Abstract
Background
There is a need for published data on real-world use of SB5, an adalimumab (ADL) biosimilar approved in Europe in 2017, on the basis of evidence from pre-clinical and analytic data as well as phase I and III clinical studies demonstrating equivalent efficacy and comparable pharmacokinetics, safety and immunogenicity profiles as the reference ADL.
Objectives
The purpose of this study was to estimate patient persistence on SB5 at 12 months post-initiation using clinical and healthcare claims data from the French Système National des Données de Santé (national healthcare claims database, SNDS) in addressing data gaps.
Methods
PERFUSE is a 12-month, observational, multi-centre cohort study of patients with rheumatic or gastrointestinal immune-mediated inflammatory diseases (IMIDs) who initiated routine SB5 treatment between October 2018 and October 2020, either as their first ADL (naïve) or transitioning from another ADL (switched). Clinical data, including disease activity scores, C-reactive protein levels, and dosing information, were collected as available from patient records captured during routine visits to specialist physicians. Persistence data were supplemented with data from the French national healthcare claims database (SNDS). Analyses of clinical data were descriptive, while persistence was assessed using a Kaplan–Meier survival analysis.
Results
Overall, 911 patients were included: 507 from rheumatology centres [116 with rheumatoid arthritis (RA), 78 psoriatic arthritis (PsA), and 313 ankylosing spondylitis (AS)] and 404 from gastroenterology centres [316 with Crohn’s disease (CD) and 88 ulcerative colitis (UC)]. Among naïve patients, 12-month remission/low activity rates were 58% for RA, 66% for PsA, 59% for AS, 94% for CD, and 85% for UC, increasing significantly from baseline for all indications (p < 0.05). Switched patients’ remission rates remained stable between baseline and month 12 (M12) for all indications (p > 0.05). Persistence (95% CI) at M12 among naïve patients was 59% (46.5, 68.8) for RA, 65% (49.7, 77.1) for PsA, 56% (48.3, 62.6) for AS, 70% (63.0, 75.7) for CD, and 42% (30.7, 53.1) for UC, compared to 60% (42.7, 73.7) for RA, 57% (37.3, 72.1) for PsA, 55% (45.8, 64.0) for AS, 63% (53.4, 71.7) for CD, and 56% (27.2, 77.6) for UC among switched patients. No significant differences were observed between naïve and switched patients (p > 0.05). SNDS pairing provided information on 68 of the 132 patients (52%) who were lost to follow-up in the clinical database, of whom 57 (84%) were confirmed persistent at M12 and 11 (16%) non-persistent. Primary treatment failure (naïve patients) and patient decision (switched patients) were the most common reasons stated for treatment discontinuation.
Conclusions
SB5 provides clinically effective treatment of both gastrointestinal and rheumatic IMIDs for naïve and switched patients, with no loss of control observed when switching. Persistence was comparable between naïve and switched populations, though the reasons for non-persistence differed.
Trial Registry
Trial registration number: Clinical Trials identifier NCT03662919. Trial registration date: 10 September 2018.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-024-00459-6.
Key Points
| SB5 provides effective treatment for both adalimumab-naïve and switched patients with rheumatic or gastrointestinal immune-mediated inflammatory diseases, with no loss of control observed among switched patients. |
| SB5 persistence at 12 months post-initiation was between 55 and 70%, except for naïve patients with ulcerative colitis, for whom it was approximately 40%. No significant differences in persistence were found between naïve and switched patients (log-rank test). |
| The main reasons for treatment discontinuation were primary treatment failure for naïve patients and patient decision for switched patients. |
Introduction
Immune-mediated inflammatory diseases (IMIDs) form a clinically diverse group of disorders affecting between 3 and 7% of the population in whom altered immune regulation causes chronic organ-specific and systemic inflammation [1, 2]. While they may present some common pathogenesis features, they also present unique pathways which define their clinical phenotype, localization, distribution and response profile. Indeed, IMIDs include rheumatoid arthritis (RA), the spondyloarthritis (SpA) family of diseases, psoriatic arthritis (PsA) and inflammatory bowel disease (IBD), such as Crohn’s disease (CD) or ulcerative colitis (UC), amongst others. IMIDs present highly significant therapeutic challenges as they remain incurable, patients often present with comorbidities, and both the primary disease and its comorbid conditions can have a significant negative impact on quality of life [1].
Treatment of IMIDs, which have historically concentrated on limiting the inflammation and the damage it causes, has evolved thanks to the development of biologics such as adalimumab, which selectively inhibit specific steps in inflammation pathways, allowing for more targeted treatments [3, 4]. Adalimumab (ADL), a tumour necrosis factor (TNF)-inhibitor which competitively binds to both soluble and transmembrane forms of TNF, thus inactivating the proinflammatory cytokine TNF by direct neutralisation, is an effective treatment of IMIDs [5–8]. However, biologics present certain drawbacks, chiefly their cost. Thus, less costly but clinically equivalent biosimilars have been developed, expanding treatment options and access for patients with IMID [9–11]. Biosimilar approval by the European Medicines Agency (EMA) requires proof of equivalence in terms of efficacy, safety and immunogenicity to the reference biologic. Approval can be extrapolated to all labelled indications on the basis of results from clinical studies in a single indication. This confers particular importance upon the role of phase IV studies in understanding the real-world use of biosimilars and providing further data on their long-term utilisation, especially in indications other than the one which served for the authorisation process.
This study focusses on SB5, an ADL biosimilar approved by the EMA in 2017 on the basis of evidence from pre-clinical and analytic data, and phase I and III clinical studies in patients with moderate-to-severe RA, which demonstrated equivalent efficacy and comparable pharmacokinetics, safety and immunogenicity profiles as the reference ADL [12, 13]. To date, there is little published evidence on the real-world use of SB5 in patients who are either ADL-naïve or switched from reference ADL or from another ADL biosimilar, and new information is welcomed by physicians and scientific organisations alike [14]. Indeed, real-world studies, using routine healthcare data, can capture the outcomes of treatments on a broader range of patients than interventional trials, complementing the controlled settings of a randomised clinical trial.
The PERFUSE study (NCT03662919) is a 12-month observational study of patients diagnosed with rheumatic or gastrointestinal IMIDs [specifically RA, PsA, ankylosing spondylitis (AS), CD and UC] who received SB5 as part of routine therapy at French specialist sites. The study provides real-world evidence on the use of SB5.
This manuscript presents the persistence, effectiveness and safety results for the overall population of the PERFUSE study adult cohort (both ADL-naïve and switched) up to 12 months post-SB5 initiation.
Methods
Study Design and Population
The PERFUSE study (NCT03662919) is a 12-month, observational, multi-centre cohort study of patients with gastrointestinal and rheumatic IMIDs, prescribed ADL biosimilar SB5 as part of routine therapy.
Enrolled patients met all the following criteria: (i) physician-confirmed diagnosis of RA, PsA or AS for the rheumatology cohort or of CD or UC for the gastroenterology cohort (ii) aged ≥ 18 years old at treatment initiation, (iii) could understand the information provided and complete questionnaires in French and (iv) initiated routine SB5 treatment between October 2018 and October 2020.
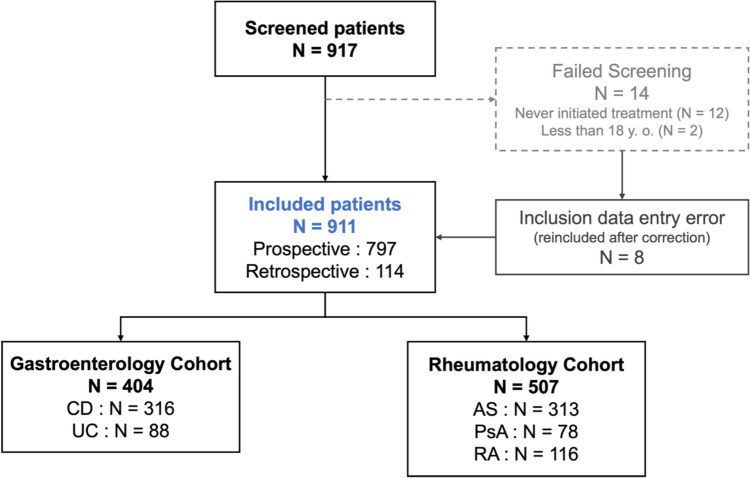
Patients with a primary diagnosis of psoriasis, juvenile idiopathic arthritis, uveitis or hidradenitis suppurativa, as well as women of childbearing potential intending to become pregnant during study follow-up and patients who could not comply with the study protocol for the duration of follow-up (i.e. patients unable to attend regular on-site check-ups or who planned to change follow-up site during the study period), were excluded. The study flowchart is shown in Fig. 1.
Fig. 1.
Study flowchart
All eligible patients were enrolled and followed by French specialist sites (tertiary care hospitals).
The primary outcome measure was SB5 treatment persistence at month 12, as reported by physicians. Secondary outcomes included a description of SB5 treatment dose, injection frequency, effectiveness, immunogenicity and safety over the same period. The data for this analysis were extracted from the study database on 18 July 2022.
Information from the study database was augmented using data from the French National healthcare claims database (Système National des Données de Santé, SNDS). The SNDS contains data on all claims submitted to the French National Health Insurance (Caisse Nationale de l’Assurance Maladie, CNAM), including dispensations and prescriptions of ADL and other biologics.
Patients were identified in the SNDS using a probabilistic pairing algorithm on the basis of available study data (age, sex, diagnoses, certain co-treatments, visit dates and location, and SB5 delivery dates). The study-specific pairing algorithm was developed and tested by the experts at the CNAM.
Data Collection
Clinical Data Collection Timepoints
Data collection timepoints were flexible and adapted to the standards of care in France: baseline (M0 = SB5 initiation), month 6 (M6 = 4–8 months) and month 12 (M12 = 10–15 months) post-initiation of SB5. In addition to the data described below, the baseline visit collected data on patients’ comorbidities, co-treatments and prior biologic treatments. To collect these data, disease-specific lists of pertinent items were designed with the collaboration of the study steering committee.
All clinical data in PERFUSE were collected retrospectively and/or prospectively, as available from patient records as part of routine clinical practice. As such, study visits coincided with routine follow-up visits and there were no protocol-specified assessments or procedures. Thus, data density and collection reflect the standards of care in France.
