visconti, P. , & Geldmann, J. (2021). Using the IUCN Red List to map threats to terrestrial vertebrates at global scale. Nature Ecology & Evolution, 5, 1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, N. , Buzan, J. , Seton, M. , Goldner, A. , Green, J. A. M. , Müller, R. D. , Markwick, P. , & Huber, M. (2014). A suite of early Eocene (∼55 Ma) climate model boundary conditions. Geoscientific Model Development, 7, 2077–2090. [Google Scholar]
- Herrel, A. , Huyghe, K. , Vanhooydonck, B. , Backeljau, T. , Breugelmans, K. , Grbac, I. , Van Damme, R. , & Irschick, D. J. (2008). Rapid large‐scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proceedings of the National Academy of Sciences of the United States of America, 105, 4792–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, B. , Therriault, T. , & Côté, I. (2017). Contrasting ecological impacts of native and non‑native marine crabs: A global meta‐analysis. Marine Ecology Progress Series, 577, 93–103. [Google Scholar]
- Intergovernmental Science‐Policy Platform on Biodiversity and Ecosystem Services (IPBES) . (2019). Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science‐Policy Platform on Biodiversity and Ecosystem Services . IPBES Secretariat. [Google Scholar]
- International Union for Conservation of Nature (IUCN) . (2018). IUCN Red List . https://www.iucnredlist.org/ [DOI] [PubMed]
- Jaureguiberry, P. , Titeux, N. , Wiemers, M. , Bowler, D. E. , Coscieme, L. , Golden, A. S. , Guerra, C. A. , Jacob, U. , Takahashi, Y. , Settele, J. , Díaz, S. , Molnár, Z. , & Purvis, A. (2022). The direct drivers of recent global anthropogenic biodiversity loss. Science Advances, 8, eabm9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing, F. , & Young, T. P. (2014). Cascading consequences of the loss of large mammals in an African savanna. Bioscience, 64, 487–495. [Google Scholar]
- Kiacz, S. , & Brightsmith, D. J. (2021). Naturalized parrots: Conservation and research opportunities. In Pruett‐Jones S. (Eds.), Naturalized parrots of the world: Distribution, ecology, and impacts of the world's most colorful colonizers (pp. 71–86). Princeton University Press. [Google Scholar]
- Kodric‐Brown, A. , & Brown, J. H. (2007). Native fishes, exotic mammals, and the conservation of desert springs. Frontiers in Ecology and the Environment, 5, 549–553. [Google Scholar]
- Kodric‐Brown, A. , Wilcox, C. , Bragg, J. G. , & Brown, J. H. (2007). Dynamics of fish in Australian desert springs: Role of large‐mammal disturbance. Diversity and Distributions, 13, 789–798. [Google Scholar]
- Kopnina, H. , & Coghlan, S. (2022). Invasion biology and its discontents. Visions for Sustainability, 18, 145–171. [Google Scholar]
- Lees, A. C. , & Bell, D. J. (2008). A conservation paradox for the 21st century: The European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mammal Review, 38, 304–320. [Google Scholar]
- Legge, S. , Kennedy, M. S. , Lloyd, R. , Murphy, S. A. , & Fisher, A. (2011). Rapid recovery of mammal fauna in the central Kimberley, northern Australia, following the removal of introduced herbivores. Austral Ecology, 36, 791–799. [Google Scholar]
- Lugo, A. E. (2004). The outcome of alien tree invasions in Puerto Rico. Frontiers in Ecology and the Environment, 2, 265–273. [Google Scholar]
- Lundgren, E. J. , Bergman, J. , Trepel, J. , Le Roux, E. , Monsarrat, S. , Kristensen, J. A. , Pedersen, R. Ø. , Pereyra, P. , Tietje, M. , & Svenning, J.‐C. (2024). Functional traits—not nativeness—shape the effects of large mammalian herbivores on plant communities. Science, 383, 531–537. [DOI] [PubMed] [Google Scholar]
- Lundgren, E. J. , Ramp, D. , Middleton, O. S. , Wooster, E. I. F. , Kusch, E. , Balisi, M. , Ripple, W. J. , Hasselerharm, C. D. , Sanchez, J. N. , Mills, M. , & Wallach, A. D. (2022). A novel trophic cascade between cougars and feral donkeys shapes desert wetlands. The Journal of Animal Ecology, 91, 2348–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren, E. J. , Ramp, D. , Ripple, W. J. , & Wallach, A. D. (2018). Introduced megafauna are rewilding the Anthropocene. Ecography, 41, 857–866. [Google Scholar]
