Abstract
The chimeric murine oncornavirus FrCasE causes a rapidly progressive paralytic disease associated with spongiform neurodegeneration throughout the neuroaxis. Neurovirulence is determined by the sequence of the viral envelope gene and by the capacity of the virus to infect microglia. The neurocytopathic effect of this virus appears to be indirect, since the cells which degenerate are not infected. In the present study we have examined the possible role of inflammatory responses in this disease and have used as a control the virus F43. F43 is an highly neuroinvasive but avirulent virus which differs from FrCasE only in 3′ pol and env sequences. Like FrCasE, F43 infects large numbers of microglial cells, but it does not induce spongiform neurodegeneration. RNAase protection assays were used to detect differential expression of genes encoding a variety of cytokines, chemokines, and inflammatory cell-specific markers. Tumor necrosis factor alpha (TNF-α) and TNF-β mRNAs were upregulated in advanced stages of disease but not early, even in regions with prominent spongiosis. Surprisingly there was no evidence for upregulation of the cytokines interleukin-1α (IL-1α), IL-1β, and IL-6 or of the microglial marker F4/80 at any stage of this disease. In contrast, increased levels of the β-chemokines MIP-1α and -β were seen early in the disease and were concentrated in regions of the brain rich in spongiosis, and the magnitude of responses was similar to that observed in the brains of mice injected with the glutamatergic neurotoxin ibotenic acid. MIP-1α and MIP-1β mRNAs were also upregulated in F43-inoculated mice, but the responses were three- to fivefold lower and occurred later in the course of infection than was observed in FrCasE-inoculated mice. These results suggest that the robust increase in expression of MIP-1α and MIP-1β in the brain represents a correlate of neurovirulence in this disease, whereas the TNF responses are likely secondary events.
CasBrE, an oncornavirus isolated from wild mice (13), as well as other neuropathogenic ecotropic oncornaviruses (43) cause chronic spongiform encephalomyelopathy after intraperitoneal inoculation of neonates, and the pathology resembles that caused by the transmissible spongiform encephalopathy agents (24, 45). The disease induced by CasBrE is characterized clinically by paralysis and tremor associated with neurogenic atrophy of skeletal muscles (1, 13). The neurodegenerative changes involve both neurons and neuroglia, leading eventually to neuronal dropout. The murine oncornaviruses are nonlytic viruses, and indeed neuronal infection in vivo appears not to be associated with any untoward effects on cellular integrity, even at the ultrastructural level (28). On the other hand, neurons which do undergo degenerative changes appear not to be infected (1, 21, 28, 45), and this has suggested that the spongiosis induced by these viruses is a consequence of indirect mechanisms. Consistent with this hypothesis is the observation that the induction of spongiosis is dependent on the infection of microglial cells (15, 28, 30, 31), the resident macrophages of the brain.
The viral sequences which determine the neurovirulence of CasBrE are located primarily within the envelope gene (11) and specifically have been localized to the surface glycoprotein (SU) (39), the subunit of Env which is involved in receptor binding. We recently compared the cellular tropisms in the brain of two chimeric viruses, the highly neurovirulent virus FrCasE and the avirulent virus F43. FrCasE contains the envelope gene of CasBrE, and F43 contains the envelope gene of the nonneurovirulent virus Friend murine leukemia virus (MuLV) 57, both on identical genetic backgrounds. The amino acid sequences of the respective envelope proteins differ by 22%. Surprisingly, both viruses infect the brain at high levels, and in fact viral burdens in the brain achieved by F43 are higher than those reached by FrCasE (3). Furthermore, both FrCasE and F43 infect large numbers of microglia in the central nervous system (CNS), yet only FrCasE causes spongiosis, which is fatal within 17 to 21 days after neonatal inoculation. Mice inoculated with F43 live for 2 to 3 months, during which time the viral burden in the brain continues to increase, yet these mice fail to exhibit signs of neurologic disease. Eventually, F43-inoculated mice develop fatal erythroleukemia. The dramatic difference in the neurovirulence of these viruses, despite shared tropism for microglial cells, suggests that the neurotoxicity induced by FrCasE is a function of an interaction between its envelope protein and microglia in which the protein is expressed. The nature of this effect is not known, but it appears to be dependent on late steps in the virus replication cycle (29).
The diseases caused by the murine oncornaviruses are thought not to have an inflammatory component, but this idea is based primarily on the absence of inflammatory cellular infiltrates in the CNS. It is now clear, however, that the manifestations of inflammatory responses in the CNS can be quite different from those in peripheral tissues (40). Infiltrating leukocytes are generally skewed toward the myelomonocytic lineages, and cells intrinsic to the brain, such as astrocytes, microglia, and neurons, are sometimes the primary sources of inflammatory mediators. There is accumulating evidence that despite the lack of cellular infiltrates, inflammatory mediators appear to play important roles in the pathogenesis of a variety of human neurodegenerative diseases, including Alzheimer's disease (34), Huntington's disease (36), Parkinson's disease (18), and the transmissible spongiform encephalopathies (5, 6).
Two studies suggest a role for inflammatory mediators in the neurodegenerative diseases induced by the murine oncornaviruses. Tumor necrosis factor alpha (TNF-α) mRNA and protein were found to be increased in the brains of mice with advanced neurologic disease caused by ts-1, a neuropathogenic variant of Moloney MuLV (7), and CasBrE (38), respectively. In the present study we have quantified the expression of a variety of inflammatory mediators at the mRNA level in the brains of mice infected with FrCasE both in advanced and in early stages of the disease. Mice inoculated with F43 were used to distinguish disease-specific responses from responses induced by virus infection per se. We found that despite the lack of leukocytic infiltrates in this disease, the β-chemokines MIP-1α and MIP-1β were upregulated early in the brain and levels of expression correlated with the extent of spongiosis. The only proinflammatory cytokines found to be upregulated in this disease were TNF-α, and TNF-β, but these responses occured late in the disease course and thus appeared not to correlate temporally with the induction of spongiosis.
MATERIALS AND METHODS
Mice and virus inoculations.
Inbred Rocky Mountain White mice (42) were bred and raised at the Rocky Mountain Laboratories (RML), and were handled according to the policies of the RML Animal Care and Use Committee. Mice were infected with virus stocks prepared as described previously (42), consisting of tissue culture supernatants of infected Mus dunni cells (25). Mice were inoculated intraperitoneally 24 to 48 h after birth with 30 μl of virus stock containing between 2 × 106 and 6 × 106 focus-forming units per ml. Virus titers were determined using a focal immunoassay described previously (8). Beginning at 11 days postinoculation (dpi), mice were evaluated clinically for signs of neurologic disease as described previously (10). Neurologic signs included abnormal adduction of the hind limbs when the mice were lifted by the tail, progressing to tremor and paralysis of both hind and forelimbs.
