Abstract
Background and Objective
The terminal complement inhibitor eculizumab is approved in the USA for the treatment of patients with acetylcholine receptor antibody-positive generalized myasthenia gravis (MG). The ELEVATE study aimed to examine clinical-practice outcome data on eculizumab effectiveness in US adults with MG (generalized or ocular). This paper reports the findings on MG exacerbations and crises and associated healthcare resource utilization, and the use of rescue therapy.
Methods
A retrospective chart review was conducted of US adults with MG who initiated eculizumab. Outcomes assessed for up to 2 years before and after eculizumab initiation included percentages and rates per patient per year (PPPY) of exacerbations and crises (the latter defined as intubation/impending intubation), healthcare resource utilization, and rescue therapy administration.
Results
A total of 119 patients diagnosed with MG were enrolled in the study; 92 patients had ≥ 3 months of data both before and during eculizumab therapy and were included in the analyses. The mean rate of MG exacerbations decreased from 0.385 PPPY before eculizumab initiation to 0.152 PPPY during eculizumab treatment (p = 0.0034); the mean rate of MG crises decreased from 0.411 to 0.056 PPPY (p = 0.0018). Rates of healthcare resource utilization and rescue therapy use also decreased significantly during eculizumab treatment.
Conclusions
This retrospective chart review analysis provides evidence for a beneficial impact of eculizumab treatment on the incidence of MG exacerbations and crises and associated healthcare resource utilization in clinical practice, and on rescue therapy use. These data further support the therapeutic benefits of eculizumab in patients with MG.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-024-00457-8.
Key Points
| This retrospective chart review analysis of data from the ELEVATE study investigated the impact of eculizumab treatment on the incidence of myasthenia gravis exacerbations and crises, associated healthcare resource utilization, and use of rescue therapy in clinical practice in patients with myasthenia gravis in the USA. |
| During eculizumab treatment, statistically significant reductions were observed in the rates of myasthenia gravis exacerbations and crises, in most measures of associated healthcare resource utilization, and in rescue therapy use, compared with the period before eculizumab treatment. |
| These results from clinical practice further support the beneficial effects and potential for a reduced socioeconomic burden associated with eculizumab treatment in patients with myasthenia gravis. |
Introduction
Patients with myasthenia gravis (MG) tend to have fluctuating symptoms and can experience exacerbations and, in patients with generalized MG (gMG), life-threatening crises requiring intubation and mechanical ventilation if the disease is inadequately controlled [1–5]. These acute events are associated with considerable healthcare resource utilization (HCRU) and can have long-term adverse effects on patient functioning and well-being [6–9]. A recent US database analysis showed that hospitalizations due to acute exacerbation or an MG crisis increased significantly between 2010 and 2019 in adults with MG [10].
The sustained efficacy and long-term tolerability of eculizumab (Soliris®; Alexion, AstraZeneca Rare Disease, Boston, MA, USA) in the treatment of adults with refractory acetylcholine receptor (AChR) autoantibody-positive (Ab+) gMG were demonstrated in the randomized, placebo-controlled, phase III REGAIN study (NCT01997229) [11] and its open-label extension (OLE; NCT02301624) [12]. Eculizumab has been approved for the treatment of adults with AChR Ab+ gMG in the USA since 2017. The aim of the ELEVATE (REal-WorLd Eculizumab EffectiVeness and ImpAct of DisconTinuation on US PatiEnt Outcomes in Myasthenia Gravis) study was to comprehensively examine patient characteristics, treatment patterns, clinical outcomes, and HCRU of adults with MG treated with eculizumab in clinical practice in the USA.
The findings of the ELEVATE study relating to symptom improvement and reductions in the use of corticosteroid and non-steroidal immunosuppressant therapies are reported elsewhere [13]. Here, we report the findings on rates of MG exacerbations and crises and associated HCRU, and the use of rescue therapy.
Methods
Study Design
In the ELEVATE study, an observational, retrospective, multisite chart review was conducted in the USA using physician-abstracted data from patient medical records. A sample of physicians from the Cardinal Health Neurology Provider Network and other providers who were known to prescribe eculizumab, from both community practices and academic centers across the USA, participated in the study (for more details see Habib et al. [13]). The index date was the date of initiation of eculizumab treatment for MG. The study investigated the period up to 2 years before eculizumab initiation and the period from initiation through up to 2 years of follow-up during eculizumab treatment or discontinuation (within 2 years of initiation).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was reviewed by the Western Institutional Review Board (Puyallup, WA, USA) and determined exempt from requiring ethical approval on the basis that the study collected only secondary data, which were limited and comprised only necessary protected health information (e.g., treatment dates, date of death), and that all data were de-identified and aggregated. As this study involved anonymized structured data, which according to applicable legal requirements do not contain data subject to privacy laws, informed consent from patients was not required.
