Abstract
Gray blight is a serious threat to the tea [Camellia sinensis (L.) O. Kuntze] production in major tea cultivating countries including India. The disease is caused by Pestalotiopsis-like species. In this study, five isolates of Pseudopestalotiopsis species isolated from symptomatic tea leaf samples in North Bengal, India were investigated. Based on the multi-locus phylogenetic analysis using concatenated sequences of three (ITS, tef-1 alpha, and tub-2) loci, cultural and micromorphological characters, and host association, the fungal isolates were identified as Pseudopestalotiopsis ampullacea F. Liu & L. Cai. The morphological analysis also revealed that the fungal isolates were evidently differentiated from other Pseudopestalotiopsis species. To date, P. ampullacea has not been reported on tea plants in India. Among the five isolates studied, isolate NKT0P03 was randomly selected for pathogenicity tests and its sensitivity to fungicides and microbial antagonists. In pathogenicity test, the isolate showed weak to high virulence reactions on 25 different tea cultivars. The pathogen showed an avirulent reaction on the cultivar TV11. In order to identify an effective management strategy against this new pathogen, synthetic fungicides and microbial biocontrol agents were evaluated in the laboratory. Results revealed that carbendazim + mancozeb, hexaconazole, propiconazole, and valextra were effective fungicides with an 85.1% to 89.8% range of inhibitory activity against P. ampullacea NKT0P03. Among microbial agents, Trichoderma harzianum, T. reesei, T. hamatum, Bacillus subtilis, and Microbacterium barkeri were efficient bioagents against P. ampullacea NKT0P03 with antagonistic activity ranging between 66.6% and 84.2%. Thus, these fungicides and microbial bioagents can be recommended as effective agents for the management of P. ampullacea causing tea gray blight after their field evaluations.
Keywords: Phylogeny, Pathogenicity, Microbial agents, Synthetic fungicides
Subject terms: Biotechnology, Microbiology, Plant sciences
Introduction
Worldwide, tea is a highly valued aromatic beverage scientifically known as Camellia sinensis (L.) O. Kuntze1. The cultivation of tea is a main source of livelihood in major tea-producing countries2. China is a major tea producer with 2.5 MT annually, followed by India (1.3 MT), Kenya, and Sri Lanka3. However, many diseases and insect pests hinder tea production. Among the diseases that infect tea, gray blight is a prevalent disease worldwide4. The disease mostly attacks old and mature leaves of tea plant in major tea growing countries such as China, India, and Sri Lanka. In southern India5 the disease caused 17% yield loss of tea production and in Japan6 it caused 10% to 20% yield losses, however yield loss estimates from China has not been conducted7. The early disease symptoms start with light yellow brown spots, which change brown and grey-white with disease progresses. The disease symptoms also varied based on the causal agents, i.e., Pestalotiopsis-like species4. The necrotic spots are sometimes irregular, circular or semi-circular, and contain abundant acervuli in rings or distributed on the adaxial leaf surface8,9. Under severe conditions, the disease destroys the entire leaf canopy of the tea plants.
Besides tea, worldwide Pestalotiopsis-like species also infect several other crops including strawberry, tomato, cardamon, coconut, mango, guava, grape, and coffee, both in postharvest and under field conditions10. The gray blight pathogen was primarily considered as Pestalotiopsis theae (Sawada) Steyaert11. However, with advancement of molecular biology, Maharachchikumbura et al.12 reviewed Pestalotiopsis species based on phylogenetic analysis of multi-locus, i.e., ITS (internal transcribed spacer), tub-2 (β-tubulin) and tef-1 alpha (translation elongation factor 1-alpha) combined with micromorphological studies and established two novel genera, Pseudopestalotiopsis and Neopestalotiopsis. Consequently, P. theae was transferred to the Pseudopestalotiopsis12. Succeeding investigations revealed that the gray blight of tea was not incited by a solitary pathogenic species, but by multiple species of Pseudopestalotiopsis, Neopestalotiopsis, and Pestalotiopsis4,7,13,14. Earlier, the genus Pseudopestalotiopsis was consisted of 11 species, however, Pestalotiopsis s. str. contained more than 500 species on various hosts, with numerous species retained under Pestalotiopsis sensu lato (s. lat.). So far, several species of Pseudopestalotiopsis have been recorded on various hosts. These includes P. camelliae-sinensis, P. cocos, P. ignota, P. indica, P. thailandica, P. kubahensis, P. ampullacea, P. chinensis, and P. theae4,12,13,15. It could therefore be difficult to accurately describe each characteristic trait of the genus Pseudopestalotiopsis given the large number of species.
In this study, we have isolated and characterized five isolates of Pseudopestalotiopsis ampullacea F. Liu & L. Cai from gray blight symptomatic leaf of tea plant in India based on phylogenetic analysis of multi-locus genes combined with micro-morphological features. Pseudopestalotiopsis ampullacea has been isolated and defined taxonomically from tea plant and other hosts including mango in China13,16. The pathogen can cause necrosis of tea and mango leaves, but the pathogenicity, geographic distribution, and effective management strategy of this pathogenic species are still unidentified13. Therefore, in the present study we have investigated the morpho-molecular analysis of P. ampullacea isolated from tea plant and its pathogenicity on various cultivars of C. sinensis. In order to find out an effective management method, beneficial microbial agents and synthetic fungicides have also evaluated against P. ampullacea under laboratory conditions.
Materials and methods
Disease samples collection, pathogen isolation, and micromorphological analysis
Diseased leaves of C. sinensis (mixed tea cultivars including TV22) with dull-brown necrotic spots were collected from different sections/divisions of tea garden of Nagrakata, Jalpaiguri, West Bengal, India (Latitude 26.9º N, Longitude 88.9º E, Elevation 262m) in pre-sterilized Ziploc bags in five replicates (each replicate contained 5 symptomatic leaves). In total, five disease samples with assigned codes NKT0P03, NKT0P04, NKT0P05, NKT0P06 and NKT0P07 were collected from different sections/divisions in X (cross) pattern. The Ziploc bags with diseased samples were transported to the Mycology laboratory, Tea Research Association, North Bengal Regional R & D Centre, Nagrakata, West Bengal and each sample was surface sterilized with 1% Sodium hypochlorite solution. After surface sterilization each leaf was cut into small pieces across the necrotic spots and were plated onto pre-sterilized potato dextrose agar medium (PDA, Himedia, India). The diseased tissue inoculated Petri plates were incubated at 28°C with 12 h white light in a BOD (Biochemical Oxygen Demand, Reico, India) incubator for 7 days in three replicates7. After the stipulated incubation, fungal colonies appeared on the diseased tissues of each sample on agar surface were separately transferred onto fresh PDA plates and incubated under the same conditions for morphological analysis. The culture obtained from each sample was also maintained on agar slant in three replicates through single spore isolation technique and preserved at 4°C for further need.