SB5 Treatment Persistence
Persistence data (i.e. patient continued use of SB5, or if applicable, date and reason of treatment discontinuation) were collected at each available collection timepoint.
Investigators specified the reason for discontinuation of SB5 according to pre-defined categories: patient decision, primary failure (defined as treatment failure observed within 14 weeks of SB5 initiation), secondary failure (defined as treatment failure observed more than 14 weeks after initiating SB5, regardless of response during the first 14 weeks), adverse event and prolonged remission. Other reasons which did not fit into the above categories were collectively labelled ‘other’. Physicians could select only one reason.
The following SNDS data for patients with a probability of an accurate pairing ≥ 80% (according to the pairing algorithm) were included in the persistence calculations: date of first SB5 dispensation, date of last available SB5 dispensation and if applicable, date and type of subsequent biologic or biosimilar therapy. SNDS data covered October 2018 to December 2021. SNDS data were primarily used to determine the status of patients for whom no data were available in physician records at M12 (i.e. censored patients): if a dispensation or prescription of SB5 was found in the SNDS after month 12, then the patient’s continued use of SB5 at month 12 is confirmed. Conversely, if a dispensation or prescription of medication incompatible with prescription of SB5 was found before month 12, then the patient was considered to have stopped SB5 before month 12. Finally, if no such data were found, the patient was considered lost to follow-up before month 12.
Disease Activity Measures
Treatment posology was collected at each available collection timepoint and patients’ response to treatment was assessed using results from routine C-reactive protein (CRP) assays as well as disease activity scores routinely used in practice and available in patients’ records. In the rheumatology cohort, the Disease Activity Score 28-joint count (DAS28), using either erythrocyte sedimentation rate (DAS28-ESR) or CRP concentration (DAS28-CRP), was used to assess disease activity in patients with both RA and PsA [15, 16] and the Bath Ankylosing Spondylitis Disease Activity index (BASDAI) was used for patients with both PsA and AS [17]. In the gastroenterology cohort, the Harvey–Bradshaw index (HBI) and Simple Clinical Colitis Activity index (SCCAI) were used for patients with CD and UC, respectively [18, 19].
Activity scores were calculated using appropriate scoring guidelines (i.e. Fleischmann et al. for the DAS28-ESR/CRP [20]; Garrett et al. for the BASDAI [17]; Harvey and Bradshaw for the HBI [18]; Walmsley et al. for the SCCAI scoring [19]; Jowett et al. for SCCAI cut-offs [21]) and categorised by disease status (high/moderate/low disease activity or remission). Cut-offs and ranges for categorical scoring are provided in Supplementary Table S1.
Safety Analysis
Safety outcomes included treatment-emergent adverse events (TEAEs) related to SB5 treatment and serious adverse events (SAEs), both related and unrelated to treatment. All adverse events (AEs) were coded using the Medical Dictionary of Regulatory Activities (MedDRA 24.1).
Immunogenicity testing based on the detection of serum anti-drug antibodies (ADAs) was performed using Lisa Tracker ELISA kits (Theradiag, Croissy-Beaubourg, France) [22]. The reasons for performing or not performing ADA testing were not reported.
Statistical Analysis
The study sample comprised all eligible patients who were seen and enrolled by sites during the 25-month inclusion period and reflects the proportions of patients seen in clinical practice. The full analysis set (FAS) is defined as all eligible enrolled patients with available baseline data (i.e. collected during the M0 visit). Results were stratified by indication (RA, PsA, AS, CD and UC) and by history of prior ADL treatment (ADL-naïve or switched from reference ADL or from another biosimilar ADL).
The primary outcome was analysed using a Kaplan–Meier (KM) survival analysis: the proportion of patients who were still treated with SB5 at M12, with secondary endpoints at months 3 (M3), 6 (M6) and 9 (M9). KM estimates of the mean and corresponding 95% confidence interval (CI) are presented. For the KM survival plots, patients switched from the reference ADL and those switched from another biosimilar ADL were analysed separately. SNDS data were used to complement the clinical database and confirm patient persistence at 12 months.
Continuous variables are reported as mean, standard deviation, minimum, first quartile (Q1), median, third quartile (Q3), maximum and 95% two-sided CIs, as appropriate. Categorical variables are summarised as frequencies and percentages.
Time series analysis of disease activity scores is presented as the difference between the values at the final timepoint (M12) and at baseline (M0) when data are available at both timepoints. Changes from baseline are considered significant when the two-sided 95% CIs do not cross zero. As disease activity scores were captured only at baseline and during routine follow-up visits, no imputation or replacement of missing values was performed.
Normality of distributions was assessed using a Shapiro–Wilk test. Differences between distributions of continuous variables were assessed using Student’s t-test when variables were normally distributed or a Wilcoxon–Mann–Whitney test otherwise. Proportions of categorical variables were compared using a chi-squared test for proportions. Differences are considered significant for p < 0.05 and are reported using stars on the relevant graphs.
SAS version 9.4 (Cary, North Carolina, USA) and Microsoft Excel (Microsoft, Washington, USA) were used for the statistical analysis.
Ethical Considerations
The study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki, Good Clinical Practice and all applicable laws or regulations.
The final versions of the study protocol, patient information material, patient and physician questionnaires and consent forms as well as any other written information to be provided to patients were approved by an independent ethics committee (Comité de Protection des Personnes, CPP) in France, in accordance with French regulations, on 25 April 2019.
Results
Included Population and Baseline Characteristics
The PERFUSE study included 404 patients (44.2%) in the gastroenterology cohort (316 patients with CD and 88 patients with UC) and 507 patients (55.8%) in the rheumatology cohort (116 patients with RA, 78 patients with PsA and 313 patients with AS), for a total population of 911 patients with IMID. Data on 797 patients (87.5%) were captured prospectively, with the rest being entered retrospectively from historical patient records (Fig. 1). SNDS data were available for 274 (30.1%) patients overall. Successful pairing rates ranged from 23.1% (n = 18) in the PsA cohort to 35.8% (n = 113) in the CD cohort (Supplementary Table S2).
Patients with RA were older on average than patients in all other indications (56.6 years), and patients with UC were the youngest (37.2 years). The RA cohort was predominantly female (n = 88, 75.9%), whereas sex in other indications was either evenly distributed (PsA) or predominantly male (AS, CD and UC). Baseline comorbidity rates ranged from 32.4% (n = 24) in naïve patients with UC to 56.7% (n = 17) in switched patients with PsA. Concomitant medication use was higher among naïve patients, reaching 84.4% among naïve patients with RA. Switched patients were slightly older than patients in the naïve cohort and had a longer disease duration at baseline, with the largest difference observed for patients with RA (6.7 years versus 20.3 years in naïve versus switched patients respectively). The percentage of patients in remission/low disease activity at baseline was significantly higher among switched patients (p < 0.01 for all indications).
Detailed baseline characteristics are presented in Tables 1 and 2 for naïve and switched patients, respectively. Complete lists of baseline comorbidities and co-treatments are provided in Supplementary Tables S3A and S3B for the rheumatology and gastroenterology cohorts, respectively.
Table 1.
Baseline characteristics—Naïve population
| Naïve patients | Rheumatology cohort | Gastroenterology cohort | |||
|---|---|---|---|---|---|
| RA N = 77 |
PsA N = 48 |
AS N = 194 |
CD N = 208 |
UC N = 74 |
|
| Data capturing, n (%) | |||||
| Fully prospective | 66 (85.7%) | 39 (81.3%) | 170 (87.6%) | 189 (90.9%) | 67 (90.5%) |
| Shifted prospective | 7 (9.1%) | 2 (4.2%) | 7 (3.6%) | 6 (2.9%) | 1 (1.4%) |
| Fully retrospective | 4 (5.2%) | 7 (14.6%) | 17 (8.8%) | 13 (6.3%) | 6 (8.1%) |
| Age, years, mean (SD) | 54.4 (13.1) | 51.5 (12.3) | 44.9 (14.3) | 36.8 (14.0) | 37.1 (14.9) |
| Female patients, n (%) | 58 (75.3%) | 29 (60.4%) | 89 (45.9%) | 113 (54.3%) | 33 (44.6%) |
| Weight, kg, mean (SD) | 70.9 (13.9) | 79.1 (16.3) | 77.3 (17.0) | 67.3 (15.1) | 71.5 (14.5) |
| Height, cm, mean (SD) | 165.2 (8.6) | 169.0 (11.7) | 170.9 (8.9) | 170.1 (9.4) | 173.1 (8.8) |
| BMI, kg/m2, mean (SD) | 25.9 (4.9) | 27.7 (5.6) | 26.4 (5.5) | 23.2 (4.5) | 23.8 (4.1) |
| Disease duration, years, mean (SD) | 6.7 (6.8) | 5.5 (7.2) | 6.9 (9.0) | 7.9 (9.3) | 6.9 (8.2) |
| Patients with comorbidities at baseline, n (%)a | 26 (33.8%) | 26 (54.2%) | 83 (42.8%) | 74 (35.6%) | 24 (32.4%) |
| Patients with co-treatments at baseline, n (%)a | 65 (84.4%) | 28 (58.3%) | 35 (18.0%) | 108 (51.9%) | 54 (73.0%) |
| Prior exposure to other biologics, n (%) | |||||
| Infliximab | 1 (1.3%) | 1 (2.1%) | 16 (8.2%) | 33 (15.9%) | 4 (5.4%) |
| Etanercept | 13 (16.9%) | 10 (20.8%) | 31 (16.0%) | – | – |
| Ustekinumab | – | 3 (6.3%) | – | 8 (3.8%) | 1 (1.4%) |
| Vedolizumab | – | – | – | 7 (3.4%) | 2 (2.7%) |
| Golimumab | 2 (2.6%) | 1 (2.1%) | 19 (9.8%) | 1 (0.5%) | 2 (2.7%) |
| Certolizumab pegol | 5 (6.5%) | 2 (4.2%) | 10 (5.2%) | – | – |
| Abatacept | 4 (5.2%) | – | 1 (0.5%) | – | – |
| Tocilizumab | 5 (6.5%) | 1 (2.1%) | – | – | – |
| Rituximab | 1 (1.3%) | – | – | – | – |
| Secukinumab | – | 6 (12.5%) | 11 (5.7%) | – | – |
| Sarilumab | 1 (1.3%) | – | – | – | – |
| Ixekizumab | – | – | 2 (1.0%) | – | – |
| JAK inhibitors | 12 (15.6%) | – | 1 (0.5%) | – | 2 (2.7%) |
| Patients taking methotrexate at baseline, n (%) | 49 (63.6%) | 22 (45.8%) | 26 (13.4%) | 13 (6.3%) | 5 (6.8%) |
| Patients taking azathioprine at baseline, n (%) | – | – | – | 56 (26.9%) | 18 (24.3%) |
| Number of patients with disease activity scores at baseline, n (%) |
DAS28-ESR 23 (29.9%) DAS28-CRP 29 (37.7%) |
BASDAI 19 (39.6%) DAS28-ESR 14 (29.2%) DAS28-CRP 8 (16.7%) |
BASDAI 117 (60.3%) |
HBI 137 (65.9%) |
SCCAI 43 (58.1%) |
| Categorical disease activity scores, n (%) | |||||
| Remission | 1 (1.9%) | 5 (12.2%) | N/A | 83 (60.6%) | N/A |
| Low/mild disease activity | 5 (9.6%) | 4 (9.8%) | 23 (19.7%) | 33 (24.1%) | 19 (44.2%) |
| Moderate disease activity | 30 (57.7%) | 10 (24.4%) | N/A | 20 (14.6%) | N/A |
| High disease activity | 16 (30.8%) | 22 (53.7%) | 94 (80.3%) | 1 (0.7%) | 24 (55.8%) |
AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity index, CD Crohn’s disease, CRP C-reactive protein, DAS28 Disease Activity Score (28 joint), ESR erythrocyte sedimentation rate, HBI Harvey–Bradshaw index, N/A not applicable, PsA psoriatic arthritis, RA rheumatoid arthritis, SD standard deviation, SCCAI Simple Clinical Colitis Activity index, shifted prospective part prospective/part retrospective, UC ulcerative colitis
aFull listings of comorbidities and co-treatments reported at baseline are provided in Supplementary Tables S3A and S3B
Table 2.
