Abstract
Pain, a multifaceted condition associated with actual or potential tissue damage, transcends nociception and is characterised as a subjective, sensory, and emotional experience. Extensive literature describing the adverse effects of untreated post-surgical pain emphasises the necessity of a comprehensive pain management protocol, incorporating both pharmacological and non-pharmacological strategies to ensure successful patient outcomes. The present study aimed to determine whether a positive dog-owner interaction influences post-operative pain perception and stress (POPPS), as well as behavioural inactive rate variability in bitches that underwent elective surgery. Randomly selected bitches (n = 18) underwent ovariohysterectomy. Eight bitches experienced a 45-min visit post-surgery (VPS) characterised by positive dog-owner interaction, while the remaining ten did not (NVPS). Utilising the validated Short Form of the Glasgow Composite Measure Pain Scale (CMPS-SF) to assess acute pain in dogs via stress-related behaviours, a significant decrease in POPPS was evident in the VPS group after the 45-min dog-owner interaction at T3 (1 h after post-sedation recovery), in contrast to the NVPS group. CMPS-SF-associated descriptive items ‘Nervous/Anxious/Fearful’ and ‘Happy Content or Happy and Bouncy’ decreased and increased, respectively, with dog-owner positive interaction in the VPS group. The inactivity rate was significantly lower in VPS bitches after the post-surgery 45-min dog-owner interaction than in NVPS bitches. This preliminary study suggests that the owner’s presence reduces POPPS and may improve the dogs’ welfare while undergoing routine surgeries.
Keywords: animal welfare, canine behavioural patterns, dog-owner interaction, ovariohysterectomy, pain management, post-operative pain
Introduction
Effective pain management in veterinary practice is crucial for successful outcomes, as highlighted in the 2022 AAHA/AAFP Pain Management Guidelines for Dogs and Cats (Gruen et al. 2022). Addressing acute post-surgical pain is vital for a patient’s recovery, and failure can lead to consequences such as sensitisation, neuropathic pain and maladaptive pain (Poleshuck et al. 2006; Nikolajsen & Minella 2009; Johansen et al. 2012; Masselin-Dubois et al. 2013). Inadequate pain control may also result in delayed healing, reduced food intake, sleep disturbances, compromised mobility, post-operative cognitive dysfunction (POCD), and changes in species-specific activities (Kitchell 1987; McGuire et al. 2006; Jirkof 2017; Nimmo et al. 2017).
Although spaying and neutering are known to cause moderate pain (Hardie et al. 1997; Siracusa et al. 2010; Slingsby et al. 2011; Srithunyarat et al. 2016), this pain is not always adequately prevented (Simon et al. 2017). Studies carried out on cats and dogs have shown significant variations in the frequency of analgesic use depending on species, sex, geographical location, and procedural stages (Farnworth et al. 2014; Lorena et al. 2014; Perret-Gentil et al. 2014; Simon et al. 2017). Factors such as insufficient assessment and recognition of pain (Hugonnard et al. 2004; Williams et al. 2005), drug-induced adverse effects, and a lack of clinical familiarity and experience in prescribing and administering opioids and NSAIDs continue to hinder their utilisation in veterinary practice.
In addition to the challenges in analgesic use, research suggests that effective pain management goes beyond pharmaceutical interventions alone, emphasising the significant influence on pain of cognitive and emotional factors (Epstein et al. 2015; Luna et al. 2015; Peters 2015). Human and animal studies consistently reveal a robust connection between psychological and emotional states and the experience of chronic or acute pain (Schlereth & Birklein 2008; Abdallah & Geha 2017; Zanini et al. 2018; Michaelides & Zis 2019; Kang et al. 2022; Sun et al. 2023). The intricate relationship between emotional states and pain is such that behavioural observation-based pain scales fail to distinguish between them (Siracusa et al. 2008), as they exert a similar influence on behaviour. Moreover, behavioural pain scales such as the GPS (Glasgow Pain Scale) and the MGPS (Modified Glasgow Pain Scale) are influenced by the psychological stress experienced by dogs during pre-surgery (Siracusa et al. 2008; de Santana et al. 2020). Therefore, values of behavioural pain scales are to be interpreted as representing psychological stress behaviours that include nociception components under specific circumstances.
Furthermore, perioperative emotional distress and short-term/sub-acute stress can significantly heighten pain levels and reduce tolerance, potentially leading to hyperalgesia and increased reliance on analgesia (Munafò & Stevenson 2001; Caumo et al. 2002; Ford et al. 2008; Jackson et al. 2016; Sun et al. 2023). In contrast, approaches that foster positive emotions and mitigate stress, fear, and anxiety, such as non-pharmacological interventions (including acupuncture, yoga, meditation, psychotherapy, and even the placebo effect), have demonstrated efficacy in reducing pain perception (Villemure & Bushnell 2002; Castiglioni et al. 2009; Bushnell et al. 2013; Nakata et al. 2014; Zanini et al. 2018). While no existing reports have specifically investigated the owner’s potential role in mitigating dogs’ pain perception, a wealth of literature underscores the significant impact owners have on their dogs’ emotional regulation (Hare & Tomasello 2005; Kaminski et al. 2012; Prato Previde & Valsecchi 2014).
Additionally, research indicates that owners’ presence and interaction during veterinary exams, known to be particularly stressful for dogs (Edwards et al. 2019), lead to a reduction in psychological and physiological stress indicators in the dogs (Csoltova et al. 2017; Stellato et al. 2020; Girault et al. 2022; Helsly et al. 2022). Moreover, a recent study by Camarasa et al. (2023) demonstrated that dogs with brachycephalic obstructive airway syndrome, discharged with owners on the same day as surgery, experienced fewer post-operative complications than those kept overnight.
This study sought to investigate the impact of dog-owner interaction on dogs’ pain perception and stress (PPS). The hypothesis posits that implementing such a protocol during ovariohysterectomy (OVH) in bitches mitigates post-operative pain perception and stress (POPPS). Additionally, we evaluated the interaction’s influence on the inactivity rate behaviours which were recognised as indicators of post-operative psychogenic stress in dogs (Siracusa et al. 2008).
Materials and methods
Ethical statement
Our research required neither licences nor permission from ethical review bodies in Spain or Mexico for the following reasons: (a) all bitches in this study (n = 18) were selected exclusively because they had an elective ovarian hysterectomy scheduled at one of the two private clinics where this study was conducted; (b) informed consent was obtained from all owners; (c) direct intervention was performed exclusively by the veterinarian surgeons in both clinics before, during or in the recovery of the surgical procedure; (d) all decisions concerning the well-being and welfare of the subjects were made exclusively by these veterinarians; and (e) each clinic used a different anaesthesia and analgesia protocol, as well as rescue analgesia, based on their individual standard operating procedures. Neither of the two clinics had used specific pain scales prior to this study.
Study design
Bitches were assigned to one of two groups (VPS vs NVPS) based on their owners’ decision upon arrival at the clinic, i.e. to have a 45-min post-surgery positive interaction with their dogs or not, respectively. All subjects were assessed for PPS using the Validated Short Form of the Glasgow Composite Measure Pain Scale (CMPS-SF; Reid et al. 2007) and recorded for 16 min at three-time-points: (T1) upon arrival at the clinic after being placed in crates in the intensive care unit (ICU) without owners’ presence (OVH was performed between 1 and 2 h after arrival); (T2) post-sedation recovery, between 2 h 30 min and 3 h 30 min post-surgery; and (T3) 1 h after T2. Positive interaction lasted 45 min for all post-surgery bitches from the VPS group. This interaction involved bitches and owners engaging in an isolated environment with minimal movement of people or other dogs and low noise levels. Owners were instructed: (a) to refrain from interfering with their dog’s natural behaviours and movements and avoid encouraging specific actions or movements in them; and (b) to express vocal affection and provide gentle caresses whenever they felt inclined or when their dog requested it.