Micro-morphological features and the day-to-day growth measurement rate of fungal colonies were carried out as described in our previous study4. For each isolate, a mycelial disc of 6 mm diameter was cut from actively growing 6-day-old culture, and kept on the centre of a fresh PDA plate, and incubated under the same conditions as previously described. The growth rate of each isolate was measured on daily basis after 5 days of inoculation and was based on mean values from three biological replicates. The micro-morphological features of conidia and appendages of each isolate were measured and photographed using a camera (Eclipse 80i; Nikon, Tokyo, Japan)-attached light microscope (Olympus Magnus). The microscopical examination of each isolate was based on randomly selected 25 each conidia and appendages.
DNA extraction and polymerase chain reaction (PCR)
In the present study, 7-day-old culture of each fungal isolate grown on PDA medium was used for the extraction of genomic DNA. The DNA of each fungal isolate was extracted using Qiagen DNA Mini Kit (Qiagen, Tokyo, Japan) adopting the manufacturer protocols. The quality of extracted DNA was assessed by recording absorbance at 260 nm using a Nanodrop 1000 spectrophotometer (Thermo Fisher, Mumbai, India). For the sequencing of genomic DNA, ITS, tef-1 alpha, and tub-2 genes were amplified using the primer pairs ITS1 and ITS217, EF1-526F and EF1-1567R17, and BT2a and BT2b18, respectively. The amplification of reaction mixture was carried out in a thermo cycler (BioRad, Pune, India) with the following PCR conditions for ITS gene: initial denaturation for 1 min at 95 °C followed by 35 denaturation cycles for 30 s at 95 °C, annealing for 30 s at 52 °C, extension for 60 s at 72 °C, and final extension for 7 min at 72 °C. The PCR conditions for tef-1 alpha and tub-2 genes were as follows: initial denaturation for 2 min at 94 °C followed by 36 cycles of denaturation for 30 s at 94 °C, annealing for 60 s at 55 °C (61 °C for tub-2), and extension for 60 s at 72 °C, and final extension for 10 min at 72 °C and hold on 4°C. The amplified products were purified separately using agarose gel (2%) with the help of electrophoresis. The bands of respective gene appeared on gel were scratched and purified for sequencing using UniPro Gel extraction kit (Macrogen, Inc., Korea). The sequencing was done by Eurofins Analytical Services India Private Limited based on Bangalore, Karnataka, India. The obtained sequences of each isolate were assembled and edited in the BioEdit Sequence Alignment Editor19. Further, for similarity test, sequences of each gene were matched to known P. ampullacea sequences available in NCBI, GenBank database.
Phylogenetic analyses
The obtained ITS, tef-1 alpha, and tub-2 sequences of all the five isolates of Pseudopestalotiopsis, i.e., NKT0P03, NKT0P04, NKT0P05, NKT0P06, and NKT0P07 were deposited in GenBank (NCBI) and used for phylogenetic analysis. The available sequences for same loci, i.e., ITS, tef-1 alpha and tub-2 genes of 19 Pseudopestalotiopsis species infecting various hosts including tea were retrieved from GenBank for the construction of phylogenetic tree and the sequence of Neopestalotiopsis sp. was used as an out group (Table 1). Multiple sequence alignment of each gene was constructed using Muscle. Phylogenetic tree was constructed and observed in MEGA XI. Phylogenetic analyses of the gene sequences involved Maximum Parsimony (MP) based on combined ITS, tef-1 alpha, and tub-2 genes sequences of the isolate NKT0P03. To estimate the confidence value for grouping within a tree, we evaluated the robustness of the most parsimonious tree by performing 1,000 bootstrap replications, each with ten replicates of random stepwise taxa additions20. In addition, with the help of MrBayes software v.3.1.221 Bayesian analysis of concatenated alignments was performed. Suitable models of nucleotide substitution were selected for analysis and the best-fit evolutionary models for each locus were assessed in MrModeltest v.2.3 using the Akaike Information Criterion22. The GTR model (no rate variation) was chosen for the concatenated alignments and then used for the Bayesian inference analysis. Analysis of six Markov chain Monte Carlo chains based on the full dataset was run for 1 × 107 generations and sampled every 1000 generations. As burn-in, the first 25% of the generations were discarded during the analysis. The generated trees were viewed and annotated in TreeGraph 223.
Table 1.
Pseudopestalotiopsis species isolated from tea plants and the reference isolates used for phylogenetic analyses.
| S.N. | Species | Isolate* | Host | Location | GenBank accession number | ||
|---|---|---|---|---|---|---|---|
| ITS | TEF | TUB | |||||
| 1. | P. camelliae sinensis | LB01 | Camellia sinensis | China | KX757707 | KY342347 | KX757720 |
| 2. | P. theae | GMCC3.9192 | C. sinensis | China | AB482210 | AB453858 | KU562851 |
| 3. | P. chinensis | LC6695 | C. sinensis | China | KX895031 | KX895249 | KX895364 |
| 4. | P. ampullacea | LC6618 | C. sinensis | China | KX895025 | KX895244 | KX895358 |
| 5. | P. ampullacea | NKT0P03 | C. sinensis | India | OQ889166 | OQ883952 | PP426614 |
| 6. | P. ampullacea | NKT0P04 | C. sinensis | India | PP218054 | PP230444 | PQ567073 |
| 7. | P. ampullacea | NKT0P05 | C. sinensis | India | PP218053 | PP230445 | PQ567074 |
| 8. | P. ampullacea | NKT0P06 | C. sinensis | India | PQ569084 | PQ567071 | PQ567075 |
| 9. | P. ampullacea | NKT0P07 | C. sinensis | India | PQ558148 | PQ567072 | PQ572174 |
| 10. | P. thailandica | MFLUCC 17-1724 | Rhizophora mucronata Lam | Thailand | NR_164472 | MK764336 | MK764358 |
| 11. | P. ignota | NN 42909 strain D97 | C. sinensis | China | KU500020 | KU500016 | - |
| 12. | P. curvatispora | MFLUCC 17-1722-23 | R. mucronata | Thailand | NG_067727 | MK764334 | MK764355 |
| 13. | P. cocos | CBS 272.29 | Cocos nucifera L. | Colombia | NG_069226 | MT957940 | KM199467 |
| 14. | P. dawaina | INPA_2912 | – | Myanmar | MN096659 | MN151310 | LC324751 |
| 15. | P. elaeidis | CBS 144023 | Acacia crassipes | China | MH554106 | MH554540 | MH554779 |
| 16. | P. gilvanii | INPA 2914 | Paullinia cupana Mart var. sorbilis | Brazil | MN385953 | MN385959 | MN385958 |
| 17. | P. indica | CBS 459.78 | Hibiscus rosa-sinensis L. | Thailand | NG_066217 | KM199560 | KM199470 |
| 18. | P. ixorae | NTUCC 17-001.2 | Ixora sp. | Taiwan | MH026056.1 | MG816337 | MG816326 |
| 19. | P. kawthaungina | F0083 | – | Myanmar | LC324753 | LC324755 | LC324754 |
| 20. | P. rhizophorae | MFLUCC 17-1560 | Rhizophora apiculata | Thailand | MN877437 | MK764335 | MK764357 |
| 21. | P. simitheae | KUMCC 17-0255 | Magnolia liliifera Blume | China | MW244023 | MW273930 | - |
| 22. | P. solicola | CBS 386.97 | – | Papua New Guinea | NR_161086 | MH554474 | MH554715 |
| 23. | P. taiwanensis | NTUCC 17-002.4 | Ixora sp. | Taiwan | MH026060 | MG816342 | MG816332 |
| 24. | P. vietnamensis | CBS 130710 | Khaya anthotheca (Welw.) C.DC. | Ghana | MH553998 | MH554425 | MH554667 |
| 25. | Neopestalotiopsis sp. | LC6288 | C. sinensis | China | KX895014 | KX895233 | KX895347 |
The bold indicates isolate obtained in this study,—not available.