Baseline characteristics—switched population
| Switched patientsa | Rheumatology cohort | Gastroenterology cohort | |||
|---|---|---|---|---|---|
| RA N = 39 |
PsA N = 30 |
AS N = 119 |
CD N = 108 |
UC N = 14 |
|
| Data capturing, n (%) | |||||
| Fully prospective | 30 (76.9%) | 21 (70.0%) | 82 (68.9%) | 72 (66.7%) | 11 (78.6%) |
| Shifted prospective | 2 (5.1%) | 2 (6.7%) | 7 (5.9%) | 14 (13.0%) | 2 (14.3%) |
| Fully retrospective | 7 (17.9%) | 7 (23.3%) | 30 (25.2%) | 22 (20.4%) | 1 (7.1%) |
| Age, years, mean (SD) | 60.7 (12.2) | 52.8 (13.6) | 48.0 (13.3) | 43.0 (14.3) | 37.9 (11.7) |
| Female patients, n (%) | 30 (76.9%) | 11 (36.7%) | 45 (37.8%) | 44 (40.7%) | 5 (35.7%) |
| Weight, kg, mean (SD) | 68.3 (12.7) | 80.4 (16.7) | 76.1 (16.4) | 75.2 (17.7) | 73.0 (19.1) |
| Height, cm, mean (SD) | 163.8 (9.4) | 170.8 (8.3) | 169.8 (9.7) | 170.9 (8.9) | 171.1 (9.5) |
| BMI, kg/m2, mean (SD) | 25.4 (4.5) | 27.7 (5.6) | 26.5 (5.7) | 25.7 (5.3) | 24.7 (5.2) |
| Disease duration, years, mean (SD) | 20.3 (12.4) | 11.4 (8.2) | 14.3 (12.4) | 12.9 (9.3) | 9.6 (7.8) |
| Patients with comorbidities at baseline, n (%)A | 20 (51.3%) | 17 (56.7%) | 58 (48.7%) | 42 (38.9%) | 3 (21.4%) |
| Patients with co-treatments at baseline, n (%)A | 23 (59.0%) | 8 (26.7%) | 10 (8.4%) | 20 (18.5%) | 2 (14.3%) |
| Prior adalimumab, n (%) | |||||
| Reference adalimumab only | 37 (94.9%) | 29 (96.7%) | 113 (95.0%) | 103 (95.4%) | 13 (92.9%) |
| Biosimilar adalimumab only | 0 (0.0%) | 1 (3.3%) | 3 (2.5%) | 2 (1.9%) | 0 (0.0%) |
| Reference and biosimilar adalimumab | 2 (5.1%) | 0 (0.0%) | 3 (2.5%) | 3 (2.7%) | 1 (7.1%) |
| Prior exposure to other biologics, n (%) | |||||
| Infliximab | 1 (2.6%) | 3 (10.0%) | 5 (4.2%) | 19 (17.6%) | 3 (21.4%) |
| Etanercept | 2 (5.1%) | 3 (10.0%) | 14 (11.8%) | 1 (0.9%) | – |
| Ustekinumab | – | – | – | 1 (0.9%) | – |
| Golimumab | – | 1 (3.3%) | – | – | – |
| Certolizumab pegol | 1 (2.6%) | 2 (6.7%) | 2 (1.7%) | – | – |
| Tocilizumab | 1 (2.6%) | – | – | – | – |
| Secukinumab | – | – | 1 (0.8%) | – | – |
| JAK inhibitors | 1 (2.6%) | – | – | – | – |
| Patients taking methotrexate at baseline, n (%) | 18 (46.2%) | 6 (20.0%) | 6 (5.0%) | 5 (4.6%) | – |
| Patients taking azathioprine at baseline, n (%) | – | – | – | 8 (7.4%) | – |
| Number of patients with disease activity scores at baseline, n (%) |
DAS28-ESR 14 (35.9%) DAS28-CRP 5 (12.8%) |
BASDAI 8 (26.7%) DAS28-ESR 3 (10.0%) DAS28-CRP 4 (13.3%) |
BASDAI 57 (47.9%) |
HBI 81 (75.0%) |
SCCAI 8 (57.1%) |
| Categorical disease activity scores, n (%) | |||||
| Remission | 15 (78.9%) | 3 (20.0%) | N/A | 68 (84.0%) | N/A |
| Low/mild disease activity | – | 9 (60.0%) | 36 (63.2%) | 7 (8.6%) | 8 (100%) |
| Moderate disease activity | 3 (15.8%) | 2 (13.3%) | N/A | 6 (7.4%) | N/A |
| Data capturing, n (%) | |||||
| Fully prospective | 30 (76.9%) | 21 (70.0%) | 82 (68.9%) | 72 (66.7%) | 11 (78.6%) |
| High disease activity | 1 (5.3%) | 1 (6.7%) | 21 (36.8%) | – | – |
AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity index, CD Crohn’s disease, CRP C-reactive protein, DAS28 Disease Activity Score (28 joint), ESR erythrocyte sedimentation rate, HBI Harvey–Bradshaw index, N/A not applicable, PsA psoriatic arthritis, RA rheumatoid arthritis, SD standard deviation, SCCAI Simple Clinical Colitis Activity index, shifted prospective part prospective/part retrospective, UC ulcerative colitis
aBoth from reference and from biosimilar adalimumab
AFull listings of comorbidities and co-treatments reported at baseline are provided in Supplementary Tables S3A & S3B
Response to Treatment
Treatment Posology
Reported treatment posologies for each cohort are presented in Table 3.
Table 3.
Treatment posology by population and by timepoint; the most frequent posology for each population at each time point is bolded
| Posology, N (%) | Rheumatology cohort | Gastroenterology cohort | |||
|---|---|---|---|---|---|
| RA | PsA | AS | CD | UC | |
| Baseline (M0) | |||||
| Naïve, N | 77 | 48 | 194 | 208 | 74 |
|
40 mg every week 40 mg Q2W 80 mg Q2W 40 mg Q3W 40 mg Q4W 80 mg at week 0 followed by 40 mg at week 2 160 mg at week 0 followed by 80 mg at week 2 |
3 (3.9%) 74 (96.1%) – – – – – |
– 48 (100%) – – – – – |
2 (1.0%) 190 (97.9%) 1 (0.5%) – 1 (0.5%) – – |
1 (0.5%) 18 (8.6%) – 1 (0.5%) 1 (0.5%) 10 (4.8%) 177 (84.6%) |
– 2 (2.7%) – – – 3 (4.1%) 69 (93.2%) |
| Switcheda, N | 39 | 29 | 112 | 108 | 13 |
|
40 mg every week 40 mg Q2W 80 mg Q2W 40 mg Q3W 40 mg Q4W 160 mg at week 0 followed by 80 mg at week 2 |
– 29 (76.3%) – 3 (7.9%) 6 (15.8%) – |
2 (6.9%) 22 (75.9%) – 3 (10.3%) 2 (6.9%) – |
4 (3.6%) 93 (83.0%) – 8 (7.1%) 7 (6.3%) – |
33 (25.6%) 73 (56.6%) 1 (0.8%) – – 1 (0.8%) |
2 (14.3%) 11 (78.6%) – – – – |
| Month 12 (M12) | |||||
| Naïve, N | 47 | 27 | 107 | 132 | 30 |
|
40 mg every week 40 mg Q2W 80 mg Q2W 40 mg Q3W 40 mg Q4W |
– 47 (100%) – – – |
3 (11.1%) 24 (88.9%) – – – |
4 (3.7%) 100 (93.5%) – 1 (0.9%) 2 (1.9%) |
26 (19.7%) 102 (77.3%) 4 (3.0%) – – |
10 (33.3%) 17 (56.7%) 2 (6.7%) – 1 (3.3%) |
| Switcheda, N | 25 | 17 | 61 | 66 | 6 |
|
40 mg every week 40 mg Q2W 80 mg Q2W 40 mg Q3W 40 mg Q4W |
1 (4.0%) 18 (72.0%) – 1 (4.0%) 5 (20.0%) |
1 (5.9%) 12 (70.6%) – 2 (11.8%) 2 (11.8%) |
5 (8.2%) 45 (73.8%) – 8 (13.1%) 3 (4.9%) |
28 (42.5%) 36 (54.5%) 1 (1.5%) 1 (1.5%) – |
1 (16.7%) 5 (83.3%) – – – |
AS ankylosing spondylitis, CD Crohn’s disease, CRP C-reactive protein, PsA psoriatic arthritis, Q2W every 2 weeks, Q3W every 3 weeks, Q4W every 4 weeks, RA rheumatoid arthritis, UC ulcerative colitis
aBoth from reference and from biosimilar adalimumab
In the rheumatology cohort, most naïve patients initiated SB5 on a maintenance dose of 40 mg every 2 weeks (Q2W), with only seven patients having a different regimen: five received weekly injections, one received injections less frequently and one patient received 80 mg Q2W. This pattern was largely unchanged at M12, with all but ten patients receiving 40 mg Q2W: seven weekly, and three every 3 or 4 weeks. In switched rheumatology patients, the proportion of patients receiving 40 mg Q2W was lower, both at baseline and at 12 months, with more patients receiving 40 mg injections less frequently (between 13.4% for patients with AS at baseline and 24% for patients with RA at 12 months).