Study animals
Eighteen randomly selected adult bitches (mean age: 36 [± 7.9] months, mean weight: 14 [± 2.4] kg) underwent elective OVH in two private clinics in Mexico City (Table 1). The group included purebred and mixed-breed dogs, all of whom had lived with human families for at least three months prior to the study. All the dogs were in good health, as confirmed by their respective veterinarians. Among the total group of 18 bitches, eight were visited post-surgery (VPS) by their owners (six underwent elective surgery at clinic A and two at clinic B), and ten were non-visited post-surgery (NVPS) by their owners (five underwent elective surgery at clinic A and five at clinic B) (Table 1).
Table 1.
Characteristics of study animals in terms of age, breed, weight, clinic and whether they were visited (VPS) or non-visited (NVPS) post-surgery by their owners
| SUBJECT | (VPS)/ (NVPS)* | AGE(MONTHS) | BREED | WEIGHT (KG) | CLINIC |
|---|---|---|---|---|---|
| 1 | VPS | 8 | MIXED | 5 | A |
| 2 | VPS | 120 | YORKIE | 2.6 | A |
| 3 | VPS | 12 | MIXED | 17.3 | A |
| 4 | VPS | 8 | PUG | 5.3 | A |
| 5 | VPS | 36 | LABRADOR | 30 | A |
| 6 | VPS | 12 | MIXED | 15 | A |
| 7 | VPS | 9 | CHIHUAHUA | 3.7 | B |
| 8 | VPS | 96 | MIXED | 17.7 | B |
| 9 | NVPS | 36 | BORDER COLLIE | 21 | A |
| 10 | NVPS | 48 | AUSTRALIAN CATTLE DOG | 27 | A |
| 11 | NVPS | 72 | LABRADOR | 27.1 | A |
| 12 | NVPS | 36 | DACHSHUND | 4.2 | A |
| 13 | NVPS | 36 | BOXER | 20 | A |
| 14 | NVPS | 12 | DACHSHUND | 6.9 | B |
| 15 | NVPS | 5 | MIXED | 3.5 | B |
| 16 | NVPS | 72 | FRENCH BULLDOG | 10.4 | B |
| 17 | NVPS | 18 | AMERICAN STAFFORDSHIRE TERRIER | 30.6 | B |
| 18 | NVPS | 17 | MIXED | 6.2 | B |
VPS: Visited post-surgery; NVPS Non-visited post-surgery
Both clinics followed specific analgesia and anaesthesia protocols: clinic A (11/18 bitches) used Xylazine HCl (Virbac México SA de CV, Mexico) (1 mg kg–1) for pre-anaesthesia, Propofol (Aculife Healthcare PVT Ltd, India) (1 mg kg–1) for anaesthesia induction, and Isoflurane (Piramal Healthcare Ltd, India) as an inhaled anaesthetic. Analgesia was provided with Tramadol HCL (Pisa Agropecuaria SA de CV, México) (3 mg kg–1) at the end of surgery and subsequently every 8 h. In contrast, clinic B (7/18 bitches) administered Tiletamine HCL and Zolazepam HCL (1 mg kg–1) and Dexmedetomidine (0.5 mg kg–1) for pre-anaesthesia, Propofol (4 mg kg–1) for anaesthesia induction, and Isoflurane as an inhaled anaesthetic. Analgesia included Buprenorphine (0.15 mg kg–1) and Meloxicam (0.1 mg kg–1) at the end of surgery and every 8 h.
PPS Assessment
Pain Perception and Stress (PPS) were evaluated using the CMPS-SF, a validated tool for assessing acute pain in dogs (Reid et al. 2007). Despite its primary focus on pain assessment, the CMPS-SF also incorporates stress-related behaviours that, in certain contexts, may indicate pain (Hellyer & Gaynor 1998; de Santana et al. 2020). In this manuscript, both pain perception (PPS) and post-operative pain perception (POPPS) encompass both pain and psychological stress.
The CMPS-SF includes six behavioural categories: vocalisation (four items); attention to the wound (five items); mobility (five items); response to touch (six items); demeanour (five items); and posture/activity (five items). Descriptive items within each category were scored on a binary system (1 for present, 0 for absent). The summed scores (with a maximum of 24 points) had a recommendation for rescue analgesia when 6 points or more were accrued (Reid et al. 2007).
The assessment of PPS was conducted by an independent behaviourist who had no direct contact with the subjects. Specifically, the evaluation of the CMPS-SF category ‘attention to wound’ was performed by the local veterinary surgeon at each clinic. Observations made at the clinic were not blinded and subsequently rechecked via video analysis.
Bitches requiring rescue analgesic treatment, based upon clinicians’ decisions, were excluded from the study. Therefore, the sample size (n = 18) represents bitches that did not require rescue analgesia.
Inactive rate
Bitches were each recorded for 16 min during T1, T2, and T3 using an iPhone 12 (Apple, Inc), with scan sampling conducted every 2 min. Behaviours were classified as either active or inactive (Table 2) in nine instantaneous scan samples at each of the three time-points, using a binary scoring system (0 for active, 1 for inactive). These observations allowed for the calculation of an inactivity rate, expressed as the percentage of the nine visual samples for each time point.
Table 2.
Active and inactive patterns of study animals as per Siracusa et al. (2008)
| Active | Standing: Positioned on four paws in contact with the ground, two on the ground, and two against the inner wall of the cage. |
| Sitting: Front leg pads are on the floor, with the front legs straight and the rump directly on the floor. | |
| Inactive | Lying ventral: Positioned on the side with the body, but not the head, in complete contact with the ground, or with the ventral side and legs in contact with the ground. |
| Lying sideways: Positioned fully on its side, with one side of the dog in complete contact with the ground. |
Statistical analysis
Data were analysed using the SAS® software (SAS® Institute Inc, Cary, NC, USA). CMPS-SF score and inactive rate were analysed using a generalised mixed model for repeated measures. A Poisson distribution for count data was applied for CMPS-SF score. A binomial distribution was applied for the inactivity rate. The model included the fixed effect of the clinic (A and B), the treatment group (VPS and NVPS), the time-point (T1, T2 and T3), and the interaction between treatment group and time-point. The dog was introduced as a repeated measure in the random statement. The least square means for these effects were estimated to provide adjusted comparisons across the groups and time-points. Pair-wise comparisons were conducted, and P-values adjusted using Tukey corrections to control for multiple testing. The significance level was set at P < 0.05.
Results
Pain Perception and Stress (PPS) assessed through the CMPS-SF
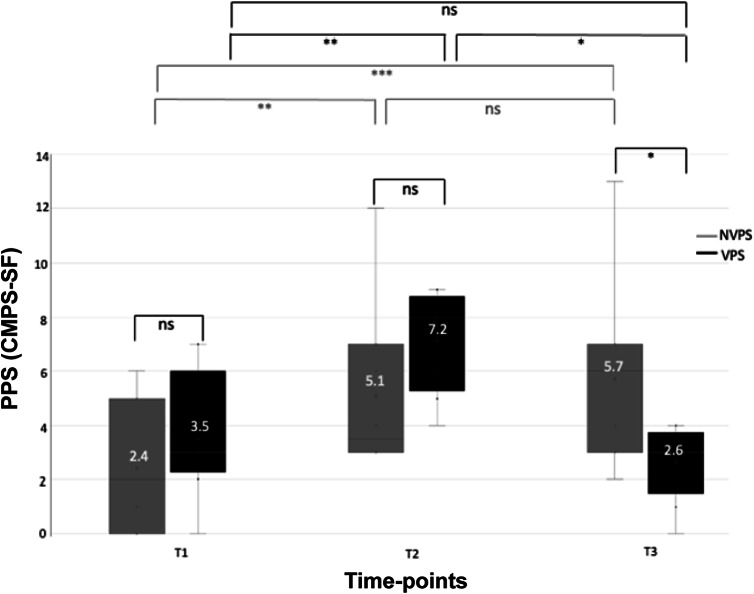
A significant interaction between the treatment group and the time-point for the CMPS-SF (P < 0.001) was detected (Figure 1). Upon arrival at the clinic (T1), the VPS and NVPS groups exhibited no significant differences in basal CMPS-SF scores (P > 0.05) (Figure 1). The OVH procedure increased the CMPS-SF scores in both groups of bitches by more than two-fold, with no significant differences between treatment groups at T2 (between 2 h 30 min and 3 h 30 min post-surgery) (Figure 1). However, the VPS group presented significantly lower CMPS-SF scores than the NVPS group at T3 (60 min after T2) following dog-owner interaction (P = 0.013; Figure 1).