Pathogenicity test against the various tea cultivars
Among all the isolates, NKT0P03 was randomly selected for pathogenicity assessment on 25 tea cultivars available with Tea Research Association, NBRRDC, Nagrakata, West Bengal through detached leaf technique as described by Chen et al.24 The detached leaf method is a non-destructive approach and is reliable to access pathogenicity25. In the laboratory bioassay, the isolate NKT0P03 was inoculated on the leaf surface through wound made by a sterilized sharp needle. Prior to conducting experiment, healthy third leaf of plucking shoot of each tea cultivar was collected from 10 to 15-year-old healthy tea bush from NBRRDC tea garden. During the collection of leaf samples from different tea cultivars, permissions were obtained from the concerned authorities of tea garden. The leaf surface of each cultivar was properly sterilized by immersing leaf in 1% sodium hypochlorite solution for 2 min. All the sterilized leaves were washed with double distilled sterilized water and aseptically air dried. Leaf of each cultivar was wounded five times on both sides of the major veined with the help of a sterile needle (0.4 mm diameter). The punctured leaf of each cultivar was kept separately in a Petri dish containing three layers of blotter papers and a 6-mm diameter mycelial disc from one-week old culture of the isolate NKT0P03 was kept over wounds using a sterilized forceps. For each cultivar, ten leaves per replicate were inoculated. Control sets (wounded) consisted of PDA discs without pathogen. The fungal disc was also inoculated on the non-wounded leaf to confirm that whether wounding is required for infection. Petri plates with leaves inoculated with pathogen and labelled with different tea cultivars were set in a CRD (complete randomized design) and incubated in a growth cabinet at 28°C with 12 h of white light and 12 h of darkness. After two days of inoculation, the fungal discs from each leaf were removed aseptically. To determine virulent nature of the pathogen, the lesion diameters produced by pathogen on each tea leaf were measured (mm) after 7 days of post-incubation. The pathogen was ranked as weak, moderate, and high virulent with respect to tea cultivar based on lesion diameters7. The percent disease incidence was computed by counting the number of symptomatic leaves over the total inoculated leaves. Further, the pathogen was re-isolated from the developed lesions and characterized through morpho-molecular analysis to fulfil Koch’s postulates26. The trial was repeated under same biological conditions, and each treatment contained three biological replicates. All methods in regards to plants were carried out in accordance with relevant guidelines. The relationship between different tea cultivars, lesion diameters, and percent disease incidence was studied by generating heat map using online software http://heatmapper.ca/27.
Evaluation of synthetic fungicides against the pathogen
Five fungicides consisting of four systemic (hexaconazole 5% EC, carbendazim 12% + mancozeb 63% WP (systemic + contact), valextra (hexaconazole 5% + validamycin 2.5% EC), propiconazole 25% EC) and one contact (copper oxychloride 50% WP) were evaluated against representative isolate NKT0P03 for antifungal activity using the artificial poison food technique of Grover and Moor28 with slight modifications. These synthetic fungicides were commercial products and composed of active ingredients and carrier materials, and were purchased from the authentic vendor of the respective company. Stock solution of technical grade formulation of each fungicide was prepared separately under aseptic conditions at 5 µg mL-1 and diluted in acetone. The PDA plates without fungicide and supplemented with acetone were kept as control sets. The test fungicides at concentrations (100 μg mL-1 for carbendazim + mancozeb and copper oxychloride, and 50 μl mL-1 for hexaconazole, propiconazole, and valextra) prescribed by Tea Board, Government of India29 were supplemented directly to the culture medium before pouring into the Petri plates. A mycelial disc (6 mm diam.) from a 6-day-old active culture of NKT0P03 was inoculated in the centres of the Petri plates containing solidified PDA medium supplemented with each fungicide, separately. The treated plates labelled with each fungicide along with control were organize in a CRD in three biological replicates and kept in an incubator at 28°C with 12 h duration of white light. The percent growth inhibition (PGI) of the pathogen by each fungicide was calculated after 6 days of post-incubation using the equation PGI = C-T/C × 100, where C and T were the mycelial diameters (mm) of isolate NKT0P03 in control and treatment plates, respectively.