Total RNA preparation and RNase protection assays.
Mice were sacrificed under deep isoflurane anesthesia by axillary incision. Brains were removed and immediately frozen in liquid nitrogen. For some experiments brain stems were separated from the rest of the brain prior to freezing. Brain stems were separated by cutting between the cerebellum and cerebrum coronally just rostral of the posterior colliculi. The cerebellum was then separated from the brain stem. Total RNA was prepared using Trizol reagent (Life Technologies) according to the manufacturer's instructions. As described previously (3) multiprobe RNase protection assays (RPA) utilized the RiboQuant system (PharMingen). Probes were labeled using [α-32P]UTP (Dupont NEN). Protected mRNA species were resolved on precast QuickPoint polyacrylamide gels (PharMingen). Bands were quantified on a STORM PhosphorImager (Molecular Dynamics) using ImageQuant software, and quantitative data were expressed as percentages of values for the protected mRNA species derived from the housekeeping gene for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The multiprobe kits from PharMingen used in the present study included MCD-1, MCK-2b, MCK-3 and MCK-5.
It should be noted that when using the MCK-5 probe set with inbred Rocky Mountain White mice, the protected IP-10 probe was smaller than indicated by PharMingen. This has been shown in other mouse strains to be a consequence of a polymorphism of the IP-10 gene resulting in a sequence mismatch between the probe and the IP-10 mRNA near the 3′ end of the probe (16). Because of the proximity of the IP-10 and MCP-1 bands in the polyacrylamide gels, the location of these bands in the multiprobe set was confirmed using either the MCP-1 or IP-10 probes alone (K. Peterson [RML], unpublished data).
Ibotenic acid injections.
Ibotenic acid (Sigma) was dissolved in phosphate-buffered saline (PBS) containing 0.02% acetic acid at a concentration of 5 μg/μl (33). At postnatal day 11 mice were anesthetized by inhalation of 2.25% isoflurane, and 10 μg of ibotenic acid in a volume of 2 μl was injected intracerebrally into the right frontal cortex, approximately 2 mm caudal of the olfactory bulb between the orbit and the midline. Injections were made freehand to a depth of 2 to 3 mm using a Hamilton 10-μl syringe and a 32-gauge needle. As reported by others (33), this dose of ibotenic acid produced consistent well-circumscribed cortical lesions and was associated with low mortality. After recovering from anesthesia, the mice were returned to their mothers. At 4 days postinjection, brains were removed as described above and right frontal lobes were separated, snap frozen, and stored at −80°C. For some mice, brains were fixed in 3.7% formaldehyde for histopathologic evaluation.
Immunohistochemistry and histoblotting of brains.
Immunohistochemistry for detection of viral envelope protein in brain sections was performed as described previously (3) using brains fixed by immersion in 3.7% formaldehyde–PBS, cyropreservation with sucrose, and sectioning on a Microm HM 505E cryostat. The antiserum was a goat anti-gp70 described previously (3), and the substrate was VIP (Vector Laboratories), which produces a deep blue color.
For an analysis of the extent and regional distribution of viral antigen in the brain, a histoblotting technique originally described by Lipkin and Oldstone (27) was used. Briefly, brains were frozen in liquid nitrogen without prior fixation. Ten-micrometer midsaggital frozen sections were transferred to polyvinylidene fluoride membranes. Sections were air dried for 30 min, and then the membranes were immersed in sodium dodecyl sulfate sample buffer (2% sodium dodecyl sulfate and 5% β-mercaptoethanol in Tris buffer [pH 6.8]). After being washed extensively in Tris-buffered saline containing 0.05% Tween 20, membranes were blocked with 10% nonfat dry milk in Tris-buffered Saline–0.05% Tween 20 overnight at 4°C and incubated for 30 min at room temperature in goat anti-gp70 antiserum diluted 1/2,000. Blots were developed with horseradish peroxidase-conjugated anti-goat immunoglobulin (ICN), followed by ECL chemiluminscent substrate (Amersham), and exposed to Kodak XOmat AR film.
Pathology analysis.
Mice were exsanguinated by axillary incision under deep isoflurane anesthesia, and the brains were removed immediately and placed in 3.7% formaldehyde–PBS for 16 to 24 h at room temperature. Brains were dehydrated and paraffin embedded, and 5-μm-thick sections were stained with hematoxylin and eosin for routine light microscopy. The photomicrographs shown in Fig. 1 and 4 were produced using Kodak Elite 160T film and were digitized using a Nikon LS-2000 scanner. The images shown in Fig. 6 were produced by directly scanning the hematoxylin-and-eosin-stained sections using a Linotype, Circon flat bed scanner (Heidelberger Druchmaschinen AG) at 4,800 dots/in.
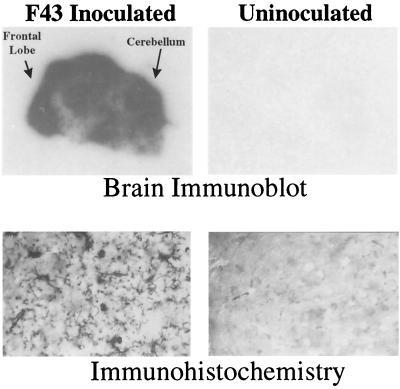
FIG. 1.
Extent of infection of the brain 28 days after intraperitoneal inoculation of neonates with the avirulent virus F43. Top panels, immunoblots of midsaggital sections of freshly frozen brain from an inoculated mouse (left panel) and an age-matched uninoculated control (right panel). Sections were blotted onto polyvinylidene fluoride membranes and probed with a goat anti-viral SU protein; bound antibody was visualized using a chemiluminsecent substrate and exposed to X-ray film (see Materials and Methods). Bottom panels, a more detailed high-power view of infected and uninfected brains. Frozen sections of formaldehyde-fixed, cryopreserved brains were stained by routine immunohistochemistry using the same anti-SU antiserum and developed with the dark-blue colored substrate VIP (Vector). These sections were not counterstained, and thus all dark-stained structures in the left panel represent virus-specific signals. This web-like array of microglia expressing SU protein was seen throughout the infected brain. These cells have been shown previously to stain with the microglia-specific antibody F4/80 (3).