Figure 1.
Boxplots showing the comparisons of PPS measured by CMPS-SF values between groups (NVPS: light grey, n = 10; VPS: dark grey, n = 8) at three different time-points: T1 (arrival at the clinic); T2 (post-sedation recovery); and T3 (60 min after T2 with or without dog-owner interaction). The number above each X in the box represents the mean of PPS (CMPS-SF). PPS: pain perception and stress; CMPS-SF: short-form Glasgow Composite Measure Pain Scale; NVPS: non-visited post-surgery; VPS: visited post-surgery; NS: non-significant; * P < 0.05; ** P < 0.01; *** P < 0.001.
Differences between periods for the VPS and NVPS groups exhibited a significant increase for both groups in CMPS-SF values at T2 as a consequence of surgery (P = 0.007 for the NVPS group and P = 0.001 for the VPS group) (Figure 1). While between T2 and T3, the NVPS group remained with stable CMPS-SF values; in the same time-frame after a 45-min dog-owner positive interaction, the VPS group exhibited a significant decrease (P < 0.001) (Figure 1), returning to CMPS-SF basal values.
Bitches underwent surgery in two different clinics, A and B. The clinic (A or B) did not significantly affect the CMPS-SF, irrespective of variations in anaesthetic/analgesic protocols. Similar patterns of PPS were detected at clinics A and B, consistent with those observed with the entire VPS and NVPS groups.
At the basal level (T1), VPS and NVPS groups in both clinics included bitches that exhibited CMPS-SF basal values around the rescue threshold (≥ 6/24) (data not shown). The OVH procedure yielded high CMPS-SF scores in the VPS and NVPS bitches in both clinics (in clinic A: 7.5 [± 0.67] in the VPS group and 5.6 [± 1.78] for the NVPS group, and in clinic B: 6.0 [± 2.0] in the VPS group and 4.6 [± 0.81] in the NVPS group). At T3 (60 min after T2), following dog-owner interaction, the VPS group presented lower CMPS-SF scores compared to the NVPS group at both clinics (at clinic A: 3.0 [± 0.45] for VPS bitches vs 6.6 [± 1.75] for the NVPS bitches and in clinic B: 1.5 [± 1.5] for VPS bitches vs 4.8 [± 0.97] for the NVPS bitches).
Differences between periods for the VPS and NVPS groups in both clinics were consistent with those observed for the entire population. Differences in anaesthetic/analgesic protocols rendered a greater POPPS increase in clinic A compared to clinic B in the VPS and NVPS groups of bitches (in clinic A: from 3.0 [± 9.3] at T1 to 7.5 [± 0.67] at T2 in the VPS group and from 2 [± 0.14] to 5.6 [± 1.78] for the NVPS group, and in clinic B: from 5.0 [± 2.0] to 6.0 [± 2.0] in the VPS group and from 2.8 [± 1.02] to 4.6 [± 0.81] in the NVPS group); however, VPS bitches of both clinics A and B showed a decrease in CMPS-SF values after dog-owner interaction at T3 of at least 4 points (at clinic A: from 7.5 [± 0.67] at T2 to 3.0 [± 0.45] at T3 and in clinic B: from 6.0 [± 2.0] to 1.5 [± 1.5] at T3). At both clinics, NVPS bitches depicted a small increase in POPPS from T2 to T3.
Individual PPS Assessment
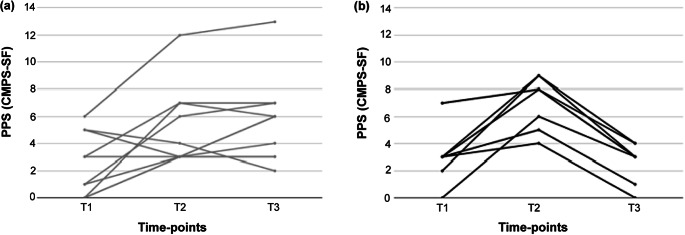
Figure 2 shows changes in PPS assessed by the CMPS-SF for each individual bitch across time-points, relative to the treatment group, NVPS, or VPS (Figure 2 left and right, respectively). Basal CMPS-SF values varied largely in both groups from scores that ranged from 0 to 7 points. Five out of 18 (27.7%) of the total group of bitches exhibited basal CMPS-SF scores between 5 and 7 points, meeting or surpassing the suggested rescue threshold of ≥ 6/24, indicating different levels of perioperative stress. OVH yielded CMPS-SF values in the NVPS group ranging from 3 to 12 points, with 40% (4/10) of them near or surpassing the rescue threshold value. In the VPS group, values ranged from 4 to 9 points, with 87.5% (7/8) of them near or surpassing the rescue threshold value (Figure 2, left and right, respectively). After 1 h at T3, NVPS bitches’ CMPS-SF values range had expanded from 2 to 12 points, with 60% (6/10) of the group with CMPS-SF meeting or surpassing the rescue threshold value. In contrast, at this time, in the VPS group, the CMPS-SF values concentrated between 0 and 4 points (Figure 2, left and right side, respectively).
Figure 2.
PPS measured with CMPS-SF for each individual bitch at three time-points: T1 (arrival at the clinic); T2 (post-sedation recovery); and T3 (60 min after T2). The measurements are presented based on the treatment group: NVPS (a) representing no dog-owner interaction, and VPS (b) representing dog-owner interaction between T2 and T3. PPS: pain perception and stress; CMPS-SF: short-form Glasgow Composite Measure Pain Scale.
CMPS-SF-associated descriptive items
The descriptive items associated with CMPS-SF are presented as percentages of bitches exhibiting each item in the VPS and NVPS groups at the T1, T2, and T3 time-points (Table 3). Due to the lack of variability in the data, no statistical analysis was conducted. At the pre-surgery time point (T1), 12.5% of dogs in the VPS group were classified as ‘Comfortable’, compared to 70% in the NVPS group. At the post-surgery time-point (T2),12.5% of the VPS bitches were classified as ‘Quiet’, compared to 80% of the NVPS bitches. By T3, 87.5% of the VPS bitches displayed behaviours described as ‘Happy and Content or Happy and Bouncy’, while none of the NVPS bitches exhibited these behaviours. Conversely, at T3, 12.5% of the VPS bitches were classified as ‘Quiet’, compared to 90% of the NVPS bitches (Table 3).
Table 3.