In the gastroenterology cohort, most naïve patients initiated SB5 on a conventional induction regimen (i.e. 160 mg at week 0 followed by 80 mg at week 2), with 13 patients initiating on 80 mg at week 0 followed by 40 mg at week 2, and 20 starting on 40 mg Q2W. By M12, most were on 40 mg Q2W, with 36 receiving weekly 40 mg injections, 1 receiving 40 mg Q4W and 6 receiving 80mg Q2W.
Switched gastroenterology patients most often initiated SB5 on a conventional maintenance regimen (i.e. 40 mg Q2W), with 35 receiving weekly 40 mg injections, 1 receiving 80mg Q2W and 1 receiving 160 mg at week 0 followed by 80 mg at week 2. At M12, most remained on 40 mg Q2W, 29 received a weekly 40 mg injections, 1 received 80 mg Q2W and 1 received 40 mg every 3 weeks.
Disease Activity Scores
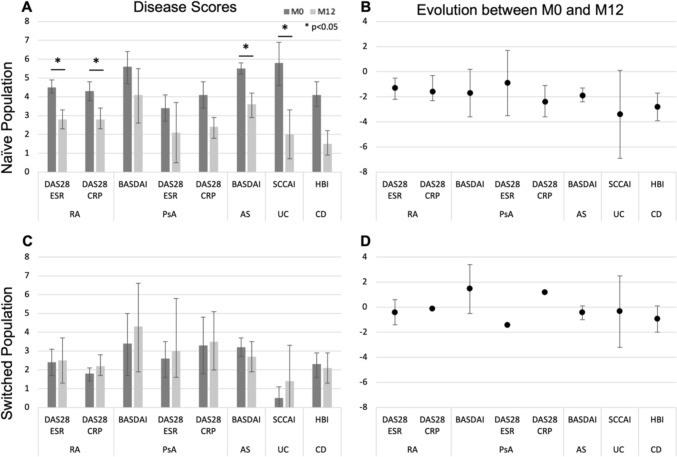
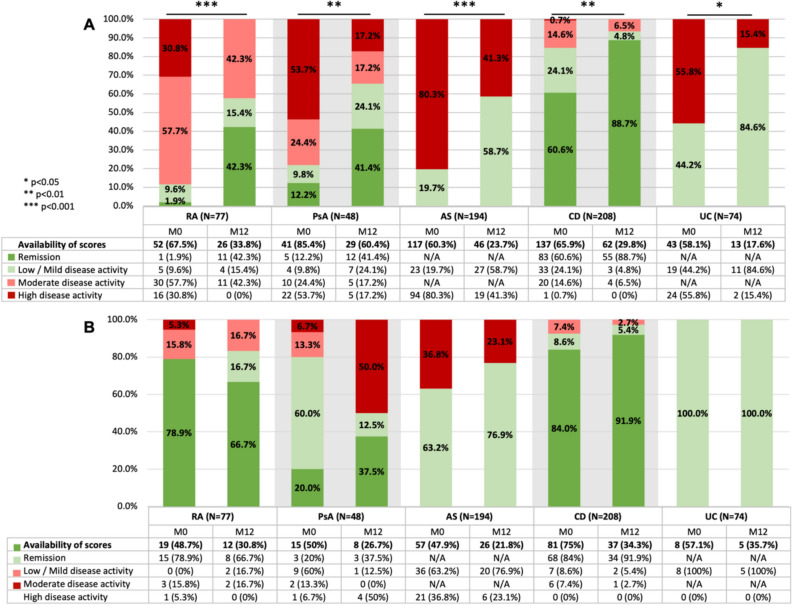
Quantitative baseline and M12 disease activity scores are presented in detail in Supplementary Table S4 and illustrated in Fig 2. Categorical disease activity scores are presented in Fig. 3.
Fig. 2.
Evolution of quantitative disease activity scores between baseline (M0, dark grey) and 12 months (M12, light grey). Error bars are 95% confidence intervals. Statistically significant (p < 0.05) population-level differences are presented with a star. A Quantitative disease activity scores for the M0 and M12 naïve populations. Results are presented for all patients with data at each timepoint. B Evolution of naïve patients’ disease activity scores between M0 and M12. Results are presented for all patients with data at both timepoints. C Quantitative disease activity scores for the M0 and M12 switched populations. Results are presented for all patients with data at each timepoint. D Evolution of switched patients’ disease activity scores between M0 and M12. Results are presented for all patients with data at both timepoints
Fig. 3.
Categorical disease activity scores at baseline (M0) and 12 months (M12). Different scores used for a single population were grouped together. Statistically significant differences in proportions are indicated with stars on the graph. A Naïve populations. B Switched populations. Categories and cut-offs are presented in Supplementary Table S1
ADL-naïve patients’ disease activity scores all decreased between baseline and M12, though not always significantly. Specifically, the proportion of patients in remission or with low disease activity increased significantly for all naïve cohorts between baseline and M12, with the greatest difference being observed in the PsA cohort (Fig. 3A). Quantitatively, significant decreases were observed in the RA (DAS28-ESR and DAS28-CRP, both p < 0.05), AS (BASDAI, p < 0.01) and CD (HBI, p < 0.01) cohorts, respectively, as well as in the PsA cohort, as measured by DAS28-CRP (p < 0.05). The observed decreases were not statistically significant for the other PsA scores (DAS28-ESR and BASDAI, both p > 0.05) nor for the UC cohort (SCCAI, p > 0.05) (Fig. 2A, B).
Switched patients’ disease activity scores did not change significantly between baseline and M12, though data density for switched patients was insufficient for statistical analysis for all but the AS (BASDAI, p > 0.05) and CD (HBI, p > 0.05) cohorts (Fig. 2C, D). No significant differences were observed in the proportion of patients in remission or with low disease activity between baseline and M12, with the baseline rates remaining mostly stable (except for PsA patients, where low population sizes prevent meaningful analysis) (Fig. 3B).
C-Reactive Protein Levels
Baseline and M12 values for CRP levels are presented in detail in Supplementary Table S5 and illustrated in Supplementary Fig. S6.
ADL-naïve patients’ mean baseline CRP levels (95% CI) were all elevated above the upper limit of normal range (> 10 mg/L). By M12, CRP levels had decreased to within the normal range (< 10 mg/L) for all indications (Fig S6A).
Switched patients’ mean baseline CRP levels were within normal ranges and remained stable at M12 for all indications (Fig S6B).
Persistence and Reasons for Discontinuation
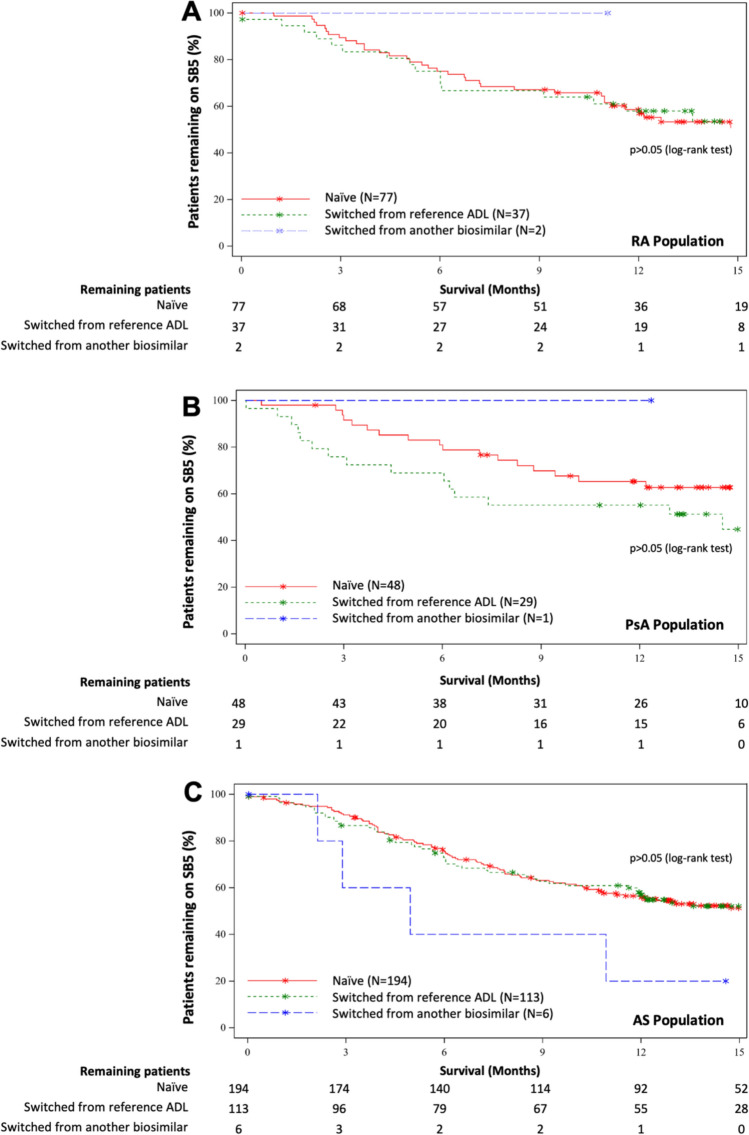
Kaplan–Meier estimates for patient persistence on SB5 at M3, M6 and M12 are presented in Supplementary Table S7, and the survival graphs are presented in Figs. 4 and 5 for rheumatological and gastroenterological indications, respectively. Reasons for discontinuation are presented in Table 4. Persistence calculations took account of SNDS data: of the 132 patients whose status at M12 was uncertain based on data from the clinical database, SNDS data provided confirmation of persistence for 57 patients (43.2%) and of non-persistence in 11 patients (8.3%) at M12 (Supplementary Table S2).