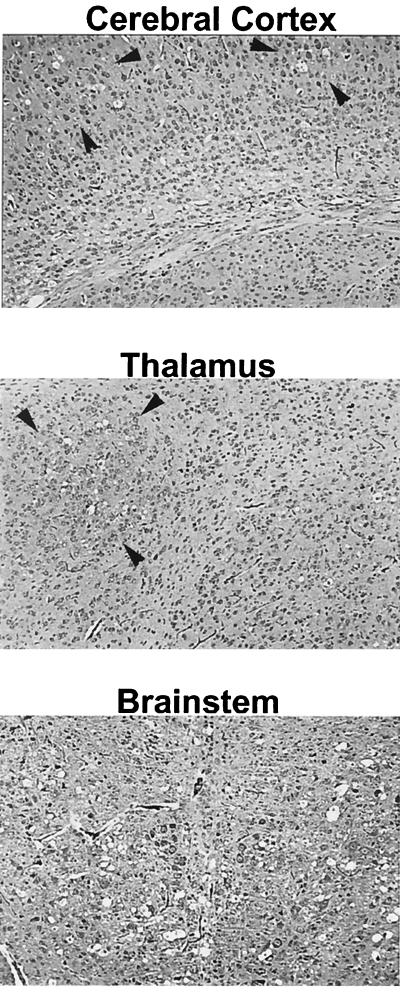
FIG. 4.
Photomicrographs of sections of various areas of the CNS 14 dpi with FrCasE. Focal spongiosis (holes), delineated by arrowheads, are seen in the cerebral cortex and thalamus. In the brain stem, however, spongiosis was already diffuse, with holes being seen throughout the entire photomicrograph. Hematoxylin and eosin staining was used. Magnification before enlargement, ×25.
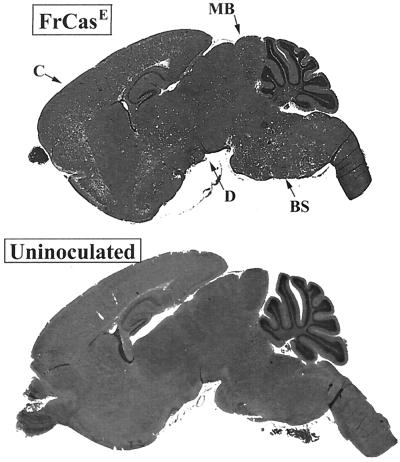
FIG. 6.
(Top) Midsaggital section of the brain of a mouse in the terminal stage of neurologic disease caused by neonatal inoculation of FrCasE. This mouse was killed 20 dpi with virus diluted 10−4. (Bottom) A comparable section of an uninoculated control mouse is shown for comparison. The white dots in the FrCasE-infected brain represent spongiform degeneration, which is extensive at this stage, being seen in the cerebral cortex (C), diencephalon (D), midbrain (MB), and brain stem (BS) regions.
Statistical analysis.
Data from the experiments containing two groups were analyzed by a nonpaired t test with Welsh correction (which does not assume equal variances). Data from the experiments containing three groups were analyzed using one-way analysis of variance, followed by Tukey's posttest. All statistical analyses were performed with Instat (GraphPad). Results presented in the graphs represent the geometric mean ± 1 standard deviation.
RESULTS
Proinflammatory responses to the nonpathogenic virus F43 are restricted to the β-chemokines.
Our initial studies focused on the nonpathogenic virus F43, which infects the brain at high levels but does not cause either spongiosis or clinical signs of neurologic disease. This virus is particularly interesting because it infects large numbers of microglial cells throughout the neuraxis. Mice which had been inoculated with F43 intraperitoneally as neonates were sacrificed at 28 dpi. The extensive infection of the brain by this virus is illustrated in Fig. 1, which shows, by histoblotting, viral envelope protein detectable throughout the brain parenchyma. By immunohistochemistry, viral protein can be seen predominantly in the web-like processes of ramified microglial cells. That these are predominently microglial cells was shown previously using double labeling with the microglia-specific antibody F4/80 (3).
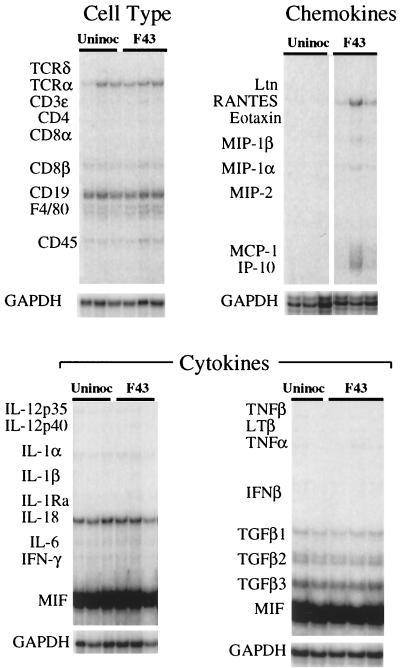
Total RNA extracted from the brains of these mice was subjected to multiprobe RPA using probe sets which detect mRNA species encoding a variety of proinflammatory cytokines and chemokines and various inflammatory-cell-type-specific molecules. Despite the extensive neuroinvasion by F43, RPA (Fig. 2) revealed little host response to this agent. There was no evidence of upregulation of proinflammatory cytokine mRNAs or of increased expression of cell-type-specific markers, including F4/80. This is particularly noteworthy because the primary target cells of this virus in the CNS are F4/80-positive microglia (3). Only the β-chemokines RANTES. MIP-1α, and MIP-1β and the α-chemokine IP-10 exhibited signs of upregulation, and these responses were variable.
FIG. 2.
Expression of genes associated with inflammatory responses in the brains of mice 28 days after neonatal intraperitoneal inoculation of the avirulent virus F43. Total RNA extracts of the brains of F43-infected and age-matched uninfected controls were analyzed by multiprobe RPA, and the autoradiograms are shown. The locations of the protected probes for inflammatory-cell-specific markers, chemokines, and cytokines are shown on the left of each panel. The signals for the GAPDH housekeeping gene for each lane are shown below each panel. Three mice are shown per group, except in the lower right panel, in which only two uninoculated controls (Uninoc) are shown. Despite the high-level and widespead infection of the brain by F43, only the mRNA species of the β-chemokines RANTES, MIP-1β, MIP-1α, and MCP-1 were variably elevated. There was no evidence for upregulation of any of the other transcripts analyzed. TCR, T-cell receptor, TGF, transforming growth factor, IFN, interferon.
Accentuated β-chemokine responses in the brains of FrCasE-infected mice.