Comparison between time-points of the percentage of VPS and NVPS bitches displaying CMPS-SF-associated descriptive items
| % Pre-surgery Bitches | % Post Surgery Bitches | % Bitches after 45 min dog-owner interaction | % Pre-surgery Bitches | % Post Surgery Bitches | % Bitches without dog-owner interaction | |
|---|---|---|---|---|---|---|
| IS THE DOG? | (VPS) T1** |
(VPS) T2** |
(VPS) T3** |
(NVPS) T1** | (NVPS) T2** |
(NVPS) T3** |
| *A 1: Vocalisation | ||||||
| Quiet | 50% | 62.50% | 75% | 80% | 70% | 70% |
| Crying | 25% | 12.50% | 25% | 20% | 20% | 30% |
| Groaning | 25% | 12.50% | 0% | 0% | 0% | 0% |
| Screaming | 0% | 12.50% | 0% | 0% | 10% | 0% |
| *A 2: Attention to wound | ||||||
| Ignoring wound | 100% | 87.50% | 100% | 100% | 90% | 90% |
| Looking wound | 0% | 0% | 0% | 0% | 10% | 0% |
| Licking wound | 0% | 12.50% | 0% | 0% | 0% | 10% |
| *B 3: Mobility | ||||||
| Normal Mobility | 100% | 87.50% | 100% | 100% | 90% | 90% |
| Lame Mobility | 0% | 12.50% | 0% | 0% | 0% | 0% |
| It refuses to move | 0% | 0% | 0% | 0% | 10% | 10% |
| *C 4: Response to touch | ||||||
| Doing Nothing | 100% | 50% | 62.50% | 100% | 60% | 60% |
| * Looking Around | 0% | 25% | 12.50% | 0% | 0% | 0% |
| Flinch | 0% | 0% | 12.50% | 0% | 0% | 0% |
| Growling | 0% | 25% | 12.50% | 0% | 40% | 30% |
| Crying | 0% | 0% | 0% | 0% | 0% | 10% |
| *D 5: Demeanour | ||||||
| Happy Content or Happy Bouncy | 25% | 25% | 87.50% | 20% | 0% | 0% |
| Quiet | 12.50% | 12.50% | 12.50% | 50% | 80% | 90% |
| Nervous/Anxious/Fearful | 62.50% | 62.50% | 0% | 30% | 20% | 10% |
| *D 6: Posture/Activity | ||||||
| Comfortable | 12.50% | 12.50% | 37.50% | 70% | 10% | 0% |
| Unsettled | 12.50% | 0% | 12.50% | 10% | 20% | 20% |
| Restless | 75% | 25% | 37.50% | 20% | 40% | 40% |
| Hunched or Tense | 0% | 62.50% | 12.50% | 0% | 30% | 40% |
CMPS-SF Behavioural categories
Time-points: (T1) arriving at the clinic; (T2) after recovering from sedation; and (T3) 60 min after T2 with (VPS) or without (NVPS) post-surgery dog-owner interaction
Surgery affected the percentage of bitches in the VPS group exhibiting the CMPS-SF-associated descriptive item ‘Hunched or Tense’, increasing from 0% at T1 to 62.5% at T2. In the NVPS group, the percentage of bitches classified as ‘Comfortable’ decreased from 70% at T1 to 10% at T2 (Table 3). Following a 45-min dog-owner interaction (T3 vs T2), changes were observed in the VPS group, with the percentage of bitches classified as ‘Happy and Content or Happy and Bouncy’ increasing from 25% at T2 to 87.5% at T3, and the percentage of those classified as ‘Nervous/anxious/fearful’ decreasing from 62.5% at T2 to 0% at T3 (Table 3). No significant changes were observed in the NVPS group between T2 and T3 (Table 3).
Additionally, differences in the percentage of bitches displaying CMPS-SF-associated descriptive items between T1 and T3 were noted. Specifically, in the VPS group, the percentage of bitches classified as ‘Happy and Content or Happy and Bouncy’ increased from 25% at T1 to 87.5% at T3, while those classified as ‘Nervous/anxious/fearful’ decreased from 62.5% at T1 to 0% at T3 (Table 3). In the NVPS group, the percentage of bitches classified as ‘Comfortable’ decreased from 70% at T1 to 0% at T3.
Variability in the inactivity rate
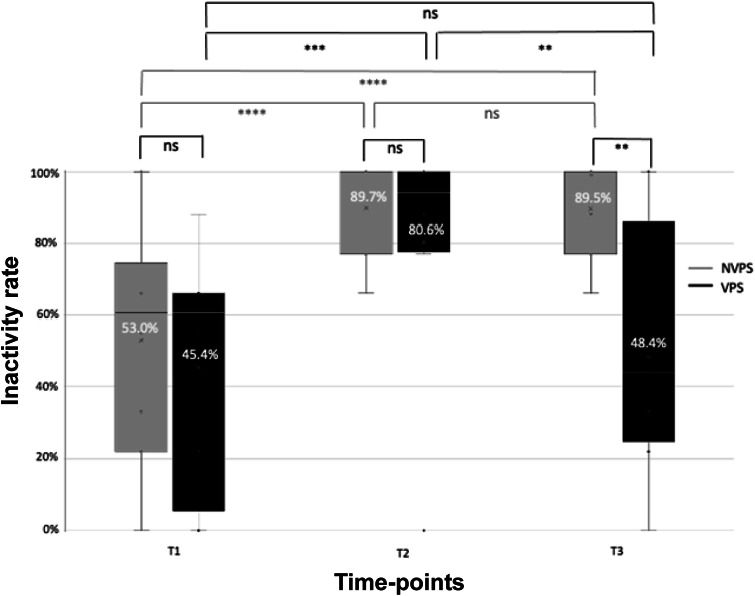
A significant interaction was detected between the treatment group and the time-point for the inactivity rate (P = 0.0117; Figure 3). Both treatment groups presented similar inactivity rates at T1 (upon arrival at the clinic) and T2 (between 2 h 20 min and 3 h 30 min post-sedation). However, at T3 (1 h after T2), VPS presented a significantly lower inactivity rate than NVPS (P = 0.0046) (Figure 3).
Figure 3.
Boxplots showing the comparisons of inactivity rate between groups (NVPS: light grey, n = 10; VPS: dark grey, n = 8) at three different time-points: T1 (arrival at the clinic); T2 (post-sedation recovery); and T3 (60 min after T2 with or without dog-owner interaction). Inactive rate is expressed as a percentage of times the dogs were observed lying in a ventral or sideways posture (9 samples at 2-min intervals per time-point). The numbers over the X in each box represent the mean value of inactivity rate for each group. NVPS: non-visited post-surgery; VPS: visited post-surgery; NS: non-significant; * P < 0.05; ** P < 0.01; *** P < 0.001.
A significant increase (P < 0.001) in the inactivity rate was observed in both groups between pre- and post-surgery (T2 vs T1) (Figure 3). The NVPS group maintained a high inactivity rate between T2 and T3 (1 h after T2) with no significant differences between treatment points (P > 0.05). After a 45-min dog-owner interaction between T2 and T3, the inactivity rate for the VPS group decreased significantly (P = 0.005), returning to its basal (T1) level (Figure 3).
No significant effect on inactivity rate was detected between bitches that underwent surgery in clinic A or B, irrespective of variations in anaesthetic/analgesic protocols. Similar patterns of inactivity rate were detected at clinics A and B, consistent with those observed with the entire VPS and NVPS groups.
Inactivity rate for individual bitches
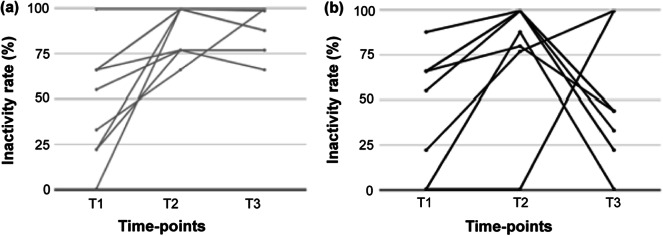
Figure 4 shows the changes in the inactivity rate for each dog across time-points and depending on the treatment group. At T3, following a 45-min interaction with owners, 75% (6/8) of the VPS group exhibited a decrease in the inactive rate, while the remaining 25% (2/8) showed an increase. Such individual variability in inactivity rate was not observed for NVPS bitches since all maintained a virtually identical inactivity rate in T3 as in T2 (Figure 4).