Patient Inclusion Criteria
Eligible patients were adults (aged ≥ 18 years) with a diagnosis of MG at the index date who initiated eculizumab between 23 October, 2017 (US Food and Drug Administration approval date for patients with AChR Ab+ gMG) and 31 December, 2019. Patients were currently or previously managed within a participating provider’s practice at the time of data collection (i.e., the provider could report complete diagnosis/treatment details, including treatments prescribed by other physicians and inpatient treatments). No exclusion criteria were specified.
Exacerbations, Crises, and HCRU
Rates of MG exacerbations and crises (the latter defined as intubation/impending intubation) and of exacerbation-related and crisis-related hospitalizations and emergency department (ED) visits were assessed in patients with ≥ 3 months of data before and during eculizumab therapy. Exacerbations and crises were evaluated as independent events. Hospitalization was defined as a hospital stay of > 23 h duration and an ED visit was defined as a visit of ≤ 23 h duration; if the patient was admitted to a general ward or intensive care unit or if the ED stay was > 23 h in duration, the visit was considered an inpatient hospitalization. More than one ED visit and hospitalization could be recorded per patient per exacerbation or crisis. Rescue therapy administration for MG exacerbations and crises (including intravenous immunoglobulin [IVIg], plasma exchange, intravenous corticosteroids, and increased oral prednisone dosage) was also assessed in patients with ≥ 3 months of data before and during eculizumab therapy.
Statistical Analysis
Outcomes were summarized for the period before eculizumab initiation (up to 2 years) and the period during eculizumab treatment (up to 2 years). Univariate analyses were conducted to compare means and proportions across groups using paired t tests and chi-square tests, respectively (the Fisher exact test was applied where the group count was fewer than five patients). Normal distribution was assumed for all tests. Bonferroni corrections were not made for multiple comparisons. To account for varying follow-up times across the study population, MG exacerbations/crises and HCRU were calculated as percentages of patients and rates per patient per year (PPPY) for all patients with ≥ 3 months of data before and during eculizumab treatment. The PPPY rates in the periods before and during eculizumab treatment were compared using chi-square tests. A two-sided α of 0.05 was used to determine statistical significance.
Results
Provider/Practice Characteristics
Patient-level data were provided by 14 experienced neurologists or neuromuscular specialists from 14 different sites (mean [standard deviation] time in practice of 18.5 [10.0] years). Most providers (79%) were from academic centers or large community practices (for further detail see Habib et al. [13]).
Study Population
In total, 119 patients were included in the ELEVATE study. Study population demographics and clinical characteristics are described elsewhere [13]. Overall, 92 patients had ≥ 3 months of data before and during eculizumab therapy and were included in the analyses of MG exacerbations, crises, and associated HCRU (see Tables 1 and 2 for details of baseline demographics and clinical characteristics). In the analysis population, 93.5% of patients were recorded as having generalized disease involvement (gMG). The Myasthenia Gravis Foundation of America clinical classification was II/III in most patients (56.5%). Seven patients (7.6%) were recorded as having Class I disease, although only five (5.4%) were reported to have ocular MG at initiation of eculizumab treatment. Myasthenia Gravis Foundation of America classification data were missing for 32.6% of the analysis population. Fourteen patients (15.2%) had a history of thymoma. Five patients (5.4%) were reported to be pregnant at the time of eculizumab initiation. No meningococcal infections were reported. Two patients (2.2%) had died by 24 months after eculizumab initiation. The follow-up periods before and after eculizumab initiation for which data were included in the analyses were of similar duration (mean [standard deviation] 19.6 [7.2] vs 21.3 [8.7] months, respectively; p = 0.14). In total, 16 patients (17.4%) discontinued eculizumab (see Table S1 of the Electronic Supplementary Material for reasons for discontinuation); 36 patients (39.1%) were still receiving eculizumab at 24 months after initiation.
Table 1.
Patient demographics
| Characteristic | Patients (N = 92) |
|---|---|
| Sex at birth, n (%) | |
| Male | 36 (39.1) |
| Female | 56 (60.9) |
| Age at MG diagnosis, years | |
| Mean (SD) | 47.6 (19.3) |
| Median (IQR) | 50.5 (35–64) |
| Race, n (%) | |
| White | 70 (76.1) |
| Black/African American | 18 (19.6) |
| Asian | 2 (2.2) |
| American Indian/Alaskan Native | 1 (1.1) |
| Other | 1 (1.1) |
| Insurance status at eculizumab initiation, n (%)a | |
| Medicare | 42 (45.7) |
| Medicaid | 8 (8.7) |
| Commercial | 45 (48.9) |
| Military | 2 (2.2) |
| Unknown | 0 |
IQR interquartile range, MG myasthenia gravis, SD standard deviation
aCategories not mutually exclusive
Table 2.