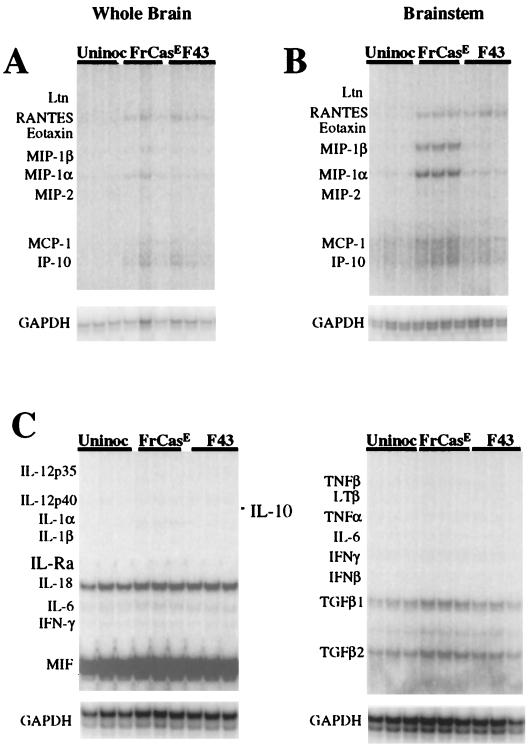
Mice inoculated with FrCasE as neonates first exhibit signs of clinical disease (reflex abnormalities of the hind limbs) at 14 to 15 dpi. We initially examined RNA from whole brain extracts of FrCasE-inoculated mice using the same probe sets shown in Fig. 2. Age-matched F43 inoculated and uninoculated mice served as controls. As in the F43 mice killed at 28 dpi, these studies revealed a remarkable dearth of upregulated genes consisting of the chemokines RANTES, MIP-1α, MCP-1, and IP-10 (Fig. 3A). Although there appeared to be some differences between the FrCasE and F43 groups, these differences were small and uninterpretable.
FIG. 3.
Comparison of RPA analyses of whole brains (A) and brain stems (B) from mice 14 dpi with the virulent virus FrCasE, the avirulent virus, F43 and age-matched uninoculated controls (Uninoc). At this early time point in the disease, MIP-1α and MIP-1β mRNA were specifically elevated in the brain stems of FrCasE-infected mice. MCP-1 was also marginally elevated, whereas RANTES was increased in both FrCasE and F43 inoculated mice. (C) Brain stem RNA analyzed by RPA for cytokine transcripts. There was no evidence for upregulation of these genes in either group of infected mice. TGF, transforming growth factor; IFN, interferon.
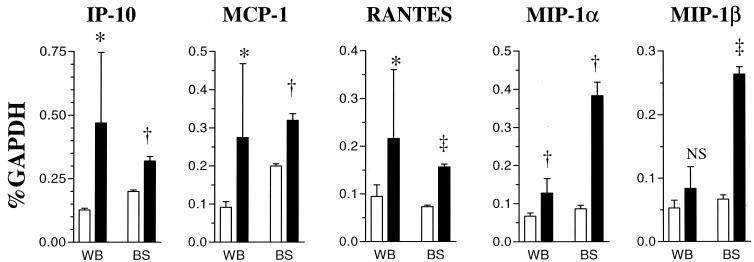
At 14 dpi, however, while spongiform lesions were detectable in many areas of the CNS, including thalamus, cerebral cortex, cerebellar nuclei (not shown), and brain stem, the lesions in all areas except the brain stem were focal in nature (Fig. 4). In the brain stem, spongiosis was already extensive (Fig. 4). RPA analysis of RNA isolated from the brain stem revealed distinct difference between the FrCasE- and F43-inoculated mice (Fig. 3B). Although the levels of RANTES mRNA were similar in the F43 and FrCasE groups, the levels of MIP-1α, MIP-1β, MCP-1, and IP-10 mRNAs were clearly higher in the mice inoculated with FrCasE than in mice inoculated with F43. Interestingly, despite the extensive spongiosis observed in the brain stem at this time point, there was no evidence for upregulation of other chemokines or cytokines (Fig. 3C). These studies, taken together, suggest that the responses of the brain to infection by FrCasE involved the same restricted set of proinflammatory mediators as observed in mice killed 28 days after infection with F43. However, the responses were accentuated in the FrCasE mice, and most importantly, increased expression of MIP-1α and MIP-1β appeared to be highest in regions of the brain having the highest concentration of spongiform lesions (i.e., brain stem > whole brain). The results of these studies are shown quantitatively in Fig. 5, which also illustrates that the RANTES, MCP-1, and IP-10 responses appeared not to exhibit the same skewing toward the brain stem as seen with MIP-1α and MIP-1β.
FIG. 5.
Enrichment of transcripts for MIP-1α and MIP-1β in the brain stems of FrCasE-inoculated mice. Quantitative analysis of chemokine mRNA levels are shown for whole brains (WB) and brain stems (BS) of mice 14 dpi with FrCasE (black bars). Age-matched uninoculated mice were the controls (white bars). Bands were quantified with a phosphorimager and normalized as a percentage of the GAPDH mRNA signal. For whole brain samples there were seven uninoculated and eight inoculated mice per group. For brain stem samples there were three mice per group. For statistical analyses, each group of inoculated mice was compared to the respective uninoculated controls. ∗, <0.05; †, <0.01; ‡, <0.001; NS, not significant.
Increased expression of TNF-α in clinically advanced neurologic disease caused by FrCasE.
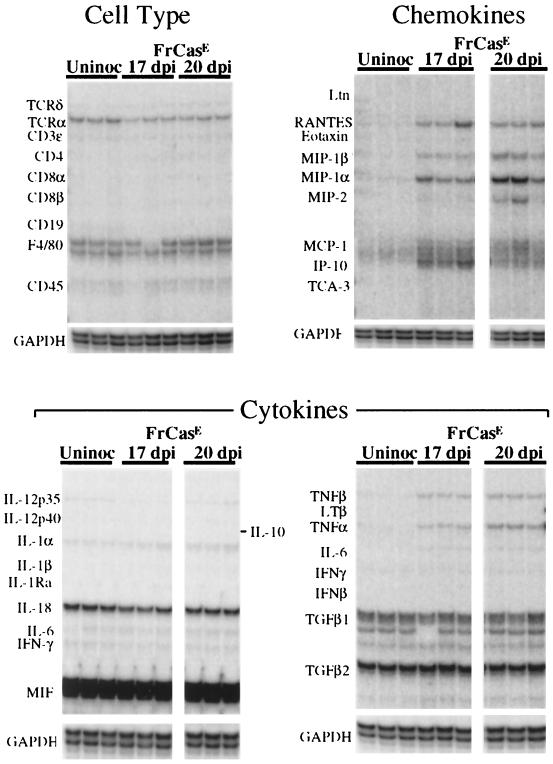
The clinical course of the disease induced by FrCasE is rather short, ranging from 3 to 6 days before mice must be sacrificed because of severe tremulous paralysis of both hind and forelimbs associated with wasting and incontinence. In an attempt to lengthen the disease course, we inoculated some mice with dilutions of FrCasE shown empirically to cause a delay in the onset of clinical signs (9). We examined two groups of mice, those that were inoculated with undiluted FrCasE and sacrificed at 17 dpi and those that were inoculated with diluted virus (10−4) and sacrificed at 20 dpi. All inoculated mice were preterminal at the time of sacrifice. The extents of the histopathology observed in these two groups, irrespective of the time of sacrifice, were indistinguishable, with spongiosis involving all levels of the neuraxis (Fig. 6).