Fig. 4.
Persistence on SB5 over time for the rheumatology cohort. Naïve patients are represented by the red line. Switched patients are represented by the green line if they switched from the reference adalimumab and by the blue line if they switched from another biosimilar adalimumab. Stars on the graph indicate censored patients
Fig. 5.
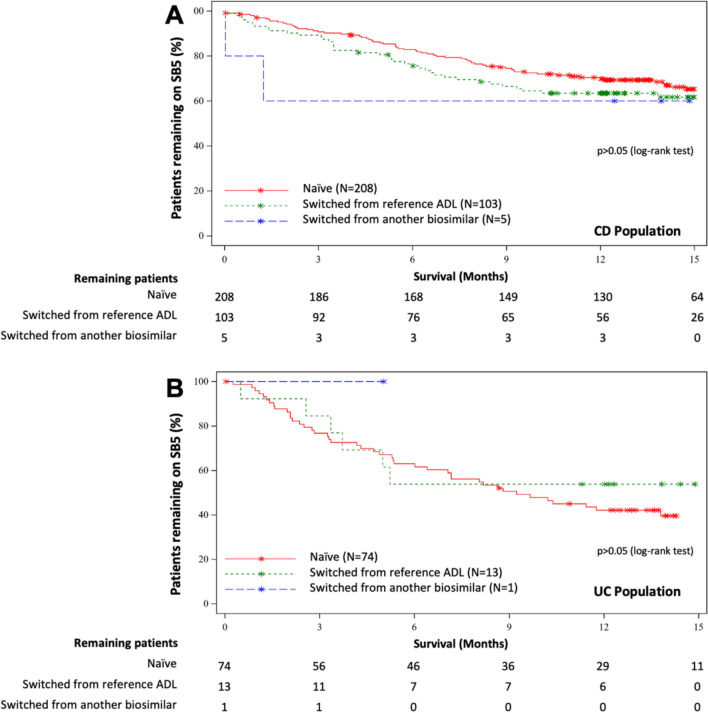
Persistence on SB5 over time for the gastroenterology cohort. Naïve patients are represented by the red line. Switched patients are represented by the green line if they switched from the reference adalimumab and by the blue line if they switched from another biosimilar adalimumab. Stars on the graph indicate censored patients
Table 4.
Reasons for discontinuation
| Naïve patients | Rheumatology cohort | Gastroenterology cohort | |||
|---|---|---|---|---|---|
| RA | PsA | AS | CD | UC | |
| N = 77 | N = 48 | N = 194 | N = 208 | N = 74 | |
| Overall | 36 (46.8%) | 18 (37.5%) | 92 (47.4%) | 74 (35.6%) | 47 (63.5%) |
| Patient decision | 1 (1.3%) | 1 (2.1%) | 14 (7.2%) | 12 (5.8%) | 4 (5.4%) |
| Investigator decision—primary failureb | 18 (23.4%) | 8 (16.7%) | 31 (16.0%) | 17 (8.2%) | 22 (29.7%) |
| Investigator decision—secondary failurec | 9 (11.7%) | 6 (12.5%) | 17 (8.8%) | 10 (4.8%) | 11 (14.9%) |
| Investigator decision—adverse event | 3 (3.9%) | 2 (4.2%) | 14 (7.2%) | 19 (9.1%) | 3 (4.1%) |
| Investigator decision—prolonged remission | 1 (1.3%) | – | 1 (0.5%) | 1 (0.5%) | 1 (1.4%) |
| Investigator decision—other | 2 (2.6%) | – | 5 (2.6%) | 4 (1.9%) | 1 (1.4%) |
| Subsequent treatment documented in SNDS | 2 (2.6%) | 1 (2.1%) | 10 (5.2%) | 11 (5.3%) | 5 (6.8%) |
| Switched patientsa | N = 39 | N = 30 | N = 119 | N = 108 | N = 14 |
|---|---|---|---|---|---|
| Overall | 17 (43.6%) | 16 (53.3%) | 59 (49.6%) | 40 (37.0%) | 6 (42.9%) |
| Patient decision | 8 (20.5%) | 8 (26.7%) | 19 (16.0%) | 16 (14.8%) | 2 (14.3%) |
| Investigator decision—primary failureb | 2 (5.1%) | 2 (6.7%) | 8 (6.7%) | 1 (0.9%) | – |
| Investigator decision—secondary failurec | – | 2 (6.7%) | 8 (6.7%) | 5 (4.6%) | – |
| Investigator decision—adverse event | 5 (12.8%) | 1 (3.3%) | 22 (18.5%) | 16 (14.8%) | 4 (28.6%) |
| Investigator decision—prolonged remission | – | – | – | – | – |
| Investigator decision—other | 1 (2.6%) | 2 (6.7%) | – | 1 (0.9%) | – |
| Subsequent treatment documented in SNDS | 1 (2.6%) | 1 (3.3%) | 2 (1.7%) | – | – |
The two most frequent reasons for discontinuation for each population are bolded
AS ankylosing spondylitis, CD Crohn’s disease, PsA psoriatic arthritis, RA rheumatoid arthritis, UC ulcerative colitis
aBoth from reference and from biosimilar adalimumab
bDefined as treatment failure observed within 14 weeks of SB5 initiation
cDefined as treatment failure observed more than 14 weeks after initiating SB5
Naïve patients’ M12 SB5 persistence rates were 58.6% (46.5, 68.8) for the RA cohort, 65.3% (49.7, 77.1) for the PsA cohort, 55.8% (48.3, 62.6) for the AS cohort, 69.9% (63.0, 75.7) for the CD cohort and 42.1% (30.7, 53.1) for the UC cohort (Figs. 4, 5). The two most frequent reasons for SB5 discontinuation among naïve patients were primary failure (between 8.2 and 29.7% of patients of any given indication) and secondary failure (between 4.8 and 14.9% of patients of any given indication), except for patients with CD, for whom the most frequent reasons were adverse events (9.1%) and primary failures (8.2%) (Table 4).
The M12 SB5 persistence rates for patients switched from reference or biosimilar ADL were 60.1% (42.7, 73.7) for the RA cohort, 56.7% (37.3, 72.1) for the PsA cohort, 55.4% (45.8, 64.0) for the AS cohort, 63.3% (53.4, 71.7) for the CD cohort and 56.3% (27.2, 77.6) for the UC cohort (Figs. 4, 5).
Very few patients had switched to SB5 from another biosimilar (n = 15), hence persistence rates for patients who exclusively switched from the reference were almost identical to those of the overall switched population (Figs. 4, 5).
The two most frequent reasons for SB5 discontinuation among switched patients were patient decision (between 14.3 and 26.7% of patients of any given indication) and adverse events (between 3.3 and 28.6% of patients of any given indication) (Table 4).
No significant differences were observed in M12 persistence rates between naïve and switched patients for any of the indications, nor between indications (p > 0.05) (Figs. 4, 5). However, the discontinuation profiles of the switched and naïve populations were significantly different (p < 0.001).
Safety and Immunogenicity Analysis
Adverse Event Reporting
Adverse events reported during the study type are listed in Table 5 and the adverse events which led to treatment discontinuation are described in Table 6, presented according to MedDRA (V24.1) System Organ Class (SOC).
Table 5.
Adverse events
| Naïve patients | Rheumatology cohort | Gastroenterology cohort | |||
|---|---|---|---|---|---|
| RA | PsA | AS | CD | UC | |
| N = 77 | N = 48 | N = 194 | N = 208 | N = 74 | |
| Subject with at least one TEAEb | 20 (26.0%) | 12 (25.0%) | 47 (24.2%) | 57 (27.4%) | 18 (24.3%) |
| Subject with at least one TESAEb | 3 (3.9%) | 3 (6.3%) | 6 (3.1%) | 6 (2.9%) | 3 (4.1%) |
| Subject with at least one treatment-emergent related non-serious AEb | 16 (20.8%) | 7 (14.6%) | 33 (17.0%) | 43 (20.7%) | 12 (16.2%) |
| Subjects with at least one TEAE leading to SB5 discontinuationb,c | 3 (3.9%) | 2 (4.2%) | 12 (6.2%) | 18 (9.1%) | 3 (4.1%) |
| Subjects with at least one TESAE leading to SB5 discontinuationb,c | – | – | 2 (1.0%) | – | – |
| Switched patientsa | N = 39 | N = 30 | N = 119 | N = 108 | N = 14 |
|---|---|---|---|---|---|
| Subject with at least one TEAEb | 9 (23.1%) | 8 (26.7%) | 43 (36.1%) | 44 (40.7%) | 6 (42.9%) |
| Subject with at least one TESAEb | 1 (2.6%) | – | 2 (1.7%) | 1 (0.9%) | – |
| Subject with at least one treatment-emergent related non-serious AEb | 8 (20.5%) | 6 (20.0%) | 39 (32.8%) | 37 (34.3%) | 6 (42.9%) |
| Subjects with at least one TEAE leading to SB5 discontinuationb,c | 5 (12.8%) | 1 (3.3%) | 22 (18.5%) | 16 (14.8%) | 4 (28.6%) |
| Subjects with at least one TESAE leading to SB5 discontinuationb,c | – | – | – | – | – |
AE adverse event, AS ankylosing spondylitis, CD Crohn’s disease, PsA psoriatic arthritis, RA rheumatoid arthritis, SAE serious adverse event, TEAE treatment-emergent adverse event, TESAE treatment-emergent serious adverse event, UC ulcerative colitis
aBoth from reference and from biosimilar adalimumab
bTEAEs are defined as any adverse events (serious and nonserious) with an onset date on or after the first day of treatment
cOnly AE leading to discontinuation for prospective patients are captured
Table 6.