- Lundgren, E. J. , Ramp, D. , Stromberg, J. C. , Wu, J. , Nieto, N. C. , Sluk, M. , Moeller, K. T. , & Wallach, A. D. (2021). Equids engineer desert water availability. Science, 372, 491–495. [DOI] [PubMed] [Google Scholar]
- Lundgren, E. J. , Ramp, D. , Wu, J. , Sluk, M. , Moeller, K. T. , Stromberg, J. C. , & Wallach, A. D. (2021). Feral equids’ varied effects on ecosystems‐Response. Science, 373, 973–974. [DOI] [PubMed] [Google Scholar]
- MacDonald, D. , King, C. M. , & Strachan, R. (2007). Introduced species and the line between biodiversity conservation and naturalistic eugenics. In Macdonald D. W. & Service K. (Eds.), Key topics in conservation biology (pp. 173–185). John Wiley & Sons. [Google Scholar]
- Mace, G. M. (2014). Whose conservation? Science, 345, 1558–1560. [DOI] [PubMed] [Google Scholar]
- Malhi, Y. , Doughty, C. E. , Galetti, M. , Smith, F. A. , Svenning, J.‐C. , & Terborgh, J. W. (2016). Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proceedings of the National Academy of Sciences of the United States of America, 113, 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini, A. , Vernesi, C. , Gandolfi, A. , & Masseti, M. (2021). No genetic signature of glacial refugia in current European fallow deer (Dama dama dama L., 1758) populations: A comment on Baker (2017). Mammalian Biology, 101, 305–312. [Google Scholar]
- Marchetti, M. P. , & Engstrom, T. (2016). The conservation paradox of endangered and invasive species. Conservation Biology, 30, 434–437. [DOI] [PubMed] [Google Scholar]
- Martens, J. M. , & Woog, F. (2017). Nest cavity characteristics, reproductive output and population trend of naturalised Amazon parrots in Germany. Journal of Ornithology, 158, 823–832. [Google Scholar]
- Mascaro, J. , Hughes, R. F. , & Schnitzer, S. A. (2012). Novel forests maintain ecosystem processes after the decline of native tree species. Ecological Monographs, 82, 221–228. [Google Scholar]
- McLachlan, J. S. , Hellmann, J. J. , & Schwartz, M. W. (2007). A framework for debate of assisted migration in an era of climate change. Conservation Biology, 21, 297–302. [DOI] [PubMed] [Google Scholar]
- Mogg, S. , Fastre, C. , Jung, M. , & Visconti, P. (2019). Targeted expansion of Protected Areas to maximise the persistence of terrestrial mammals. bioRxiv. 10.1101/608992 [DOI]
- Monsarrat, S. , Novellie, P. , Rushworth, I. , & Kerley, G. (2019). Shifted distribution baselines: Neglecting long‐term biodiversity records risks overlooking potentially suitable habitat for conservation management. Philosophical Transactions of the Royal Society B: Biological Sciences, 374, 20190215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsarrat, S. , & Svenning, J.‐C. (2022). Using recent baselines as benchmarks for megafauna restoration places an unfair burden on the Global South. Ecography, 2022(4). 10.1111/ecog.05795 [DOI] [Google Scholar]
- Olson, D. M. , Dinerstein, E. , Wikramanayake, E. D. , Burgess, N. D. , Powell, G. V. N. , Underwood, E. C. , D'amico, J. A. , Itoua, I. , Strand, H. E. , Morrison, J. C. , Loucks, C. J. , Allnutt, T. F. , Ricketts, T. H. , Kura, Y. , Lamoreux, J. F. , Wettengel, W. W. , Hedao, P. , & Kassem, K. R. (2001). Terrestrial ecoregions of the world: A new map of life on earth. Bioscience, 51, 933–938. [Google Scholar]
- Pauchard, A. , Meyerson, L. A. , Bacher, S. , Blackburn, T. M. , Brundu, G. , Cadotte, M. W. , Courchamp, F. , Essl, F. , Genovesi, P. , Haider, S. , Holmes, N. D. , Hulme, P. E. , Jeschke, J. M. , Lockwood, J. L. , Novoa, A. , Nuñez, M. A. , Peltzer, D. A. , Pyšek, P. , Richardson, D. M. , … Zenni, R. D. (2018). Biodiversity assessments: Origin matters. PLoS Biology, 16, e2006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretti, J. H. (1998). Nativism and nature: Rethinking biological invasion. Environmental Values, 7, 183–192. [Google Scholar]
- Pereyra, P. J. (2020). Rethinking the native range concept. Conservation Biology, 34, 373–377. [DOI] [PubMed] [Google Scholar]
- Powers, R. P. , & Jetz, W. (2019). Global habitat loss and extinction risk of terrestrial vertebrates under future land‐use‐change scenarios. Nature Climate Change, 9, 323–329. [Google Scholar]