Patient clinical characteristics
| Characteristic | Patients (N = 92) |
|---|---|
| Age at initiation of eculizumab therapy, years | |
| Mean (SD) | 56.5 (16.2) |
| Median (IQR) | 56 (43–68) |
| Time from diagnosis to eculizumab initiation, years, mean (SD) | 8.9 (9.9) |
| MG-ADL score before eculizumab initiationa | |
| Mean (SD) | 7.6 (3.6) |
| Median (IQR) | 8.0 (5.0–10.0) |
| MGFA classification at eculizumab initiation, n (%) | |
| Class I | 7 (7.6) |
| Class II | 23 (25.0) |
| Class III | 29 (31.5) |
| Class IV | 3 (3.3) |
| Class V | 0 |
| Unknown | 30 (32.6) |
| AChR antibody status tested before eculizumab initiation, n (%) | 91 (98.9) |
| Seropositive | 90 (97.8) |
| Seronegative | 1 (1.1) |
| Unknown | 1 (1.1) |
| Meningococcal vaccination ≥ 14 days before eculizumab initiation, n (%) | |
| Yes | 89 (96.7) |
| Nob | 3 (3.3) |
| Treatment for MG before eculizumab initiation,c n (%) | |
| Prednisone | 56 (60.9) |
| NSIST | 50 (54.3) |
| Rituximab | 4 (4.3) |
| Pyridostigmine | 75 (81.5) |
| Chronic IVIg | 35 (38.0) |
| Chronic PLEX | 7 (7.6) |
| None of the above | 2 (2.2) |
AChR acetylcholine receptor, IQR interquartile range, IVIg intravenous immunoglobulin, MG myasthenia gravis, MG-ADL Myasthenia Gravis-Activities of Daily Living, MGFA Myasthenia Gravis Foundation of America, NSIST non-steroidal immunosuppressant therapy, PLEX plasma exchange, SD standard deviation
aMost recent score before eculizumab initiation; n = 71
bOf the three patients reported as not receiving a meningococcal vaccination, two received prophylactic antibiotics in order to start eculizumab immediately and discontinued the antibiotics ≥ 14 days after vaccination, and one patient was reported to have not received prophylactic antibiotics
cIn the 24 months before eculizumab initiation
MG Exacerbations and Crises
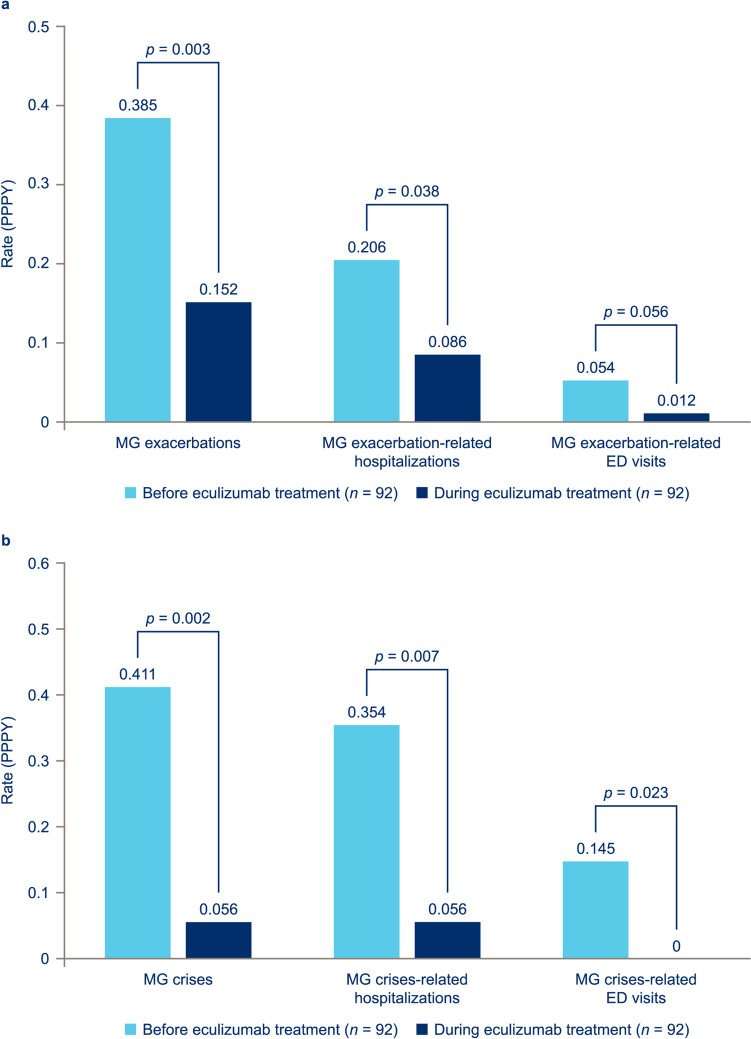
Eculizumab treatment was associated with statistically significant improvements in the incidence of MG exacerbations and crises. The proportion of patients experiencing MG exacerbations (with or without ED visits or hospitalization; excluding crises) decreased from 45.7% in the period up to 2 years before eculizumab initiation to 21.7% during the eculizumab treatment period (p = 0.0006). Similarly, the proportion experiencing a myasthenic crisis (with or without ED visits or hospitalization) decreased from 27.2 to 7.6% (p = 0.0005). The exacerbation rates, annualized for the follow-up duration, decreased by 61.0%, from 0.385 PPPY before eculizumab initiation to 0.152 PPPY during eculizumab treatment (p = 0.0034) [Fig. 1a]. The annualized crisis rate decreased by 86.0%, from 0.411 PPPY before eculizumab initiation to 0.056 PPPY during eculizumab treatment (p = 0.0018) [Fig. 1b].