Total RNA was extracted from whole brains of these mice and was subjected to multiprobe RPA (Fig. 7). In addition to the chemokines which were upregulated at 14 dpi (RANTES, MIP-1α, MIP-1β, MCP-1, and IP-10), there was also evidence for upregulation of expression of MIP-2 as well as the cytokines TNF-α and TNF-β. All genes found to be upregulated at 17 dpi were also upregulated at 20 dpi. Thus, although the responses in early stages of lesion development were restricted to the chemokines, a limited number of additional genes were upregulated in late-stage disease. It is noteworthy that although there was clear-cut upregulation of TNF-α and TNF-β in the inoculated groups, other responses which might be consistent with microglial activation were not observed. Thus, there was no evidence for upregulation of interleukin-1α (IL-1α), IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-6, F4/80, or CD45 (Fig. 7). This was particularly curious in view of (i) the observation that this virus, like F43, replicates in microglia (28) and (ii) the widespread spongiform neurodegeneration observed in these mice and the notion that microglial cells are generally considered to be early responders to neuronal insult (23).
FIG. 7.
Expression of genes associated with inflammatory responses in the brains of mice with advanced neurologic disease. All mice were preterminal, with severe tremor and both hind- and forelimb paralysis. Mice were killed 17 days after neonatal inoculation of FrCasE or 20 days after inoculation of diluted FrCasE, and RNA was extracted from whole brains. RPA analysis shows, in addition to the chemokines found to be upregulated at 14 dpi (upper right panel), the upregulation of TNF-α and TNF-β (lower right panel) as well as the chemokine MIP-2. It should be noted, however, that even at this late time point in the disease, there was no evidence for increased expression of other cytokine genes, including those for the IL-1 family or gamma interferon (IFNγ) (lower panels). IL-6 appeared to be marginally increased in the infected groups (lower right panel), but this response was not seen consistently (lower left panel). In addition, there was no evidence for increased expression of inflammatory-cell-specific markers (upper left panel). Note the constitutive expression of T-cell receptor α (TCRα) in the brains of all mice (see Discussion). TGF, transforming growth factor.
Fifteen-day-old mice respond to neuronal death induced by ibotenic acid.
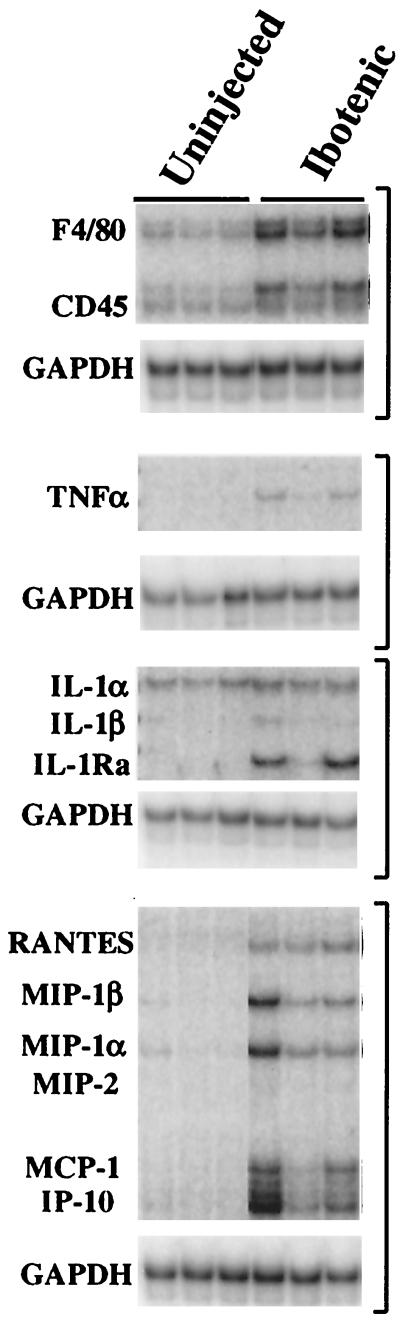
In view of the surprisingly restricted nature of the inflammatory responses observed in the FrCasE-infected mice, even in the presence of substantial spongiform degeneration, it was important to determine whether this might be related to the young age of the host. It has been reported, for instance, that even traumatic injury to the neonatal mouse brain evokes little of the MCP-1 response or astrogliosis observed with similar injury to the adult brain (14). We therefore injected the neurotoxin ibotenic acid intracerebrally at postnatal day 11 (corresponding to the time when the first signs of spongiosis are observed in FrCasE-infected mice [10]). The mice were killed 4 days later (corresponding to the 14-dpi time point for the FrCasE-inoculated mice shown in Fig. 3). Ibotenic acid is a glutamatergic neurotoxin which binds to the N-methyl-d-aspartate receptor and, like other excitotoxins, kills neurons by both apoptosis and necrosis (41). Histopathologic examination (not shown) revealed a well-circumscribed area of cell death. RPA on RNA extracted from frontal lobes (Fig. 8) revealed that in addition to the genes upregulated in the FrCasE-infected mice (i.e., chemokines and TNF-α), there was also upregulation of F4/80, CD45, and IL-1Ra, suggesting an associated activation of microglia. It appears, therefore, that the restricted nature of the inflammatory response seen in the FrCasE-infected brains could not be attributed to the young age of the mice.
FIG. 8.
RPA analysis of frontal lobes 4 days after intracerebral injection of 10 μg of ibotenic acid. a glutamatergic neurotoxin. Mice were injected on postnatal day 11 and sacrificed on postnatal day 15. Shown are those genes which were upregulated in the treated group. TNF-α and the same chemokines found to be upregulated in FrCasE-inoculated mice were also upregulated in the ibotenic acid-treated mice. In addition, however, F4/80, CD45, and IL-1Ra mRNA levels were increased in the ibotenic acid-treated mice. These genes were never found to be upregulated in FrCasE-infected mice, even in those mice with advanced disease.