Description of TEAE by SOC leading to treatment discontinuation (both serious and non-serious)
| Naïve patients | Rheumatology cohort | Gastroenterology cohort | |||
|---|---|---|---|---|---|
| RA | PsA | AS | CD | UC | |
| N = 77 | N = 48 | N = 194 | N = 208 | N = 74 | |
| Patients with least one TEAEb leading to SB5 discontinuation, n (%) | 3 (3.9%) | 2 (4.2%) | 14 (7.2%) | 19 (9.1%) | 3 (4.1%) |
| General disorders and administration site conditions | 1 (1.3%) | – | 6 (3.1%) | 9 (4.3%) | 2 (2.7%) |
| Skin and subcutaneous tissue disorders | 1 (1.3%) | – | 1 (0.5%) | 9 (4.3%) | 2 (2.7%) |
| Musculoskeletal and connective tissue disorders | 1 (1.3%) | – | 2 (1.0%) | 3 (1.4%) | – |
| Infections and infestations | – | – | 1 (0.5%) | – | – |
| Investigations | – | 1 (2.1%) | 2 (1.0%) | – | – |
| Nervous system disorders | – | 1 (2.1%) | 2 (1.7%) | 1 (0.5%) | – |
| Hepatobiliary disorders | – | – | – | 1 (0.5%) | – |
| Gastrointestinal disorders | – | – | 1 (0.5%) | – | – |
| Blood and lymphatic system disorders | – | – | 1 (0.5%) | – | – |
| Ear and labyrinth disorders | – | – | 1 (0.5%) | – | – |
| Injury, poisoning and procedural complications | – | – | 1 (0.5%) | – | – |
| Respiratory, thoracic and mediastinal disorders | – | – | 1 (0.5%) | – | – |
| Switched patientsa | N = 39 | N = 30 | N = 119 | N = 108 | N = 14 |
|---|---|---|---|---|---|
| Patients with least one TEAEb leading to SB5 discontinuation, n (%) | 5 (12.8%) | 1 (3.3%) | 22 (18.5%) | 16 (14.8%) | 4 (28.6%) |
| General disorders and administration site conditions | 4 (10.3%) | – | 19 (16.0%) | 11 (10.2%) | 4 (28.6%) |
| Skin and subcutaneous tissue disorders | – | – | 2 (1.7%) | 3 (2.8%) | – |
| Musculoskeletal and connective tissue disorders | – | – | 2 (1.7%) | 1 (0.9%) | 1 (7.1%) |
| Infections and infestations | 1 (2.6%) | 1 (3.3%) | 1 (0.8%) | 1 (0.9%) | – |
| Nervous system disorders | – | – | 1 (0.5%) | – | – |
| Gastrointestinal disorders | – | – | 2 (1.7%) | – | 1 (7.1%) |
Note: The number of reported and classified TEAEs may exceed the number of patients as it was possible to report multiple concurrent TEAEs leading to SB5 discontinuation for a given patient
AS ankylosing spondylitis, CD Crohn’s disease, PsA psoriatic arthritis, RA rheumatoid arthritis, SOC system organ class, TEAE treatment-emergent adverse event, UC ulcerative colitis
aFrom reference and/or from other biosimilar adalimumab
bTEAEs are defined as any adverse events (serious and non-serious) with an onset date on or after the first day of treatment
TEAEs were reported for 154 (25.6%) of naïve patients overall. Specifically, TEAEs were observed in 20 (26.0%) patients with RA, 12 (25.0%) patients with PsA, 47 (24.2%) patients with AS, 57 (27.4%) patients with CD and 18 (24.3%) patients with UC, respectively. Of these patients, 38 (24.6%) discontinued SB5 treatment following a TEAE.
Of the naïve patients who experienced TEAEs, 21 (13.6%) experienced a SAE: 3 (3.9%) patients with RA, 3 (6.3%) patients with PsA, 6 (3.1%) patients with AS, 6 (2.9%) patients with CD and 3 (4.1%) UC patients, respectively. However, only two patients (both in the AS cohort) discontinued SB5 treatment following an SAE (Table 5).
The most frequent TEAEs leading to treatment discontinuation were general disorders and administration site conditions (n = 18; 3.0%) as well as skin and subcutaneous tissue disorders (n = 13; 2.2%) (Table 6).
TEAEs were observed in 110 (35.5%) of switched patients overall: 9 (23.1%) patients with RA, 8 (26.7%) patients with PsA, 43 (36.1%) patients with AS, 44 (40.7%) patients with CD and 6 (42.9%) patients with UC. Of patients reporting a TEAE, 48 (43.6%) discontinued SB5 treatment after the event.
Of the switched patients who experienced TEAEs, four (3.6%) experienced a SAE: one (2.6%) patient with RA, 2 (1.7%) patients with AS and 1 (0.9%) patient with CD, with none leading to treatment discontinuation (Table 5).
The TEAEs which led to treatment discontinuation were mostly general disorders and administration site conditions (n = 38; 34.5%), which were markedly more frequent than other AE categories (Table 6).
Immunogenicity
A summary of immunogenicity testing is provided in Table 7.
Table 7.
Immunogenicity analysis
| AS | PsA | CD | UC | Total | |||
|---|---|---|---|---|---|---|---|
| Switcheda | Naïve | Naïve | Switcheda | Naïve | Switcheda | ||
| Baseline, n | 1 | 5 | 4 | 5 | 11 | 2 | 28 |
| ADA-negative, n | 1 | 5 | 4 | 5 | 11 | 2 | 28 |
| After baseline, n | 0 | 13 | 15 | 11 | 23 | 5 | 67 |
| ADA-negative, n | 0 | 12 | 14 | 10 | 23 | 5 | 64 |
| ADA-positive, n | 0 | 1 | 1 | 1 | 0 | 0 | 3 |
Number of tests performed at/after baseline. Tests were considered positive for an anti-drug-antibody concentration > 10 mg/L. No tests were performed on switched patients with PsA
ADA anti-drug antibody, AS ankylosing spondylitis, CD Crohn’s disease, PsA psoriatic arthritis, RA rheumatoid arthritis, UC ulcerative colitis
aBoth from reference and from biosimilar adalimumab
In total, 95 immunogenicity tests were performed by 14 sites. Of these, 28 were performed at baseline (all negative) and 67 were performed post-baseline (all but 3 were negative, with a positive result confirmed for 1 naïve patient with AS, 1 naïve patient with PsA and 1 switched patient with CD).
Discussion
This final 12-month analysis of the PERFUSE cohort of patients with IMID shows that SB5 provides effective disease control for both ADL-naïve patients and patients who switched from reference or biosimilar ADL, with no discernible loss of control observed in the latter group and with disease activity scores within clinically acceptable ranges for all populations at 12 months post-initiation of SB5. This analysis also presents 12-month SB5 persistence data for each of the included indications: treatment persistence at 12 months was around 60% for all populations, both naïve and switched, except for naïve patients with UC whose persistence was lower, at around 40%. The main reasons for non-persistence among naïve patients were primary and secondary failure, whereas among switched patients they were patient decision and adverse events. In terms of safety, the most frequent adverse events leading to SB5 discontinuation were general and administration site disorders related to the injection itself or skin disorders.
Our findings on treatment persistence in the CD cohort were in line with the available literature regarding ADL biosimilars, with Derikx et al. reporting 12-month persistence rates of 60.3 and 70.8% among naïve and switched patients, respectively, and Lukas et al. finding a rate of 64.8% in a population of patients who switched to SB5 [23, 24]. This is also in line with studies on biosimilars of other molecules [25]. Persistence amongst patients with UC was lower than for patients with CD; little data on ADL biosimilars is available on patients with UC specifically, although a small study of 23 patients found a drug survival rate of 50% at 12 months [26]. Overall, these rates were also lower than those observed historically for the reference ADL, which are closer to 80% at 12 months for patients with CD and 55–70% for patients with UC [30, 31].
Concerning rheumatology patients, available literature is sparse, though recent evidence in RA and PsA found 12-month persistence rates of 85.1 and 85.8%, respectively, significantly higher than those observed in this study [27, 28]. Furthermore, a 48-week analysis of the PROPER study found a persistence rate of 80.0% among patients with rheumatic IMIDs [29].
The type and frequency of observed reasons for discontinuation were similar to those observed in available literature on ADL and its biosimilars: an observational study by Deprez et al. reported that the most frequent reason for discontinuation among 110 patients switched to SB5 was adverse events, with 50% of these being injection-site related [32].
The high rate of discontinuation following adverse events and patient decision could possibly be indicative of a nocebo effect [34], especially as they tended to occur earlier than discontinuations for other reasons (Supplementary File S9). This would explain the preponderance of adverse events coded as ‘injection site disorders’ and ‘skin disorders’. However, this could also suggest that AE reporting, especially for AEs occurring in the first few months of follow-up, may not have been objective and consistent across all sites, hence interpretation of these data is not unequivocal. This is in line with the observations of the PROPER Study, in which differential AE reporting between sites was evident [35]. The higher incidence of discontinuation following patient-decision- or administration-site-related adverse event in switched versus naïve patients could also indicate a preference for patients to switch back to a familiar brand/device, regardless of objective injection experience.
Another explanation may be an excipient of the SB5 formulation at the time of study conduct, the citrate buffer, which has previously been associated with greater perception of injection site pain [33]. As for naïve patients, the observed rates of treatment failure (both primary and secondary) were in line with previous studies, though rates have been found to vary [23, 26]. That said, certain adverse events may be considered a loss of response in other studies and vice versa due to different definitions, which may explain some of the observed variance [36].
Disease activity scores all evolved as expected between baseline and M12, decreasing in naïve patients and remaining low in switched patients, leading to high proportions of patients in remission or with low disease activity at the end of the observation period. Observed remission rates are in line with previous studies on both patients with IBD and rheumatology patients, respectively, with studies having observed rates ranging from 50 to 90% [37–39]. However, the small number of data points, especially at M12, precludes the possibility of robust statistical analyses.