Fig. 1.
Mean per patient per year (PPPY) rates of a myasthenia gravis (MG) exacerbations and b MG crises and related healthcare resource utilization in patients with ≥ 3 months’ data before and after eculizumab initiation. Crisis was defined as intubation/impending intubation. Hospitalization was defined as any stay of > 23 h duration; an emergency department (ED) visit was defined as any visit of ≤ 23 h duration (if the patient was admitted to a general ward or intensive care unit or remained in the ED for > 23 h, then the visit was considered an inpatient hospitalization). The PPPY rates of exacerbations/crises in the periods before and during eculizumab treatment were compared using chi-square tests
MG-Related HCRU
With the exception of annualized rates of exacerbation-related ED visits, all HCRU endpoints showed statistically significant improvements following eculizumab initiation.
Hospitalizations
The proportion of patients undergoing MG exacerbation-related hospitalizations decreased from 27.2% in the period up to 2 years before eculizumab initiation to 15.2% during eculizumab treatment (p = 0.047); MG crisis-related hospitalizations decreased from 22.8 to 7.6% (p = 0.0041). The mean annualized rate of exacerbation-related hospitalizations decreased by more than half, from 0.206 PPPY before eculizumab initiation to 0.086 PPPY during eculizumab treatment (p = 0.0384; Fig. 1a). The mean annualized rate of crisis-related hospitalizations decreased to less than one-fifth, from 0.354 PPPY before eculizumab initiation to 0.056 PPPY during eculizumab treatment (p = 0.0066; Fig. 1b).
ED Visits
The proportion of patients with exacerbation-related ED visits decreased from 13.0% in the period up to 2 years before eculizumab initiation to 2.2% during eculizumab treatment (p = 0.0054); crisis-related ED visits decreased from 8.7 to 0.0% (p = 0.0067). The mean annualized rate of exacerbation-related ED visits decreased by four-fifths after eculizumab initiation, from 0.054 PPPY before eculizumab initiation to 0.012 PPPY during eculizumab treatment; however, the difference did not reach statistical significance (p = 0.0558; Fig. 1a). The mean annualized rate of crisis-related ED visits was reduced from 0.145 PPPY before eculizumab initiation to 0 PPPY during eculizumab treatment (p = 0.0234; Fig. 1b).
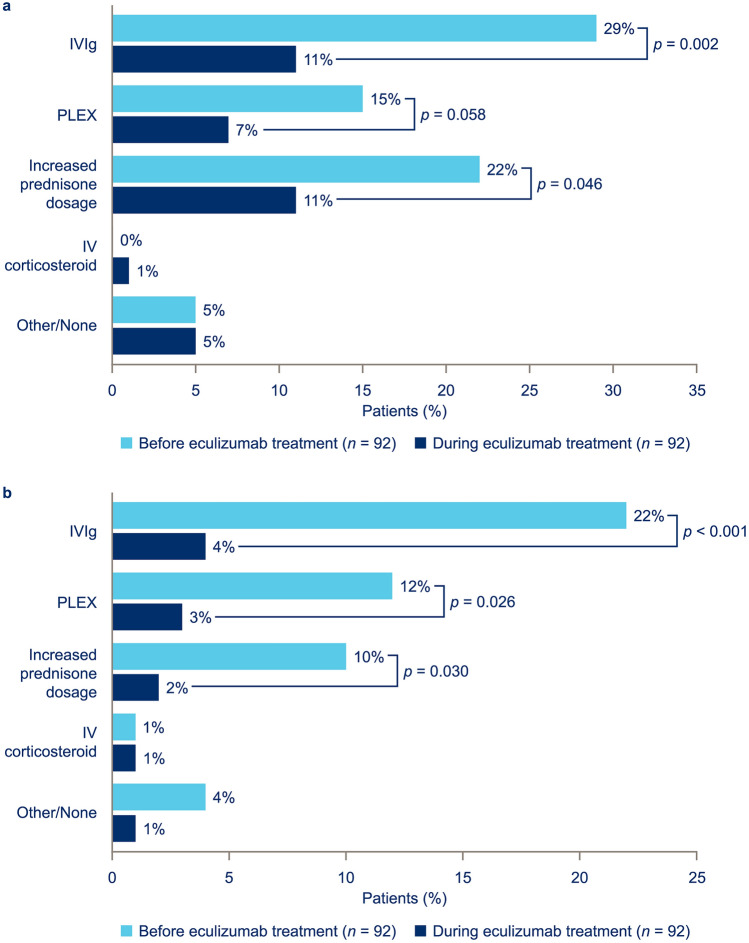
Rescue Therapy Administration
Compared with the 2-year period before eculizumab initiation, significantly fewer patients were administered rescue therapy for MG-related exacerbations in the 2-year period following eculizumab initiation. Use of IVIg was reduced from administration in 29.3 to 10.9% of patients (p = 0.0018) and increases in oral prednisone dosage were reduced from 21.7 to 10.9% of patients (p = 0.0460) [Fig. 2a]. Additionally, significantly fewer patients were administered rescue therapy for crises; use of IVIg decreased from administration in 21.7 to 4.3% of patients (p = 0.0005), plasma exchange decreased from 12.0 to 3.3% of patients (p = 0.0261), and increases in the oral prednisone dosage were reduced from 9.8 to 2.2% of patients (p = 0.0295) [Fig. 2b].