Figure 4.
Inactivity rate measured for each individual bitch at three time-points: T1 (arrival at the clinic); T2 (post-sedation recovery); and T3 (60 min after T2). The measurements are presented based on the treatment group: NVPS (a) representing no dog-owner interaction, and VPS (b) representing dog-owner interaction between T2 and T3.
Discussion
This study aimed to examine the influence of positive interactions between bitches and their owners on post-operative pain perception and stress (POPPS), as well as inactivity rate. Pain perception and stress (PPS) were evaluated using scores from the Short Form of the Glasgow Composite Measure Pain Scale (CMPS-SF), a validated behavioural assessment tool for assessing acute pain in dogs (Reid et al. 2007). The CMPS-SF considers stress-related behaviours that may indicate pain in specific contexts (Hellyer & Gaynor 1998; de Santana et al. 2020), thus demonstrating high sensitivity to psychological stress as well.
The findings presented here strongly indicate that positive interactions between bitches that have undergone OVH surgery and their owners, following anaesthesia recovery, have a statistically significant effect on reducing POPPS and decreasing inactivity patterns.
As previously discussed by Luna et al. (2015) and de Santana et al. (2020), dogs’ experiences of stress have been demonstrated to significantly impact CMPS-SF scores. Our results agree; here, upon arrival at the clinic, 27.8% (5/18) of the total dog group displayed CMPS-SF scores ranging from 5/24 to 7/24 points. It is important to note that while these scores fall near the CMPS-SF rescue threshold of ≥ 6/24, they do not indicate perceived pain, as all dogs were healthy and had not undergone surgery or received medication. During this time, the CMPS-SF scores of the entire dog group were influenced by item descriptors such as ‘Crying’, ‘Growling’, ‘Quiet’, ‘Nervous, Anxious, or Fearful’, and ‘Restless’, all of which encompass emotional components. The OVH procedure significantly increased the VPS and NVPS CMPS-SF scores (2 h 30 min–3 h 30 min after surgery). No significant differences were observed between both groups in POPPS and in the inactivity rate, indicating that both responded to surgery without relevant differences. However, a positive 45-min post-surgery (T3 vs T2) interaction with their owners consistently resulted in a significant decrease in POPPS. This result was observed identically in both clinics. Each clinic deployed different analgesia and anaesthesia protocols. Despite subjects being treated with Tramadol hydrochloride exhibiting a more pronounced post-operative increase in CMPS-SF score compared to those receiving a multimodal approach with Buprenorphine and Meloxicam (Hellyer et al. 2007; Dongaonkar et al. 2019), we found that VPS bitches from both clinics experienced a statistically significant POPPS decrease compared to the NVPS group. The POPPS decrease, between T2 and T3, in the VPS group was characterised by changes in the percentage of bitches displaying the CMPS-SF-associated descriptive items ‘Happy and Content or Happy and Bouncy’ and ‘Nervous/Anxious/Fearful’.
POPPS in the VPS bitches decreased to the extent that the group exhibited a lower CPMS-SF value at the end of the study compared to the one for the same group upon arrival at the clinic. There were also changes in the percentage of bitches displaying the CMPS-SF-associated descriptive items ‘Happy and Content or Happy and Bouncy’ and ‘Nervous/Anxious/Fearful’, both of which have relevant emotional components and been described as part of the perioperative stress response in dogs subjected to elective surgery (Siracusa et al. 2008).
In the context of dogs, owners may play a role as distractors, mitigating stress and addressing emotional and cognitive components, thereby alleviating POPPS, which aligns with previous reports on non-pharmacological pain modulation interventions in humans, such as yoga or meditation, with promising results (Sharar et al. 2008; Nakata et al. 2014; Peters 2015; Li et al. 2019). As previously reported (Mariti et al. 2013; Petersson et al. 2017), our findings also highlight the crucial role owners play in promoting dogs’ well-being in stressful situations.
Parallels have been drawn between the bond that is shared by dogs and their owners and that of an infant and their caregiver, with shared behavioural and neuroendocrine characteristics (Hare & Tomasello 2005; Kaminski et al. 2012; Prato Previde & Valsecchi 2014; Nagasawa et al. 2015; Petersson et al. 2017). The reduction in POPPS observed in our study may echo findings in humans, where the presence and interaction of parents with their children during invasive procedures effectively reduced their pain scores (Filippa et al. 2021; Azar et al. 2022). We hypothesise that the observed reduction in POPPS among dogs that interacted with their owners may involve the neuropeptide, oxytocin, similar to infant-human interactions (Filippa et al. 2021).
Previous research has shown that the presence and affective interaction of dogs with owners can increase concentrations of oxytocin and other substances, including β-endorphin, prolactin, β-phenylethylamine, and dopamine in both dogs and humans (Odendaal & Meintjes 2003; Nagasawa et al. 2009; Handlin et al. 2012; Romero et al. 2014). Due to their evolutionary history (Miklósi 2009), it has been suggested that dogs and humans possess a unique ability to activate each other’s oxytocinergic systems, resulting in oxytocin-linked effects (Beetz et al. 2012). Even more so, animal models strongly support the idea that oxytocin has an analgesic effect, demonstrating increased pain tolerance and attenuation of acute pain (Rash et al. 2014). Additionally, the positive effects of oxytocin in alleviating manifestations of fear, stress, or anxiety can contribute significantly to minimising the perception of pain (Heinrichs et al. 2003; Poisbeau et al. 2018). More studies are needed to further explore this hypothesis.
Concerning the potential that any positive post-OVH human-dog interaction (not involving owners) could reduce POPPS, though not explored here (an avenue we consider valuable for future research), previous studies indicate that post-OVH-surgery bitches respond to handlers with a reduced tendency to move or actively interact with them (Hardie et al. 1997; Siracusa et al. 2008), which could mean that not all humans are the same from the dogs’ perspective and as has been demonstrated in previous studies (Mariti et al. 2013). However, differences between previous protocols and ours, such as the type of interaction and the small amount of time handlers spend with dogs, could be relevant and should be further explored.
Recognising that pain and stress can induce changes in animal behaviour, such as aggression, alterations in body posture, activity levels, and movement frequency (Hellyer et al. 2007; Camps et al. 2012; Lefman & Prittie 2019), our study aimed to investigate the influence of post-surgery dog-owner interaction on specific inactive behaviours, i.e. lying ventrally or sideways. Previous research has underscored the substantial impact of post-operative psychogenic stress on these behaviours in dogs undergoing elective surgery (Hardie et al. 1997; Siracusa et al. 2008).
We evaluated the inactivity rate between different time-points for each of the bitches in the study. Dog-owner interaction (T3 vs T2) yielded a significant difference in the inactivity rate in VPS bitches compared to NVPS. While the majority of the VPS bitches experienced a decrease in inactivity rate, a small proportion, which were in a rigid stance at T2, probably as a result of pain (Johnson 1991), exhibited an increase in inactivity rate after being reunited with their owner. Variability, as a consequence of dog-owner interaction, appeared as constant in the VPS group, in contrast to NVPS bitches where almost no variability in the inactivity rate between T2 and T3 was detected. Behavioural changes induced by dog-owner interaction during veterinary examination have been previously reported (Csoltova et al. 2017; Stellato et al. 2020; Girault et al. 2022; Helsly et al. 2022), although in those instances, dogs had only been exposed to stressful situations, not painful interventions.