The evolution of CRP levels mirrored that of disease activity scores, with concentrations below the upper levels of normal at the end of the observation period [40], further confirming the clinical effectiveness of SB5 among patients who persisted on SB5 treatment until month 12.
The scores and assays used in this study were appropriate to allow for detection of clinically relevant differences in disease activity. The validated disease activity scores are those used in clinical practice and correlate well with other existing options. Additionally, CRP remains the most common biomarker for evaluation of therapeutic disease management of patients with IMID [41].
While this study provides novel 12-month persistence and safety data, certain limitations need to be considered, primarily related to the real-world observational design. Whilst such studies offer significant opportunities to examine practice and utilisation outside the controlled, randomised clinical trial setting, they also permit freedom of patient treatment and follow-up, which can lead to a paucity of data points. Data collection and thus density is dependent upon site practices. In this study we can see that enrolled patients were not always seen by study sites during the anticipated visit windows, nor were disease activity scoring, CRP measurement and ADA assays routinely performed [42]. Concerning cohort-specific data points, in the gastroenterology cohorts endoscopic healing data were not reported, and though calprotectin levels were reported by investigators when available, low data density did not allow for meaningful analysis. Similarly, in the rheumatology cohort, ESR values were generally not reported. Low data density may suggest that these assays are not routinely performed at French sites, or are only evaluated when the physician deems them necessary, depending upon their clinical assessment of their patients’ health status. These differing densities may offer insights into the care pathways of patients.
This analysis did not include patient-reported outcome measures, nor a predictive factor analysis to attempt to further understand the persistence results. Furthermore, additional confounders which could potentially provide a more complete understanding of patient experience and of other potential factors leading to treatment discontinuation were not identified. However, recent results from a separate analysis focussing on patient experience in the gastroenterology cohort found that most of the measured dimensions of patient experience had relatively small effects on 12-month persistence [43].
Finally, integrating SNDS data provided the confirmation of the persistence status of 68 patients (15.4%) out of the 441 patients for whom there was no record of 12-month exposure in the clinical database. This led to a small change in persistence for each indication (approximately one percentage point for each indication) and did not change the statistical significance of any of the persistence analyses (Supplementary Table S2). In total, 30.1% of patients were successfully identified in the SNDS thanks to the probabilistic pairing approach, which used patient characteristics as well as patient visit dates and SB5 injection dates as a proxy for SB5 dispensation dates. While this approximation was necessary, as dispensation dates were not reported by physicians, the algorithm could not unequivocally identify a higher proportion of study patients; discrepancies between SB5 dispensation and injection dates were too great to allow matching with a sufficiently high degree of certainty. Though the observed impact of SNDS pairing was minimal in our case, had the 234 patients with SNDS data all been lost to follow-up, the change to persistence would have been much more noticeable. Indeed, this study confirmed the possibility and showed the potential of including data from the French SNDS into a cohort study. This study has also highlighted the need for specific types of data points which would improve probabilistic pairing strategies.
For even better pairing results, a deterministic pairing using each patient’s social security number could be implemented. This would, however, have required the collection and secure storage of patients’ social security numbers, which was not practically feasible for this study, as SNDS analysis was not foreseen at study initiation; at the time of introduction of the SNDS component, some patients had already reached the end of the study or were lost to follow-up and retrospective capture of the necessary personal information was not feasible.
Conclusions
SB5 provides clinically effective treatment of both gastrointestinal and rheumatic IMIDs for adalimumab-naïve and switched patients, with no loss of control observed when switching. Persistence was comparable between naïve and switched populations, though the reasons for non-persistence were different: switched patients discontinued mainly due to patient decision or an adverse event, whereas naïve patients discontinued chiefly following primary or secondary treatment failure. The study provides new insights into the use of SB5 in France, including persistence data bolstered by the inclusion of data from the French national healthcare claims database. Indeed, this study’s design may serve as a starting point for the development of more hybrid multicentric cohorts integrating SNDS data to provide even more, much anticipated, real-world data.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to acknowledge Sanoïa e-Health Services for data management services and writing and editorial support for this paper, acknowledge eXYSTAT for data management and analysis support, thank Dr. Guillaume Desjeux for his expertise pertaining to the analysis of SNDS data and thank all the investigators who contributed to data collection and all the participants without whom this study would not have been possible.
Declarations
Funding
This study was sponsored by Biogen Int GmbH (Baar, Switzerland). Funding was provided by Biogen. Open access fees were sponsored by Biogen Int GmbH (Baar, Switzerland).
Conflict of interest
Bruno Fautrel: received speaker/consulting fees from AbbVie, Biogen, Boehringer Ingelheim, BMS, Celgene, Janssen, Lilly, Medac, MSD, NORDIC Pharma, Novartis, Pfizer, Roche, Sobi and UCB, and grant/research support from AbbVie, MSD and Pfizer. Yoram Bouhnik: received consultancy and/or speaker fees from AbbVie, Biogaran, Biogen, Boehringer Ingelheim, CTMA, Ferring, Gilead, Hospira, ICON, Inception IBD, Janssen, Lilly, Mayoly Spindler, Merck, Merck Sharp & Dohme, Norgine, Pfizer, Robarts Clinical Trials, Roche, Sanofi, Shire, Takeda, UCB and Vifor Pharma. Carine Salliot: received consultancy and/or speaker fees from Novartis, Roche-Chugai, Pfizer and Galapagos, and grant/research support from Roche-Chugai and Novartis. Frank Carbonnel: received speaker fees from Abbvie, Astra, Biocodex, Biogen, Ferring, Janssen, MSD, Pfizer, Pileje, Takeda, Tillotts; and is a member of advisory boards for Amgen, Arena, BMS, Celltrion, Enterome, Ferring, Janssen, Medtronic, Pfizer, Pharmacosmos, Roche and Tillotts. Mathurin Fumery: received consultancy and/or speaker fees from AbbVie, Biogen, Boehringer Ingelheim, CTMA, Ferring, Gilead, Hospira, Janssen, Lilly, Merck Sharp & Dohme, Pfizer, Takeda, Tillots and Celgene. Chirstophe Bernardeau: nothing to disclose with respect to their contributions to this work. Yves Maugars: nothing to disclose with respect to their contributions to this work. Mathurin Flamant: received consultancy and/or speaker fees from AbbVie, Amgen, Biogen, Ferring, Janssen, Mayoly Spindler, Merck Sharp & Dohme, Pfizer, Takeda, Tillots and Pharma. Fabienne Coury: received speaker/consulting fees from Abbvie, Amgen, BMS, Janssen, Lilly, MSD, Novartis, Pfizer, Roche-Chugai, Sanofi and UCB, and grant/research support from Abbvie, Biogen, Celgene, Chugai, Novartis, Pfizer and UCB. Ben Braithwaite: no conflicts of interest to disclose. Salima Hateb: is an employee of and may hold stock in Biogen. Janet Addison: is an employee of and may hold stock in Biogen.
Availability of data and material
The datasets generated and analysed during the current study are not publicly available as data for this study contain potentially identifying information.
Ethics approval
All necessary ethical considerations and measures were taken to ensure the protection of all participants: patients were eligible for the study only if they were able to understand and sign a consent form. This study is listed on clinicaltrials.gov under the identifier NCT03662919 and was approved by the appropriate bodies in terms of quality of methodology, data security and scientific merit. The final amendment to the protocol for this study was approved by an independent ethics committee (Comité de Protection des Personnes, CPP) in France on 25 April 2019, in accordance with French regulations (CPP SUD-EST II; study ref. 2018-06; internal ref. 19.03.29.73319). Furthermore, this study conformed to all regulations concerning the use of personal data. All procedures were carried out in accordance with the ethical rules and the principles of the Declaration of Helsinki and its later amendments.
Consent to participate
Informed consent was obtained from all individual participants included in the study in the form of a non-opposition form signed and archived by the physician in accordance with French regulations.
Code availability
The code used to perform data management and run the analyses uses proprietary modules owned by eXYSTAT. Thus, the code cannot be made publicly available.