Fig. 2.
Rescue therapy administration: a myasthenia gravis (MG) exacerbation-related rescue therapy and b MG crisis-related rescue therapy, in patients with ≥ 3 months’ data before and during eculizumab therapy. Crisis was defined as intubation/impending intubation. Use of rescue therapy before and during eculizumab treatment was compared using a chi-square test. IV intravenous, IVIg intravenous immunoglobulin, PLEX plasma exchange
Discussion
This analysis of ELEVATE study data on MG exacerbations and crises as well as MG-related HCRU has demonstrated the benefits associated with eculizumab treatment in adult patients with MG in the USA.
Inadequately controlled MG can result in exacerbations and, in patients with gMG, life-threatening crises requiring intubation and mechanical ventilation [1–5]. High rates of exacerbations have been reported in other MG studies. For example, in a retrospective study in the USA, 58% of patients with gMG were found to have experienced an exacerbation in the first 6 years after diagnosis [14]. In another retrospective study, 71% of treatment-refractory patients with gMG and 32% of those who were not refractory had experienced a disease exacerbation during a 1-year follow-up period, whereas 21 and 6%, respectively, had experienced at least one myasthenic crisis [4]. An analysis of US national hospitalization data estimated that, in 2019, there were approximately 5300 hospitalizations for acute exacerbations (without a crisis) and 1650 hospitalizations for an MG crisis [10].
In the ELEVATE study population included in this analysis, rates of myasthenic exacerbations and crises and associated HCRU were statistically significantly reduced during eculizumab treatment compared with the period before treatment. These clinical-practice data are comparable with results from the REGAIN OLE, which reported that the exacerbation rate was reduced by 75% and the rate of MG-related hospitalizations by over 80% after eculizumab initiation compared with the period before eculizumab treatment [12]. The phase III clinical trial of ravulizumab (CHAMPION MG, NCT03920293) and a clinical-practice study have confirmed the benefit provided by complement C5 inhibitors in reducing the incidence of clinical deterioration in patients with AChR Ab+ gMG [15, 16]. A small retrospective chart review of real-world eculizumab treatment in patients in the USA with MG defined as treatment refractory also reported that the mean number of exacerbations per patient was significantly reduced following eculizumab initiation compared with the 12 months before eculizumab treatment [17]. Correspondingly, HCRU associated with MG exacerbations and crises decreased significantly during eculizumab treatment [17]. Although not assessed in the current study, it is likely that such reductions will have an impact on the socioeconomic burden of disease. Further research is warranted in this area as this continues to represent a major knowledge gap.
It is clear that exacerbations and crises incur substantial HCRU and costs [18]; however, it should also be borne in mind that these acute episodes may have a considerable impact on patients’ long-term function and well-being [7, 8, 19, 20]. For example, a retrospective study of the hospital records of 53 US patients found that 83% could perform their activities of daily living independently before hospitalization for a crisis, but only 42% retained independence at the time of hospital discharge [6]. A multicenter retrospective study of 250 German patients reported that 50% lived independently at home before an MG crisis, compared with only 12% after a crisis [21]. Data from the cross-sectional US Nationwide Inpatient Survey (2001–2) showed that only 28% of patients were discharged home after experiencing a crisis, whereas 48% were transferred to a short-term hospital or other setting such as a skilled nursing facility or intermediate care [22].
The current analysis has shown that eculizumab initiation was followed by a statistically significant decrease in the use of rescue therapy involving plasma exchange, IVIg, intravenous corticosteroids, or increases in oral prednisone dosage. These results are in accordance with those of the REGAIN study and its OLE, which reported that the rate of rescue therapy use substantially decreased from 67.5 events per 100 patient-years in patients receiving placebo in REGAIN to 23.1 events per 100 patient-years in eculizumab-treated patients in the OLE [12]. Rescue therapy, while generally well tolerated, is associated with a range of possible adverse effects that may have serious long-term clinical consequences [23–26], as well as increased healthcare costs [9]. Any reduction in the use of rescue therapy is therefore likely to have clinical and economic benefits.