Evaluation of microbial bioagents against pathogen
In this study, pre-characterized eight microbial biocontrol agents30,31, namely Trichoderma harzianum, T. reesei, T. hamatum, Pseudomonas guariconensis, Microbacterium barkeri, Bacillus subtilis, Bosea thiooxidans, and Lysinibacillus fusiformis isolated from tea rhizosphere and available with Mycology Lab, NBRRDC, Nagrakata were evaluated for antagonistic activity against representative isolate NKT0P03 following the dual culture test of Castillo et al.32. The mycelial discs (6 mm diam.) of each Trichoderma isolate and isolate NKT0P03 from 7-day-old cultures were placed 6 cm apart in parallel onto PDA medium in a Petri plate (9 cm diam.). For the bacterial antagonists, a loopful of a 48-h old active broth culture of each antagonist (with spore density 1 × 109 CFU mL-1) was inoculated on one edge of the PDA plate (without antibiotics) and mycelial disc (6 mm diameter) of pathogen on another side of the plate. The treated plates were labelled with each antagonistic microbe in triplicates. The treated and control (without antagonist) plates were set in a CRD and incubated for 7 days in a BOD incubator at 28°C under 12 h white light. The PGI of pathogenic isolate NKT0P03 by each antagonistic microbe was calculated with the formula:
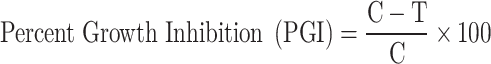 |
where C and T were the colony diameters (mm) of NKT0P03 in control and dual culture plates, respectively. Through microscopic study it was confirmed that whether microbial bioagents produced spores on NKT0P03 colonies or prohibited it from spore production33.
Data analysis
Data were analysed using the values of three replicates, including, daily growth rates and presented as mean ± Standard Error (SE). Each experiment was repeated and data were combined for analysis since the variances of repeated experiments did not differ significantly. To assess the assumptions of one-way analysis of variance (ANOVA) we performed normality test and Shapiro–Wilk test showed non-significant results at 1% significance level. Data were analysed through ANOVA using SAS 9.4 software and mean values were compared using Tukey’s Honestly Significant Difference (HSD) (p ≤ 0.05) test.
Results
In 2022, symptoms of gray blight were examined on tea cultivars planted in tea garden of Nagrakata, India. Pathogen was causing gray blight on both young and mature leaves. Symptom generally starts with minute dull-brown circular to irregular lesions (Fig. 1a,b) with concentric ring up to 2.8 cm diameter bright-gray with brown-margin when mature, or casing up to half of the leaf; dotted with acervuli. It eventually led to significant losses of maintenance foliage of tea bushes as the disease progressed (Fig. 2). In the advanced stage, necrotic spots grew larger and turned bright-gray (Fig. 1c). This gray blight symptom was in accordance with the previous studies7,24.
Fig. 1.
Symptoms developed by Pseudopestalotiopsis ampullacea and its cultural characteristics on agar plate, images (a) and (b) are showing initial necrotic lesions developed by the pathogen, (c) is advanced symptom, and its conidiomata (d) obverse (e) and reverse (f) colony morphology of representative isolate NKT0P03 on agar plate.
Fig. 2.
Tea garden (Nagrakata) showing leaf defoliation caused by gray blight (left; arrow indicates defoliated plants) and a tea plant infected with gray blight (right; arrow indicates gray blight symptomatic leaves).
Cultural and micromorphological characteristics of the pathogen
After 7 days of post-incubation all the five isolates showed similar cultural and morphological characters under the microscopic examinations. Colonies of each isolate of the pathogen on PDA grew up to 72.0 ± 2.5 mm in diameter with moderate-whitish arial growth (Fig. 1e), with lobate edge, and a slight-yellowish on the reverse side (Fig. 1f). The conidiomata produced on the surface of mycelial mat were black and assembled closely, spread throughout the culture after 10 days of post-incubation (Fig. 1d). Conidiomata pycnidial on PDA plate, globose or clavate, erumpent to semi-immersed, solitary or aggregated, black to dark-brown, exuding dark-brown to black-globose conidial masses.
In all the five isolates, conidiophores reduced to conidiogenous cells. Conidiogenous cell colourless, ampulliform, integrated or discrete, minutely verruculose or smooth, per-currently multiplying 1 to 3 times. Each isolate had five-celled fusoid, slightly curved to straight conidia divided by 4-septa which were darker than the rest of the cells with 22.4–29.5 μm (length) × 6.8–8.9 μm (width) (av. ± SD = 26.6 ± 2.3 × 7.4 ± 0.7 μm); basal cell 2.6–5.3 μm long (av. ± SD = 4.3 ± 0.9 μm), conic to obconic or hemispherical with truncate base, colourless or pale brown, thin–walled and rugose; three median cells 15.7–19.2 μm (av. ± SD = 17.5 ± 1.0 μm) long, doliiform, wall minutely verruculose, concolourous, septa darker than other conidial cells, conidia were constructed at the septum (2nd cell from base, 4.8– 8.2 μm long; 3rd cell 4.7–7.2 μm long; 4rth cell 4.2–7.1 μm long); apical cell colourless, 3.2–4.9 μm long, thin-walled, subcylindrical or obconic with a truncate base, slightly rugose; with 2 to 3 (mostly 3) unbranched, filiform tubular apical appendages, arising from the apical crest, 17.8–25.6 μm long; solitary basal appendage, unbranched, centric, slightly curved or straight or, tubular, 2.6–5.3 μm long, arise from the basal crests. The basal cells were transparent, obconic with a truncate base and the middle three cells were light to dark-brown (Fig. 3a–f).
Fig. 3.
Conidial morphology of P. ampullacea isolates NKT0P03 (a, b), NKT0P04 (c), NKT0P05 (d), NKT0P06 (e), and NKT0P07 (f) on PDA medium after 8 days incubation (scale bar = 10 µm).
Sequencing and phylogenetic analysis
The PCR and sequencing analysis produced ≥ 523 bp of ITS, ≥ 843 bp of tef-1 alpha, and ≥ 522 bp of tub-2 gene sequences for all the five isolates. The BLAST analysis also implied that the sequences of ITS, tef-1 alpha, and tub-2 genes of all the five isolates investigated in the present study shared 98% to 100% similarity with P. ampullacea available in GenBank database. The obtained identical sequences of these three genes of all the five isolates, i.e., NKT0P03, NKT0P04, NKT0P05, NKT0P06, and NKT0P07 were submitted to NCBI GenBank under the accession numbers given in Table 1. Based on the micromorphological and molecular analysis of ITS, tef-1 alpha and tub-2 sequences, each isolate was identified as P. ampullacea. To confirm that the overall tree topology of the individual dataset and the tree obtained from the combined alignment was similar, the topologies for the trees obtained for each gene were manually compared. The tree obtained through MP analyses was consistent with the tree obtained through the Bayesian analyses. The result of phylogenetic analysis is shown in Fig. 4. The dataset of MP consisted of 1888 characters (ITS 523, tef-1 alpha 843, and tub-2 522) of which 1615 were constant, 165 variable characters were parsimony-uninformative and 108 characters were parsimony-informative. The Bayesian analysis resulted in 2000 trees after 2,000,000 generations after the topological convergence. After removing 500 trees representing the burn-in phase, the remaining trees were used to calculate posterior probabilities for the majority rule consensus tree. The final alignment included 25 strains, representing eight clades in Pseudopestalotiopsis species. Phylogenetic support values varied between the two methods, with Bayesian posterior probabilities being higher than MP bootstrap support rates (Fig. 4). Multi-locus phylogenetic analyses with five isolates of P. ampullacea and other Pseudopestalotiopsis species retrieved from the NCBI GenBank database were separated into two clades. The isolates NKT0P03, NKT0P04, NKT0P05, NKT0P06, and NKT0P07 clustered with type isolate P. ampullacea LC6618 in one clade with high boot strap support of 100% and remaining Pseudopestalotiopsis isolates clustered in other clades with seven sister clades. Further, the sequences of Neopestalotiopsis sp. used as an out-group formed a single clade separating from all the isolates (Fig. 4).