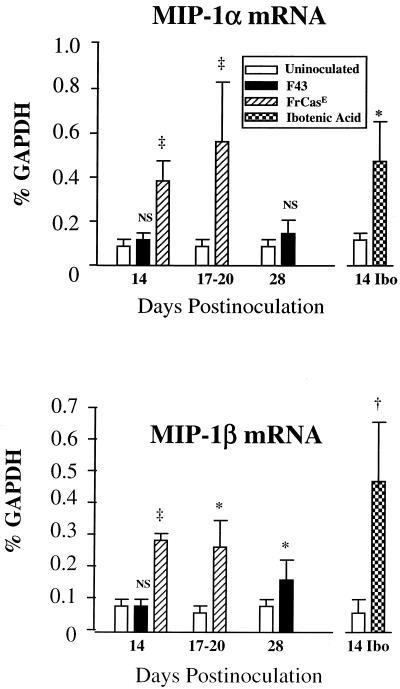
In order to place the MIP-1α and MIP-1β responses induced by virus infection in context, we compiled the quantitative data on these chemokine mRNAs (Fig. 9). Two points can be made from this bar graph: (i) the upregulation of MIP-1α and MIP-1β mRNAs in the FrCasE groups were similar in amplitude to that observed in the ibotenic acid-treated mice, and (ii) although, as seen in Fig. 2, F43 induced upregulation of both MIP-1α and MIP-1β mRNAs, the amplitude of the responses was three- to fivefold lower than that seen in the FrCasE-inoculated mice.
FIG. 9.
MIP-1α and MIP-1β mRNA responses to FrCasE infection were three- to fivefold greater than those induced by F43 and were comparable to the responses measured in mice injected with ibotenic acid. Shown are compilations of quantitative determinations of the relative levels of these mRNA species found in brain stem 14 days postinoculation and in whole brain extracts 17 to 20 days and 28 days postinoculation. For comparison, the levels of MIP-1α and MIP-1β mRNAs in frontal lobes of 15-day-old mice injected intracerebrally 4 days earlier with ibotenic acid were quantified. The numbers of mice per group ranged from three to eight. Symbols representing P values are defined in the legend to Fig. 5.
DISCUSSION
Despite the lack of inflammatory cell infiltrates in the neurodegenerative disease caused by the murine retrovirus FrCasE, we found evidence of increased expression, at the mRNA level, of a variety of inflammatory mediators in the brain. These included the chemokines MIP-1α. MIP-1β, MIP-2, MCP-1, RANTES, and IP-10 and the cytokines TNF-α and TNF-β. The upregulation of TNF-α and -β mRNAs in the brains of mice with advanced disease caused by FrCasE is consistent with reports that increased expression of TNF is a correlate of the spongiform encephalopathies caused by the oncornaviruses CasBrE (38) and ts-1 Moloney MuLV (7), as well as the transmissible spongiform encephalopathy agents (6). Neither TNF-α nor TNF-β mRNA was found to be upregulated in the brains of mice inoculated with the neuroinvasive but avirulent virus F43. Thus, upregulation of TNF-α and TNF-β mRNAs would appear to correlate with neurovirulence. However, when we examined FrCasE-infected mice at an earlier stage of the disease, there was no evidence of upregulation of these genes, despite the presence of extensive spongiosis. This lack of temporal correlation suggests that increased expression of TNF-α and -β more likely represents a response to the spongiosis rather than its cause.
Among the chemokines, the upregulation of RANTES mRNA appeared to represent a generic response to virus infection, since the response was comparable in both F43- and FrCasE-inoculated mice. IP-10 also appeared to belong in this category, as its expression was upregulated approximately threefold in F43-inoculated mice, even at the 14-day time point (not shown). However, this did not appear to be the case for other chemokines. MIP-1α, MIP-1β, and MCP-1 mRNAs each were incrementally upregulated in F43-infected mice at 28 dpi, but these genes appeared not to be upregulated at 14 dpi. Yet at this early time point, levels of each of these mRNA species were clearly increased in the FrCasE-infected mice, although their distributions within the brain were not identical. Unlike the case for MCP-1, there was a positive correlation between the levels of MIP-1α and MIP-1β mRNAs and the relative level of spongiosis. Thus, while MCP-1 mRNA was clearly increased in whole brain extracts at 14 dpi, this was not the case for MIP-1α and MIP-1β mRNAs, which were only marginally increased. Yet both MIP-1α and MIP-1β mRNAs were increased dramatically in the brain stem, the site where spongiosis was most concentrated at 14 dpi. The incremental increase in MIP-1 mRNAs in whole brain extracts at this early time point was therefore a consequence of dilutional effects, since the brain stem made up less than 10% of the whole brain extacts. This regional specificity was not seen for MCP-1, RANTES, or IP-10 mRNA (Fig. 5). Thus, it appears from these observations that early in the disease, MIP-1α and MIP-1β were the only proinflammatory mediators in our panel to be upregulated in a lesion-specific fashion. Furthermore, the magnitude of the responses was robust, since it was comparable to that observed in mice injected intracerebrally with the glutamatergic neurotoxin ibotenic acid.
While upregulation of MIP-1α and MIP-1β coincided with the appearance of spongiosis, it is not clear whether these responses actually preceded the appearance of lesions. At 14 dpi with FrCasE, spongiosis was of a focal nature except in the brain stem, where lesions were already extensive. Three to 6 days later (in mice with advanced disease), spongiosis was widespread throughout the brain and MIP-1α and MIP-1β transcripts were increased as well in whole brain extracts (Fig. 7). Thus, the upregulation of MIP-1α and β in this disease could, like that of TNF-α and TNF-β, simply represent a generic, albeit early, response to the neuronal and neuroglial damage induced by FrCasE. Even subtle alterations in myelin integrity induced by intraperitoneal injection of the toxin triehyltin (35) have been shown to cause upregulation of MIP-1α. Furthermore, in the present study, the chemokine responses of the brain after injection of ibotenic acid were qualitatively and quantitatively similar to those induced by FrCasE. Thus, from the perspective of the chemokine response profile alone, it is difficult to distinguish the neurotoxicity caused by ibotenic acid from that caused by infection of the brain by FrCasE. These observations, then, support the notion that these responses were a reaction to the neuronal damage and point out the difficulty in drawing causal relationships from correlative data, even in a well-controlled animal model. It will now be important to test more directly the role of these chemokines in the pathogenesis of the spongiosis using appropriate knockout mice.