The patient sample included in the ELEVATE study was derived from a range of clinical-practice settings and geographic sites in the USA [13]. The clinical and demographic characteristics of the population (including race, disease severity, history of thymoma, and ‘refractory’ status) were more diverse than those of the REGAIN study, meaning that the results of the two studies should be compared with caution; however, the findings from the ELEVATE study provide support for generalizing the results to a wider patient population with MG. The clinical benefits of complement C5 inhibitors are further supported by efficacy and safety results from the phase III trial of ravulizumab [15, 27]. In addition, a recently reported post-marketing surveillance study conducted in Japan over a period of more than 10 years in over 1000 patients with paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, or AChR Ab+ gMG confirmed that the safety profile of eculizumab in clinical practice was consistent with that in clinical trials [28]. Further information on the outcomes associated with eculizumab and ravulizumab treatment in clinical practice in patients with AChR Ab+ gMG is anticipated from the ongoing registry study (NCT04202341) [29, 30] and updated ELEVATE analyses. Insights from direct comparisons with other approved targeted MG therapies in clinical practice would also help guide treatment decisions. Such data are currently sparse; however, a small study of clinical-practice outcomes in Italy (n = 63), with a median follow-up time of 36 weeks, showed that patients treated with eculizumab were significantly less likely than those treated with efgartigimod (a neonatal Fc receptor blocker) to experience an MG deterioration/crisis or to suspend treatment [16]. In addition, patients treated with eculizumab had significantly greater reductions in the Quantitative Myasthenia Gravis total score and prednisone dose than those treated with efgartigimod.
Although the results reported here are based on medical record data from a wide range of clinical settings and geographic locations in the USA, there are some limitations. Physicians were required to record only data available in the electronic medical records; therefore, some events may have been underreported if they were not captured. In addition, patients had variable follow-up periods at the time of data cut-off; however, this was accounted for by reporting results as PPPY.
Conclusions
The findings from this study provide evidence of a beneficial impact of eculizumab treatment in reducing the rates of MG exacerbations and crises, associated HCRU, and the use of rescue treatment in diverse clinical-practice settings in the USA, suggesting a reduced socioeconomic burden. This furthers the observations from prior reports demonstrating the positive role of complement inhibitor therapy in patients with MG.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the investigators and patients who made this study possible. They also thank Sivani Paskaradevan (formerly Alexion, AstraZeneca Rare Disease), Korie Handwerger, Adrian Kielhorn, Jennifer Tyson, and Karen Yee (Alexion, AstraZeneca Rare Disease) for critical review of the manuscript; Hsing-Ting Yu and Jill Kaufman (both Cardinal Health) for their contributions to project management and data acquisition, respectively; Bela Bapat (formerly Cardinal Health) for help with study design; and Harlen Hays, Parisa Asgarisabet, Prathamesh D. Pathak, Sonali Dharurkar (all Cardinal Health), and Justin Lee (Alexion, AstraZeneca Rare Disease) for help with data analysis. Medical writing assistance in developing the manuscript under the direction of the authors was provided by Julie Ponting of Piper Medical Communications, funded by Alexion, AstraZeneca Rare Disease.
Declarations
Funding
Research funding for this study and funding for medical writing assistance and open-access fees were provided by Alexion, AstraZeneca Rare Disease.
Conflict of interest
Richard J. Nowak has received research support from Alexion, AstraZeneca Rare Disease, argenx, Genentech, Grifols, Immunovant, Inc., Momenta Pharmaceuticals, the Myasthenia Gravis Foundation of America, the National Institutes of Health (National Institute of Neurological Disorders and Stroke and National Institute of Allergy and Infectious Diseases), Ra Pharmaceuticals (now UCB), and Viela Bio Inc. (now Horizon Therapeutics); and consultancy fees from Alexion, AstraZeneca Rare Disease, argenx, Cour Pharmaceuticals, Grifols, Immunovant, Inc., Momenta Pharmaceuticals, Ra Pharmaceuticals (now UCB), Roivant Sciences, and Viela Bio (now Horizon Therapeutics). Ali A. Habib has served as a medical advisor and speaker on behalf of Alexion and AstraZeneca Rare Disease; he has received research support from Alexion, AstraZeneca Rare Disease, and Cabaletta Bio; and has received research support and honoraria from argenx, UCB, Pfizer, Genentech, Viela Bio, and Regeneron. Andrew J. Klink is a salaried employee of and owns stock in Cardinal Health, which received funding from Alexion, AstraZeneca Rare Disease for work performed on this study. Srikanth Muppidi has served on advisory board meetings for Alexion, AstraZeneca Rare Disease, argenx, Horizon Therapeutics, and Ra Pharmaceuticals (now UCB). Anju Parthan was an employee of Alexion, AstraZeneca Rare Disease, and owned stock in AstraZeneca at the time the study was conducted and analyzed. S. Chloe Sader is an employee of Alexion, AstraZeneca Rare Disease and owns stock in AstraZeneca. Alexandrina Balanean is a salaried employee of Cardinal Health, which received funding from Alexion, AstraZeneca Rare Disease for work performed on this study. Ajeet Gajra was a salaried employee of and owned stock in Cardinal Health, which received funding from Alexion, AstraZeneca Rare Disease for work performed on this study, at the time the study was conducted. James F. Howard Jr has received research support (paid to institution) from Ad Scientiam, Alexion, AstraZeneca Rare Disease, argenx, Cartesian Therapeutics, the Centers for Disease Control and Prevention (Atlanta, GA, USA), the Myasthenia Gravis Foundation of America, the Muscular Dystrophy Association, the National Institutes of Health (including the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases), PCORI, and UCB Pharma; honoraria from Alexion AstraZeneca Rare Disease, Amgen, argenx, Biohaven Ltd, Biologix Pharma, CheckRare CME, F. Hoffman-LaRoche, Medscape CME, Merck EMD Serono, NMD Pharma, Novartis Pharma, PeerView CME, Physicians’ Education Resource CME, PlatformQ CME, Regeneron Pharmaceuticals, Sanofi US, UCB Pharma, and Zai Lab; and non-financial support from Alexion, AstraZeneca Rare Disease, argenx, Biohaven Ltd, Toleranzia AB, UCB Pharma, and Zai Lab.