- Pringle, R. M. , Abraham, J. O. , Anderson, T. M. , Coverdale, T. C. , Davies, A. B. , Dutton, C. L. , Gaylard, A. , Goheen, J. R. , Holdo, R. M. , Hutchinson, M. C. , Kimuyu, D. M. , Long, R. A. , Subalusky, A. L. , & Veldhuis, M. P. (2023). Impacts of large herbivores on terrestrial ecosystems. Current Biology, 33, R584–R610. [DOI] [PubMed] [Google Scholar]
- Pruett‐Jones, S. (2021). Naturalized parrots of the world: Distribution, ecology, and impacts of the world's most colorful colonizers. Princeton University Press. [Google Scholar]
- Rejmánek, M. , & Simberloff, D. (2017). Origin matters. Environmental Conservation, 44, 97–99. [Google Scholar]
- Reo, N. J. , & Ogden, L. A. (2018). Anishnaabe Aki: An indigenous perspective on the global threat of invasive species. Sustainability Science, 13, 1443–1452. [Google Scholar]
- Ripple, W. J. , Newsome, T. M. , Wolf, C. , Dirzo, R. , Everatt, K. T. , Galetti, M. , Hayward, M. W. , Kerley, G. I. H. , Levi, T. , Lindsey, P. A. , Macdonald, D. W. , Malhi, Y. , Painter, L. E. , Sandom, C. J. , Terborgh, J. , & Van Valkenburgh, B. (2015). Collapse of the world's largest herbivores. Science Advances, 1, e1400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi, R. , & Lomolino, M. V. (2017). Rapid dwarfing of an insular mammal—The feral cattle of Amsterdam Island. Scientific Reports, 7, 8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoff, M. (2020). Fact and value in invasion biology. Conservation Biology, 34, 581–588. [DOI] [PubMed] [Google Scholar]
- Sales, L. P. , Galetti, M. , Carnaval, A. , Monsarrat, S. , Svenning, J.‐C. , & Pires, M. M. (2022). The effect of past defaunation on ranges, niches, and future biodiversity forecasts. Global Change Biology, 28, 3683–3693. [DOI] [PubMed] [Google Scholar]
- Saunders, G. , Cooke, B. , McColl, K. , Shine, R. , & Peacock, T. (2010). Modern approaches for the biological control of vertebrate pests: An Australian perspective. Biological Control: Theory and Applications in Pest Management, 52, 288–295. [Google Scholar]
- Schipper, J. , Chanson, J. S. , Chiozza, F. , Cox, N. A. , Hoffmann, M. , Katariya, V. , Lamoreux, J. , Rodrigues, A. S. L. , Stuart, S. N. , Temple, H. J. , Baillie, J. , Boitani, L. , Lacher, T. E. , Mittermeier, R. A. , Smith, A. T. , Absolon, D. , Aguiar, J. M. , Amori, G. , Bakkour, N. , … Young, B. E. (2008). The status of the world's land and marine mammals: Diversity, threat, and knowledge. Science, 322, 225–230. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, M. A. (2018). Do non‐native species contribute to biodiversity? PLoS Biology, 16, e2005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer, M. A. , & Lawler, J. J. (2023). Conserving biodiversity in the face of rapid climate change requires a shift in priorities. Wiley Interdisciplinary Reviews Climate Change, 14, e798. [Google Scholar]
- Schlaepfer, M. A. , Sax, D. F. , & Olden, J. D. (2011). The potential conservation value of non‐native species. Conservation Biology, 25, 428–437. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, M. A. , Sherman, P. W. , Blossey, B. , & Runge, M. C. (2005). Introduced species as evolutionary traps. Ecology Letters, 8, 241–246. [Google Scholar]
- Shaffer, H. B (2018). Urban biodiversity arks. Nature Sustainability, 1, 725–727. [Google Scholar]
- Simberloff, D. , & Gibbons, L. (2004). Now you see them, now you don't! – Population crashes of established introduced species. Biological Invasions, 6, 161–172. [Google Scholar]
- Soulé, M. E. (1985). What is conservation biology? Bioscience, 35, 727–734. [Google Scholar]
- Svenning, J.‐C. (2020). Rewilding should be central to global restoration efforts. One Earth, 3, 657–660. [Google Scholar]