In the present study, the lack of inflammatory cell infiltrates in the brain was supported by the absence of any demonstrable change in expression of lymphocyte-specific genes such as those for CD3, CD4, and CD-8 or of macrophage/monocyte markers CD45 and F4/80. It should be noted that T-cell receptor α chain mRNA appeared to be constitutively expressed in the brains of all mice, irrespective of whether they were infected or not (Fig. 2 and 7). This transcript has been observed previously in both the brain and kidney (32) but appears to be expressed by nonlymphoid cells.
How, then, can one explain this lack of cellular infiltration in the face of increased expression of a variety of chemokines in the brain? It has been shown that intracranial injection of recombinant chemokines alone is sufficient to induce cellular infiltration (4). Furthermore, transgenic mice overexpressing MCP-1 in the brain also exhibit inflammatory cell infiltates (12). Chemokine expression, however, is only one of several factors which promote passage of leukocytes across the blood-brain barrier. The upregulation of cell adhesion molecules (VCAM and ICAM) on the endothelial cell membrane and increased expression of integrins on the surface of activated leukocytes also affect the efficiency of transmigration. In addition, the accumulation at least of T lymphocyes in the CNS requires antigen recognition (reviewed in reference 17). It should be noted that the mice in the present study were inoculated as neonates and exhibit a profound immunologic hyporesponsiveness to the virus (20). Thus, despite the presence of abundant viral antigen in the nervous system, this age-related immunologic tolerance alone could explain the lack of T lymphocytes in the brains of these mice. It will be important to determine whether the upregulation of chemokine mRNAs is reflective of increased expression at the protein level and, if so, what cells are actually expressing these mediators. Immunohistochemical studies will be required to address these issues.
Increased expression of β-chemokines in the brain appears to be a correlate of human immunodeficiency virus-associated dementia (22) and Simian immunodeficiency virus encephalitis (44) and is also observed in neurologic diseases induced by a variety of other viruses, including lymphocytic choriomeningitis virus (2), mouse hepatitis virus (26), Theiler's virus (19), and Bornavirus (37). In each of these examples, there is other evidence of an inflammatory component, including influx of mononuclear cells from the periphery and/or activation of microglia and astrocytes. We have found none of these other signs of inflammation in the disease caused by FrCasE. Even in the advanced stages of paralytic disease associated with widespread spongiosis, there was no evidence for upregulation of F4/80 or cytokines such as IL-1α, IL-1β, or IL-1Ra, which might suggest microglial activation. In addition, previous immunohistochemical studies have failed to find upregulation of CD11b (28, 30), another marker of microglial activation. We considered the possibility that the apparent lack of microglial response might be related to the young age of the mice. However, this did not appear to be the case, since intracerebral injection of ibotenic acid into mice of comparable age induced upregulation of F4/80 as well as CD45 and IL-1Ra. The apparent lack of microglial activation in the disease caused by FrCasE remains unexplained but suggests the possibility that the virus itself may downmodulate microglial responses.
These studies have found that the spongiosis induced by FrCasE is associated with signs of an early, albeit blunted, inflammatory response manifested principally by the upregulation of chemokine but not cytokine mRNAs. It will be important now to look for other signs of both peripheral and CNS inflammation, such as the upregulation of acute-phase proteins and perhaps the expression of complement components and matrix metalloproteinases in the brain.
ACKNOWLEDGMENTS
We thank W. Ian Lipkin (University of California, Irvine) for his enthusiasm and encouragement in studying the nonvirulent virus F43 and Glenn Rall (Fox Chase) and Blaise Favara (RML) for critiquing the manuscript. We also thank Gary Hettrick (RML) for assistance in generating the digitized micrograph used for Fig. 6.
REFERENCES
- 1.Andrews J M, Gardner M B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974;33:285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Asensio V C, Kincaid C, Campbell I L. Chemokines and the inflammatory response to viral infection in the central nervous system with a focus on lymphocytic choriomeningitis virus. J Neurovirol. 1999;5:65–75. doi: 10.3109/13550289909029747. [DOI] [PubMed] [Google Scholar]
- 3.Askovic S, McAtee F J, Favara C, Portis J L. Brain infection by neuroinvasive but avirulent murine oncornaviruses. J Virol. 2000;74:465–473. doi: 10.1128/jvi.74.1.465-473.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell M D, Taub D D, Perry V H. Overriding the brain's intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience. 1996;74:283–292. doi: 10.1016/0306-4522(96)00083-8. [DOI] [PubMed] [Google Scholar]
- 5.Betmouni S, Perry V H, Gordon J L. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience. 1996;74:1–5. doi: 10.1016/0306-4522(96)00212-6. [DOI] [PubMed] [Google Scholar]
- 6.Campbell I L, Eddleston M, Kemper P, Oldstone M B, Hobbs M V. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J Virol. 1994;68:2383–2387. doi: 10.1128/jvi.68.4.2383-2387.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choe W, Stoica G, Lynn W, Wong P K. Neurodegeneration induced by MoMuLV-ts1 and increased expression of Fas and TNF-alpha in the central nervous system. Brain Res. 1998;779:1–8. doi: 10.1016/s0006-8993(97)00929-3. [DOI] [PubMed] [Google Scholar]
- 8.Czub M, Czub S, McAtee F J, Portis J L. Age-dependent resistance to murine retrovirus-induced spongiform neurodegeneration results from central nervous system-specific restriction of virus replication. J Virol. 1991;65:2539–2544. doi: 10.1128/jvi.65.5.2539-2544.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czub M, McAtee F J, Portis J L. Murine retrovirus-induced spongiform encephalomyelopathy: host and viral factors which determine the length of the incubation period. J Virol. 1992;66:3298–3305. doi: 10.1128/jvi.66.6.3298-3305.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czub S, Lynch W P, Czub M, Portis J L. Kinetic analysis of spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab Invest. 1994;70:711–723. [PubMed] [Google Scholar]