Fig. 4.
Phylogenetic tree obtained using the MP method based on a combined datasets of ITS, tef-1 alpha, and tub-2 genes sequences of 25 strains showing the relationship of isolates NKT0P03, NKT0P04, NKT0P05, NKT0P06 & NKT0P07 with Pseudopestalotiopsis species, and with the Neopestalotiopsis species as the outgroup taxon. The numbers in the bootstrap test (1000 replicates) are indicated above and below the branches. Bootstrap support values for MP greater than 50% are shown left at the nodes and Bayesian posterior probabilities greater than or equal to 0.95 are indicated right at the nodes. Scale bar indicates expected number of changes per site.
Pathogenicity test against the various tea cultivars
In pathogenicity test, isolate P. ampullacea NKT0P03 was pathogenic on all tea cultivars except TV11 (Fig. 5), and 30.0% to 93.3% of the leaves inoculated with pathogen exhibited gray blight symptoms (Table 2, Fig. 6). Symptoms initiated two days after inoculation as small-brown or dark-spots around the wound on the leaf surface. Six days after inoculation, these spots enlarged to adjacent healthy tissues with diameter of the lesions in cultivars ranged from 5.8 to 26.5 mm (Table 2; and Fig. 5). At the end of the incubation, black acervuli were produced on the surface of the spots developed on the leaf surface. Although P. ampullacea NKT0P03 was pathogenic on leaves, it showed differences in virulence (Table 2, Fig. 6). Pathogen caused higher lesion diameters on the leaves of cultivars TV1, TV9, Naxalbari and Oxblood after seven days of post-inoculation, revealing that these cultivars were very susceptible to the P. ampullacea NKT0P03, while did not show pathogenicity on the cultivar TV11. Pathogen showed high to weak virulence on the other tea cultivars screened in the present study as demonstrated in Table 2. ANOVA analysis showed a significant (P < 0.001) difference between cultivars with higher, moderate, and weak virulence reactions. Symptoms produced by P. ampullacea NKT0P03 on leaf of various tea cultivars were similar to those examined in the field conditions. During the experiment, wounded and non-wounded leaves inoculated with respective agar disc and mycelial disc did not exhibit any symptoms after one week of post-incubation. The fungal colonies re-isolated from the developed spots revealed the identical micro-morphological features and 100% sequence similarities with the P. ampullacea NKT0P03 used for the inoculations. The recovery frequency of P. ampullacea NKT0P03 was 100% from the inoculated lesions, whereas for the control set a recovery of 0% was reported.
Fig. 5.
Typical symptoms developed by representative isolate P. ampullacea NKT0P03 on the leaves of various tea cultivars, cultivar TV11 is showing immune reaction.
Table 2.
Pathogenicity of the isolate P. ampullacea NKT0P03 on various tea cultivars after 7 days post inoculation.
| Tea cultivars | Scientific name | Origin | Lesion diameter in mm$ | Infected tea leaves (%)$ | Virulence* |
|---|---|---|---|---|---|
| TV1 | Camellia sinensis | India | 25.5 ± 2.8a | 86.7 ± 0.6a | High |
| TV2 | C. sinensis var. assamica | India | 22.5 ± 2.5a | 66.7 ± 1.2b | High |
| AV2 | C. sinensis | India | 18.5 ± 1.4b | 60.0 ± 0.5b | High |
| TV7 | C. sinensis | India | 11.7 ± 0.5c | 40.0 ± 1.3d | Weaker |
| TV8 | C. sinensis var. assamica | India | 9.8 ± 0.6d | 53.3 ± 1.6c | Moderate |
| TV9 | C. sinensis var. cambodiensis | India | 26.5 ± 0.8a | 93.3 ± 0.5a | High |
| TV11 | C. sinensis var. assamica | India | 00.0 ± 0.0 | 00.0 ± 0.0f. | Avirulent |
| TV12 | C. sinensis var. assamica | India | 16.0 ± 0.8b | 53.3 ± 0.8c | Moderate |
| TV13 | C. sinensis var. assamica | India | 24.0 ± 1.4a | 66.7 ± 1.3b | High |
| TV14 | C. sinensis var. assamica | India | 15.0 ± 0.0c | 53.3 ± 1.4c | Moderate |
| TV16 | C. sinensis var. assamica | India | 15.8 ± 0.2b | 56.7 ± 0.8c | Moderate |
| TV17 | C. sinensis var. assamica | India | 20.5 ± 2.3b | 66.7 ± 1.5b | High |
| TV18 | C. sinensis var. cambodiensis | India | 11.7 ± 0.5c | 46.7 ± 1.3d | Moderate |
| TV19 | C. sinensis var. cambodiensis | India | 12.5 ± 0.0c | 46.7 ± 2.3d | Moderate |
| TV20 | C. sinensis var. cambodiensis | India | 8.8 ± 2.1d | 33.3 ± 1.5e | Weaker |
| TV21 | C. sinensis var. assamica | India | 10.3 ± 0.9 | 53.3 ± 0.5c | Moderate |
| TV22 | C. sinensis var. cambodiensis | India | 7.2 ± 0.8d | 30.0 ± 0.5e | Weaker |
| TV23 | C. sinensis var. cambodiensis | India | 5.8 ± 1.9d | 40.0 ± 0.0e | Weaker |
| TV24 | C. sinensis var. cambodiensis | India | 6.3 ± 1.6d | 33.3 ± 0.5e | Weaker |
| TV25 | C. sinensis var. cambodiensis | India | 10.3 ± 0.2d | 46.7 ± 1.2d | Moderate |
| TV26 | C. sinensis var. cambodiensis | India | 11.7 ± 0.5c | 50.0 ± 1.3c | Moderate |
| TV29 | C. sinensis var. cambodiensis | India | 9.2 ± 0.6d | 33.3 ± 1.2e | Weaker |
| TV30 | C. sinensis var. cambodiensis | India | 9.2 ± 0.6d | 46.7 ± 1.3d | Moderate |
| Naxalbari | C. sinensis (Naxalbari) | India | 26.5 ± 0.8a | 73.3 ± 1.2a | High |
| Oxblood | C. sinensis (Oxblood) | India | 25.5 ± 2.8a | 66.7 ± 1.5b | High |
| SEm | 1.03 | 0.33 | |||
| F value | 52.32 | 33.59 | |||
| CV (< 0.01) | 12.3 | 11.13 | |||
| CD (< 0.01) | 2.91 | 0.95 |
aColumns with the same letter do not differ significantly per Tukey’s test (P ≤ 0.05). *Virulence of the isolate was evaluated by measuring the percent infected leaf 7 days after inoculation. $Values are mean of three replicates ± standard error. SEm Standard error mean, CV Coefficient of variation, CD Critical difference.