Bitches in our study displayed behaviours associated with stress upon arrival at the clinic as they were separated from their owners and placed in isolation within the ICU. These actions have been shown to contribute to elevated stress levels in dogs in hospitalisation settings (Siracusa et al. 2008, 2010; Srithunyarat et al. 2016; Lloyd 2017; Edwards et al. 2019). However, surprisingly, upon arrival at the clinic, differences were detected in the percentage of bitches in the VPS and NVPS groups displaying the ‘comfortable’ and ‘restless’ CMPS-SF-associated descriptive items. The observed differences between the two groups at T1, coupled with our approach of allowing owners to decide whether to visit their dogs after ovariohysterectomy (OVH) upon arrival at the clinic, may suggest a relationship between owners who opted to see their dogs and the psychological stress experienced by the dogs when separated. Previous research has highlighted the mutual perception and emotional interplay between owners and dogs (Handlin et al. 2012; Buttner et al. 2015; Petersson et al. 2017; Sundman et al. 2019). It is plausible to hypothesise that owners who chose to visit their dogs might have stronger attachments to them and could experience heightened anxiety when leaving them at the clinic. This emotional bond could potentially contribute to the observed differences in the CMPS-SF-associated descriptive items between the groups.
While interpreting our findings, it is important to exercise caution due to the limited number of animals included and the potential lack of standardisation resulting from the study’s group selection approach based upon owners’ decisions. However, concurrently examining the impact of owners on their dogs’ POPPS and the inactivity rate using two distinct parameters, coupled with the statistical similarity observed in both, enhances the reliability of the study.
Animal welfare implications and Conclusion
While challenges in spaying and neutering pain control have been extensively studied, achieving consistent and effective prevention remains elusive. The findings in this paper suggest that owner-assisted pain and stress management may prove beneficial across clinical settings. This study delves into the impact of positive dog-owner interaction on POPPS and inactivity rate in bitches undergoing ovariohysterectomy (OVH). The aim is to highlight the potential clinical advantages of such interactions in POPPS management. Our hypothesis, which posited that positive dog-owner interaction after surgery could significantly alleviate POPPS in OVH-operated bitches, found robust support in statistically significant results. The importance of owners’ presence and interaction with their dogs in mitigating POPPS, potentially by serving as emotional and cognitive distractors, was further reinforced by observing the same effect across highly effective and less effective analgesia/anaesthesia protocols.
Notably, alterations in the ‘Happy and Content or Happy and Bouncy’, ‘Quiet’, and ‘Nervous/Anxious/Fearful’ CMPS-SF-associated descriptive items emerged as the most prominent variables influenced by dog-owner interaction or its absence. These findings align with the understanding that these items carry a profound emotional component, underscoring the well-established affective bond between dogs and humans. Bitches that engaged in a 45-min interaction with their owners and experienced reduced POPPS also demonstrated a decrease in inactivity rate, which further underscored the role of this interaction in promoting a more comfortable and emotionally enriched post-operative recovery process for dogs. Exploring the role of owners in post-surgery pain relief presents an avenue for ground-breaking research. Investigating these interactions’ emotional, behavioural, and physiological aspects can deepen our comprehension of the human-dog bond and may pave the way for innovative post-operative pain management strategies.
Acknowledgements
This work received financial support from the Autonomous University of Barcelona. We thank Fidel Payan and Ana Lorena Gutierrez for the critical review of this manuscript.
Competing interest
None.
References
- Abdallah CG and Geha P 2017. Chronic pain and chronic stress: Two sides of the same coin? Chronic Stress (Thousand Oaks) 1: 2470547017704763. 10.1177/2470547017704763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar M, Aksucu G and Caglar S 2022. The effect of parental presence on pain levels of children during invasive procedures: A systematic review. Pain Managment Nursing 23(5): 682–688. 10.1016/j.pmn.2022.03.01 [DOI] [PubMed] [Google Scholar]
- Beetz A, Uvnäs-Moberg K, Julius H and Kotrschal K 2012. Psychosocial and psychophysiological effects of human-animal interactions: the possible role of oxytocin. Frontiers in Psychology 3: 234. 10.3389/fpsyg.2012.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M and Low LA 2013. Cognitive and emotional control of pain and its disruption in chronic pain. Nature Reviews. Neuroscience 14(7): 502–511. 10.1038/nrn3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner AP, Thompson B, Strasser R and Santo J 2015. Evidence for a synchronization of hormonal states between humans and dogs during competition. Physiology & Behavior 147: 54–62. 10.1016/j.physbeh.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Camarasa JJ, Gordo I, Bird FG, Vallefuoco R, Longley M and Brissot HN 2023. Owner-assisted recovery and early discharge after surgical treatment in dogs with brachycephalic obstructive airway syndrome. The Journal of Small Animal Practice 64(11): 680–686. 10.1111/jsap.13647 [DOI] [PubMed] [Google Scholar]
- Camps T, Amat M, Mariotti VM, Le Brech S and Manteca X 2012. Pain-related aggression in dogs: 12 clinical cases. Journal of Veterinary Behavior: Clinical Applications and Research 7(2): 99–102. 10.1016/j.jveb.2011.08.002 [DOI] [Google Scholar]
- Castiglioni JA, Russell MI, Setlow B, Young KA, Welsh JC and Steele-Russell I 2009. An animal model of hypnotic pain attenuation. Behavioural Brain Research 197(1): 198–204. 10.1016/j.bbr.2008.08.020 [DOI] [PubMed] [Google Scholar]
- Caumo W, Schmidt AP, Schneider CN, Bergmann J, Iwamoto CW, Adamatti LC, Bandeira D and Ferreira MB 2002. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiologica Scandinavica 46(10): 1265–1271. 10.1034/j.1399-6576.2002.461015.x [DOI] [PubMed] [Google Scholar]
- Csoltova E, Martineau M, Boissy A and Gilbert C 2017. Behavioral and physiological reactions in dogs to a veterinary examination: Owner-dog interactions improve canine well-being. Physiology & Behavior 177: 270–281. 10.1016/j.physbeh.2017.05.013 [DOI] [PubMed] [Google Scholar]
- de Santana NG, Malm C, Maia MZ, Megda TT, Beier SL, Mamão LD and Franco TC 2020. Evaluation of post-operative pain and stress in dogs after elective ovariohysterectomy under hospitalization. Brazilian Journal of Veterinary Research and Animal Science 57(3): 1–10. 10.11606/ISSN.1678-4456.BJVRAS.2020.162908 [DOI] [Google Scholar]
- Dongaonkar KR, Gulavane SU, Chariar VM and Shelar KR 2019. Laparoscopic ovariectomy in dogs in late gestation. BMC Veterinary Research 15(19). 10.1186/s12917-018-1770-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards PT, Smith BP, McArthur ML and Hazel SJ 2019. Fearful Fido: Investigating dog experience in the veterinary context in an effort to reduce distress. Applied Animal Behaviour Science 213: 14–25. 10.1016/j.applanim.2019.02.009 [DOI] [Google Scholar]