- Svenning, J.‐C. , Pedersen, P. B. M. , Donlan, C. J. , Ejrnæs, R. , Faurby, S. , Galetti, M. , Hansen, D. M. , Sandel, B. , Sandom, C. J. , Terborgh, J. W. , & Vera, F. W. M. (2016). Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proceedings of the National Academy of Sciences of the United States of America, 113, 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, E. T. , Tonasso‐Calvière, L. , Chauvey, L. , Schiavinato, S. , Fages, A. , Seguin‐Orlando, A. , Clavel, P. , Khan, N. , Pérez Pardal, L. , Patterson Rosa, L. , Librado, P. , Ringbauer, H. , Verdugo, M. , Southon, J. , Aury, J.‐M. , Perdereau, A. , Vila, E. , Marzullo, M. , Prato, O. , … Orlando, L. (2022). The genomic history and global expansion of domestic donkeys. Science, 377, 1172–1180. [DOI] [PubMed] [Google Scholar]
- Trepel, J. , le Roux, E. , Abraham, A. J. , Buitenwerf, R. , Kamp, J. , Kristensen, J. A. , Tietje, M. , Lundgren, E. J. , & Svenning, J.‐C. (2024). Meta‐analysis shows that wild large herbivores shape ecosystem properties and promote spatial heterogeneity. Nature Ecology & Evolution, 8(4), 705–716. 10.1038/s41559-024-02327-6 [DOI] [PubMed] [Google Scholar]
- Twardek, W. M. , Taylor, J. J. , Rytwinski, T. , Aitken, S. N. , Macdonald, A. L. , Van Bogaert, R. , & Cooke, S. J. (2023). The application of assisted migration as a climate change adaptation tactic: An evidence map and synthesis. Biological Conservation, 280, 109932. [Google Scholar]
- Vellend, M. , Harmon, L. J. , Lockwood, J. L. , Mayfield, M. M. , Hughes, A. R. , Wares, J. P. , & Sax, D. F. (2007). Effects of exotic species on evolutionary diversification. Trends in Ecology & Evolution, 22, 481–488. [DOI] [PubMed] [Google Scholar]
- Vermeij, G. J. (1991). When biotas meet: Understanding biotic interchange. Science, 253, 1099–1104. [DOI] [PubMed] [Google Scholar]
- Vizentin‐Bugoni, J. , Tarwater, C. E. , Foster, J. T. , Drake, D. R. , Gleditsch, J. M. , Hruska, A. M. , Kelley, J. P. , & Sperry, J. H. (2019). Structure, spatial dynamics, and stability of novel seed dispersal mutualistic networks in Hawai'i. Science, 364, 78–82. [DOI] [PubMed] [Google Scholar]
- Wallach, A. D. , Bekoff, M. , Batavia, C. , Nelson, M. P. , & Ramp, D. (2018). Summoning compassion to address the challenges of conservation. Conservation Biology, 32, 1255–1265. [DOI] [PubMed] [Google Scholar]
- Wallach, A. D. , Lundgren, E. , Batavia, C. , Nelson, M. P. , Yanco, E. , Linklater, W. L. , Carroll, S. P. , Celermajer, D. , Brandis, K. J. , Steer, J. , & Ramp, D. (2020). When all life counts in conservation. Conservation Biology, 34, 997–1007. [DOI] [PubMed] [Google Scholar]
- Wallach, A. D. , Lundgren, E. J. , Ripple, W. J. , & Ramp, D. (2018). Invisible megafauna. Conservation Biology, 32, 962–965. [DOI] [PubMed] [Google Scholar]
- Wallach, A. D. , Ramp, D. , Benítez‐López, A. , Wooster, E. I. F. , Carroll, S. , Carthey, A. J. R. , Rogers, E. I. E. , Middleton, O. , Zawada, K. J. A. , Svenning, J.‐C. , Avidor, E. , & Lundgren, E. (2022). Savviness of prey to introduced predators. Conservation Biology, 37, e14012. [DOI] [PubMed] [Google Scholar]
- Wallach, A. D. , Ripple, W. J. , & Carroll, S. P. (2015). Novel trophic cascades: Apex predators enable coexistence. Trends in Ecology & Evolution, 30, 146–153. [DOI] [PubMed] [Google Scholar]
- Weijola, V. , Vahtera, V. , Koch, A. , Schmitz, A. , & Kraus, F. (2020). Taxonomy of Micronesian monitors (Reptilia: Squamata: Varanus): Endemic status of new species argues for caution in pursuing eradication plans. Royal Society Open Science, 7, 200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, P. A. (2005). Impact of feral water buffalo and fire on growth and survival of mature savanna trees: An experimental field study in Kakadu National Park, northern Australia. Austral Ecology, 30, 625–647. [Google Scholar]
- Werner, P. A. , Cowie, I. D. , & Cusack, J. S. (2006). Juvenile tree growth and demography in response to feral water buffalo in savannas of northern Australia: An experimental field study in Kakadu National Park. Australian Journal of Botany, 54, 283–296. [Google Scholar]
- West, C. K. , Greenwood, D. R. , Reichgelt, T. , Lowe, A. J. , Vachon, J. M. , & Basinger, J. F. (2020). Paleobotanical proxies for early Eocene climates and ecosystems in northern North America from middle to high latitudes. Climate of the Past, 16, 1387–1410. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