Fig. 6.

Heat map showing relationship between tea cultivars, lesion diameter (LD), and percent disease incidence (DI). The heatmap was generated using online software http://heatmapper.ca/24.
Efficacy of synthetic fungicides against P. ampullacea NKT0P03
The mycelial growth of P. ampullacea NKT0P03 inhibited by each fungicide was recorded 6 days after incubation under laboratory conditions. All the fungicides showed fungitoxicity against the pathogen and successfully inhibited mycelial growth. However, mycelial growth inhibited by systemic fungicides was significantly differed (P < 0.001) from contact fungicide, i.e., copper oxychloride in ANOVA analysis (DF = 5, F value = 86.54, CV (< 0.001) = 21.95). The mycelial inhibitions of P. ampullacea NKT0P03 due to carbendazim + mancozeb, hexaconazole, propiconazole, and valextra were 89.8%, 85.1%, 89.8%, and 86.8%, respectively. Moreover, copper oxychloride exhibited only 69.1% mycelial growth inhibition against P. ampullacea NKT0P03.
Efficacy of microbial bioagents against P. ampullacea NKT0P03
Among the microbial bioagents screened, fungal antagonists showed higher inhibitory activity than bacterial antagonists and differed significantly (P < 0.001) in ANOVA analysis. The percent mycelial inhibitions of P. ampullacea NKT0P03 due to T. harzianum, T. reesei, and T. hamatum were 70.04%, 84.2%, and 77.06%, respectively. The inhibitory activities of bacterial antagonists were ranged between 18.98% and 69.23% are reported in Table 3.
Table 3.
Inhibitory activity of microbial bioagents on mycelial growth rates of P. ampullacea NKT0P03.
| Microbial isolates | Origin | Source | GenBank accession number | Inhibitory activity (%) |
|---|---|---|---|---|
| Trichoderma harzianum | Tindharia TG | Soil | OQ703058 (ITS) and PP496405 (TEF) | 70.05 ± 2.1b |
| T. reesei | Pathrajhora TG | Soil | ON138957 (ITS) and ON695921 (TEF), | 84.2 ± 0.7a |
| T. hamatum | Aryaman TG | Soil | OQ695467 (ITS) | 77.06 ± 1.2a |
| Pseudomonas guariconensis | Selim hill TG | Soil | OQ179938 (16S rRNA) | 18.9 ± 2.6e |
| Bosea thiooxidans | Kurti TG | Soil | ON627835 (16S rRNA) | 53.8 ± 3.5c |
| Lysinibacillus fusiformis | Nagrakata TG | Soil | ON130945 (16S rRNA) | 38.6 ± 3.3d |
| Bacillus subtilis | Okayti TG | Soil | ON128449 (16S rRNA) | 69.23 ± 1.7b |
| Microbacterium barkeri | Patharjhora TG | Soil | OR251496 (16S rRNA) | 66.59 ± 1.6b |
| SEm | 1.64 | |||
| F value | 176.47 | |||
| CV (< 0.01) | 8.03 | |||
| CD (< 0.01) | 4.91 |
aColumn with the same letter do not differ significantly per Tukey’s test (P ≤ 0.05). $Values are mean of three replicates ± standard error. SEm Standard error mean, CV Coefficient of variation, CD Critical difference.
Discussion
Knowingly, more than 20 Pestalotiopsis-like species belong to the genera Pseudopestalotiopsis, Neopestalotiopsis, and Pestalotiopsis cause gray blight on tea plants34. In the present study, P. ampullacea was micromorphologically and phylogenetically different from other species of the genus Pseudopestalotiopsis. It was closely associated to P. chinensis and P. indica, but distinct in producing petite apical appendages (17.8–25.6 μm vs. 30–40 μm in P. indica, 24–41 μm in P. chinensis) and further from P. indica by its smaller conidia (22.4–29.5 μm vs. 31.5–37μm)13. Sporulation of P. ampullacea was higher on the PDA medium, with many conidiomata produced after 10 days of post-incubation.
In a previous study, three novel species of Pseudopestalotiopsis, such as P. ampullacea, P. camelliae-sinensis F. Liu & L. Cai, and P. chinensis F. Liu & L. Cai inciting gray blight on tea plants were described in China13. Pseudopestalotiopsis camelliae-sinensis has also been reported in our previous study4. Till date, worldwide species of Pseudopestalotiopsis causing gray blight on tea include P. ampullacea, P. camelliae-sinensis, P. chinensis, P. theae, P. indica, P. thailandica, P. ignota, P. annellata, and P. curvatispora4,7,13,14,35. Although, P. camelliae-sinensis, P. theae, P. thailandica, and P. curvatispora have been reported from India4,35, however till date, there is no record on occurrence of P. ampullacea on tea host in India. In this study, five isolates of P. ampullacea isolated from gray blight symptomatic tea leaves were characterized, and this is the first report of P. ampullacea inciting gray blight disease on C. sinensis in India. This species formed conidia containing five cells, including three concolorous pigmented median cells, and two-to-three apical appendages ascending from the apical cells, thus accommodating under the genus Pseudopestalotiopsis based on the morphological features, supporting the classification as proposed by Maharachchikumbura et al.12. Based on morphological characteristics, this species differed from any previously reported Pseudopestalotiopsis species. However, it matched with the P. ampullacea first time described by Liu et al.13. Furthermore, multi-locus sequence datasets phylogenetic analysis also supported the results of morphological analysis. The phylogenetic tree comprises of clades containing known species and NKT0P03, NKT0P04, NKT0P05, NKT0P06, and NKT0P07 isolates of P. ampullacea identified in our study. Species within each clade were well supported by boot strap value.