- Epstein ME, Rodanm I, Griffenhagen G, Kadrlik J, Petty MC, Robertson SA and Simpson W 2015. AAHA/AAFP pain management guidelines for dogs and cats. Journal of Feline Medicine and Surgery 17(3): 251–272. 10.1177/1098612X15572062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnworth M, Adams NJ, Keown A, Waran N and Stafford K 2014. Veterinary provision of analgesia for domestic cats (Felis catus) undergoing gonadectomy: a comparison of samples from New Zealand, Australia and the United Kingdom. New Zealand Veterinary Journal 62(3): 117–122. 10.1080/00480169.2013.852447 [DOI] [PubMed] [Google Scholar]
- Filippa M, Monaci MG, Spagnuolo C, Serravalle P, Daniele R and Grandjean D 2021. Maternal speech decreases pain scores and increases oxytocin levels in preterm infants during painful procedures. Scientific Reports 11(1): 17301. 10.1038/s41598-021-96840-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford GK, Moriarty O, McGuire BE and Finn DP 2008. Investigating the effects of distracting stimuli on nociceptive behaviour and associated alterations in brain monoamines in rats. European Journal of Pain 12(8): 970–979. 10.1016/j.ejpain.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Girault C, Priymenko N, Helsly M, Duranton C and Gaunet F 2022. Dog behaviours in veterinary consultations: Part 1. Effect of the owner’s presence or absence. Veterinary Journal 280: 105788. 10.1016/j.tvjl.2022.105788 [DOI] [PubMed] [Google Scholar]
- Gruen ME, Lascelles BDX, Colleran E, Gottlieb A, Johnson J, Lotsikas P, Marcellin-Little D and Wright B 2022. AAHA Pain Management Guidelines for Dogs and Cats. Journal of the American Animal Hospital Association 58(2): 55–76. 10.5326/JAAHA-MS-7292 [DOI] [PubMed] [Google Scholar]
- Handlin L, Nilsson A, Ejdebäck M, Hydbring-Sandberg E and Uvnäs-Moberg K 2012. Associations between the psychological characteristics of the human-dog relationship and oxytocin and cortisol levels. Anthrozoös: A Multidisciplinary Journal of The Interactions of People & Animals 25(2): 215–228. 10.2752/175303712X13316289505468 [DOI] [Google Scholar]
- Hardie EM, Hansen BD and Carroll GS 1997. Behavior after ovariohysterectomy in the dog: what’ s normal? Applied Animal Behaviour Science 51(1-2): 111–128. 10.1016/S0168-1591(96)01078-7 [DOI] [Google Scholar]
- Hare B and Tomasello M 2005. Human-like social skills in dogs? Trends in Cognitive Sciences 9(9): 439–444. 10.1016/j.tics.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C and Ehlert U 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry 54(12): 1389–1398. 10.1016/s0006-3223(03)00465-7 [DOI] [PubMed] [Google Scholar]
- Hellyer P and Gaynor J 1998. Acute postsurgical pain in dogs and cats. Compendium on Continuing Education for The Practicing Veterinarian 20(2): 140–153. https://typeset.io/papers/acute-postsurgical-pain-in-dogs-and-cats-36fjnn5oig?citations_has_pdf=true [Google Scholar]
- Hellyer P, Rodan I, Brunt J, Downing R, Hagedorn JE and Robertson SA 2007. AAHA/AAFP pain management guidelines for dogs and cats. Journal of the American Animal Hospital Association 43(5): 235–248. 10.5326/0430235 [DOI] [PubMed] [Google Scholar]
- Helsly M, Priymenko N, Girault C, Duranton C and Gaunet F 2022. Dog behaviours in veterinary consultations: Part II. The relationship between the behaviours of dogs and their owners. Veterinary Journal 281: 105789. 10.1016/j.tvjl.2022.105789 [DOI] [PubMed] [Google Scholar]
- Hugonnard M, Leblond A, Keroack S, Cadoré JL and Troncy E 2004. Attitudes and concerns of French veterinarians towards pain and analgesia in dogs and cats. Veterinary Anaesthesia and Analgesia 31(3): 154–163. 10.1111/j.1467-2987.2004.00175.x [DOI] [PubMed] [Google Scholar]
- Jackson T, Tian P, Wang Y, Lezzi T and Xie W 2016. Toward identifying moderators of associations between presurgery emotional distress and postoperative pain outcomes: A meta-analysis of longitudinal studies. The Journal of Pain 17(8): 874–888. 10.1016/j.jpain.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Jirkof P 2017. Side effects of pain and analgesia in animal experimentation. Lab Animal 46(4): 123–128. 10.1038/laban.1216 [DOI] [PubMed] [Google Scholar]
- Johansen A, Romundstad L, Nielsen CS, Schirmer H and Stubhaug A 2012. Persistent postsurgical pain in a general group: Prevalence and predictors in the Tromsø study. Pain 153(7): 1390–1396. 10.1016/j.pain.2012.02.018 [DOI] [PubMed] [Google Scholar]
- Johnson JM 1991. The veterinarian’s responsibility: Assessing and managing acute pain in dogs and cats. The Compendium on Continuing Education for the Practicing Veterinarian (USA). https://www.nasphv.org/Documents/VeterinaryStandardPrecautions.pdf (accessed 12 August 2024).
- Kaminski J, Schulz L and Tomasello M 2012. How dogs know when communication is intended for them. Developmental Science 15(2): 222–232. 10.1111/j.1467-7687.2011.01120.x [DOI] [PubMed] [Google Scholar]
- Kang EH, Park SH, Oh YI and Seo KW 2022. Assessment of salivary alpha-amylase and cortisol as a pain related stress biomarker in dogs pre-and post-operation. BMC Veterinary Research 18(31). 10.1186/s12917-021-03114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchell RL 1987. Problems in defining pain and peripheral mechanisms of pain. Journal of the American Veterinary Medical Association 191(10): 1195–1199. https://www.cabidigitallibrary.org/doi/full/10.5555/19882277511 (accessed 12 August 2024). [PubMed] [Google Scholar]
- Lefman SH and Prittie JE 2019. Psychogenic stress in hospitalized veterinary patients: Causation, implications, and therapies. Journal of Veterinary Emergency and Critical Care 29(2): 107–120. 10.1111/vec.12821 [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Jiang J and Yuan S 2019. Effects of yoga on patients with chronic nonspecific neck pain; A PRISMA systematic review and meta-analysis. Medicine 98(8): e14649. 10.1097/MD.0000000000014649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JKF 2017. Minimising stress for patients in the veterinary hospital: Why it is important and what can be done about it. Veterinary Sciences 4(2): 22. 10.3390/vetsci4020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorena SE, Luna SP, Lascelles BD and Corrente JE 2014. Current attitudes regarding the use of perioperative analgesics in dogs and cats by Brazilian veterinarians. Veterinary Anaesthesia and Analgesia 41(1): 82–89. 10.1111/vaa.12104 [DOI] [PubMed] [Google Scholar]
- Luna SP, Kelawala NH, da Maia Lima AF, Saarto EE, Restitutti FC and da Silva NE 2015. Effect of aquapuncture on postoperative analgesia after ovariohysterectomy in dogs. Semina 36(1):1979–1989. 10.5433/1679-0359.2015v36n3Supl1p1979 [DOI] [Google Scholar]
- Mariti C, Ricci E, Zilocchi M and Gazzano A 2013. Owners as a secure base for their dogs. Behaviour 150(11): 1275–1294. 10.1163/1568539X-00003095 [DOI] [Google Scholar]
- Masselin-Dubois A, Attal N, Fletcher D, Jayr C, Albi A, Fermanian J, Bouhassia D and Baudic S 2013. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. The Journal of Pain 14(8): 854–864. 10.1016/j.jpain.2013.02.013 [DOI] [PubMed] [Google Scholar]
- McGuire L, Heffner K, Glaser R, Needleman B, Malarkey W, Dickinson S, Lemeshow S, Cook C, Muscarella P, Melvin WS, Ellison EC and Kiecolt-Glaser JK 2006. Pain and wound healing in surgical patients. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine 31(2): 165–172. 10.1207/s15324796abm3102_8 [DOI] [PubMed] [Google Scholar]
- Michaelides A and Zis P 2019. Depression, anxiety and acute pain: links and management challenges. Postgraduate Medicine 131(7): 438–444. 10.1080/00325481.2019.1663705 [DOI] [PubMed] [Google Scholar]