- 11.DesGroseillers L, Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984;52:448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuentes M E, Durham S K, Swerdel M R, Lewin A C, Barton D S, Megill J R, Bravo R, Lira S A. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- 13.Gardner M B, Henderson B E, Officer J E, Rongey R W, Parker J C, Oliver C, Estes J D, Huebner R J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973;51:1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glabinski A R, Balasingam V, Tani M, Kunkel S L, Strieter R M, Yong V W, Ransohoff R M. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- 15.Gravel C, Kay D G, Jolicoeur P. Identification of the infected target cell type in spongiform myeloencephalopathy induced by the neurotropic Cas-Br-E-murine leukemia virus. J Virol. 1993;67:6648–6658. doi: 10.1128/jvi.67.11.6648-6658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallensleben W, Biro L, Sauder C, Hausmann J, Asensio V C, Campbell I L, Staeheli P. A polymorphism in the mouse crg-2/IP-10 gene complicates chemokine gene expression analysis using a commercial ribonuclease protection assay. J Immunol Methods. 2000;234:149–151. doi: 10.1016/s0022-1759(99)00197-0. [DOI] [PubMed] [Google Scholar]
- 17.Hickey W F. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch E C, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson's disease: a role in neurodegeneration? Ann Neurol. 1998;44:S115–S120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman L M, Fife B T, Begolka W S, Miller S D, Karpus W J. Central nervous system chemokine expression during Theiler's virus-induced demyelinating disease. J Neurovirol. 1999;5:635–642. doi: 10.3109/13550289909021292. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman P M, Robbins D S, Morse H C., III Role of immunity in age-related resistance to paralysis after murine leukemia virus infection. J Virol. 1984;52:734–738. doi: 10.1128/jvi.52.3.734-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay D G, Gravel C, Robitaille Y, Jolicoeur P. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc Natl Acad Sci USA. 1991;88:1281–1285. doi: 10.1073/pnas.88.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelder W, McArthur J C, Nance-Sproson T, McClernon D, Griffin D E. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 23.Kreutzberg G W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 24.Lampert P W, Gajdusek D C, Gibbs C J., Jr Subacute spongiform virus encephalopathies. Scrapie, Kuru and Creutzfeldt-Jakob disease: a review. Am J Pathol. 1972;68:626–652. [PMC free article] [PubMed] [Google Scholar]
- 25.Lander M R, Chattopadhyay S K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic. amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane T E, Asensio V C, Yu N, Paoletti A D, Campbell I L, Buchmeier M J. Dynamic regulation of α- and β-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J Immunol. 1998;160:970–978. [PubMed] [Google Scholar]
- 27.Lipkin W I, Oldstone M B. Analysis of endogenous and exogenous antigens in the nervous system using whole animal sections. J Neuroimmunol. 1986;11:251–257. doi: 10.1016/0165-5728(86)90009-3. [DOI] [PubMed] [Google Scholar]
- 28.Lynch W P, Czub S, McAtee F J, Hayes S F, Portis J L. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron. 1991;7:365–379. doi: 10.1016/0896-6273(91)90289-c. [DOI] [PubMed] [Google Scholar]
- 29.Lynch W P, Portis J L. Neural stem cells as tools for understanding retroviral neuropathogenesis. Virology. 2000;271:227–233. doi: 10.1006/viro.2000.0338. [DOI] [PubMed] [Google Scholar]
- 30.Lynch W P, Robertson S J, Portis J L. Induction of focal spongiform neurodegeneration in developmentally restricted mice by implantation of murine retrovirus-infected microglia. J Virol. 1995;69:1408–1419. doi: 10.1128/jvi.69.3.1408-1419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch W P, Snyder E Y, Qualtiere L, Portis J L, Sharpe A H. Late virus replication events in microglia are required for neurovirulent retrovirus-induced spongiform neurodegeneration: evidence from neural progenitor-derived chimeric mouse brains. J Virol. 1996;70:8896–8907. doi: 10.1128/jvi.70.12.8896-8907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madrenas J, Pazderka F, Parfrey N A, Halloran P F. Thymus-independent expression of a truncated T cell receptor-alpha mRNA in murine kidney. J Immunol. 1992;148:612–619. [PubMed] [Google Scholar]
- 33.Marret S, Mukendi R, Gadisseux J F, Gressens P, Evrard P. Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol. 1995;54:358–370. doi: 10.1097/00005072-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 34.McGeer P L, McGeer E G. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 35.Mehta P S, Bruccoleri A, Brown H W, Harry G J. Increase in brain stem cytokine mRNA levels as an early response to chemical-induced myelin edema. J Neuroimmunol. 1998;88:154–164. doi: 10.1016/s0165-5728(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 36.Messmer K, Reynolds G P. Increased peripheral benzodiazepine binding sites in the brain of patients with Huntington's disease. Neurosci Lett. 1998;241:53–56. doi: 10.1016/s0304-3940(97)00967-1. [DOI] [PubMed] [Google Scholar]
- 37.Morimoto K, Hooper D C, Bornhorst A, Corisdeo S, Bette M, Fu Z F, Schafer M K, Koprowski H, Weihe E, Dietzschold B. Intrinsic responses to Borna disease virus infection of the central nervous system. Proc Natl Acad Sci USA. 1996;93:13345–13350. doi: 10.1073/pnas.93.23.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagra R M, Heyes M P, Wiley C A. Viral load and its relationship to quinolinic acid, TNF alpha, and IL-6 levels in the CNS of retroviral infected mice. Mol Chem Neuropathol. 1994;22:143–160. doi: 10.1007/BF03160102. [DOI] [PubMed] [Google Scholar]
- 39.Paquette Y, Hanna Z, Savard P, Brousseau R, Robitaille Y, Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci USA. 1989;86:3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry V H, Anderson P B, Gordon S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993;16:268–273. doi: 10.1016/0166-2236(93)90180-t. [DOI] [PubMed] [Google Scholar]
- 41.Portera-Cailliau C, Price D L, Martin L J. Excitotoxic neuronal death in the immature brain is an apoptosis-necrosis morphological continuum. J Comp Neurol. 1997;378:70–87. [PubMed] [Google Scholar]
- 42.Portis J L, Czub S, Garon C F, McAtee F J. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol. 1990;64:1648–1656. doi: 10.1128/jvi.64.4.1648-1656.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portis J L, Lynch W P. Dissecting the determinants of neuropathogenesis of the murine oncornaviruses. Virology. 1998;247:127–136. doi: 10.1006/viro.1998.9240. [DOI] [PubMed] [Google Scholar]
- 44.Sasseville V G, Smith M M, Mackay C R, Pauley D R, Mansfield K G, Ringler D J, Lackner A A. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- 45.Swarz J R, Brooks B R, Johnson R T. Spongiform polioencephalomyelopathy caused by a murine retrovirus. II. Ultrastructural localization of virus replication and spongiform changes in the central nervous system. Neuropathol Appl Neurobiol. 1981;7:365–380. doi: 10.1111/j.1365-2990.1981.tb00239.x. [DOI] [PubMed] [Google Scholar]