Ethics approval
The study was reviewed by the Western Institutional Review Board and determined exempt from requiring ethical approval on the basis that the study collected only secondary data, which were limited and comprised only necessary protected health information (e.g., treatment dates, date of death), and that all data were de-identified and aggregated.
Consent to participate
As this study involves anonymized structured data, which according to applicable legal requirements do not contain data subject to privacy laws, obtaining informed consent from patients was not required.
Consent for publication
Not applicable.
Availability of data and material
Alexion, AstraZeneca Rare Disease will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods such as data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study an informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://www.alexionclinicaltrialtransparency.com/data-requests/.
Code availability
Not applicable.
Author contributions
Study concept: RJN, AJK, SM, AP, SCS, AG. Study design: RJN, AJK, SM, AP, SCS, AB, AG, JFH Jr. Data acquisition: AAH, JFH Jr. Data analysis: RJN, AJK, AP, SCS, AB, AG. Data interpretation: all authors. Drafting/revision of the article for content: all authors. All authors read and approved the final version.
References
- 1.Schneider-Gold C, Hagenacker T, Melzer N, Ruck T. Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord. 2019;12:1756286419832242. 10.1177/1756286419832242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvestri NJ, Wolfe GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis. 2014;15(4):167–78. 10.1097/CND.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 3.Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16–22. 10.1177/1941875210382918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel-Nitz NM, Boscoe A, Wolbeck R, Johnson J, Silvestri NJ. Burden of illness in patients with treatment refractory myasthenia gravis. Muscle Nerve. 2018;58(1):99–105. 10.1002/mus.26114. [DOI] [PubMed] [Google Scholar]
- 5.Gummi RR, Kukulka NA, Deroche CB, Govindarajan R. Factors associated with acute exacerbations of myasthenia gravis. Muscle Nerve. 2019;60(6):693–9. 10.1002/mus.26689. [DOI] [PubMed] [Google Scholar]
- 6.Thomas CE, Mayer SA, Gungor Y, Swarup R, Webster EA, Chang I, et al. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. 1997;48(5):1253–60. 10.1212/wnl.48.5.1253. [DOI] [PubMed] [Google Scholar]
- 7.Desai R, Abbas SA, Fong HK, Lodhi MU, Doshi R, Savani S, et al. Burden and impact of takotsubo syndrome in myasthenic crisis: a national inpatient perspective on the under-recognized but potentially fatal association. Int J Cardiol. 2020;299:63–6. 10.1016/j.ijcard.2019.09.054. [DOI] [PubMed] [Google Scholar]
- 8.Heatwole C, Johnson N, Holloway R, Noyes K. Plasma exchange versus intravenous immunoglobulin for myasthenia gravis crisis: an acute hospital cost comparison study. J Clin Neuromuscul Dis. 2011;13(2):85–94. 10.1097/CND.0b013e31822c34dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips G, Abreu C, Goyal A, Li Y, Whangbo A, Gelinas D, et al. Real-world healthcare resource utilization and cost burden assessment for adults with generalized myasthenia gravis in the United States. Front Neurol. 2021;12: 809999. 10.3389/fneur.2021.809999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habib AA, Sacks N, Cool C, Durgapal S, Dennen S, Everson K, et al. Hospitalizations and mortality from myasthenia gravis: trends from 2 US national datasets. Neurology. 2024;102(2): e207863. 10.1212/wnl.0000000000207863. [DOI] [PubMed] [Google Scholar]
- 11.Howard JF Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976–86. 10.1016/s1474-4422(17)30369-1. [DOI] [PubMed] [Google Scholar]