- Miklósi Á 2009. Evolutionary approach to communication between humans and dogs. Veterinary Research Communications 33(1): 53–59. 10.1007/s11259-009-9248-x [DOI] [PubMed] [Google Scholar]
- Munafò MR and Stevenson J 2001. Anxiety and surgical recovery reinterpreting the literature. Journal of Psychosomatic Research 51(4): 589–596. 10.1016/s0022-3999(01)00258-6 [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Kikusui T, Onaka T and Ohta M 2009. Dog’s gaze at its owner increases owner’s urinary oxytocin during social interaction. Hormones and Behavior 55(3): 434–441. 10.1016/j.yhbeh.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, Onaka T, Mogi K and Kikusui T 2015. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 348(6232): 333–336. 10.1126/science.1261022 [DOI] [PubMed] [Google Scholar]
- Nakata H, Sakamoto K and Kakigi R 2014. Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Frontiers in Psychology 5(1489): 1–12. 10.3389/fpsyg.2014.01489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolajsen L and Minella CE 2009. Acute postoperative pain as a risk factor for chronic pain after surgery. European Journal of Pain Supplements 3(2): 29–32. 10.1016/j.eujps.2009.07.011 [DOI] [Google Scholar]
- Nimmo SM, Foo ITH and Paterson HM 2017. Enhanced recovery after surgery: Pain management. Journal of Surgical Oncology 116(5): 583–591. 10.1002/jso.24814 [DOI] [PubMed] [Google Scholar]
- Odendaal JS and Meintjes RA 2003. Neurophysiological correlates of affiliative behaviour between humans and dogs. Veterinary Journal 165(3): 296–301. 10.1016/s1090-0233(02)00237-x [DOI] [PubMed] [Google Scholar]
- Perret-Gentil F, Doherr MG, Spadavecchia C and Levionnois OL 2014. Attitudes of Swiss veterinarians towards pain and analgesia in dogs and cats. Schweizer Archiv fur Tierheilkunde 156(3): 111–117. 10.1024/0036-7281/a000560 [DOI] [PubMed] [Google Scholar]
- Peters ML 2015. Emotional and cognitive influences on pain experience. Modern Trends in Pharmacopsychiatry 30: 138–152. 10.1159/000435938 [DOI] [PubMed] [Google Scholar]
- Petersson M, Uvnäs-Moberg K, Nilsson A, Gustafson LL, Hydbring-Sandberg E and Handlin L 2017. Oxytocin and cortisol levels in dog owners and their dogs are associated with behavioural patterns: An exploratory study. Frontiers in Psychology 8: 1796. 10.3389/fpsyg.2017.01796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisbeau P, Grinevich V and Charlet A 2018. Oxytocin signaling in pain: Cellular, circuit, system, and behavioural levels. Current Topics in Behavioral Neurosciences 35: 193–211. 10.1007/7854_2017_14 [DOI] [PubMed] [Google Scholar]
- Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI and Dworkin RH 2006. Risk factors for chronic pain following breast cancer surgery: A prospective study. The Journal of Pain 7(9): 626–634. 10.1016/j.jpain.2006.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato-Previde E and Valsecchi P 2014. The Social Dog: Behavior and Cognition pp 165–189. Elsevier Inc: London, UK. 10.1016/B978-0-12-407818-5.00006-1 [DOI] [Google Scholar]
- Rash JA, Aguirre-Camacho A and Campbell TS 2014. Oxytocin and pain a systematic review and synthesis of findings. The Clinical Journal of Pain 30(5): 453–462. 10.1097/AJP.0b013e31829f57df [DOI] [PubMed] [Google Scholar]
- Reid J, Nolan A, Hughes J, Lascelles D, Pawson P and Scott E 2007. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Animal Welfare 16(S1): 97–104. 10.1017/S096272860003178X [DOI] [Google Scholar]
- Romero T, Nagasawa M, Mogi K, Hasegawa T and Kikusui T 2014. Oxytocin promotes social bonding in dogs. Proceedings of the National Academy of Sciences of the United States of America 111(25): 9085–9090. 10.1073/pnas.1322868111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth T and Birklein F 2008. The sympathetic nervous system and pain. Neuromolecular Medicine 10(3): 141–147. 10.1007/s12017-007-8018-6 [DOI] [PubMed] [Google Scholar]
- Sharar SR, Miller W, Teeley A, Soltani M, Hoffman HG, Jensen MP and Patterson DR 2008. Applications of virtual reality for pain management in burn-injured patients. Expert Review of Neurotherapeutics 8(11): 1667–1674. 10.1586/14737175.8.11.1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon BT, Scallan EM, Carroll G and Steagall PV 2017. The lack of analgesic use (oligoanalgesia) in small animal practice. The Journal of Small Animal Practice 58(10): 543–554. 10.1111/jsap.12717 [DOI] [PubMed] [Google Scholar]
- Siracusa C, Manteca X, Cerón J, Martínez-Subiela S, Cuenca R, Lavín S and Pastor J 2008. Perioperative stress response in dogs undergoing elective surgery: variations in behavioural, neuroendocrine, immune and acute phase responses. Animal Welfare 17(3): 259–273. 10.1017/S0962728600032188 [DOI] [Google Scholar]
- Siracusa C, Manteca X, Cuenca R, del Mar Alcalá M, Alba A, Lavín S and Pastor J 2010. Effect of a synthetic appeasing pheromone on behavioural, neuroendocrine, immune, and acute-phase perioperative stress responses in dogs. Journal of the American Veterinary Medical Association 237(6): 673–681. 10.2460/javma.237.6.673 [DOI] [PubMed] [Google Scholar]
- Slingsby LS, Taylor PM and Murrell JC 2011. A study to evaluate buprenorphine at 40μg kg (-1) compared to 20 μg kg (-1) as a post-operative analgesic in the dog. Veterinary Anaesthesia and Analgesia 38(6): 584–593. 10.1111/j.1467-2995.2011.00656.x [DOI] [PubMed] [Google Scholar]
- Srithunyarat T, Höglund OV, Hagman R, Olsson U, Stridsberg M, Lagerstedt A and Pettersson A 2016. Catestatin, vasostatin, cortisol, temperature, heart rate, respiratory rate, scores of the short form of the Glasgow composite measure pain scale and visual analog scale for stress and pain behavior in dogs before and after ovariohysterectomy. BMC Research Notes 9: 381. 10.1186/s13104-016-2193-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellato AC, Dewey CE, Widowski TM and Niel L 2020. Small animals and exotic evaluation of associations between owner presence and indicators of fear in dogs during routine veterinary examinations. Journal of the American Veterinary Medical Association 257(10): 1031–1040. 10.2460/javma.2020.257.10.1031 [DOI] [PubMed] [Google Scholar]
- Sun J, Xu W, Ye H, Tang D, Jiang Y, Pan J, Zhu J, Zhou M and Chen L 2023. Stress induces prolonged pain recovery after surgery: involvement of glucocorticoid related pathway. The International Journal of Neuropsychopharmacology 26(4): 268–279. 10.1093/ijnp/pyad010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundman A-S, Van Poucke E, Svensson Holm, AC, Farejo A, Theodorsson E, Jensen P and Roth LSV 2019. Long-term stress levels are synchronized in dogs and their owners. Scientific Reports 9(1): 7391. 10.1038/s41598-019-43851-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C and Bushnell MC 2002. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain 95(3): 195–199. 10.1016/S0304-3959(02)00007-6 [DOI] [PubMed] [Google Scholar]
- Williams VM, Lascelles BD and Robson MC 2005. Current attitudes to, and use of, peri-operative analgesia in dogs and cats by veterinarians in New Zealand. New Zealand Veterinary Journal 53(3): 193–202. 10.1080/00480169.2005.36504 [DOI] [PubMed] [Google Scholar]
- Zanini S, Voltolini A, Gragnano G, Fumagalli E and Pagnini F 2018. Changes in pain perception following psychotherapy: The mediating role of psychological components. Pain Research and Management 873084. 10.1155/2018/8713084 [DOI] [PMC free article] [PubMed] [Google Scholar]