In the present study, the isolate P. ampullacea NKT0P03 showed pathogenicity to wounded tea leaves; conversely, symptoms were not typically encountered on unwounded leaves. Earlier researchers also reported similar results7,36, signifying that wounding may be essential for the gray blight symptom development. Pseudopestalotiopsis ampullacea has also been reported as a gray blight causal agent on Camellia chrysantha (Hu) Tuyama37 and Mangifera indica L.16 in China. The virulence of P. ampullacea NKT0P03 isolate was also varied to some extent on various tea cultivars, which corroborated with the findings of earlier investigators4,7,24,36, they reported variability in pathogenicity of Pestalotiopsis-like species on different tea cultivars. This suggests that pathogenicity may be more dependent on the virulent nature of pathogen or susceptibility of host species. In order to understand the mechanism underlying variability in the pathogenicity of P. ampullacea towards different tea cultivars, further research is required using recent omics approaches. Additionally, cultivar TV11 showed less susceptibility against P. ampullacea NKT0P03, while other tea cultivars showed moderate to high susceptibility against pathogen. The variation in pathogenicity on various tea cultivars may be due to variation in phytochemicals or resistant genes found in tea cultivars1, however, further study is warranted to know which gene(s) is responsible for resistance or susceptible in tea cultivars against P. ampullacea. Further, this study segregates the tea cultivars less susceptible to this species, which after larger scale evaluation against other Pestalotiopsis-like species can be used in the tea breeding program to develop gray blight-resistant cultivars.
Fungicides and microbial bioagents are the most commonly used methods to mitigate gray blight31,32. In order to search the management solution for this species, various synthetic fungicides and microbial bioagents were evaluated under in vitro conditions. Screening results revealed that carbendazim + mancozeb, hexaconazole, propiconazole, and valextra were effective fungicides against P. ampullacea NKT0P03. As far as microbial bioagents are concerned, T. harzianum, T. reesei, T. hamatum, M. barkeri, and B. subtilis showed potential antagonistic activity. To date, however, T. reesei, T. hamatum, M. barkeri, and B. subtilis have not been used for gray blight management. Further, worldwide these fungicides38,39 and microbial bioagents,40–43 were widely used for the management of diseases caused by necrotrophic pathogens in a wide variety of arable and plantation crops. Besides, in our previous study T. reesei has also been found efficient against gray blight caused by P. theae and Fusarium dieback in tea31,44. Thus, the efficacy of fungicides, microbial bioagents, and the pathogen’s susceptibility to certain tea cultivars are significant information for sustainable gray blight management associated with P. ampullacea after their field evaluations.
Acknowledgements
Authors are thankful to Deputy Director, Tea Research Association, North Bengal Regional R & D Center, Nagrakata, West Bengal for providing necessary lab facilities.
Author contributions
AKP conceived the study and original draft preparation; SY conducted the experiments, morphological examination, molecular biology work after significant input from AKP; MH conducted the phylogenetic reconstructions and heat map; HKS assisted SY in experiments and microscopical examination; all authors discussed the findings and contributed to the final draft.
Funding
This study was supported by Science and Engineering Research Board, Government of India, New Delhi, India via grant number SRG/2021/00299.
Data availability
Sequence data have been deposited in GenBank with accession numbers OQ889166, PP218054, PP218053, OQ883952, PP230444, PP230445, PP426614, PQ558148, PQ569084, PQ567071, PQ567072, PQ567073, PQ567074, PQ567075, and PQ572174.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
During the collection of leaves and disease samples from C. sinensis, permissions were obtained from the concerned authorities of tea garden.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pandey, A. K., Sinniah, G., Babu, A. & Tanti, A. How the global tea industry copes up with fungal diseases-challenges and opportunities. Plant Dis.105, 1868–1879 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Wambulwa, M. C., Meegahakumbura, M. K., Kamunya, S. & Wachira, F. N. From the wild to the cup: Tracking footprints of the tea species in time and space. Front Nutr8, 706770 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FAO (2017) World tea production in 2017; Crops/World Regions/Production Quantity from pick lists. Food and Agriculture Organization of the United Nations, Statistics Division (FAOSTAT). Archived from the original on 11 April 2020.
- 4.Pandey, A. K. et al. Characterization and identification of fungicide insensitive Pestalotiopsis-like species pathogenic to tea crop in India. World J. Microbiol. Biotechnol.39, 34 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Joshi, S. D., Sanjay, R., Baby, U. I. & Mandal, A. K. A. Molecular characterization of Pestalotiopsis spp. associated with tea (Camellia sinensis) in southern India using RAPD and ISSR markers. Indian J. Biotechnol.8, 377–383 (2009). [Google Scholar]
- 6.Horikawa, T. Yield loss of new tea shoots due to grey blight caused by Pestalotia longiseta Spegazzini. Bull. Shizuoka Tea Exp. Stn.12, 1–8 (1986). [Google Scholar]
- 7.Wang, S., Mi, X., Wu, L., Zhang, L. & Wei, C. Characterization and pathogenicity of Pestalotiopsis-like species associated with gray blight disease on Camellia sinensis in Anhui Province, China. Plant Dis.103, 2786–2797 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Wang, Q. et al. Neopestalotiopsis longiappendiculata as the agent of grey blight disease of Camellia spp.. J. Phytopathol.170, 770–777 (2022). [Google Scholar]
- 9.Chen, Y. et al. Characterization of Pestalotiopsis chamaeropis causing gray blight disease on tea leaves (Camellia sinensis) in Chongqing. China Can. J. Plant. Pathol.43, 413–420 (2020). [Google Scholar]
- 10.Maharachchikumbura, S. S. N., Guo, L. D., Chukeatirote, E., Bahkali, A. H. & Hyde, K. D. Pestalotiopsis—Morphology, phylogeny, biochemistry and diversity. Fungal Divers50, 167–187 (2011). [Google Scholar]
- 11.Keith, L., Ko, W. H., & Sato, D. M. Identification guide for diseases of tea (Camellia sinensis). Plant Disease series PD-33. University of Hawaii, Honolulu, HI (2006).