Author contributions
Bruno Fautrel: as the coordinating investigator for rheumatology, made substantial contributions to the conception and design of the work, the acquisition and interpretation of data for the work and reviewed all draft versions of the manuscript for important intellectual content. Yoram Bouhnik: as the coordinating investigator for gastroenterology, made substantial contributions to the conception and design of the work, the acquisition and interpretation of data for the work and reviewed all draft versions of the manuscript for important intellectual content. Carine Salliot: as an investigator, made substantial contributions to the acquisition and interpretation of data for the work and reviewed all draft versions of the manuscript for important intellectual content. Mathurin Fumery: as an investigator, made substantial contributions to the acquisition and interpretation of data for the work and reviewed all draft versions of the manuscript for important intellectual content. Chirstophe Bernardeau: as an investigator, made substantial contributions to the acquisition and interpretation of data for the work and reviewed all draft versions of the manuscript for important intellectual content. Mathurin Flamant: as an investigator, made substantial contributions to the acquisition and interpretation of data for the work and reviewed all draft versions of the manuscript for important intellectual content. Fabienne Coury: as an investigator, made substantial contributions to the acquisition and interpretation of data for the work and reviewed all draft versions of the manuscript for important intellectual content. Ben Braithwaite: as the lead writer and biostatistics coordinator for this work, made substantial contributions to the design of the work, acquisition, analysis and interpretation of data for the work and produced all draft versions of the manuscript. Salima Hateb: as a study coordinator for the sponsor, made substantial contributions to the conception and design of the work, the acquisition and interpretation of data for the work and reviewed all draft versions of the manuscript for important intellectual content. Janet Addison: as the sponsor’s lead scientist for this work, made substantial contributions to the conception and design of the work, the analysis and interpretation of data for the work and critically reviewed, commented upon and edited all draft versions of the manuscript. The first draft of the manuscript was written by Ben Braithwaite and all authors commented on previous versions of the manuscript. All authors read, provided input and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021;21:680–6. 10.1038/s41577-021-00603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H-H, Chao W-C, Chen Y-H, Hsieh T-Y, Lai K-L, Chen Y-M, et al. Risk of immune-mediated inflammatory diseases in newly diagnosed ankylosing spondylitis patients: a population-based matched cohort study. Arthritis Res Ther. 2019;21:196. 10.1186/s13075-019-1980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Privitera G, Pugliese D, Lopetuso LR, Scaldaferri F, Neri M, Guidi L, et al. Novel trends with biologics in inflammatory bowel disease: sequential and combined approaches. Ther Adv Gastroenterol. 2021;14:17562848211006668. 10.1177/17562848211006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shams S, Martinez JM, Dawson JRD, Flores J, Gabriel M, Garcia G, et al. The therapeutic landscape of rheumatoid arthritis: current state and future directions. Front Pharmacol. 2021;12: 680043. 10.3389/fphar.2021.680043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis CR, Azmat CE. Adalimumab. StatPearls: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 6.Vitello A, Grova M, Pugliese D, Rizzello F, Lanzarotto F, Lavagna A, et al. Effectiveness of adalimumab for ulcerative colitis: a multicentre, retrospective study of clinical practice in Italy. Dig Liver Dis. 2022;54:352–7. 10.1016/j.dld.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EHS, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–89. 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:iii–iv, xi–xiii, 1–229. 10.3310/hta10420. [DOI] [PubMed]
- 9.Patel D, Shelbaya A, Cheung R, Aggarwal J, Park SH, Coindreau J. Cost-effectiveness of early treatment with originator biologics or their biosimilars after methotrexate failure in patients with established rheumatoid arthritis. Adv Ther. 2019;36:2086–95. 10.1007/s12325-019-00986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glintborg B, Ibsen R, Bilbo REQ, Hetland ML, Kjellberg J. Does a mandatory non-medical switch from originator to biosimilar etanercept lead to increase in healthcare use and costs? A Danish register-based study of patients with inflammatory arthritis. RMD Open. 2019;5: e001016. 10.1136/rmdopen-2019-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Adrichem RCS, Voorneveld HJE, Waverijn GJ, Kok MR, Bisoendial RJ. The non-medical switch from reference adalimumab to biosimilar adalimumab is highly successful in a large cohort of patients with stable inflammatory rheumatic joint diseases: a real-life observational study. Rheumatol Ther. 2022;9:1109–18. 10.1007/s40744-022-00465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin D, Kim Y, Kim HS, Fuhr R, Körnicke T. FRI0110 a phase I pharmacokinetic study comparing SB5, an adalimumab biosimilar, and adalimumab reference product (Humira®) in healthy subjects. Ann Rheum Dis. 2015;74:459–60. 10.1136/annrheumdis-2015-eular.1419. [Google Scholar]
- 13.Weinblatt ME, Baranauskaite A, Niebrzydowski J, Dokoupilova E, Zielinska A, Jaworski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2018;70:40–8. 10.1002/art.40336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhlig T, Goll GL. Reviewing the evidence for biosimilars: key insights, lessons learned and future horizons. Rheumatology (Oxford). 2017;56:iv49–62. 10.1093/rheumatology/kex276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 16.Wells G, Becker J-C, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League against rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–60. 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 18.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 19.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32. 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann RM, van der Heijde D, Gardiner PV, Szumski A, Marshall L, Bananis E. DAS28-CRP and DAS28-ESR cut-offs for high disease activity in rheumatoid arthritis are not interchangeable. RMD Open. 2017;3: e000382. 10.1136/rmdopen-2016-000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jowett SL, Seal CJ, Phillips E, et al. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38(2):164–71. 10.1080/00365520310000654. [DOI] [PubMed] [Google Scholar]
- 22.Francois F, Naimi L, Roblin X, Berger A-E, Paul S. Adalimumab and anti-adalimumab LISA-TRACKER immunoassays performance criteria for therapeutic drug monitoring of adalimumab-amgen biosimilar (ABP501). BMC Immunol. 2021;22:81. 10.1186/s12865-021-00473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derikx LAAP, Dolby HW, Plevris N, Lucaciu L, Rees CS, Lyons M, et al. Effectiveness and safety of adalimumab biosimilar SB5 in inflammatory bowel disease: outcomes in originator to SB5 switch, double biosimilar switch and Bio-Naïve SB5 observational cohorts. J Crohn’s Colitis. 2021;15:2011–21. 10.1093/ecco-jcc/jjab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukas M, Kolar M, Reissigova J, Duricova D, Machkova N, Hruba V, et al. A switch from originator-adalimumab to the biosimilar SB5 in patients with Crohn’s disease: an analysis of two propensity score-matched cohorts. Scand J Gastroenterol. 2022;57:814–24. 10.1080/00365521.2022.2041082. [DOI] [PubMed] [Google Scholar]
- 25.Haugeberg G, Bakland G, Rødevand E, Hansen IJW, Diamantopoulos A, Pripp AH. Effectiveness and persistence in SB4- and reference etanercept-treated rheumatoid arthritis patients in ordinary clinical practice in Norway. Arthritis Care Res. 2023. 10.1002/acr.25092. [DOI] [PubMed] [Google Scholar]
- 26.McDermott E, Murphy S, Keegan D, O’Donoghue D, Mulcahy H, Doherty G. Efficacy of adalimumab as a long term maintenance therapy in ulcerative colitis. J Crohns Colitis. 2013;7:150–3. 10.1016/j.crohns.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Bruni C, Gentileschi S, Pacini G, Bardelli M, Tofani L, Bartoli F, et al. Switching from originator adalimumab to biosimilar SB5 in a rheumatology cohort: persistence on treatment, predictors of drug interruption and safety analysis. Ther Adv Musculoskelet Dis. 2021;13:1759720X211033679. 10.1177/1759720X211033679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khraishi MM, Remple VP, Silverberg S, Stewart JC, Florica B, Bessette L. Canadian adalimumab postmarketing observational epidemiological study assessing effectiveness in psoriatic arthritis (COMPLETE-PsA): 12-month results of comparative effectiveness of adalimumab and nbDMARDs. J Rheumatol. 2022;49:454–64. 10.3899/jrheum.200609. [DOI] [PubMed] [Google Scholar]
- 29.Müller-Ladner U, Gaffney K, Jadon D, Freudensprung U, Addison J, et al. AB0348: the PROPER study: a 48-week analysis of a pan-EU real-world study of SB5 biosimilar following transition from reference adalimumab in patients with rheumatoid arthritis, axial spondyloarthritis or psoriatic arthritis. Ann Rheum Dis. 2022;81(Suppl 1):1299–300. [Google Scholar]
- 30.Sartini A, Scaioli E, Liverani E, Bellanova M, Ricciardiello L, Bazzoli F, et al. Retention rate, persistence and safety of adalimumab in inflammatory bowel disease: a real-life, 9-year, single-center experience in Italy. Dig Dis Sci. 2019;64:863–74. 10.1007/s10620-018-5329-4. [DOI] [PubMed] [Google Scholar]
- 31.Blesl A, Binder L, Högenauer C, Wenzl H, Borenich A, Pregartner G, et al. Limited long-term treatment persistence of first anti-TNF therapy in 538 patients with inflammatory bowel diseases: a 20-year real-world study. Aliment Pharmacol Ther. 2021;54:667–77. 10.1111/apt.16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deprez N, De Somer T, Baert D, Deceuninck M, Huys I, Mattens V, et al. Evaluation of the safety and effectiveness after switch from adalimumab originator to biosimilar SB5 in patients with inflammatory bowel disease in a real-life setting. AGEB. 2022;85:557–64. 10.51821/85.4.10724. [DOI] [PubMed] [Google Scholar]
- 33.Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical switching from originators to biosimilars: does the nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther. 2020;7:35–64. 10.1007/s40744-019-00190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St Clair-Jones A, Prignano F, Goncalves J, Paul M, Sewerin P. Understanding and minimising injection-site pain following subcutaneous administration of biologics: a narrative review. Rheumatol Ther. 2020;7:741–57. 10.1007/s40744-020-00245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller-Ladner U, Dignass A, Gaffney K, Jadon D, Matucci-Cerinic M, Lobaton T, et al. The PROPER study: a 48-week, Pan-European, real-world study of biosimilar SB5 following transition from reference adalimumab in patients with immune-mediated inflammatory disease. BioDrugs. 2023;37(6):873–89. 10.1007/s40259-023-00616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallejo-Yagüe E, Keystone EC, Kandhasamy S, Micheroli R, Finckh A, Burden AM. Primary and secondary non-response: in need of operational definitions in observational studies. Ann Rheum Dis. 2021;80:961–4. 10.1136/annrheumdis-2021-220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Beloso N, Altabás-González I, Samartín-Ucha M, Gayoso-Rey M, De Castro-Parga ML, Salgado-Barreira Á, et al. Switching between reference adalimumab and biosimilars in chronic immune-mediated inflammatory diseases: a systematic literature review. Br J Clin Pharmacol. 2022;88:1529–50. 10.1111/bcp.15101. [DOI] [PubMed] [Google Scholar]
- 38.Mastronardi M, Curlo M, Cavalcanti E, Burattini O, Cuppone R, Tauro R, et al. Administration timing is the best clinical outcome predictor for adalimumab administration in Crohn’s disease. Front Med (Lausanne). 2019;6:234. 10.3389/fmed.2019.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cingolani L, Barberio B, Zingone F, Ferronato A, Bertani L, Costa F, et al. Adalimumab biosimilars, ABP501 and SB5, are equally effective and safe as adalimumab originator. Sci Rep. 2021. 10.1038/s41598-021-89790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–31. 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blyth A, Simmonds O, Blackler L, Kirkham B, Ng N, Cope A, et al. 238 biologic dose optimisation in clinical practice. Rheumatology. 2018. 10.1093/rheumatology/key075.462. [Google Scholar]
- 42.Simon GE, Platt R, Watanabe JH, Bindman AB, John London A, Horberg M, et al. When can we rely on real-world evidence to evaluate new medical treatments? Clin Pharmacol Ther. 2022;111:30–4. 10.1002/cpt.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouhnik Y, Carbonnel F, Fumery M, et al. The PERFUSE study: the experience of patients receiving adalimumab biosimilar SB5. Digest Liver Dis. 2023. 10.1016/j.dld.2023.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.