- 12.Muppidi S, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019;60(1):14–24. 10.1002/mus.26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habib AA, Klink AJ, Muppidi S, Parthan A, Sader SC, Balanean A, et al. United States clinical practice experience with eculizumab in myasthenia gravis: symptoms, function, and immunosuppressant therapy use. J Neurol. 2024;271(9):6114–26. 10.1007/s00415-024-12569-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abuzinadah AR, Alanazy MH, Butt NS, Barohn RJ, Dimachkie MM. Exacerbation rate in generalized myasthenia gravis and its predictors. Eur Neurol. 2021;84(1):43–8. 10.1159/000512077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vu T, Meisel A, Mantegazza R, Annane D, Katsuno M, Aguzzi R, et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid. 2022;1: EVIDoa2100066. 10.1056/EVIDoa2100066. [DOI] [PubMed] [Google Scholar]
- 16.Pane C, Di Stefano V, Cuomo N, Sarnataro A, Vinciguerra C, Bevilacqua L, et al. A real-life experience with eculizumab and efgartigimod in generalized myasthenia gravis patients. J Neurol. 2024;271(9):6209–19. 10.1007/s00415-024-12588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katyal N, Narula N, Govindarajan R. Clinical experience with eculizumab in treatment-refractory acetylcholine receptor antibody-positive generalized myasthenia gravis. J Neuromuscul Dis. 2021;8(2):287–94. 10.3233/JND-200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landfeldt E, Pogoryelova O, Sejersen T, Zethraeus N, Breiner A, Lochmuller H. Economic costs of myasthenia gravis: a systematic review. Pharmacoeconomics. 2020;38(7):715–28. 10.1007/s40273-020-00912-8. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Li T, Wang Q, Xu A, Wu B. Post-traumatic stress disorder symptoms after respiratory insufficiency in patients with myasthenia gravis. Psychol Health Med. 2021;26(2):221–7. 10.1080/13548506.2020.1807577. [DOI] [PubMed] [Google Scholar]
- 20.Law N, Davio K, Blunck M, Lobban D, Seddik K. The lived experience of myasthenia gravis: a patient-led analysis. Neurol Ther. 2021;10(2):1103–25. 10.1007/s40120-021-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann B, Angstwurm K, Mergenthaler P, Kohler S, Schonenberger S, Bosel J, et al. Myasthenic crisis demanding mechanical ventilation: a multicenter analysis of 250 cases. Neurology. 2020;94(3):e299–313. 10.1212/WNL.0000000000008688. [DOI] [PubMed] [Google Scholar]
- 22.Souayah N, Nasar A, Suri MF, Kirmani JF, Ezzeddine MA, Qureshi AI. Trends in outcomes and hospitalization charges among mechanically ventilated patients with myasthenia gravis in the United States. Int J Biomed Sci. 2009;5(3):209–14. [PMC free article] [PubMed] [Google Scholar]
- 23.Rodnitzky RL, Goeken JA. Complications of plasma exchange in neurological patients. Arch Neurol. 1982;39(6):350–4. 10.1001/archneur.1982.00510180028007. [DOI] [PubMed] [Google Scholar]
- 24.Mokrzycki MH, Kaplan AA. Therapeutic plasma exchange: complications and management. Am J Kidney Dis. 1994;23(6):817–27. 10.1016/s0272-6386(12)80135-1. [DOI] [PubMed] [Google Scholar]
- 25.Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence-based indications and safety profile. Pharmacol Ther. 2004;102(3):177–93. 10.1016/j.pharmthera.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;9:1299. 10.3389/fimmu.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meisel A, Annane D, Vu T, Mantegazza R, Katsuno M, Aguzzi R, et al. Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. J Neurol. 2023;270(8):3862–75. 10.1007/s00415-023-11699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura JI, Kawaguchi T, Ito S, Murai H, Shimono A, Matsuda T, et al. Real-world safety profile of eculizumab in patients with paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, or generalized myasthenia gravis: an integrated analysis of post-marketing surveillance in Japan. Int J Hematol. 2023;118(4):419–31. 10.1007/s12185-023-03630-x. [DOI] [PubMed] [Google Scholar]
- 29.Greene E, Cutter G, Muppidi S, Juel V, Rodrigues E, Korideck H, et al. Myasthenia gravis activities of daily living (MG-ADL) response to eculizumab treatment in patients from the generalized myasthenia gravis registry (P1–5.020). Neurology. 2023;100(17 Suppl. 2):2071. 10.1212/wnl.0000000000202295. [Google Scholar]
- 30.Pulley M, Macwan S, Nowak R, Mozaffar T, Rodrigues E, Korideck H, et al. Change in concomitant therapies for generalized myasthenia gravis in patients receiving eculizumab: a retrospective analysis of registry data (P1-5.002). Neurology. 2023;100(17 Suppl. 2):2183. 10.1212/wnl.0000000000202372. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.