- 12.Maharachchikumbura, S. S. N., Hyde, K. D., Groenewald, J. Z., Xu, J. & Crous, P. W. Pestalotiopsis revisited. Stud. Mycol.79, 121–186 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, F., Hou, L., Raza, M. & Cai, L. Pestalotiopsis and allied genera from Camellia, with description of 11 new species from China. Sci Rep7, 866 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai, I. et al. Cryptic diversity, molecular systematics, and pathogenicity of genus Pestalotiopsis and allied genera causing gray blight disease of tea in Taiwan, with a description of a new Pseudopestalotiopsis species. Plant Dis.105, 425–443 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Lateef, A. A., Sepiah, M. & Bolhassan, M. H. Description of Pseudopestalotiopsis kubahensis sp. nov., a new species of microfungi from Kubah National Park, Sarawak, Malaysia. Curr. Res. Environ. Appl. Mycol.5, 376–381 (2015). [Google Scholar]
- 16.Shu, J. et al. Identification and characterization of Pestalotioid fungi causing leaf spots on mango in southern China. Plant Dis.104, 1207–1213 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Rehner, S. A. & Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia97, 84–98 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Glass, N. L. & Donaldson, G. C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol.61, 1323–1330 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series, Vol. 41 95–98 (1999).
- 20.Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol.10, 512–526 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics19, 1572–1574 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Nylander, J. A. A. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre (Uppsala University, 2004). [Google Scholar]
- 23.Stöver, B. C. & Müller, K. F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform.11, 7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen, Y. J. et al. Pestalotiopsis-like species causing gray blight disease on Camellia sinensis in China. Plant Dis.102, 98–106 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Amponsah, N. T., Jones, E. E., Ridgway, H. J. & Jaspers, M. V. Identification, potential inoculum sources and pathogenicity of botryosphaeriaceous species associated with grapevine dieback disease in New Zealand. Eur. J. Pl Pathol.131, 467–482 (2011). [Google Scholar]
- 26.Solarte, F. A., Muñoz, C. G., Maharachchikumbura, S. & Álvarez, E. Diversity of Neopestalotiopsis and Pestalotiopsis spp., causal agents of guava scab in Colombia. Plant Dis.102, 49–59 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Sasha, B., David, A., Ana, M., Yongjie, L., Jason, R.G., Adam, M., & David, S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. (epub ahead of print) (2016). 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed]
- 28.Grover, R. K. & Moore, J. D. Toximetric studies of fungicides against brown rot organism-Sclerotinia fructicola and S. laxa. Phytopathol52, 876–880 (1962). [Google Scholar]
- 29.Plant Protection Code, Policy on usage of Plant Protection Formulations in Tea Plantations of India, Version 14, Tea Board of India. Online Publication PPC_Version_14_pdf3115.pdf (teaboard.gov.in) (2022).
- 30.Pandey, A. K., Dinesh, K., Yadav, S., Sharma, H. & Babu, A. Functional traits and phylogenetic analysis of top-soil inhabiting rhizobacteria associated with tea rhizospheres in North Bengal, India. Curr. Res. Microb. Sci.5, 100200 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey, A. K., Samota, M. K., Tanti, A. J. & Babu, A. Trichoderma reesei induces defense-related biochemical markers associated with resistance to Fusarium dieback in tea crop. Biol. Contr.180, 105200 (2023). [Google Scholar]
- 32.Castillo, F. D. H. et al. In vitro antagonist action of Trichoderma strains against Sclerotinia sclerotiorum and Sclerotium cepivorum. Am. J. Agric. Biol. Sci.6, 410–417 (2011). [Google Scholar]
- 33.Campanile, G., Ruscelli, A. & Luisi, N. Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. Eur. J. Plant Pathol.117, 237–246 (2007). [Google Scholar]
- 34.Maharachchikumbura, S. S. N., Guo, L. D., Liu, Z. Y. & Hyde, K. D. Pseudopestalotiopsis ignota and Ps. camelliae spp. nov. associated with gray blight disease of tea in China. Mycol. Prog.15, 1–7 (2016). [Google Scholar]
- 35.Bora, P., Bora, L. C., Bhuyan, R. P., Hashem, A. & Abd-Allah, E. F. Bioagent consortia assisted suppression in grey blight disease with enhanced leaf nutrients and biochemical properties of tea (Camellia sinensis). Biol. Contr.170, 104907 (2022). [Google Scholar]
- 36.Chen, Y. et al. Characterization of Pestalotiopsis chamaeropis causing gray blight disease on tea leaves (Camellia sinensis) in Chongqing, China. Can. J. Plant. Pathol.43, 413–420 (2021). [Google Scholar]
- 37.Zhao, J. et al. First report of Pseudopestalotiopsis and Neopestalotiopsis species causing leaf spot of Camellia chrysantha in China. Plant Dis.104, 3071 (2020). [Google Scholar]
- 38.William, V. M., Wilson, A. N., Fortunus, A. K., Stanslaus, A. L. & Donatha, D. T. Characterization and chemical management of cashew Fusarium wilt disease caused by Fusarium oxysporum in Tanzania. Crop Prot.139, 105379 (2021). [Google Scholar]
- 39.Xu, C. et al. Identification, characterization and chemical management of Alternaria alternata causing blackcurrant leaf spot in China. J. Appl. Microbiol.342, 124–135 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Abbas, A. et al. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. Comptes Rendus Biol.342, 124–135 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Karan, R., Renganathan, P. & Balabaskar, P. Integrated use of propiconazole, Pseudomonas fluorescens and Bacillus subtilis for the management of rice brown leaf spot. Ann. Plant Soil Res.24, 97–100 (2022). [Google Scholar]
- 42.Suárez-Estrella, F. et al. Seed priming by application of Microbacterium spp. strains for control of Botrytis cinerea and growth promotion of lettuce plants. Sci. Hort.313, 111901 (2023). [Google Scholar]
- 43.Swehla, A., Pandey, A. K. & Nair, R. M. Bioactivity of Trichoderma harzianum isolates against fungal root rot pathogens with special reference to Macrophomina phaseolina causing dry root rot of mungbean. Indian Phytopath73, 787–792 (2020). [Google Scholar]
- 44.Pandey, A. K., Kumar, A., Samota, A. & Tanti, A. Trichoderma reesei as an elicitor triggers defense responses in tea plant and delays gray blight symptoms. Pesticide Biochem. Physiol.188, 105279 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data have been deposited in GenBank with accession numbers OQ889166, PP218054, PP218053, OQ883952, PP230444, PP230445, PP426614, PQ558148, PQ569084, PQ567071, PQ567072, PQ567073, PQ567074, PQ567075, and PQ572174.







